Abstract
Background: One third of epileptic patients are resistant to several anti-epileptic drugs (AED). P-glycoprotein (P-gp) is an efflux transporter encoded by ATP-binding cassette subfamily B member 1 (ABCB1) gene that excludes drugs from the cells and plays a significant role in AEDs resistance. Over-expression of P-gp could be a result of polymorphisms in ABCB1 gene. We studied the association of T129C and T1236C single-nucleotide polymorphisms (SNP) of ABCB1 gene with drug-resistant epilepsy in Iranian epileptics. Methods: DNA samples were obtained from 200 healthy controls and 332 epileptic patients, of whom 200 were drug responsive and 132 drug resistant. The frequencies of the genotypes of the two SNP were determined by polymerase chain reaction followed by restriction fragment length polymorphism. Results: No significant association was found between T129C and T1236C genotypes and drug-resistant epilepsy neither in adults nor in children. However, the risk of drug resistance was higher in female patients with 1236CC (P = 0.02) or CT (P = 0.008) genotype than in those with TT genotype. The risk of drug resistance was also higher in patients with symptomatic epilepsies with 1236CC (P = 0.02) or CT (P = 0.004) genotype than in those with TT genotype. The risk of drug resistance was lower in patients with idiopathic epilepsies with 129TT genotype (P = 0.001) than in those with CT genotype. Conclusion: Our results indicate that T1236C polymorphism is associated with drug resistance in Iranian female epileptic patients. Replication studies with large sample sizes are needed to confirm our results.
Key Words: ATP-binding cassette subfamily B member 1 (ABCB1), Drug-resistant epilepsy, Single nucleotide polymorphism
INTRODUCTION
Epilepsy is the second most common neurological disorder after stroke [1]. Although new anti-epileptic drugs (AED) were made available since the late 1980s, refractoriness to treatment is still an important issue in epilepsy care. Only two-thirds of patients are seizure free under pharmacological treatment [2].
It has been hypothesized that over-expression of drug efflux transporters at the blood-brain barrier, by reducing AED accumulation in the seizure foci, contributes to drug resistance in epilepsy [3, 4]. P-glycoprotein (P-gp) is an energy-dependent efflux pump that expels several AED [3, 5-7]. This protein is the product of ATP-binding cassette subfamily B member1 (ABCB1), also known as the multidrug resistance 1 gene [3]. It has been suggested that increased brain expression of efflux transporters such as P-gp could be a result of genetic factors such as polymorphisms in ABCB1 gene [8]. Association between single nucleotide polymorphisms (SNP) in the ABCB1 gene and refractory epilepsy has been studied by many researchers at different nucleotide positions such as T129C and T1236C in exon 12, G2677T in exon 21 and C3435T in exon 27 [9-15]. However, the results have not been identical as yet to confirm the association. Therefore, the association of SNP in ABCB1 gene with drug-resistant epilepsy needs to be investigated in different ethnic groups, because there may be significant variations in gene frequencies that affect ethnic populations differently.
Moreover, in drug-resistant epilepsy, multiple aspects including clinical factors (etiology, early age at seizure onset, type of epileptic syndrome and seizure, and structural brain abnormalities or lesions) should be considered [16-19]. In most studies performed on ABCB1 polymorphism and drug-resistant epilepsy in addition to variation in phenotype definition (definition of resistance and response to AED), patients with multiple types of epilepsy consuming multiple AED have been enrolled in the studies. The multiplicity of factors involved may affect the results and lead to distorted/various findings [20]. Hypothetically, if drug-resistant epileptics are classified to subgroups with similar specifications (e.g. clinical), influencing drug resistance, and the association between polymorphisms and drug-resistant epilepsy is analyzed in the similar subgroups, this may lead to more precise and uniform results.
According to the report of Iran Epilepsy Association, there have been about 80,000 registered epileptic patients in Iran until the end of 2007, of whom 25,000 patients are resistant to drug therapy. The possible association of ABCB1 SNP with drug-resistant epilepsy in Iranian population has not been studied yet. If any association exists, it may help the early diagnosis of drug-resistant epileptics, increase the success of therapy and reduce the cost imposed on patients and health care system. We have recently found that Iranian patients with AED-resistant epilepsy are more likely to have the CC genotype at the ABCB1 C3435T polymorphism (article under review). Therefore, this study proceeded to the association of two other most investigated SNP of the ABCB1 gene, T129C (rs2188524) and T1236C (rs1128503), with drug-resistant epilepsy in Iranian epileptic patients.
MATERIALS AND METHODS
Subjects. The study was approved by the Ethics Committee of Pasteur Institute of Iran and conforms to the Declaration of Helsinki. A total of 132 patients with drug-resistant seizures and 200 patients with drug-responsive seizures were enrolled in this study. All subjects were Iranian and all patients who were received AED treatment for at least a year were recruited from epilepsy clinic of Loghman Hospital, Shahid Beheshti University of Medical Sciences (Tehran, Iran). The control subjects were recruited from Pasture Institute of Iran. All subjects participated in this study voluntarily. Written informed consents were obtained from all subjects following a complete description of the study. A 5-ml venous blood sample was taken for DNA extraction and genotyping. Subject information and genotype data were identified by a code to ensure that the genotyping was done blindly.
Phenotyping. Three phenotypic groups were defined as drug-responsive epileptics, drug-resistant epileptics and normal (non-epileptic) subjects. Patients who had not experienced any seizure for at least a year up to the date of enrollment, and received a stable dose of an AED were considered drug responsive [2]. Patients who had at least one seizure per month or 10 seizures over the previous year despite treatment with two or more AED at the maximally tolerated doses and therapeutic serum drug concentrations were considered drug resistant [10, 13]. People without present or past history of epilepsy were considered as normal.
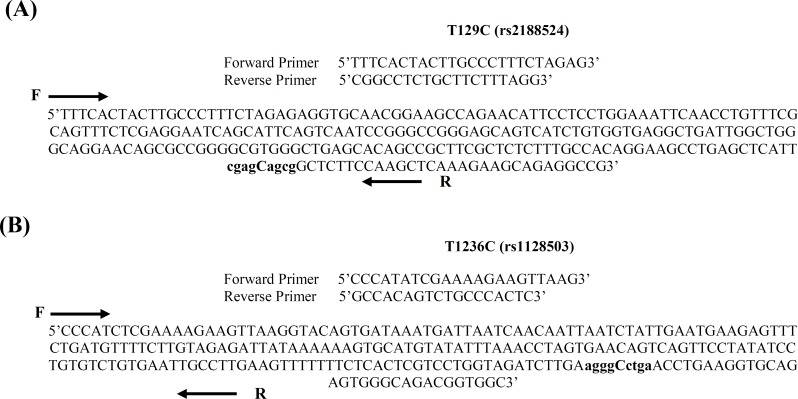
Genotyping. Genomic DNA was extracted from peripheral blood leukocytes using standard salting out extraction method [21] and diluted to a final concentration of 20 ng/µl with 1 × TE buffer (pH 7.5). PCR followed by restriction fragment length polymorphism (RFLP) analysis was used to obtain the genotypes of the two groups. Genomic DNA (200 ng) was amplified in a 25-µl reaction containing 10 pmol of forward and reverse primers (listed in Table 1), 1 × PCR buffer (10 mM Tris hydrochloride, pH 8.5, 50 mM potassium chloride), 0.2 mM deoxynucleotide triphosphates, 1.5 mM magnesium chloride, and 1 U of Taq DNA polymerase (Cinnagen, Iran). The PCR conditions were as follows: an initial denaturation step at 94°C for 5 min, followed by 35 cycles of denaturation at 94°C for 40 s, annealing for 40 s at 58°C for T129C and 62°C for T1236C, an extension step at 72°C for 30 s, and a final extension step at 72°C for 5 min. PCR products were digested with respective restriction endonucleases at 37°C for 16 h in enzyme buffers. Digested PCR products were run on 2.5% agarose gel and the bands were visualized under ultraviolet light after staining with ethidium bromide. The RFLP genotyping methods were verified by a 100% concordance rate after sequencing eight PCR products of each genotype. The SNP genotyping method including primer sequences, PCR products length, restriction endonucleases and genotype determination on the gel is summarized in Table 1. Primer sequences and SNP at nucleotide positions T129C (A) and T1236C (B) in ABCB1 gene are demonstrated in Figure 1.
Table 1.
Primers and restriction endonucleases used for SNP genotyping
| SNP | Primer sequences | PCR product (bp) | Restriction endonucleases | Genotype determination on the gel |
|---|---|---|---|---|
| T129C (rs2188524) | Forward: 5'TTTCaCTACTTGCCCTTTCTAGAG3' Reverse: 5'CGGCCTCTGCTTCTTTGAG3' |
258 | MspA1I | TT: 258 bp TC: 258 bp/226 bp/36 bp CC: 226 bp/32 bp |
| T1236C (rs1128503) |
Forward: 5'GCCACaGTCTGCCCACTC3' Reverse: 5'CCCATaTCGAAAAGAAATTAAG3 |
240 | HaeIII | TT: 240 bp TC: 240 bp/204 bp/36 bp CC: 204 bp/36 bp |
Data analysis. To evaluate the influence of patient age, patients were divided into children (≤12 years) and adolescent-adults (>12 years) subgroups. To evaluate the influence of the etiology of epilepsy, patients were divided into idiopathic and symptomatic epilepsy subgroups. Seizures were classified as generalized tonic-clonic, partial, and complex partial. The SPSS for Windows version 11.5 software was used for statistical analysis. The Hardy-Weinberg equilibrium (HWE) for genotype frequency distributions was verified using the chi-square goodness-of-fit test. The differences in genotype frequencies in different subgroups between drug-responsive and drug-resistant patients were tested by binary logistic regression. The Unpaired Student's t-test was used to compare the age for two groups. The level of significance for all statistical tests was 0.05.
Fig. 1.
Primer sequences and single nucleotide polymorphisms at nucleotide positions T129C (A) and T1236C (B) in ABCB1 gene
Table 2.
Demographic characteristics of epileptic patients
| Subgroups | Categories | Drug-responsive patients |
Drug-resistant
patients |
OR
(95% CI ) |
P |
|---|---|---|---|---|---|
| Patient age in years (Mean ± SD) | 27 ± 13 | 28.8 ± 11 | - | 0.19 | |
| Patient age groups | <12 years >12 years |
10 (5%) 190 (95%) |
4 (3%) 128 (97%) |
1.68 (0.52-5.48) 1 |
0.39 |
| Gender | Male Female |
96 (48%) 104 (52%) |
79 (59.8%) 53 (40.2%) |
1.62 (1.03-2.52) 1 |
0.04 |
| Type of seizure | Complex partial | 1 (0.5%) | 0 | ||
| Generalized tonic-clonic | 199 (99.5%) | 0 | |||
| Generalized tonic-clonic + complex partial | 0 | 118 (89.4%) | |||
| Generalized tonic-clonic + partial | 0 | 14 (10.6%) | |||
| Type of epilepsy | Complex partial | 122 (61%) | 126 (95.5%) | 13.08 (5.49-31.16) | <0.001 |
| Generalized tonic-clonic | 76 (38%) | 6 (4.5%) | 1 | ||
| Juvenile myoclonic | 2 (1%) | 0 | |||
| Etiology of epilepsy | Idiopathic Symptomatic |
78 (59%) 122 (61%) |
6 (4.5%) 126 (95.5%) |
13.43 (5.64-31.92) 1 |
<0.001 |
| Number of dministered antiepileptic drugs | 1 2 3 4 5 |
191 (99.5%) 9 (0.5%) 0 0 0 |
0 18 (13.6%) 88 (66.6%) 25 (18.9%) 1 (0.8%) |
||
OR, odds ratios; CI, confidence interva
RESULTS
Demographic data. Demographic characteristics of the epileptic patients are demonstrated in Table 2. There is no significant difference between drug-responsive and drug-resistant patients regarding the age. However, frequency of male subjects with drug-resistant epilepsy was significantly more than drug-resistant females. Significant difference between drug-resistant and drug-responsive patients was found in type of epilepsy (P<0.001). Drug-resistant patients had a larger proportion of patients with symptomatic epilepsy (95.5%) compared to drug-responsive patients, in which 61% of the patients had symptomatic epilepsy (P<0.001). The AED which had been administered to the patients were phenytoin, phenobarbital, primidone, carbama-zepine, valproate, oxcarbazepine, levetiracetam, lamotrigine, clonazepam and topiramate. Nine out of 200 drug-responsive patients were treated by two AED and 191 patients by one AED. Drug-resistant patients received 2-4 above-mentioned AED at the maximum tolerated doses and only one patient was treated by 5 AED.
Analysis of genotype frequencies. The PCR-RFLP method identified wild type heterozygous or homozygous variation at the two polymorphic sites (Figs. 2 and 3). Genotype success rate was 100% for SNP. Our results indicate that both SNP are polymorphic in the Iranian population (Tables 3 and 4). The genotype frequencies of both SNP in both normal subjects and epileptic patients were followed by HWE. The genotype frequency of both T129C and T1236C polymorphisms did not differ significantly between drug-responsive and drug-resistant patients for CC, CT or TT genotypes (Table 4). When patients were stratified by patient age, no significant association was observed between ABCB1-T129C and -T1236C polymorphisms and drug resistance in adults (Table 4). In children, sample size was too small to be able to analyze the genotype frequencies. However, when patients were stratified by gender, significant association was observed between ABCB1-T1236C polymorphism and drug resistance in females (Table 5). The odds ratios indicate a higher risk of drug resistance in the female patients with the CC or CT genotypes than in those with the TT genotype. The genotype frequency of T129C polymorphism did not differ significantly between drug-responsive and drug-resistant male or female patients for CC, CT or TT genotypes (Table 5). With regard to epilepsy etiology, a significant association was found between ABCB1-1236C>T polymorphism and drug resistance in patients with symptomatic epilepsy (Table 6). The odds ratios indicate a higher risk of drug resistance in the patients with the CC or CT genotypes than in those with the TT genotype. Moreover, a significant association was found between ABCB1-129T>C polymorphism and drug response in patients with idiopathic epilepsy but not in those with symptomatic epilepsy (Table 6). The odds ratios indicate a higher risk of drug response in the patients with the TT genotype than in those with the CT genotype.
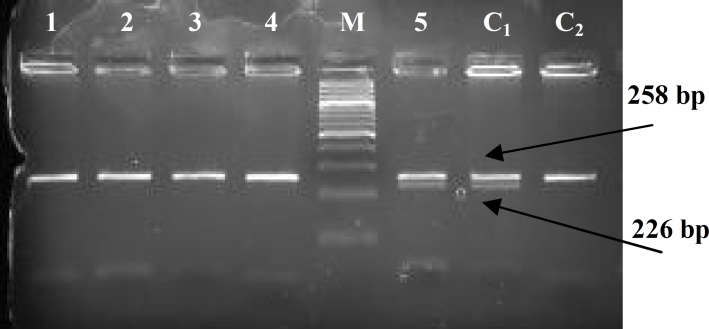
Fig. 2.
Genotype determination of epileptic patients for ABCB1-T129C polymorphism based on digestion of a 258-bp amplified DNA fragment with MspA1I, analyzed by 2.5% gel agarose electrophoresis. Lanes 1-4, fragments with no digestion site (TT genotype); lane 5, digestion resulted in three DNA fragments of 258 bp, 226 bp and 32 bp (TC genotype); M, 100 bp DNA ladder; C1 and C2, sequenced fragments with and without site for digestion, as positive controls.
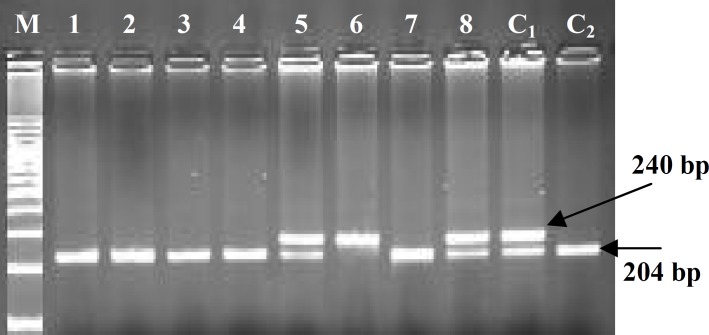
Fig. 3.
Genotype determination of epileptic patients for ABCB1-T1236C polymorphism based on digestion of a 240 bp amplified DNA fragment with HaeIII, analyzed by 2.5% agarose gel electrophoresis. Lanes 1-4 and 7, digestion resulted in two DNA fragments of 204 bp and 36 bp (CC genotype); lanes 5 and 8, digestion resulted in three DNA fragments of 240 bp, 204 bp and 36 bp (TC genotype); lane 6, fragment with no digestion site (TT genotype); M, 100 bp DNA ladder; C1 and C2 sequenced fragments with site for digestion in one or both alleles, as positive controls.
Table 3.
Genotype and allele frequencies at nucleotide positions T129C and T1236C of ABCB1 gene in normal non-epileptic subjects.
| Nucleotide position |
Genotype
frequency |
Allele
frequency |
|---|---|---|
| ABCB1-T129C (rs2188524) | CC 0 (0%) CT 11 (5.5%) TT 189 (94.5%) |
C 11 (2.75%) T 289 (97.25%) |
| ABCB1-T1236C (rs1128503) | CC 43 (21.5%) CT 95 (47.5) TT 62 (31.00%) |
T 219 (54.7%) C 181 (45.25%) |
Table 4.
Genotype frequencies and drug-resistance odds ratio for ABCB1-T129C and -T1236C polymorphisms in Iranian epileptic patients.
| P |
OR
(95% CI) |
Drug-resistant
patients |
Drug-responsive
patients |
Genotype | Subgroups of age |
|---|---|---|---|---|---|
| 0.75 | 1.21 (0.36-4.06) 1 |
0 5 (3.75%) 127 (96.25%) |
0 7 (3.5%) 193 (96.5%) |
CC CT TT |
ABCB1-T129C (n = 332) |
| 0.75 | 1.21 (0.36-4.06) 1 |
0 5 (3.84%) 125 (96.15%) |
0 6 (3.19%) 182 (96.8%) |
CC CT TT |
Adults (n = 318) |
| small sample size | 0 0 4 (100%) |
0 1 (10%) 9 (90%) |
CC CT TT |
Children (n = 14) |
|
| 0.11 0.19 |
1.73 (0.88-3.41) 1.39 (0.85-3.41) 1 |
24 (18.18%) 65 (49.24%) 43 (32.57%) |
28 (14%) 87 (43.5%) 85 (42.5%) |
CC CT TT |
ABCB1-T1236C (n = 332) |
| 0.11 0.19 |
1.73 (0.88-3.41) 1.39 (0.85-3.41) 1 |
23 (17.96%) 62 (48.43%) 43 (33.59%) |
25 (13.15%) 84 (44.21%) 81 (42.63%) |
CC CT TT |
Adults (n = 318) |
| small sample size | 1 (25%) 3 (75%) 0 |
3 (30%) 3 (30%) 4 (40%) |
CC CT TT |
Children (n = 14) |
Table 5.
Genotype frequencies of ABCB1-T129C and -T1236C polymorphisms and odds ratios in male and female epileptic patients.
| P |
OR
(95% CI) |
Drug-resistant
patients |
Drug-responsive
patients |
Genotype | Subgroups of gender |
|---|---|---|---|---|---|
| ABCB1-T129C (n = 332) | |||||
| 0.78 | 1.23 (0.30-5.07) 1 |
0 4 (5.06%) 75 (94.93%) |
0 4 (4.16%) 92(95.83%) |
CC CT TT |
Male (n = 175) |
| 0.65 | 0.71 (0.07-6.38) 1 |
0 1 (1.88%) 52 (98.11%) |
0 3 (2.9%) 101 (97.1%) |
CC CT TT |
Female (n =157) |
| ABCB1-T1236C (n = 332) | |||||
| 0.53 0.41 |
0.76 (0.33-1.77) 0.75 (0.38-1.49) 1 |
16(20.25%) 36(45.56%) 27 (34.17%) |
21 (21.87%) 48 (50%) 27 (28.12%) |
CC CT TT |
Male (n = 175) |
| 0.02 0.008 |
4.14 (1.31-13.16) 2.70 (1.30-5.61) 1 |
8 (15.09%) 29 (54.71%) 16 (30.18%) |
7 (6.73%) 39 (37.50%) 58 (55.76%) |
CC CT TT |
Female (n =157) |
OR, odds ratios; CI, confidence interval
Table 6.
Genotype frequencies of ABCB1-T129C and -T1236C polymorphisms and odds ratios in patients with idiopathic or symptomatic epilepsy.
| P |
OR
(95% CI) |
Drug-resistant
patients |
Drug-responsive
patients |
Genotype | Etiology of epilepsy |
|---|---|---|---|---|---|
| ABCB1-T129C (n = 332) | |||||
| 0.001 | 25.00 (3.48-179.80) 1 |
0 3 (50%) 3 (50%) |
0 3 (3.8%) 75 (96.2%) |
CC CT TT |
Idiopathic (n = 84) |
| 0.77 | 1.22 (0.32-4.65) 1 |
0 5 (4.0%) 121 (96.0%) |
0 4 (3.3%) 118 (96.7%) |
CC CT TT |
Symptomatic (n=248) |
| ABCB1-T1236C (n = 332) | |||||
| Small sample size | 0 0 6 (100%) |
12 (15.4%) 41 (52.6%) 25 (32.0%) |
CC CT TT |
Idiopathic (n= 84) |
|
| 0.02 0.004 |
2.43 (1.15-5.17) 2.29 (1.31-4.00) 1 |
24 (19.0 %) 65 (51.6%) 37 (29.4%) |
16 (13.1%) 46 (37.7%) 60 (49.2%) |
CC CT TT |
Symptomatic (n =248) |
OR, odds ratios; CI, confidence interval
DISCUSSION
Results of the present study demonstrate that by analyzing all patients as a whole, T129C and T1236C polymorphisms of ABCB1 gene are not associated with drug resistance in Iranian epileptics.
The effect of T129C (rs2188524) and T1236C (rs1128503) polymorphisms of ABCB1 gene on AED resistance has been studied by many researchers. In some of these studies, an association was found between T1236C polymorphism and drug-resistant epilepsy [9-11]. However, in other studies such association was not observed for T1236C [12, 14] or for T129C [11]. Differences in results among studies have been mostly attributed to phenotype definition, small sample size, overlap in substrate specificity between P-gp and other drug efflux transporters and also to inclusion of AED that might not be P-gp [8, 20]. In the present study, drug resistance was defined as failure of two or more AED with seizure frequency of 10 per year. This is similar to criteria of drug resistance used by Hung et al. [10]. However, in contrast to that study, we did not find any association between T1236C polymorphism and drug resistance in epileptic patients. Therefore, it seems that there are other important factors influencing drug-resistant epilepsy that their unequal presence in various studies leads to contradictory results. In drug-resistant epilepsy, multiple aspects including clinical factors (etiology, early age at seizure onset, type of epileptic syndrome and seizure, and structural brain abnormalities or lesions) should be considered [16-19]. In most studies performed on ABCB1 polymorphism and drug-resistant epilepsy, in addition to different phenotype definition, patients with multiple types of epilepsy who consume multiple AED have been enrolled in the studies. These unequal factors may affect the results and leads to unequal findings [20].
To unmask the effect of some of these factors, we stratified the patients by age, gender, and etiology of epilepsy and analyzed the association between polymorphisms and drug resistance in the subgroups. When patients were stratified by age, no significant association was observed between ABCB1-T129C and T1236C polymorphisms and drug resistance neither in adults nor in children. The result of analysis in adults was similar to the case that all patients were analyzed as a whole because the main proportions of the subjects in our study were adults and the number of children was low. With regard to patient gender, the risk of drug resistance was higher in the female patients with 1236CC or CT genotypes than in those with TT genotype. The genotype frequency of T129C polymorphism did not differ significantly between drug-responsive and drug-resistant male or female patients for CC, CT or TT genotypes. With regard to epilepsy etiology, the risk of drug resistance was higher in the patients with the genotype 1236CC or CT than in those with the TT genotype with symptomatic epilepsy. Moreover, the risk of drug response was higher in the patients with 129TT than in those with the CT genotype with idiopathic epilepsy.
It is well known that not all AED are substrates of human P-gp [20]. Studies using in vitro models indicated that phenytoin, phenobarbital, lamotrigine and levetiracetam, but not valproate and carbamazepine, are transported by human P-gp [22, 23]. Therefore, it is suggested that inclusion of the patients treated with drugs that are not substrate for P-gp is likely to reduce any association between ABCB1 polymorphism and drug-resistant epilepsy [8]. In our study, from 132 patients with drug-resistant epilepsy who were received several AED such as phenytoin, phenobarbital, primidone, carbamazepine, valproate, oxcarbazepine, levetiracetam, lamotrigine, clonazepam and topiramate, just 10 patients took carbamazepine and valproate. Exclusion of the patients treated with carbamazepine and valproate did not affect the final result of T129C and T1236C polymorphisms and drug resistance. This is in line with the common clinical observations that many patients with drug-resistant epilepsy fail to respond to many different drugs, including AED that are (such as phenytoin and lamotrigine) and that are not (e.g. carbamazepine) substrates of P-gp transport [8].
Our results indicate a higher risk of drug resistance in Iranian female epileptic patients with the ABCB1-1236CC genotype than in those with the 1236TT genotype. A large study of replication is needed to confirm our results.
ACKNOWLEDGEMENTS
Financial support by grant no. 332 from Pasteur Institute of Iran is acknowledged. We thank Dr Mohammad Karimi and Dr Maziar Shojaee, Assistant Professors of Neurology, Loghman Hospital, for providing epileptic patients.
References
- 1.Porter RJ, Meldrum BS. Antiseizure drugs . In: Katzung BG, editor. Basic and Clinical Pharmacology. New York USA: McGraw- Hill ; 2001. p. 395. [Google Scholar]
- 2.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 3.Sisodiya SM. Mechanisms of antiepileptic drug resistance. Curr Opin Neurol. 2003;16:197–201. doi: 10.1097/01.wco.0000063771.81810.6c. [DOI] [PubMed] [Google Scholar]
- 4.Loscher W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci . 2005;6:591–602. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- 5.Potschka H, Fedrowitz M, Loscher W. P-glycoprotein and multidrug resistance-associated protein are involved in the regulation of extracellular levels of the major antiepileptic drug carbamazepine in the brain. Neuroreport . 2001;12:3557–3560. doi: 10.1097/00001756-200111160-00037. [DOI] [PubMed] [Google Scholar]
- 6.Potschka H, Loscher W. In vivo evidence for P-glycoprotein-mediated transport of phenytoin at the blood-brain barrier of rats. Epilepsia. 2001;42:1231–1240. doi: 10.1046/j.1528-1157.2001.01901.x. [DOI] [PubMed] [Google Scholar]
- 7.Loscher W, Potschka H. Role of multidrug transporters in pharmacoresistance to antiepileptic drugs. J Pharmacol Exp Ther. 2002;301:7–14. doi: 10.1124/jpet.301.1.7. [DOI] [PubMed] [Google Scholar]
- 8.Loscher W, Delanty N. MDR1/ABCB1 polymorphisms and multidrug resistance in epilepsy: in and out of fashion. Pharmacogenomics. 2009;10:711–713. doi: 10.2217/pgs.09.47. [DOI] [PubMed] [Google Scholar]
- 9.Zimprich F, Sunder-Plassmann R, Stogmann E, Gleiss A, Dal-Bianco A, Zimprich A, Plumer S, Baumgartner C, Mannhalter C. Association of an ABCB1 gene haplotype with pharmacoresistance in temporal lobe epilepsy. Neurology. 2004;63:1087–1089. doi: 10.1212/01.wnl.0000141021.42763.f6. [DOI] [PubMed] [Google Scholar]
- 10.Hung CC, Tai JJ, Lin CJ, Lee MJ, Liou HH. Complex haplotypic effects of the ABCB1 gene on epilepsy treatment response. Pharmacogenomics. 2005;6:411–417. doi: 10.1517/14622416.6.4.411. [DOI] [PubMed] [Google Scholar]
- 11.Seo T, Ishitsu T, Ueda N, Nakada N, Yurube K, Ueda K, Nakagawa K. ABCB1 polymorphisms influence the response to antiepileptic drugs in Japanese epilepsy patients. Pharmacogenomics. 2006;7:551–561. doi: 10.2217/14622416.7.4.551. [DOI] [PubMed] [Google Scholar]
- 12.Kim YO, Kim MK, Woo YJ, Lee MC, Kim JH, Park KW, Kim EY, Roh YI, Kim CJ. Single nucleotide polymorphisms in the multidrug resistance 1 gene in Korean epileptics. Seizure. 2006;15:67–72. doi: 10.1016/j.seizure.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Kwan P, Baum L, Wong V, Ng PW, Lui CH, Sin NC, Hui AC, Yu E, Wong LK. Association between ABCB1 C3435T polymorphism and drug-resistant epilepsy in Han Chinese. Epilepsy Behav. 2007;11:112–117. doi: 10.1016/j.yebeh.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Lakhan R, Misra UK, Kalita J, Pradhan S, Gogtay NJ, Singh MK, Mittal B. No association of ABCB1 polymorphisms with drug-refractory epilepsy in a north Indian population. Epilepsy Behav . 2009;14:78–82. doi: 10.1016/j.yebeh.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Kim DW, Lee SK, Chu K, Jang IJ, Yu KS, Cho JY, Kim SJ. Lack of association between ABCB1 ABCG2 and ABCC2 genetic polymorphisms and multidrug resistance in partial epilepsy. Epilepsy Res. 2009;84:86–90. doi: 10.1016/j.eplepsyres.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Regesta G, Tanganelli P. Clinical aspects and biological bases of drug-resistance epilepsies. Epilepsy Res. 1999;34:109–122. doi: 10.1016/s0920-1211(98)00106-5. [DOI] [PubMed] [Google Scholar]
- 17.Kwan P, Brodie MJ. Refractory epilepsy: a progressive intractable but preventable condition? Seizure. 2002;11:77–84. doi: 10.1053/seiz.2002.0593. [DOI] [PubMed] [Google Scholar]
- 18.Loscher W. How to explain multidrug resistance in epilepsy? Epilepsy Curr. 2005;5:107–112. doi: 10.1111/j.1535-7511.2005.05311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.French JA. Refractory epilepsy: clinical overview. Epilepsia. 2007;48(Suppl 1):3–7. doi: 10.1111/j.1528-1167.2007.00992.x. [DOI] [PubMed] [Google Scholar]
- 20.Loscher W, Klotz U, Zimprich F, Schmidt D. The clinical impact of pharmacogenetics on the treatment of epilepsy. Epilepsia. 2009;50:1–23. doi: 10.1111/j.1528-1167.2008.01716.x. [DOI] [PubMed] [Google Scholar]
- 21.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cucullo L, Hossain M, Rapp E, Manders T, Marchi N, Janigro D. Development of a humanized in vitro blood-brain barrier model to screen for brain penetration of antiepileptic drugs. Epilepsia. 2007;48:505–516. doi: 10.1111/j.1528-1167.2006.00960.x. [DOI] [PubMed] [Google Scholar]
- 23.Luna-Tortos C, Fedrowitz M, Loscher W. Several major antiepileptic drugs are substrates for human P-glycoprotein. Neuro-pharmacology. 2008;55:1364–1375. doi: 10.1016/j.neuropharm.2008.08.032. [DOI] [PubMed] [Google Scholar]