Abstract
Objective:
The purpose of this study was to test a two-phased nutrition and exercise education, coping skills training, and exercise intervention program for overweight or obese low-income ethnic minority 2nd to 4th grade children and their parents in rural North Carolina, USA.
Methods:
A cluster randomized controlled trial was carried out with 358 children (7–10 years) and a parent for each child (n=358). General linear mixed models were used to determine the effects of the intervention on weight, adiposity, health behaviors, and eating and exercise self-efficacy by examining changes in children and parents from baseline to completion of the study (18 months).
Results:
At 18 months, children in the experimental group did not have a significantly decreased body mass index (BMI) percentile (P=0.470); however, they showed a reduction in the growth rate of their triceps (P=0.001) and subscapular skinfolds (P<0.001) and an improvement in dietary knowledge (P=0.018) and drank less than one glass of soda per day (P=0.052) compared with the control group. Parents in the experimental group had decreased BMI (P=0.001), triceps (P<0.001) and subscapular skinfolds (P<0.001) and increased nutrition (P=0.003) and exercise (P<0.001) knowledge and more often drank water or unsweetened drinks (P=0.029). At 18 months, children in the experimental group did not show significant improvement in eating (P=0.956) or exercise self-efficacy (P=0.976). Experimental parents demonstrated improved socially acceptable eating self-efficacy (P=0.013); however, they did not show significant improvement in self-efficacy pertaining to emotional eating (P=0.155) and exercise (P=0.680).
Conclusion:
The results suggest that inclusion of children and parents in the same intervention program is an effective way to decrease adiposity and improve nutrition behaviors in both children and parents and improve weight and eating self-efficacy in parents.
Keywords: overweight, obesity, children, parents, intervention
Introduction
Overweight and obesity have increased markedly in both children and adults over the past three decades.1 In the United States, currently 36% of children and 68% of adults are overweight or obese.1 Medical expenditures related to overweight and obesity now exceed $300 billion per year.2 Overweight and obesity are major contributors to premature morbidity and mortality due to type 2 diabetes and cardiovascular disease.3, 4
The majority of children and adults in the United States consume excessive energy-dense foods and drink sweetened beverages,5, 6 do not engage in recommended amounts of physical activity7 and engage in high rates of sedentary behavior.8, 9 Treatments for overweight and obese children are designed to slow down the rate of weight gain while achieving normal growth and development.10, 11 Management of overweight and obesity in adults focuses on weight loss and maintenance.1 Nutrition, exercise and behavioral interventions targeting the child alone, or with a parent, have been conducted primarily with non-Hispanic white, middle-income 8–16-year-old children in clinic settings with varying sample sizes (n=31–190).12, 13, 14, 15, 16, 17, 18, 19, 20 Few studies have focused on overweight or obese children and parents belonging to an ethnic minority population in order to manage their weight,21, 22, 23 and even fewer studies have been conducted with overweight or obese children and parents of an ethnic minority in rural settings.24, 25
The study reported here examined the effects of a two-phase community-based intervention program on weight, adiposity, health behaviors and self-efficacy in overweight or obese children and parents. The primary outcome for children was stabilization of percentile growth of body mass index (BMI) from baseline to 18 months. The primary outcome for parents was a decrease in BMI from baseline to 18 months. Secondary outcomes in both children and parents included a decrease in adiposity and an improvement in health behaviors and self-efficacy from baseline to 18 months. It was hypothesized that children and parents in the experimental group would show significantly greater reductions in weight and adiposity and significantly greater improvements in health behaviors and self-efficacy compared with children and parents in the control group from baseline to 18 months.
Materials and Methods
Study design
The Family Partners for Health study was a 5-year, cluster randomized controlled trial in which an overweight or obese parent was partnered with his/her overweight or obese 2nd–4th grade child, and the efficacy of an innovative nutrition and exercise education, coping skills training and exercise intervention program was tested on them. The institutional review board at the University of North Carolina at Chapel Hill approved the study. Detailed descriptions of the study design and recruitment and retention procedures have been published elsewhere.26, 27
Sites
Eight rural elementary schools in two counties were used as sites for recruitment and delivery of the intervention after school hours. The schools were similar in ethnic and racial mix, in structure and size, were in rural settings and were 5–10 miles from each other. There were eight enrollment periods, from August 2007 to April 2010, with an average of 44–45 children and 44–45 parents enrolled into either the experimental or the control group in two schools in each period. Schools were randomized to either the experimental or the control group for the first enrollment and exchanged conditions for the second enrollment. The sequence of each school was randomized before the start of the study and was stratified by county. A total of 18 months had passed and the first group had completed their time in the study prior to the second enrollment in each school. This design preserved a balance of treatment groups within each site to avoid confounding site effects with intervention effects. Participants and staff were blinded to group assignment from enrollment until implementation.
Sample
Inclusion criteria for children included the ability to speak, write and read English; enrollment in the 2nd–4th grade; age 7–10 years; BMI⩾85th percentile for age and gender; at least one biological parent with a BMI⩾25 kg/m2; self-consent and their parent's consent for their participation. Children in the 2nd–4th grade were chosen because they were in the concrete operational stage and parents have more influence on their nutrition and exercise behaviors during this stage of development.13, 14 Inclusion criteria for parents were ability to speak, write and read English; BMI⩾25 kg/m2; a 2nd–4th-grade child with a BMI⩾85th percentile for age and gender; residence with the child; and consent to participate. Dyads were excluded if either the parent or the child had a heart murmur, congenital heart disease, family history of sudden death, claustrophobia or if they were participating in another weight management program. Those of Asian descent were excluded because of lower BMI cutoffs for overweight and obesity.28 If the parent and child met the inclusion criteria, a research assistant reviewed the child's and parent's consent with them.
Intervention
The nutrition and exercise education and coping skills intervention was based on social cognitive theory and was designed to increase self-efficacy and improve health behaviors.29 Parents' cognitive-affective processes were viewed as driving their role modeling of health behaviors.30 Parents and children who developed skills in goal setting, problem solving and conflict resolution were expected to be more capable of making healthy nutrition and exercise behavior change and to better manage their weight.31 Practicing coping skills was expected to improve health behaviors and eating and exercise self-efficacy.29, 32 By improving health behaviors and self-efficacy, the experimental children and parents were expected to show a decrease in weight and adiposity.
The children and parents in the experimental group received a two-phase intervention.26 In Phase I, the children and parents attended all classes together and received 60 min of nutrition and exercise education and coping skills training, followed by 45 min of exercise once a week for 12 weeks (Table 1).10, 33, 34 These education classes were taught by the same nurse practitioner or registered dietitian, and the exercise classes were taught by the same certified exercise trainer. At the start of each class, the parents and children reported progress on their nutrition or exercise goal from the week before, and at the end of the class the parents and children indicated a nutrition or exercise goal they were going to work on in the coming week.
Table 1. Phase I and Phase II intervention classes.
| Class topics | Interval | Time |
|---|---|---|
| Phase I classes (intensive intervention) | ||
| 1. NE—understanding calories, proteins, carbohydrates and fats and goal setting | Weekly | 60 min |
| 2. NE—how portion control can make a difference and goal setting | Weekly | 60 min |
| 3. NE—how to make healthy substitutes with food and goal setting | Weekly | 60 min |
| 4. NE—choosing healthy food when eating out and goal setting | Weekly | 60 min |
| 5. EE—the importance of exercise and goal setting | Weekly | 60 min |
| 6. CST—increasing exercise and cognitive restructuring and goal setting | Weekly | 60 min |
| 7. CST—improving nutrition and exercise behaviors and social problem solving and goal setting | Weekly | 60 min |
| 8. CST—motivating each other in a positive manner and assertiveness training and goal setting | Weekly | 60 min |
| 9. CST—understanding barriers to healthy choices and social problem solving and goal setting | Weekly | 60 min |
| 10. CST—getting back on track after relapse and assertiveness training and goal setting | Weekly | 60 min |
| 11. CST—working through conflict and conflict resolution and goal setting | Weekly | 60 min |
| 12. Final class—putting it all together and goal setting | Weekly | 60 min |
| Phase II classes (continued support) | ||
| 1. Nutrition and exercise problem solving and goal setting | Monthly | 60 min |
| 2. Nutrition and exercise problem solving and goal setting | Monthly | 60 min |
| 3. Nutrition and exercise problem solving and goal setting | Monthly | 60 min |
| 4. Nutrition and exercise problem solving and goal setting | Monthly | 60 min |
| 5. Nutrition and exercise problem solving and goal setting | Monthly | 60 min |
| 6. Nutrition and exercise problem solving and goal setting | Monthly | 60 min |
| 7. Nutrition and exercise problem solving and goal setting | Monthly | 60 min |
| 8. Nutrition and exercise problem solving and goal setting | Monthly | 60 min |
| 9. Nutrition and exercise problem solving and goal setting | Monthly | 60 min |
Abbreviations: CST, coping skills training; EE, exercise education; NE, nutrition education.
Parents and children attended exercise sessions together, which included basketball, soccer, walking, cardio kickboxing, Dance Dance Revolution (Konomi, Tokyo, Japan), strength training and information on how to increase their exercise at home. Both children and parents received a pedometer as a source of motivation and were encouraged to incrementally increase their activity to 10 000 steps per day.
In Phase II, the children and parents met once a month for 9 months for 60 min of class and 45 min of exercise. They met in small groups with the same interventionist to problem-solve issues they were having with nutrition and exercise (Table 1). The parents and children were asked to share a nutrition or exercise goal that they wanted to work on during the month. The experimental children and parents thus received 21 contacts over 12 months. They were then followed up for 6 more months after the completion of Phase II.
Data collection
Data collection procedures have been published elsewhere.26 Data were collected at the time of enrollment into the study, at 0 months; post Phase I, at 3 months; post Phase II, at 12 months; and after 6 months of no contact with the study staff, at 18 months. A control group of children and parents received usual care and had data collected at the same time points as the experimental children and parents. After completion of the final data collection, they were offered the Phase I classes.
Weight outcomes
All measurements were taken in a private room with two research assistants. Height was measured twice while wearing street clothes without shoes, using a stadiometer, calibrated at 1/8-centimeter (cm) intervals. Weight was measured twice to the nearest 0.1 kg in street clothes without shoes, using a Tanita WB110A Digital Scale (Tanita, Arlington Heights, IL, USA), and averaged. For children, BMI percentiles were calculated with a computer using height, weight, age and gender, and for parents BMI was calculated with a computer using height and weight (kg/m2).35
Adiposity outcomes
Waist circumference was measured three times and averaged using a Figure Finder measuring tape with a lock (Novel Products Inc., Rockton, IL, USA).36 Triceps and subscapular skinfolds were measured according to the National Health and Nutrition Examination Survey Procedures on the right side of the body, three times, and averaged.37, 38
Health behavior outcomes
The Child Health Behavior Survey (20 items)39 and the Adult Health Behavior Survey (23 items)39 were used to collect information on usual food and beverage intake. Responses were scored from zero (none) to 5 (all or most days of the week). All answers in each question were rescored as either a healthy choice (1) or an unhealthy choice (2) according to national nutritional guidelines.39 For example, the first question asks: on a typical day, how much soda do you drink? The answer choices were none, less than one glass, one glass, two glasses, three glasses or four or more glasses. The healthier choices included none, less than one glass and one glass and were assigned a score of 1. The unhealthier choices included two glasses, three glasses or four or more glasses and were assigned a score of 2. Mean values and percentages were then calculated for each group across the time points.
The Health Promoting Lifestyle Profile II,40 with four subscales, was used to measure nutrition, exercise, health responsibility and stress management in parents. The 48 items have four responses (never, sometimes, often or routinely) on a four-point Likert scale. Mean subscale scores were calculated. Alpha coefficients have ranged from 0.78 to 0.93 for the subscales.40 Internal consistency values for the current study were as follows: nutrition α=0.80; exercise α=0.85; health responsibility α=0.82; and stress α=0.76.
The Child and Adolescent Health (CATCH) questionnaire was used to measure health behaviors in children.55 The CATCH contains 130 items in seven subscales using a three-point Likert scale. Reliability and validity of the instrument was previously determined in 5000 children.41 Internal consistency values for the current study were as follows: dietary intention α=0.80; usual food choices α=0.77; dietary knowledge α=0.75; dietary habits α=0.16; support for physical activity α=0.68; parent reinforcement for food choices α=0.69; teacher reinforcement for food choices α=0.83; friend reinforcement for food choices α=0.81; diet self-efficacy α=0.73; and exercise self-efficacy α=0.49.
Physical activity of the children and parents was measured using the Actical Omni-directional accelerometer (Phillips Respironics, Bend, OR, USA). Participants were instructed to wear the accelerometer on their right hip from the time they woke up on Wednesday morning through midnight on Saturday and remove it only during bathing and sleep during each of the four data collection periods. For parents, epochs were defined per 60 s and for children per 30 s.
Self-efficacy outcomes
The eating self-efficacy scale42 was used on parents to measure the effects of negative affect (emotional eating) and socially acceptable circumstances (holidays) on eating. This 25-item scale asks respondents to rate their difficulty in controlling their eating from 1 (no difficulty) to 7 (a lot of difficulty). Alpha coefficients were 0.94 for the negative affect subscale and 0.85 for the socially acceptable circumstances subscale in previous studies.31, 43 Internal consistency values for the current study were as follows: positive affect scale α=0.93 and negative affect scale α=0.97.
Bandura's Exercise Self-Efficacy Scale32 was used to measure exercise self-efficacy in parents. This instrument contains 18 questions rated on a 100-point scale with a 10-unit range from zero (cannot do at all) to 50 (moderately certain can do) to 100 (certain can do).32 A mean score was calculated by summing the questions and dividing by 18. The alpha coefficient of the total scale was 0.94 in adult men and women.44 Internal consistency in the current study was α=0.95. The last two subscales of the CATCH45 were used to measure eating and exercise self-efficacy in children. Internal consistency values in the current study were as follows: dietary self-efficacy α=0.73 and exercise self-efficacy α=0.49.
Parents completed a baseline demographic questionnaire and an update questionnaire at each subsequent data collection to obtain data on whether their child or they had been diagnosed with asthma or diabetes, had started steroids or psychiatric medications, had joined another weight management program or if they had stopped smoking.
Data analysis
All analyses were performed using SAS, version 9 (SAS, Cary, NC, USA). An intent-to-treat approach was used. Separate general linear mixed models were used to determine the effects of the intervention on weight status, adiposity, health behaviors and self-efficacy of children and parents. These random intercept models were used to compare each outcome difference between the intervention and control groups at each post-baseline time point.46 Fixed effects included indicators for intervention, post-baseline time, and pairs of simultaneous intervention and control groups, as well as baseline value for the corresponding measure, which adjusted for any imbalances at baseline, and the intervention-by-time interaction. Random effects included a subject-specific intercept, and the enrollment cohort to account for cluster randomization, where estimable. Planned contrasts between the experimental and control groups addressed pairwise change at each post-baseline time point. The Hochberg correction controlled the overall error rate at 0.05 for multiple comparisons across the follow-up times within each outcome.47 Within the experimental group and at each post-baseline time point, Pearson correlation coefficients were computed for parents' changes (from baseline) in weight status, adiposity, health behaviors and self-efficacy, along with their children's corresponding changes from baseline, to determine how strongly the dyads were associated.
For analysis of the accelerometry data, a duration of more than 60 consecutive minutes of zero counts was considered noncompliant and removed.7 The hours from midnight to 5:00 am were removed because participants did not wear their accelerometer during sleep, and night workers were removed from the analyses. A day was included if at least 10 h of data remained for weekdays and 8 h for Saturdays. The participant had to have data for at least 2 weekdays to be included in weekday results. Three measures were analyzed: mean counts per hour proportion of sedentary time and proportion of time spent in moderate-to-vigorous activity. These measures were computed separately for weekdays and for Saturday for each participant. They were compared between the experimental and control groups at the four time points and for parents and children separately. In accordance with Colley and Tremblay48 and Puyau et al.,49 <100 counts per minute were considered ‘sedentary.' Time spent in moderate-to-vigorous activity was determined using cutoff points we had previously developed for obese adults50 and those developed by Colley for children.48
Results
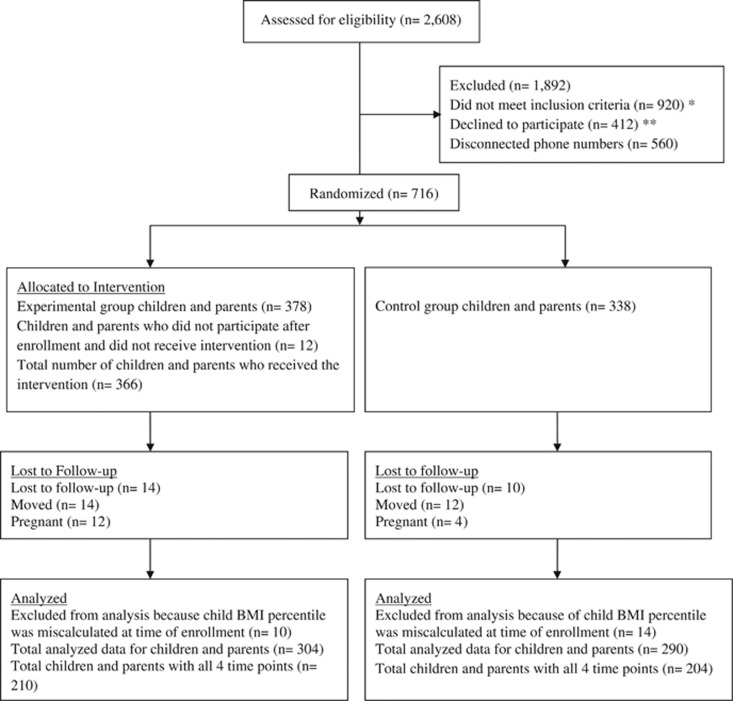
Cluster randomization resulted in 53% (n=378) of the children and parents being assigned to the experimental group and 47% (n=338) to the control group (Figure 1). Table 2 shows demographics overall and by group. There were significant differences in baseline characteristics with respect to parents' BMI (experimental 36.41 kg/m2 (0.61); control 39.13 kg/m2 (0.65); P=0.003), waist circumference (experimental 106.91 cm (1.32); control 114.17 cm (1.40); P=0.002) and subscapular skinfolds (experimental 35.33 mm (1.15); control 38.61 mm (1.19); P=0.048). There were also significant differences in baseline characteristics with respect to children's BMI percentiles (experimental 95.22% (0.37); control 96.59% (0.39); P=0.011), waist circumference (experimental 76.07 cm (1.28); control 80.62 cm (1.33); P=0.014) and subscapular skinfolds (experimental 21.30 mm (0.91); control 24.07 mm (0.95); P=0.036) (See Supplementary Table). These differences may have been the result of the cluster approach and were accounted for in the analyses. To assess the extent of selection bias owing to attrition, the mean values for parent BMI and for child BMI percentiles were compared between those participants who did not contribute data beyond the Phase I intervention and those who did. There were no significant differences between these groups, either overall or by experimental group (P>0.35).
Figure 1.
*Reasons for not meeting the inclusion criteria were as follows: non-English speaker (n=258); parent, child or both did not meet the BMI criteria (BMI of 25 kg/m2 and above for parent and BMI⩾85th percentile for child) (n=618); currently pregnant (n=18); or family had previously enrolled in the program (n=26). **Reasons for declining to participate included the following: conflicting work schedules; evening church activities; or child was participating in sports/scouts/or other extracurricular activities during the scheduled intervention times. ***Reasons parents gave when requesting to drop out of the study included the following: conflicting work schedule or change in work hours from day shift to evening or night shift; cancer diagnosis; incarceration; or death of a spouse.
Table 2. Baseline Demographic Characteristics of Experimental and Control Groups.
| Variable |
Total |
Group |
P | ||||
|---|---|---|---|---|---|---|---|
| N | Percent |
Experimental |
Control |
||||
| N | Percent | N | Percent | ||||
| Parent | |||||||
| Agea | 36.9 (±8.1) | 36.9 (±8.1) | 36.8 (±8.1) | 0.864 | |||
| Gender | |||||||
| Male | 25 | 7.23 | 13 | 7.07 | 12 | 7.41 | 0.532 |
| Female | 321 | 92.77 | 171 | 92.93 | 150 | 92.59 | |
| Marital status | |||||||
| Married | 156 | 45.09 | 86 | 46.74 | 70 | 43.21 | 0.685 |
| Divorced/separated | 67 | 19.37 | 31 | 16.85 | 36 | 22.22 | |
| Never married | 95 | 27.46 | 50 | 27.17 | 45 | 27.78 | |
| Living with someone | 28 | 8.08 | 17 | 9.24 | 11 | 6.79 | |
| Employment | |||||||
| Full-Time | 212 | 61.27 | 111 | 60.32 | 101 | 62.35 | 0.972 |
| Part-Time | 36 | 10.41 | 20 | 10.87 | 16 | 9.88 | |
| Homemaker | 43 | 12.43 | 25 | 13.59 | 18 | 11.11 | |
| Unemployed | 55 | 15.89 | 28 | 15.22 | 27 | 16.66 | |
| Occupation | |||||||
| Professional | 75 | 21.67 | 39 | 21.20 | 36 | 22.22 | 0.351 |
| Technical | 271 | 78.33 | 145 | 78.80 | 126 | 77.78 | |
| Education level | |||||||
| Less than high school | 32 | 9.25 | 14 | 7.61 | 18 | 11.03 | 0.752 |
| High school diploma | 118 | 34.10 | 65 | 35.33 | 53 | 32.71 | |
| College degree | 196 | 56.65 | 105 | 57.06 | 91 | 56.18 | |
| Race | |||||||
| African American | 218 | 63.01 | 117 | 63.59 | 101 | 62.60 | 0.968 |
| White | 106 | 30.64 | 55 | 29.89 | 51 | 31.25 | |
| Other | 22 | 6.35 | 12 | 6.52 | 10 | 6.15 | |
| Ethnicity | |||||||
| Hispanic | 17 | 4.91 | 10 | 5.43 | 7 | 4.32 | 0.412 |
| Not hispanic | 329 | 95.09 | 174 | 94.57 | 155 | 95.68 | |
| Income | |||||||
| <$20 000 | 115 | 33.24 | 65 | 35.33 | 50 | 30.86 | 0.268 |
| $20 000–$39 999 | 132 | 38.15 | 60 | 32.61 | 72 | 44.44 | |
| ⩾$40 000 | 60 | 17.35 | 37 | 20.1 | 23 | 14.21 | |
| Do not wish to respond | 39 | 11.26 | 22 | 11.96 | 17 | 10.49 | |
| Biological parent | |||||||
| Yes | 301 | 87.50 | 157 | 85.33 | 144 | 90.00 | 0.126 |
| No | 43 | 12.50 | 27 | 14.67 | 16 | 10.00 | |
| Children | |||||||
| Agea | 9.1 (±0.95) | 9.2 (±0.96) | 9.0 (±0.93) | 0.203 | |||
| Gender | |||||||
| Male | 154 | 44.50 | 83 | 45.10 | 71 | 43.80 | 0.448 |
| Female | 192 | 55.50 | 101 | 54.90 | 91 | 56.20 | |
| Education level | |||||||
| 2nd grade | 66 | 19.10 | 35 | 19.00 | 31 | 19.10 | 0.467 |
| 3rd grade | 145 | 41.90 | 72 | 39.10 | 73 | 45.10 | |
| 4th grade | 135 | 39.00 | 77 | 41.90 | 58 | 35.80 | |
| Race | |||||||
| African American | 222 | 64.20 | 117 | 63.60 | 105 | 64.80 | 0.957 |
| White | 93 | 26.90 | 50 | 27.20 | 43 | 26.50 | |
| Other | 31 | 8.90 | 17 | 9.20 | 14 | 8.70 | |
| Ethnicity | |||||||
| Hispanic | 26 | 7.51 | 13 | 7.07 | 13 | 8.02 | 0.446 |
| Not hispanic | 320 | 92.49 | 171 | 92.93 | 149 | 91.98 | |
values denoted as mean (±s.d.).
There were no significant differences in BMI percentiles in the experimental group children compared with the control group children at any time point (Table 3). Parents in the experimental group did not show a significantly greater decrease in BMI compared with control parents at the end of Phase I, but their BMI decreased significantly more at the end of Phase II and this difference was maintained at 18 months.
Table 3. Children's and parent's weight, adiposity and health behavior outcomes.
| Variable |
Post Phase I intervention |
P |
Post Phase II intervention |
P |
Completion of study |
P | |||
|---|---|---|---|---|---|---|---|---|---|
|
Experimental |
Control |
Experimental |
Control |
Experimental |
Control |
||||
| Adjusted mean (s.e.)a | Adjusted mean (s.e.)a | Adjusted mean (s.e.)a | |||||||
| Child BMI percentile | 95.2 (0.40) | 95.9 (0.42) | 0.288 | 94.6 (0.42) | 95.6 (0.43) | 0.287 | 94.6 (0.44) | 95.1 (0.43) | 0.470 |
| Child waist circumferenceb | 78.7 (0.43) | 79.5 (0.46) | 0.236 | 82.0 (0.48) | 84.3 (0.49) | 0.003 | 84.9 (0.49) | 86.4 (0.48) | 0.060 |
| Child triceps skinfoldsc | 23.7 (0.44) | 24.8 (0.46) | 0.104 | 24.2 (0.48) | 25.4 (0.48) | 0.104 | 22.9 (0.49) | 25.4 (0.48) | 0.001 |
| Child subscapular skinfoldsc | 20.8 (0.46) | 23.1 (0.48) | 0.002 | 22.7 (0.49) | 24.4 (0.50) | 0.018 | 22.1 (0.51) | 25.2 (0.50) | <0.001 |
| Parent BMI | 37.6 (0.20) | 37.9 (0.20) | 0.117 | 37.3 (0.21) | 38.2 (0.21) | 0.004 | 37.3 (0.21) | 38.5 (0.21) | 0.001 |
| Parent waist circumferenceb | 108.0 (0.65) | 109.9 (0.67) | 0.033 | 107.6 (0.69) | 112.2 (0.70) | <0.001 | 109.9 (0.70) | 112.9 (0.69) | 0.005 |
| Parent triceps skinfoldsc | 34.8 (0.55) | 36.1 (0.58) | 0.101 | 33.8 (0.61) | 36.3 (0.62) | 0.009 | 31.8 (0.63) | 35.5 (0.61) | <0.001 |
| Parent subscapular skinfoldsc | 33.8 (0.55) | 35.2 (0.57) | 0.068 | 34.2 (0.60) | 36.5 (0.60) | 0.013 | 32.9 (0.61) | 36.3 (0.60) | <0.001 |
| Child health behaviors | |||||||||
| Dietary intention scale range (−13 to +13)d | 3.13 (0.49) | 1.88 (0.52) | 0.080 | 4.19 (0.54) | 1.68 (0.55) | 0.003 | 3.20 (0.55) | 1.82 (0.54) | 0.080 |
| Usual food choices scale range (−14 to +14)d | 0.65 (0.56) | −0.45 (0.59) | 0.176 | 2.18 (0.60) | −0.28 (0.61) | 0.012 | 1.50 (0.61) | 0.08 (0.61) | 0.176 |
| Dietary knowledge scale range (−14 to +14)d | 7.51 (0.34) | 6.90 (0.36) | 0.417 | 8.23 (0.37) | 8.06 (0.37) | 0.755 | 9.97 (0.38) | 8.51 (0.37) | 0.018 |
| Dietary habits scale range (−9 to +9)d | 1.19 (0.24) | 0.77 (0.26) | 0.280 | 1.20 (0.27) | 0.58 (0.27) | 0.309 | 1.18 (0.28) | 0.84 (0.27) | 0.280 |
| Physical activity support scale range (−18 to +18)d | 11.19 (0.38) | 10.20 (0.40) | 0.139 | 12.89 (0.41) | 11.38 (0.42) | 0.030 | 13.45 (0.42) | 12.63 (0.42) | 0.163 |
| Parent reinforcement (food) scale range (−7 to +7)d | 2.74 (0.28) | 1.96 (0.30) | 0.119 | 3.25 (0.31) | 2.65 (0.32) | 0.174 | 3.53 (0.32) | 2.57 (0.32) | 0.097 |
| Teacher reinforcement (food) scale range (−7 to +7)d | −0.92 (0.37) | −0.67 (0.39) | 0.771 | −0.34 (0.40) | 0.25 (0.41) | 0.771 | 0.98 (0.42) | 0.81 (0.41) | 0.771 |
| Friend reinforcement (food) scale range (−7 to +7)d | −1.60 (0.37) | −1.54 (0.40) | 0.900 | −0.98 (0.41) | −0.15 (0.42) | 0.900 | −0.52 (0.43) | −0.94 (0.42) | 0.900 |
| Parent health behaviors | |||||||||
| Nutrition knowledge scale range (1 to 4)d | 2.70 (0.04) | 2.39 (0.57) | <0.001 | 2.58 (0.04) | 2.37 (0.40) | 0.002 | 2.62 (0.04) | 2.43 (0.04) | 0.003 |
| Exercise knowledge scale range (1 to 4)d | 2.46 (0.05) | 2.05 (0.05) | <0.001 | 2.33 (0.05) | 2.03 (0.05) | <0.001 | 2.27 (0.05) | 1.96 (0.50) | <0.001 |
| Health responsibility scale range (1 to 4)d | 2.67 (0.04) | 2.46 (0.05) | 0.003 | 2.65 (0.05) | 2.52 (0.05) | 0.055 | 2.67 (0.05) | 2.54 (0.05) | 0.055 |
| Stress management scale range (1 to 4)d | 2.52 (0.05) | 2.36 (0.05) | 0.038 | 2.45 (0.05) | 2.34 (0.05) | 0.135 | 2.41 (0.05) | 2.31 (0.05) | 0.135 |
Abbreviation: BMI, body mass index.
The scale range scores were predetermined by the instrument scoring instructions for all subscales above.
The P-value for experimental versus control comparison was Hochberg-corrected. Bold values indicate P<0.05.
Fixed effects included indicators for intervention, post-baseline time and pairs of simultaneous intervention and control groups, as well as baseline value for the corresponding measure, which adjusted for any imbalances at baseline, and the intervention-by-time interaction.
Expressed in centimeters.
Expressed in millimeters.
A higher positive score is better.
The effects of the intervention on slowing down the growth of waist circumference in the experimental group children were not significant after Phase I; however, these children showed significantly less growth in waist circumference compared with the control group children after Phase II, but the difference was no longer significant at 18 months (Table 3). Parents in the experimental group showed a decrease in waist circumference after Phase I and Phase II, and an increase at 18 months.
Children in the experimental group did not significantly slow down the growth of or decrease their triceps skinfolds at the end of Phases I and II; however, by 18 months they had decreased their skinfolds significantly more compared with the control group (Table 3). The experimental group children slowed down the growth of their subscapular skinfolds significantly more than did the control group at all post-intervention time points. The experimental group parents did not significantly decrease their triceps or subscapular skinfolds at the end of Phase I; however, by the end of Phase II they had decreased their skinfolds significantly more than did control parents, and they maintained that difference at 18 months.
Children in the experimental group reported significantly greater improvement in dietary knowledge compared with the control group at 18 months (Table 3). The experimental group parents reported significantly greater improvements in nutrition and exercise knowledge and health responsibility compared with the control group at all post-intervention time points. The experimental group children and parents did not report significant differences from the control group on accelerometer measurements at any post-intervention time points.
Following the Phase II intervention and at 18 months, children in the experimental group reported that they were marginally significantly more likely than the control group to drink less than one glass of soda per day (Table 4). Parents in the experimental group reported that they increased the consumption of drinking water or unsweetened drinks significantly more compared with the control group after the Phase I intervention and at 18 months. Parents in the experimental group reported that they were significantly more likely to eat unsweetened breakfast cereal at all post-intervention time points.
Table 4. Children's and parent's nutritional intake and self-efficacy outcomes.
| Variable |
Post Phase I intervention |
Post Phase II intervention |
Completion of study |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Experimental (%) | Control (%) | P | Experimental (%) | Control (%) | P | Experimental (%) | Control (%) | P | |
| Children's nutrition | |||||||||
| Drink ⩽1 glass of soda per day | 66 | 56 | 0.103 | 69 | 57 | 0.059 | 71 | 58 | 0.052 |
| Drink ⩽1 glass of sweet drinks per day | 48 | 45 | 0.658 | 50 | 46 | 0.511 | 51 | 46 | 0.478 |
| Drink ⩽4−6 ounces of soda or sweet drinks per day | 47 | 46 | 0.953 | 39 | 45 | 0.352 | 42 | 45 | 0.611 |
| Drink 3 glasses of milk a day | 79 | 69 | 0.043 | 76 | 76 | 0.908 | 76 | 78 | 0.776 |
| Drink skimmed, nonfat, 1/2% or 1% milk | 58 | 55 | 0.600 | 54 | 49 | 0.466 | 48 | 40 | 0.256 |
| Drink ⩾4 glasses of water per day | 34 | 37 | 0.618 | 38 | 37 | 0.907 | 39 | 45 | 0.400 |
| Drink water or unsweetened drinks when thirsty | 50 | 44 | 0.303 | 52 | 55 | 0.559 | 44 | 54 | 0.149 |
| Eat ⩾4 servings of vegetables per day | 9 | 12 | 0.358 | 8 | 9 | 0.781 | 8 | 7 | 0.645 |
| Eat 1−2 servings of fruit per day | 52 | 50 | 0.775 | 52 | 55 | 0.589 | 61 | 52 | 0.168 |
| Eat ⩽1 serving of french fries or chips per day | 47 | 40 | 0.286 | 39 | 50 | 0.093 | 44 | 40 | 0.559 |
| Eat fast food ⩽1 time per week | 34 | 41 | 0.225 | 37 | 38 | 0.771 | 37 | 41 | 0.577 |
| Supersize drinks or food never or sometimes | 22 | 20 | 0.630 | 9 | 16 | 0.143 | 9 | 11 | 0.644 |
| Eat fruit, vegetables, low-fat cheese for a snack | 56 | 43 | 0.032 | 51 | 40 | 0.108 | 48 | 37 | 0.613 |
| Buy extra food/drink ⩾3 times per week at school | 71 | 78 | 0.200 | 75 | 83 | 0.129 | 88 | 81 | 0.296 |
| Eat breakfast 5 –7 times a week | 70 | 62 | 0.132 | 78 | 58 | <0.001 | 70 | 69 | 0.896 |
| Eat whole wheat or dark bread | 38 | 41 | 0.606 | 46 | 44 | 0.744 | 42 | 42 | 0.920 |
| Eat unsweetened breakfast cereal | 27 | 32 | 0.448 | 43 | 33 | 0.137 | 43 | 32 | 0.284 |
| Do not prepare fried foods at home | 18 | 15 | 0.448 | 20 | 17 | 0.082 | 13 | 12 | 0.703 |
| Eat fried foods at home ⩽1 time per week | 39 | 35 | 0.513 | 42 | 38 | 0.523 | 49 | 38 | 0.132 |
| Eat baked goods at home ⩽1 time per week | 41 | 43 | 0.705 | 43 | 37 | 0.323 | 46 | 41 | 0.444 |
| Parent's nutrition | |||||||||
| Drink ⩽1 glass of soda per day | 79 | 60 | <0.001 | 73 | 63 | 0.096 | 72 | 70 | 0.661 |
| Drink ⩽1 glass of sweet drinks per day | 68 | 54 | 0.020 | 64 | 51 | 0.039 | 69 | 60 | 0.175 |
| Drink ⩽4–6 ounces of soda or sweet drinks per day | 45 | 28 | 0.003 | 47 | 32 | 0.020 | 40 | 31 | 0.150 |
| Drink 3 glasses of milk a day | 3 | 1 | 0.312 | 2 | 3 | 0.710 | 4 | 3 | 0.650 |
| Drink skimmed, nonfat, 1/2% or 1% milk | 31 | 19 | 0.016 | 26 | 21 | 0.343 | 29 | 21 | 0.128 |
| Drink ⩾4 glasses of water per day | 56 | 36 | <0.001 | 52 | 42 | 0.124 | 53 | 48 | 0.437 |
| Drink water or unsweetened drinks when thirsty | 75 | 62 | 0.020 | 76 | 68 | 0.190 | 83 | 71 | 0.029 |
| Eat ⩾4 servings of vegetables per day | 9 | 4 | 0.103 | 11 | 5 | 0.094 | 12 | 10 | 0.665 |
| Eat 1–2 servings of fruit per day | 76 | 68 | 0.169 | 70 | 77 | 0.289 | 72 | 71 | 0.861 |
| Eat ⩽1 serving of french fries or chips per day | 93 | 76 | <0.001 | 82 | 83 | 0.929 | 85 | 83 | 0.609 |
| Eat fast food ⩽1 time per week | 64 | 43 | <0.001 | 57 | 40 | 0.012 | 56 | 51 | 0.421 |
| Supersize drinks or food never or sometimes | 99 | 94 | 0.036 | 98 | 98 | 0.999 | 98 | 98 | 0.954 |
| Eat fruit, vegetables, low-fat cheese for a snack | 48 | 38 | 0.109 | 51 | 38 | 0.080 | 48 | 37 | 0.107 |
| Eat breakfast 5–7 times a week | 58 | 48 | 0.115 | 54 | 49 | 0.475 | 43 | 48 | 0.379 |
| Eat whole wheat or dark bread | 72 | 51 | <0.001 | 66 | 60 | 0.354 | 66 | 56 | 0.107 |
| Eat unsweetened breakfast cereal | 64 | 49 | 0.014 | 66 | 53 | 0.046 | 63 | 45 | 0.009 |
| Do not prepare fried foods at home | 28 | 17 | 0.028 | 32 | 17 | 0.007 | 31 | 21 | 0.089 |
| Eat fried foods at home ⩽1 time per week | 44 | 28 | 0.013 | 40 | 31 | 0.165 | 30 | 32 | 0.791 |
| Fry with corn, canola, vegetable or olive oil | 87 | 85 | 0.655 | 88 | 87 | 0.694 | 84 | 78 | 0.241 |
| Bake cookies or pies at home | 34 | 38 | 0.463 | 38 | 33 | 0.421 | 36 | 35 | 0.879 |
| Eat baked goods at home ⩽1 time per week | 81 | 81 | 0.997 | 85 | 83 | 0.705 | 80 | 79 | 0.846 |
| Bake with corn, canola, vegetable or olive oil | 56 | 51 | 0.484 | 58 | 41 | 0.018 | 48 | 43 | 0.470 |
| Use artificial sweeteners 4–6 times per week | 24 | 17 | 0.196 | 20 | 20 | 0.873 | 22 | 18 | 0.482 |
| Eating and exercise self-efficacy (Adjusted mean (s.e.)) | |||||||||
| Parent's eating self-efficacy | |||||||||
| Social circumstances scale range (1 to 7)b | 3.28 (0.08) | 3.48 (0.08) | 0.073 | 3.15 (0.09) | 3.41 (0.09) | 0.068 | 3.11 (0.09) | 3.46 (0.09) | 0.013 |
| Emotional eating scale range (1 to 7)b | 2.52 (0.08) | 2.66 (0.08) | 0.240 | 2.40 (0.09) | 2.63 (0.09) | 0.155 | 2.42 (0.09) | 2.64 (0.09) | 0.155 |
| Parent exercise self-efficacy scale range (0–100)c | 45.46 (1.46) | 44.50 (1.54) | 0.680 | 43.82 (1.58) | 42.89 (1.62) | 0.680 | 41.19 (1.60) | 43.00 (1.60) | 0.680 |
| Child eating self-efficacy scale range (−15 to +15)d | −12.02 (0.24) | −12.32 (0.25) | 0.956 | −12.02 (0.26) | −12.00 (0.26) | 0.956 | −12.21 (0.27) | −12.05 (0.26) | 0.956 |
| Child exercise self-efficacy scale range (−5 to +5)d | −3.96 (0.11) | −3.92 (0.11) | 0.976 | −3.98 (0.12) | −3.89 (0.12) | 0.976 | −4.00 (0.13) | −4.01 (0.12) | 0.976 |
The scale range scores were predetermined by the instrument scoring instructions for all subscales above.
The P-value for experimental versus control comparison was Hochberg-corrected. Bold values indicate P<0.05.
Fixed effects included indicators for intervention, post-baseline time and pairs of simultaneous intervention and control groups, as well as a baseline value for the corresponding measure, which adjusted for any imbalances at baseline, and the intervention-by-time interaction.
A lower score is better with 1=no difficulty controlling eating and 7=much difficulty controlling eating.
A higher score is better with 0=certain cannot do; 40–60=moderately certain can do; 100=certain can do.
A higher positive score is better.
The experimental group children did not report any significant differences from the control group in eating or exercise self-efficacy at the completion of the study (Table 4). At 18 months, experimental group parents did report significantly more improvement in eating self-efficacy in social circumstances, but they did not report any significant differences in either emotional eating or exercise self-efficacy at the completion of the study.
There was no significant relationship between the BMI percentiles of parents and children of the experimental group at any post-intervention time point (Table 5). There were significant relationships between experimental parents' and experimental children's waist circumference, triceps and subscapular skinfolds at most post-intervention time points.
Table 5. Correlations between children's and parent's outcomes in the experimental group.
| Variable |
Post Phase I intervention |
Post Phase II intervention |
Completion of study |
|||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| Parent BMI and child BMI percentile | 0.007 | 0.932 | 0.047 | 0.615 | −0.114 | 0.235 |
| Parent and child waist circumference | 0.328 | <0.001 | 0.259 | 0.005 | 0.409 | <0.001 |
| Parent and child triceps skinfolds | 0.429 | <0.001 | 0.533 | <0.001 | 0.332 | <0.001 |
| Parent and child subscapular skinfolds | 0.151 | 0.065 | 0.368 | <0.001 | 0.292 | 0.002 |
| Parent and child nutrition knowledge | 0.203 | 0.012 | 0.002 | 0.984 | 0.024 | 0.802 |
| Parent and child eating self-efficacy | −0.067 | 0.411 | −0.131 | 0.152 | −0.111 | 0.239 |
| Parent and child exercise self-efficacy | 0.121 | 0.139 | 0.162 | 0.075 | −0.098 | 0.297 |
Abbreviation: BMI, body mass index. Bold values indicate P<0.05.
Discussion
Obesity in ethnically diverse low-income children and adults continues to increase.3, 4, 51 Interventions that improve children's and parents' nutrition and exercise knowledge and teach coping skills are needed. This study was designed to provide ethnically diverse low-income children and parents with a strong foundation in nutrition and exercise knowledge and help them learn problem solving.
There was no statistically significant difference between the experimental and control groups in terms of the primary outcome, BMI percentile in children. The goal of management of obesity in children is to improve nutrition and exercise behaviors and slow down the rate of weight and adiposity gain while maintaining normal growth and development.52 However, recent evidence suggests that BMI alone may not be sufficient for evaluating interventions for child obesity and that neglecting other outcomes such as adiposity and health behaviors may be restrictive.53 Parents in the experimental group demonstrated a significant decrease in their primary outcome, BMI, whereas the control group parents showed an increase in BMI. Weight loss and maintenance are difficult with high rates of recidivism; therefore, treating the entire family may be a more successful approach and should be tested in future studies.54, 55
Children in the experimental group did not significantly decrease or slow down the growth of their waist circumference; however, they showed a significant decrease in triceps skinfolds and slowed down the growth of their subscapular skinfolds. These changes may be one of the initial parameters of weight control, occurring before BMI percentile change in this population.56 Parents in the experimental group showed an increase in waist circumference, although not as much as the control group and, similar to the children, they showed a significant decrease in skinfolds.
Children in the experimental group increased their dietary knowledge and decreased their soda intake to less than one glass a day. These findings are similar to those of previous studies.57, 58 However, our children were younger and more ethnically diverse, suggesting that younger children may benefit from simple messages on nutrition. Parents in the experimental group increased their nutrition and exercise knowledge and drank more water and unsweetened beverages and more often chose unsweetened cereal for breakfast.
The intervention had little effect on the exercise habits of children or parents in the experimental group. These results could be related to the fact that the intervention provided supervised exercise only once a week. Exercise behaviors appear to be hard to change, particularly in low-income households and single-parent families and for adults working multiple jobs.59 A number of factors may influence children's activity, such as being a ‘latch-key' child, neighborhood safety, lack of facilities or opportunities, or lack of parental support.60
Children in the experimental group did not significantly improve their eating or exercise self-efficacy at 18 months. In this age group, children frequently do not have a choice of what they eat and when they exercise. At 18 months, the experimental group parents did show significant improvement in eating self-efficacy in social circumstances, but there was no improvement in emotional eating or in exercise self-efficacy. Changing self-efficacy may take more time, and eating self-efficacy may be easier to change than exercise self-efficacy.29
At 18 months, waist circumference, triceps skinfolds and subscapular skinfolds were significantly related in the experimental children and parents. The children slowed down their increase in waist circumference, triceps and subscapular skinfolds and the parents decreased their waist circumference and triceps and subscapular skinfolds. The relationship between parent and child adiposity has been previously noted, and the data from this study confirm that changes in adiposity in parents and children are closely linked.12, 13, 14, 15
Limitations include the fact that the data do not reflect a representative sample of all African-American, non-Hispanic white and bilingual Hispanic children and parents. Data were self-reported, except for weight, adiposity and physical activity measurements, and potential bias is always possible with self-reported data. Some of the measures were imbalanced between groups at baseline, presumably owing to the nature of the cluster randomization, and were accounted for in the analyses. It is unclear why the alpha coefficient on the dietary habits of children was so low. Our study was funded to evaluate self-efficacy measures and behaviors as outcomes; however, we also recognize that others may evaluate them as potential mediators.
Despite these limitations, the study provides important information on weight, adiposity, health behaviors and self-efficacy in a large group of overweight and obese low-income multiethnic children and parents in rural North Carolina. Our results suggest that it is possible to affect weight in parents as well as adiposity and some health behaviors in children and parents when they participate together in a community-based program. After the Phase I intervention, children and parents in the experimental group made many significant changes in health behaviors; however, when the intervention decreased in intensity, both children and parents lost many of those gains. Children and parents may need more intensive intervention, booster sessions and longer monitoring. This study suggests that partnering overweight parents and children may be a beneficial approach to managing weight and making healthy nutrition and exercise choices.
Acknowledgments
The trial was funded by the National Institute of Health and the National Institute of Nursing Research (1R01NR010254-05). Deep gratitude and appreciation is expressed to Dr Margaret Grey and Dr Kathleen Knafl as consultants and to Ms Siying Li and Ms Diana Lam for their work on the accelerometry data; we also thank the school superintendents, teachers, parents and children for their generosity and time.
Author contributions
DCB, TAS, RGM, AHS and GM had a role in designing and planning of the study, the analyses, and drafting the article. DCB, TAS, RGM, AHS, MN, EGH, NA, DJA and GM had a role in implementing the study, critically reviewing the study results and revising the article. All the authors approved this version for publication.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Nutrition & Diabetes website (http://www.nature.com/nutd)
Supplementary Material
References
- Centers for Disease Control and Prevention Estimated County-Level Prevalence of Diabetes and Obesity-United States, 2007. Morbidity and Mortality Weekly Report. 2009;58:1259–1263. [PubMed] [Google Scholar]
- Behan DF, Cox SH, Lin Y, Pai J, Pedersen HW, Yi M. Obesity and its relation to mortality and morbidity costs. Society of Actuaries. 2010. pp. 1–61.
- American Diabetes Association Standards of Medical Care in Diabetes 2013. Diabetes Care. 2013;36:S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Heart Association Heart Disease and Stroke Statistics - 2013 Update. Circulation. 2013;127:e7–e243. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piernas C, Popkin BM. Increased portion sizes from energy-dense foods affect total energy intake at eating occassions in US children and adolescents: Patterns and trends by age, group, and sociodemographic characteristics, 1977-2006. Am J Clin Nutr. 2011;94:1324–1332. doi: 10.3945/ajcn.110.008466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney EN, Rolfes SR.In: Whitney EN, Rolfes SR (eds). Understanding Nutrition Update (With 2010 Dietary Guidelines). Wadsworth Cengage Learning: Belmont, CA, USA, 2011.
- Troiano RP, Berrigan D, Dodd K, Masse L, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RRea. Amount of time spent in sedentary behaviors in the United States, 2003-2004. Am J Epidemiol. 2008;167:875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtain LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- U.S. Department Health and Human Services . Dietary Guidelines for Americans 2005: Chapter 3 Weight Management. U.S. Department Health and Human Services: Washington, DC, USA; 2005. [Google Scholar]
- National Center for Health Statistics . Health, United States, 2011: Special feature on socioeconomic status and health. National Center for Health Statistics: Hyattsville, MD, USA; 2012. pp. 1–583. [PubMed] [Google Scholar]
- Summerbell CD, Ashton V, Campbell KJ, Edmunds L, Kelly S, Waters E. Interventions for Treating Obesity in Children: Cochrane Review. Wiley Publishers: Oxford; 2004. [DOI] [PubMed] [Google Scholar]
- Kitzman-Ulrich H, Wilson DK, St George SM, Lawman H, Segal M, Fairchild A. The integration of a family systems approach to understanding youth obesity, physical activity, and dietary programs. Clin Child Fam Psycol Rev. 2010;13:231–253. doi: 10.1007/s10567-010-0073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzmann KM, Dalton WT, Stanley CM, Beech BM, Reeves TP, Buscemi J, et al. Lifestyle interventions for youth who are overweight: a meta-analystic review. Health Psychol. 2010;29:91–101. doi: 10.1037/a0017437. [DOI] [PubMed] [Google Scholar]
- Brownell KD, Kelman JH, Stunkard AJ. Treatment of obese children with and without their mothers: changes in weight and blood pressure. Pediatrics. 1983;71:515–523. [PubMed] [Google Scholar]
- Epstein LH, Valoski A, Wing RR, McCurley J. Ten-year outcomes of behavioral family-based treatment for childhood obesity. Health Psychol. 1994;13:373–383. doi: 10.1037//0278-6133.13.5.373. [DOI] [PubMed] [Google Scholar]
- Golan M, Kaufman V, Shahar DR. Childhood obesity treatment: targeting parents exclusively v. parents and children. Br J Nutr. 2006;95:1008–1015. doi: 10.1079/bjn20061757. [DOI] [PubMed] [Google Scholar]
- Israel AC, Guile CA, Baker JE, Silverman WK. An evaluation of enhanced self-regulation training in the treatment of childhood obesity. J Pediatr Psychol. 1994;19:737–749. doi: 10.1093/jpepsy/19.6.737. [DOI] [PubMed] [Google Scholar]
- Munsch S, Roth B, Michael T, Meyer AH, Bidert E, Roth Sea. Randomized controlled comparison of two cognitive behavioral therapies for obese children: mother versus mother-child cognitive behavioral therapy. Psychother Psychosom. 2008;77:235–246. doi: 10.1159/000129659. [DOI] [PubMed] [Google Scholar]
- Nowicka P, Pietrobelli A, Flodmark CE. Low-intensity family therapy intervention is useful in a clinical setting to treat obese and extremely obese children. Int J Pediatr Obes. 2007;2:211–217. doi: 10.1080/17477160701379810. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Stunkard AJ, Rich L, Rubin CJ, Sweidel G, McKinney S. Obesity in black adolescent girls: a controlled clinical trial of treatment by diet, behavior modification, and parental support. Pediatrics. 1990;85:345–352. [PubMed] [Google Scholar]
- Williamson DA, Martin PD, White MA, Newton RL, Walden HM, York-Crowe E. Efficacy of an internet-based behavioral weight loss program for overweight adolescent African-American girls. Eat Weight Disord. 2005;10:193–203. doi: 10.1007/BF03327547. [DOI] [PubMed] [Google Scholar]
- Williamson DA, Walden HM, White MA, York-Crowe E, Newton RL, Alfonso A. Two-year internet-based randomized controlled trial for weight loss in African-American girls. Obesity. 2006;14:1231–1243. doi: 10.1038/oby.2006.140. [DOI] [PubMed] [Google Scholar]
- Janicke DM, Sallinen BJ, Perri MG, Lutes LD, Huerta M, Silverstein JH. Comparison of parent only vs family-based interventions for overweight children in underserved rural settings: outcomes from project STORY. Arch Pediatr Adolesc Med. 2008;162:1119–1125. doi: 10.1001/archpedi.162.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton D, Le Gros K, Norton L, Stanton-Cook S, Morgon J, Masterman P. Randomised controlled trial: a parent-based group education programme for overweight children. J Paediatr Child Health. 2007;43:799–805. doi: 10.1111/j.1440-1754.2007.01150.x. [DOI] [PubMed] [Google Scholar]
- Berry DC, McMurray RG, Schwartz TA, Skelly A, Sanchez M, Neal M, et al. Rationale, design, methodology and sample charactristics for the Family Partners for Health Study: a cluster randomized controlled study. BMC Public Health. 2012;12:250. doi: 10.1186/1471-2458-12-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DC, Neal M, Hall EG, McMurray RG, Schwartz TA, Skelly AH, et al. Recruitment and retention strategies for a community-based weight management study for multi-ethnic elementary school children and parents. Public Health Nurs. 2012;30:80–86. doi: 10.1111/phn.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazare JA, Smith JD, Borel AL, Haffner SM, Balkau B, Ross R, et al. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the international study of prediction of intra-abdominal adiposity and its relationship with cardiometabolic risk/intra-abdominal adiposity. Am J Clin Nutr. 2012;96:714–726. doi: 10.3945/ajcn.112.035758. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Prentice Hall: Englewood Cliffs, NJ, USA; 1986. [Google Scholar]
- Teti DM, O'Connell MA, Reiner CD. Parenting sensitivity, parental depression and child health: the mediational role of parental self-efficacy. Early Dev Parenting. 1996;5:237–250. [Google Scholar]
- Berry D, Savoye M, Melkus G, Grey M.An intervention for multiethnic overweight and obese parents and their overweight children Appl Nurs Res 2063–71.(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy: The Exercise of Control. W.H. Freeman: New York, NY, USA; 1997. [Google Scholar]
- Forman SG. Coping Skills Training for Children and Adolescents. Jossey-Bass: San Francisco, CA, USA; 1993. [Google Scholar]
- American College of Sports Medicine Guidelines for Exercise Testing and Prescription7th ed.Lippincott Williams and Wilkins: New York, NY, USA; 2006 [Google Scholar]
- Centers for Disease Control and Prevention What is BMI? United States Department of Health and Human Services. http://www.cdc.gov/nccdphp/dnpa/bmi/bmi-adult.htm .
- Haffner SM, Howard G, Mayer E. Insulin sensitivity and acute insulin response in African-Americans, Non-Hispanic whites, and Hispanics with NIDDM: the Insulin Resistance Atherosclerosis Study. Diabetes. 1997;46:63–69. doi: 10.2337/diab.46.1.63. [DOI] [PubMed] [Google Scholar]
- National Heart, Lung,and Blood Institute Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The evidence report National Institutes of Health: Washington, DC, USA; 1998. NIH Publications No. 98-4083. [Google Scholar]
- National Heart, Lung and Blood Institute . The Practical Guide to Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. National Institutes of Health: Washington, DC, USA; 2000. [Google Scholar]
- Department of Health and Human Services . Health Behavior Survey: Physical Activity and Nutrition (PAN) Behaviors Monitoring Form. Department of Health and Human Services: Raleigh, NC, USA; 2004. [Google Scholar]
- Walker SN, Sechrist KR, Pender NJ. The health-promoting lifestyle profile: development and psychometric characteristics. Nurs Res. 1987;36:76–81. [PubMed] [Google Scholar]
- Nadar PR, Stone EJ, Lytle LA, Perry CL, Osganian SK, Kelder S, et al. Three-year maintenance of improved diet and physical activity: The CATCH cohort. Arch Pediatr Adolesc Med. 1999;153:695–704. doi: 10.1001/archpedi.153.7.695. [DOI] [PubMed] [Google Scholar]
- Glynn SM, Ruderman J. The development and validation of an eating self-efficacy scale. Cog Ther Res. 1986;10:403–420. [Google Scholar]
- Berry D, Grey M, Melkus G, Savoye M. Preliminary testing of an intervention for multiethnic overweight and obese parents of at risk for overweight and overweight children. Obes Res. 2005;13:A88. doi: 10.1016/j.apnr.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YH, Jang HJ, Pender NJ. Psychometric evaluation of the exercise self-efficacy scale among Korean adults with chronic diseases. Res Nurs Health. 2001;24:68–76. doi: 10.1002/1098-240x(200102)24:1<68::aid-nur1008>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Parcel GS, Edmundson E, Perry CL, Feldman HA, O'Hara-Tompkins N, Nader PR, et al. Measurement of self-efficacy for diet-related behaviors among elementary school children. J Sch Health. 1995;65:23–27. doi: 10.1111/j.1746-1561.1995.tb03335.x. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:968–974. [PubMed] [Google Scholar]
- Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. [Google Scholar]
- Colley RC, Tremblay MS. Moderate and vigorous physical activity intensity cut-points for actical accelerometer. J Sports Sci. 2011;29:783–789. doi: 10.1080/02640414.2011.557744. [DOI] [PubMed] [Google Scholar]
- Puyau M, Adolph AL, Vohra PA, Butte NP. Validation and calibration of physical activity monitors in children. Obes Res. 2002;10:150–157. doi: 10.1038/oby.2002.24. [DOI] [PubMed] [Google Scholar]
- Giffuni J, McMurray RG, Schwartz T, Berry D. Actical sccelerometry cut-points for quantifying levels of exertion: comparing normal and overweight adults. Int J Exerc Sci. 2012;5:170–182. [PMC free article] [PubMed] [Google Scholar]
- Herman WH. The economic cost of diabetes: is it time for a new treatment paradigm. Diabetes Care. 2013;36:775–776. doi: 10.2337/dc13-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics Organizational principles to guide and define the child health system and/or improve the health of all children; committee on children Pediatrics 2003112424–430.12897303 [Google Scholar]
- Kolotourou M, Radley D, Chadwick D, Smith L, Orfanos S, Kapetanakis V, et al. Is BMI alone a sufficient outcome to evaluate interventions for child obesity. Child Obes. 2013;9:350–356. doi: 10.1089/chi.2013.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchi CR, Russell C, White M.Shifting processes model: a conceptual model for sustainable weight management Fam Syst Health 2013. e-pub ahead of print 12 August 2012. [DOI] [PubMed]
- Moore BJ, Frame IJ, Baehr N.Preventing childhood obesity: it takes a nationIn Akabas SR, Lederman SA, Moore BJ (eds) Textbook of Obesity1st ednWiley-Blackwell: Ames, IA, USA; 2012424–462. [Google Scholar]
- Mihalopoulos NL, Holubkov R, Young P, Dai S, Labarthe DR. Expected changes in clinical measures of adiposity during puberty. J Adolesc Health. 2010;47:360–366. doi: 10.1016/j.jadohealth.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JA, Crespo NC, Sallis JF, Patterson RE, Elder JP. Dietary-related and physical activity-related predictors of obesity in children: a 2-year prospective study. Int J Obes. 2012;8:110–115. doi: 10.1089/chi.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbeling CB, Feldman HA, Osganian SK, Chomitz VR, Ellenbogen SJ, Ludwig DS. Effects of decreasing sugar-sweetened beverage consumption on body weight in adolescents: a randomized, controlled pilot study. Pediatrics. 2006;117:673–680. doi: 10.1542/peds.2005-0983. [DOI] [PubMed] [Google Scholar]
- Bauman AE, Reis RS, Sallis JF, Wells JC, Loos RJ, Martin BW, et al. Correlates of physical activity: why are some people physically active and others not. Lancet. 2012;380:258–271. doi: 10.1016/S0140-6736(12)60735-1. [DOI] [PubMed] [Google Scholar]
- Deforche B, Haerens L, de Bourdeaudhuij L. How to make overweight children exercise and follow the recommendations. Int J Pediatr Obes. 2011;6 (Suppl 1:35–41. doi: 10.3109/17477166.2011.583660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.