Abstract
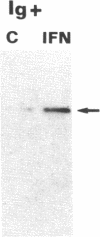
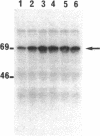
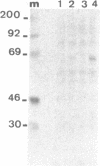
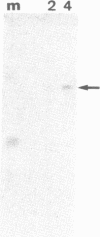
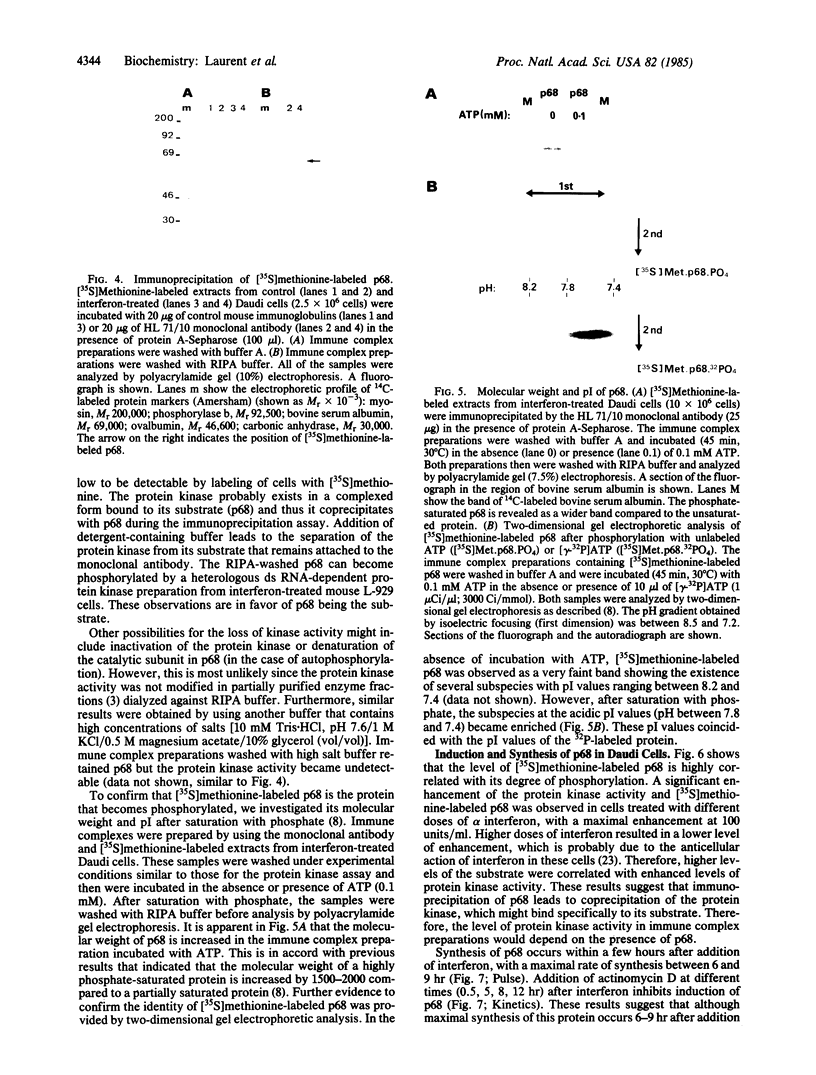
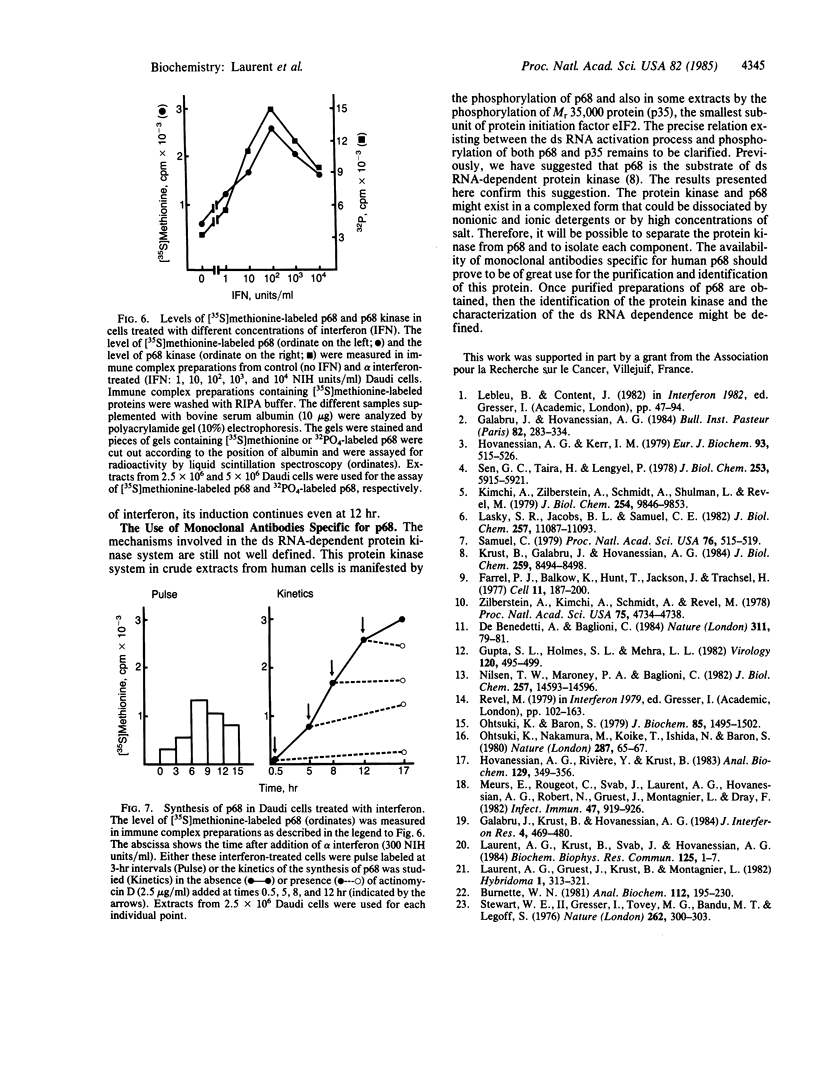
Extracts from interferon-treated human cells show an enhanced level of a double-stranded RNA-dependent protein kinase activity that is manifested by the phosphorylation of an endogenous Mr 69,000-72,000 protein in its phosphate-saturated state. By using a highly purified protein kinase fraction from interferon-treated human Daudi cells, we can now describe the preparation of murine monoclonal antibodies directed against this phosphoprotein, the Mr of which in its native state is found to be 68,000. These monoclonal antibodies (class IgG1) can identify the electrophoresed protein (p68) in polyacrylamide gels by the electrophoretic transfer blotting technique. Immunoprecipitates formed after incubation of extracts from interferon-treated human cells with the monoclonal antibodies can be conveniently recovered by protein A-Sepharose. Such immune complex preparations have associated protein kinase activity--i.e., addition of [gamma-32P]ATP results in the phosphorylation of p68 and added substrates, calf thymus histone, and eukaryotic initiation factor 2. Immune complex preparations from [35S]methionine-labeled extracts show the specific immunoprecipitation of p68. In addition, several other [35S]methionine-labeled proteins are bound unspecifically in these immune complexes prepared under similar experimental conditions as for the assay of protein kinase activity. These unspecifically bound proteins can be washed out by using a buffer containing detergents or high concentrations of KCl and magnesium acetate. Immune complex preparations washed similarly with these buffers still retain p68 but lose their capacity to phosphorylate p68 or exogenous substrates. These results indicate that p68 by itself has no protein kinase activity. The induction of [35S]methionine-labeled p68 in Daudi cells occurs with as little as 1 unit of human alpha interferon, with maximal synthesis between 6 to 9 hr after the addition of interferon. Actinomycin D blocks this induction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- De Benedetti A., Baglioni C. Inhibition of mRNA binding to ribosomes by localized activation of dsRNA-dependent protein kinase. Nature. 1984 Sep 6;311(5981):79–81. doi: 10.1038/311079a0. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Galabru J., Krust B., Hovanessian A. G. Interferon-mediated protein kinase activity in different fractions of mouse L-929 cells. J Interferon Res. 1984 Fall;4(4):469–480. doi: 10.1089/jir.1984.4.469. [DOI] [PubMed] [Google Scholar]
- Gupta S. L., Holmes S. L., Mehra L. L. Interferon action against reovirus: activation of interferon-induced protein kinase in mouse L929 cells upon reovirus infection. Virology. 1982 Jul 30;120(2):495–499. doi: 10.1016/0042-6822(82)90051-4. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Kerr I. M. The (2'-5') oligoadenylate (pppA2'-5'A2'-5'A) synthetase and protein kinase(s) from interferon-treated cells. Eur J Biochem. 1979 Feb 1;93(3):515–526. doi: 10.1111/j.1432-1033.1979.tb12850.x. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Rivière Y., Krust B. Assay of interferon-mediated protein kinase activity from plasma and tissue extracts. Anal Biochem. 1983 Mar;129(2):349–356. doi: 10.1016/0003-2697(83)90561-4. [DOI] [PubMed] [Google Scholar]
- Kimchi A., Zilberstein A., Schmidt A., Shulman L., Revel M. The interferon-induced protein kinase PK-i from mouse L cells. J Biol Chem. 1979 Oct 10;254(19):9846–9853. [PubMed] [Google Scholar]
- Krust B., Galabru J., Hovanessian A. G. Further characterization of the protein kinase activity mediated by interferon in mouse and human cells. J Biol Chem. 1984 Jul 10;259(13):8494–8498. [PubMed] [Google Scholar]
- Lasky S. R., Jacobs B. L., Samuel C. E. Mechanism of interferon action. Characterization of sites of phosphorylation in the interferon-induced phosphoprotein P1 from mouse fibroblasts: evidence for two forms of P1. J Biol Chem. 1982 Sep 25;257(18):11087–11093. [PubMed] [Google Scholar]
- Laurent A. G., Gruest J., Krust B., Montagnier L. Characterization of monoclonal antibody specific for human alpha interferon. Hybridoma. 1982;1(3):313–322. doi: 10.1089/hyb.1.1982.1.313. [DOI] [PubMed] [Google Scholar]
- Laurent A. G., Krust B., Svab J., Hovanessian A. G. Characterisation of the interferon-mediated protein kinase by polyclonal antibodies. Biochem Biophys Res Commun. 1984 Nov 30;125(1):1–7. doi: 10.1016/s0006-291x(84)80324-1. [DOI] [PubMed] [Google Scholar]
- Lebleu B., Content J. Mechanisms of interferon action: biochemical and genetic approaches. Interferon. 1982;4:47–94. [PubMed] [Google Scholar]
- Meurs E., Rougeot C., Svab J., Laurent A. G., Hovanessian A. G., Robert N., Gruest J., Montagnier L., Dray F. Use of an anti-human leukocyte interferon monoclonal antibody for the purification and radioimmunoassay of human alpha interferon. Infect Immun. 1982 Sep;37(3):919–926. doi: 10.1128/iai.37.3.919-926.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Baglioni C. Inhibition of protein synthesis in reovirus-infected HeLa cells with elevated levels of interferon-induced protein kinase activity. J Biol Chem. 1982 Dec 25;257(24):14593–14596. [PubMed] [Google Scholar]
- Ohtsuki K., Baron S. An interferon-induced, ribosome-associated protein kinase which reduces the activity of initiation factor. J Biochem. 1979 Jun;85(6):1495–1502. doi: 10.1093/oxfordjournals.jbchem.a132478. [DOI] [PubMed] [Google Scholar]
- Ohtsuki K., Nakamura M., Koike T., Ishida N., Baron S. A ribosomal protein mediates eIF-2 phosphorylation by interferon-induced kinase. Nature. 1980 Sep 4;287(5777):65–67. doi: 10.1038/287065a0. [DOI] [PubMed] [Google Scholar]
- Sen G. C., Taira H., Lengyel P. Interferon, double-stranded RNA, and protein phosphorylation. Characteristics of a double-stranded RNA-activated protein kinase system partially purified from interferon treated Ehrlich ascites tumor cells. J Biol Chem. 1978 Sep 10;253(17):5915–5921. [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Gresser I., Tovey M. G., Bandu M., Le Goff S. Identification of the cell multiplication inhibitory factors in interferon preparations as interferons. Nature. 1976 Jul 22;262(5566):300–302. doi: 10.1038/262300a0. [DOI] [PubMed] [Google Scholar]
- Zilberstein A., Kimchi A., Schmidt A., Revel M. Isolation of two interferon-induced translational inhibitors: a protein kinase and an oligo-isoadenylate synthetase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4734–4738. doi: 10.1073/pnas.75.10.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]