Abstract
Background
Numerous studies have shown that attention deficit/hyperactivity disorder (ADHD) is associated higher risk of cannabis use disorders (CUD). However, these studies are limited in that most did not: (a) differentiate the role of hyperactivity-impulsivity (HI) and inattention (IN); (b) control for associated psychopathology; and (c) consider more fine-grained CUD-related measures. Our aim was to clarify the unique and interactive contributions of inattention and hyperactivity symptoms to age of cannabis initiation and DSM-IV cannabis dependence, craving, and severity of problems related to cannabis use while statistically controlling for symptoms of comorbid psychopathology in a non-clinical sample of young adults.
Methods
Cannabis variables, current use of cigarettes and alcohol, current and childhood ADHD, and comorbid internalizing and externalizing psychopathology were assessed in 376 male and female undergraduates.
Results
Results indicate that current and childhood IN were independently associated with more severe cannabis use, craving, and problem use-related outcomes in young adulthood (p’s<.01) and that childhood HI symptoms were associated with earlier initiation of cannabis (p<.01). Further, current IN symptoms moderated the relationships between level of use and more severe outcomes (p’s<.01), such that higher IN strengthened positive associations among use and problem cannabis use. Associations with ADHD symptom dimensions and current use of alcohol and cigarettes were also present.
Conclusions
Thus, current and childhood inattention symptoms as well as childhood hyperactive-impulsive symptoms emerged as significant factors in cannabis-related outcomes in young adults, even after statistically controlling for important confounding variables.
Keywords: ADHD, substance use, substance dependence, inattention, hyperactivity
1. INTRODUCTION
Cannabis is the most commonly used illicit drug for individuals aged 12 and over, with 19% of 18–25 year olds reporting current use (SAMHSA, 2011). Second only to the number of individuals receiving treatment for problematic alcohol use, 827,000 young people receive treatment for problematic cannabis use each year (SAMHSA, 2011). Thus, there is a need to characterize factors that contribute to and exacerbate problem use.
Attention deficit/hyperactivity disorder (ADHD), which frequently co-occurs with cannabis use disorders (CUD; i.e. either abuse or dependence, Lee et al., 2011), is characterized by the triad of inattention, hyperactivity, and impulsivity. Although the majority of ADHD studies have focused on children, ADHD symptoms can persist into adolescence and adulthood, and recent meta-analyses suggest that the prevalence of ADHD is similar in children (5.7%) and adults (5.0%; Polanczyk et al., 2007; Willcutt, 2012). The co-occurrence of CUD and ADHD is relevant to both etiological and clinical research. There is evidence that substance use and ADHD may share common etiological influences (Young et al., 2009). Thus, explicating the phenomenology of the CUD-ADHD comorbidity can inform phenotypes relevant for genetically-informed investigations. In addition, identification of specific aspects of ADHD that increase risk for the development and maintenance of CUD may permit more targeted treatments for both disorders at various stages of their expression.
In a recent meta-analysis including 27 prospective studies assessing later substance use disorders (SUD), childhood ADHD prospectively predicted adolescent/adult nicotine, alcohol, cannabis and cocaine use disorders (Lee, et al., 2011). Thus, there is solid evidence to demonstrate that childhood ADHD increases risk for later CUD. Some early studies suggested that cannabis might be the preferred drug of abuse among individuals with ADHD (Biederman et al., 1995). However, the results of another meta-analysis of the association of ADHD and CUD showed high levels of heterogeneity (Charach et al., 2011), suggesting that, although an association appears to be present, single studies were highly influential to the meta-analytic results. Thus, while extant findings provide general support for the CUD-ADHD relation, several features of this association require further attention in order to clarify the unique role of inattention and hyperactivity-impulsivity symptoms in risk for CUD.
First, few studies have adequately controlled for symptoms of other psychopathology including comorbid externalizing and internalizing problems, which have both been found to predict substance abuse/dependence (e.g., Hayatbakhsh et al., 2007). Controlling for comorbid symptoms is critical, in part, because common influences across ADHD and externalizing phenotypes (e.g., inconsistent parenting, peer influences) may work synergistically with neurodevelopmental influences in order to contribute to risk. In addition, few studies have considered comorbid internalizing psychopathology, which may be either risk conferring or protective for SUD in individuals with ADHD symptoms (Levy, 2004). Studies that did control for the co-occurrence of other psychopathology primarily focused on externalizing problems and found that the unique predictive contribution of ADHD symptoms was less evident (Lee et al., 2011), suggesting that the role of ADHD in risk for SUD has been overstated (Looby, 2008).
In addition, prior studies may not have optimally modeled ADHD symptoms, typically using a categorical diagnosis of ADHD. Given that there is strong evidence to suggest that ADHD may exist on the extreme end of a continuum behavior (Frazier et al., 2007), many researchers have advocated for the analysis of continuous measures of ADHD symptoms rather than categorical diagnoses which may underestimate associations with ADHD and substance use outcomes of interest (e.g., Elkins et al., 2007; Kollins et al., 2005). In addition, as the presentation of ADHD symptoms may vary as they persist into adulthood (Barkley et al., 2008), it is also critical to asses both childhood and current symptoms in a single study, which few studies have done.
In addition to the psychometric considerations that support the potential utility of a dimensional approach to defining ADHD, a recent meta-analysis indicated that ADHD symptoms are best described by two correlated, but separable symptom dimensions (Willcutt et al., 2012). Specifically, exploratory and confirmatory factor analyses consistently found distinct factors of inattention (IN) and hyperactivity-impulsivity (HI) symptoms, and the external validity of these dimensions was supported by differential relations with key aspects of social, academic, and neuropsychological functioning. However, results were mixed regarding the associations between these symptom dimensions and SUD, and some studies suggest that these associations may differ depending on the specific substance and outcome in question. With regard to cannabis, studies have found that only IN are independently predictive of marijuana use (Molina and Pelham, 2003; Upadhyaya and Carpenter, 2008), whereas another found that only HI are independently predictive of a CUD (Elkins et al., 2007).
Finally, studies of the associations of ADHD with CUD have typically utilized a dichotomous CUD measure. However, similar to the literature on IN and HI symptoms, studies of CUD suggest that specific facets of CUD such as craving and age of initiation may differentially predict important outcomes including severity of illness and risk for relapse (Cornelius et al., 2008). An approach that may be more reflective of the underlying pathology of SUD is the use of CUD-related outcomes, such as the age of initiation, craving, and the severity of problems related to use, that may be indicators of severity and/or problem use prior to the full onset of dependence. Because little is known about the relation between ADHD symptoms and these aspects of CUD, the inclusion of these measures may facilitate the development of a more comprehensive characterization of the spectrum of cannabis phenotypes that are associated with symptoms of ADHD. In addition to testing multiple cannabis phenotypes, testing parallel associations among ADHD and use of other substances (e.g., cigarettes and alcohol) that have been previously associated with ADHD (Lee, et al., 2011) would further contextualize any reported ADHD-CUD relations.
In sum, it is important to examine associations among CUD and ADHD using a dimensional approach that includes both continuous measures of CUD-related outcomes and an examination of the role of IN and HI ADHD symptoms. Further, examining these associations in non-clinical samples can tap subclinical variation across the ADHD continuum that could be important to SUD etiology and treatment. In the current study, we test the unique and interactive contributions of IN and HI (childhood and current) to important CUD-related outcomes in a non-clinical sample of young adults, while covarying comorbid internalizing and externalizing problems. We tested whether: (1) childhood and current IN and HI symptoms differentially associate with age of initiation, dependence, craving, and severity of use-related problems; (2) ADHD symptoms associate with CUD outcomes independently when comorbid internalizing and externalizing problems are controlled; and (3) these relationships are moderated by level of current cannabis use. Secondary analyses tested associations with ADHD and measures of current cigarette and alcohol use.
2. METHOD
2.1 Participants and Procedures
Participants were recruited as part of a longitudinal study of cannabis use among college-aged individuals. University of Colorado freshmen 18–19 years old (n=377) were recruited via flyers and university mass emails. Eligibility was determined based on past cannabis use. Participants were recruited as never users (i.e., have never tried cannabis), infrequent cannabis users (i.e., use cannabis four times or less per month for more than 1 year, but less than three years) and frequent cannabis users (i.e., use cannabis an average of 5 days a week for at least the past year). Because of the larger study’s interest in neurophysiological responses, individuals were excluded if they reported a history of 3 or more lifetime head injuries, neurological disorder, or the use of prescription medication (with the exception of oral contraceptives or medical cannabis).
Eligible participants were enrolled in a total of 6 sessions across three years. Participants were instructed to abstain from alcohol for 24 hours, recreational drugs (including cannabis) for 6 hours, and caffeine and cigarettes for 1 hour prior to the sessions. The current analyses focus on self-report data collected during the baseline session (measures described below). 376 individuals had complete substance use and ADHD data. Table 1 lists the demographic, substance use, ADHD, and psychopathology symptom characteristics for the sample by cannabis use group.
Table 1.
Sample Characteristics by Smoking Group
| Never | Infrequent | Frequent | |
|---|---|---|---|
|
|
|||
| n=127 | n=146 | n=103 | |
| Gender (% female) | 50% | 52% | 51% |
| Age in years, M(SD) | 18.30 (.46) | 18.40 (.52) | 18.35 (.50) |
| Ethnicity (% White) | 70.9% | 71.1% | 76.4% |
|
| |||
| Psychopathology Symptom Ratings | M (SD) | M (SD) | M (SD) |
| Childhood ADHD IN | .45 (.45) | .68 (.58) | .63 (.49) |
| Childhood ADHD HI | .61 (.55) | .75 (.60) | .81 (.53) |
| Current ADHD IN | .47 (.32) | .62 (.42) | .74 (.41) |
| Current ADHD HI | .62 (.38) | .71 (.38) | .72 (.34) |
| Number with ≥ 6 lifetime IN symptoms1, N (%) | 10 (7.8) | 15 (10.2) | 16 (15.5) |
| Number with ≥ 6 lifetime HI symptoms1, N (%) | 14 (11.0) | 20 (13.7) | 19 (18.4) |
| Number reporting having been given a lifetime diagnosis of ADHD, N (%) | 8 (6.3) | 14 (9.6) | 15 (14.6) |
| ASR Internalizing T-score | 50.7 (9.5) | 52.2 (11.3) | 52.7 (10.2) |
| ASR Internalizing symptoms | 12.0 (7.9) | 13.8 (10.2) | 13.9 (9.9) |
| Number with Internalizing T-score ≥ 65, N (%) | 7 (5.5) | 18 (12.3) | 12 (11.7) |
| ASR Externalizing T-score | 49.4 (8.5) | 53.7 (9.0) | 58.8 (6.8) |
| ASR Externalizing symptoms2 | 8.5 (6.0) | 10.3 (7.5) | 12.8 (7.2) |
| Number with Externalizing T-score ≥ 65, N (%) | 5 (3.9) | 16 (11.0) | 17 (16.5) |
|
| |||
| Substance Use | M (SD) | M (SD) | M (SD) |
| Tobacco | |||
| Total number of cigarettes, past 30 days | 1.8 (15.5) | 2.9 (16.6) | 40.3 (82.8) |
| Alcohol | |||
| Total number of drinking days, past 30 days | 2.0 (2.7) | 5.0 (3.9) | 8.9 (4.4) |
| Total number of alcoholic drinks, past 30 days | 9.4 (17.2) | 29.8 (33.0) | 60.1 (41.4) |
| Cannabis | |||
| Age of initiation | -- | 16.4 (1.3) | 14.5 (1.4) |
| Age of regular smoking | -- | 17.3 (1.1) | 16.3 (1.2) |
| Total number of smoking days, past 30 days | -- | 1.7 (1.9) | 25.8 (3.8) |
| Total grams consumed, past 30 days | -- | 0.9 (1.4) | 24.5 (16.5) |
| Marijuana Dependence Symptoms (MDS) | -- | 0.95 (1.2) | 4.3 (2.3) |
| Cannabis Craving | -- | 1.5 (0.6) | 3.3 (1.4) |
| Marijuana Problems Index (MPI) | -- | 1.2 (0.3) | 2.0 (0.5) |
Note. IN=Inattention; HI=Hyperactivity-Impulsivity; ASR=Adult Self-Report. Alcohol and cannabis use reflect the number of days the substance was used in the 30 days prior to the first laboratory appointment and have ranges of 0–30. Tobacco use reflects the total number of cigarettes smoked in the 30 days prior to the first laboratory appointment. Other possible ranges are (1) Craving 1–7, (2) Marijuana Problems (MPI) 1–5, (3) Marijuana Dependence (MDS) 0–10, (4) Inattention/Hyperactivity 0–3, (5) Internalizing 0–78, (6) Externalizing 0–66,
Number of individuals who endorsed six or more childhood or current symptoms as occurring “often” or “very often.”
Externalizing symptom count excludes three items referring to substance use and law breaking. Higher values indicate greater use, craving, problems, dependence, and psychopathology symptoms;
2.2 Measures
2.2.1. Current substance use
The calendar-assisted Timeline Follow-Back interview (TLFB; Dennis et al., 2004) was used to assess past 30-day use of cannabis, alcohol, and cigarettes. The self-report TLFB has high agreement with biochemical measures of substance use (e.g., 87.3%–90.9% agreement for cannabis across studies; Hjorthoj et al., 2012). Cannabis use was defined as number of days that cannabis was used; alcohol use was defined as the number of alcoholic drinks consumed; cigarettes use was defined as the number of cigarettes smoked.
2.2.2. Cannabis Dependence
The Marijuana Dependence Scale (MDS; Stephens et al., 2000) is a 10-item scale that assesses DSM-IV cannabis dependence symptoms within the past 12 months. Individuals responded ‘yes’ or ‘no’ to each dependence item (e.g., “When I smoked marijuana, I often smoked more or for longer periods of time than I intended”; “I need to smoke more marijuana to achieve the same ‘high’; α=.84).
2.2.3. Cannabis Craving
Cannabis craving was assessed using an 8-item measure adapted from the Alcohol Urge Questionnaire (Bohn et al., 1995). Participants rate the extent to which they were craving a hit of cannabis “right now” (e.g., “All I want to do now is have a hit”). These items were assessed using a 7-point likert scale ranging from “Strongly Disagree” to “Strongly Agree”; α=.90). The mean score of all items provided an index of overall craving.
2.2.4. Cannabis-related problems
The Marijuana Problems Index (MPI; Johnson and White, 1989) is a 29-item questionnaire asking how many times an event or experience occurred as a result of smoking cannabis within the past 12 months in a number of domains, including school, social relations, and personal issues. These items were assessed using a 5-point likert scale (ranging from “Never” to “More than 10 times”; α=.94). The mean score of all items provided an index of problems.
2.2.5. ADHD symptoms
Consistent with prior studies of ADHD in adults, childhood and current ADHD symptoms were assessed via self-report (e.g., Barkley et al., 2011; Gudjonsson et al., 2009). On the Current Symptom Scale the participant indicates how often each of the 18 DSM-IV ADHD symptoms is true on a 4-point Likert scale (0–3) with the anchors “Not at All”, “Once in a While”, “Often” and “Very Often” (Barkley and Murphy, 1998). The Childhood Symptom Scale asks the individual to rate the extent to which each symptom was true during childhood (i.e., 5–12 years of age; Barkley and Murphy, 1998). The mean score of the nine items that comprise each symptom dimension provided the IN and HI indices.
2.2.6. Comorbid Psychopathology Symptoms
Participants were administered the Achenbach System of Empirically Based Assessment (ASEBA) Adult Self-report (ASR; Achenbach and Rescorla, 2003). The ASR is a standardized, self-report measure of current internalizing and externalizing psychopathology symptoms that is the adult parallel to the Child Behavior Checklist (CBCL). Three items referring to substance use and law breaking (uses drugs, gets drunk, trouble with the law) were excluded from calculation of the externalizing score to ensure that any overlap between externalizing and cannabis use was not due to the former simply measuring use of illegal substances. These internalizing and externalizing scales on the ASR have been well validated and have adequate psychometric characteristics (Achenbach and Rescorla, 2003).
2.3. Data Preparation and Analyses
The distribution of each variable was examined for skewness and kurtosis, and the natural logarithm was computed for variables with skewness and kurtosis greater than or equal to 3. Variables adjusted were: MDS, MPI, cannabis craving, and current use of cannabis, alcohol, and cigarettes. To facilitate interpretation of the multiple regression analyses, all measures were first mean-centered. These adjusted scores were used for all analyses. Due to the large number of statistical tests, we considered results significant with p < 0.01.
Pearson correlations were initially computed among substance use measures and psychopathology symptoms. In order to test the unique and interactive associations among childhood and current IN and HI symptoms and cannabis-related outcomes, multiple regression analyses were conducted in which each substance use outcome was predicted by IN, HI, and the IN x HI interaction. Where there was a significant association among IN or HI and a cannabis outcome, secondary multiple regression models tested if current cannabis use moderated the association. These models were constructed to include current cannabis use, IN, HI, all possible two-way interactions, and the three-way interaction as predictors of the cannabis outcome. Significant interactions were examined using simple slope analyses (Aiken and West, 1991). Gender, current internalizing, and current (non-substance use related) externalizing symptom counts were included as covariates in all models of current symptoms. Because measures of comorbid psychopathology in childhood were not available, only gender was included as a covariate in the childhood ADHD models.
3. RESULTS
3.1. Correlations
Alcohol, cannabis, and cigarette use were all significantly correlated (Alcohol/cannabis: r=.50; alcohol/cigarettes: r=.28; cannabis/cigarettes: r=.38, all p<.001). ADHD symptom dimensions were also significantly correlated (Current IN/Current HI: r=.55; Childhood IN/Childhood HI: r=.69; Current IN/Childhood IN: r=.48; Current HI/Childhood HI: r=.57, all p<.001). Table 2 presents the correlations among substance use and current and childhood ADHD symptoms.
Table 2.
Correlations among substance use and ADHD symptoms
| Zero-order Correlations
|
|||||||
|---|---|---|---|---|---|---|---|
| Cigarette Use+ | Alcohol Use+ | Cannabis Measures | |||||
|
| |||||||
| Use+ | Dependence | Craving | Problems | Age of Initiation | |||
|
| |||||||
| ADHD Symptoms | |||||||
| Childhood IN | .06 | .21** | .11 | .15* | .13* | .17** | .07 |
| Childhood HI | .07 | .22** | .11 | .14* | .11 | .16* | −.07 |
| Current IN | .26** | .22** | .25** | .27** | .28** | .35** | −.05 |
| Current HI | .12 | .11 | .09 | 07 | .10 | .12 | −.03 |
| Comorbid Psychopathology | |||||||
| ASR Internalizing | .09 | −.06 | .05 | .12 | .11 | .18** | .03 |
| ASR Externalizing | .17** | .24** | .21** | .24** | .26** | .33** | −.06 |
p< 0.01;
p< 0.001 level (2-tailed);
Cigarette, Alcohol, and Cannabis Use based on self-report of use in past 30 days on Timeline Follow Back: total number of cigarettes, total number of alcoholic drinks, and total days used cannabis.
IN=Inattention; HI=Hyperactivity-Impulsivity; ASR=Adult Self-Report.
3.2. Regressions testing associations with current and childhood ADHD and cannabis, alcohol, and smoking measures
Table 3 (first block) summarizes multiple regression analyses in which current IN and HI predicted substance use outcomes while controlling for current internalizing and externalizing psychopathology. Current IN was independently associated with more severe outcomes on nearly all measures, indicating that elevations of IN in young adults are associated with higher levels of cannabis dependence, craving, and problems related to cannabis use and higher levels of cannabis, alcohol, and cigarette use. In contrast, current HI were not independently associated with any of the substance use measures. Neither current IN nor HI were significantly associated with age of cannabis initiation. There were no significant current IN x HI interactions.
Table 3.
Multiple regressions examining symptoms of inattention (IN) and hyperactivity/impulsivity (HI) in predicting substance use outcomes
| Multiple Regressions testing current symptoms
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | IN | HI | IN x HI Interaction | Model | |||||
|
|
|
|
|
||||||
| B | 95% CI | B | 95% CI | B | 95% CI | R2 | F | ||
| Substance Use Measure | |||||||||
| Cannabis Dependence | 376 | .35** | [.18, .52] | −.09 | [−.25, .07] | −.14 | [−.31, .02] | .13 | 8.8** |
| Age of initiation1 | 244 | −.61 | [−1.7, .51] | −.17 | [−1.3, 1.0] | .45 | [−.61, 1.5] | .02 | .65 |
| Craving for cannabis | 376 | .14* | [.06, .22] | −.06 | [−.13, .02] | −.03 | [−.11, .05] | .12 | 8.4** |
| Problems related to cannabis use | 376 | .10** | [.06, .15] | −.03 | [−.07, .01] | −.04 | [−.08, .00] | .19 | 14.4** |
| Current cannabis use | 376 | .64** | [.32, .96] | −.03 | [−.32, .26] | −.28 | [−.59, .03] | .10 | 6.8** |
| Current alcohol use | 376 | .80** | [.44, .1.2] | .16 | [−.17, .49] | −.48 | [−.83,−.14] | .15 | 10.5** |
| Current cigarette use | 376 | .49* | [.15, .82] | −.10 | [−.40, .21] | −.06 | [−.38,.26] | .08 | 5.3** |
|
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Multiple Regressions testing childhood symptoms
| |||||||||
| N | IN | HI | IN x HI Interaction | Model | |||||
|
|
|
|
|
||||||
| B | 95% CI | B | 95% CI | B | 95% CI | R2 | F | ||
| Cannabis Dependence | 376 | .12 | [−.02, .25] | .07 | [−.04, .17] | −.05 | [−.14, .05] | .03 | 2.9+ |
| Age of initiation1 | 244 | .22 | [.55, 1.0] | −.97* | [−1.7, .28] | .40 | [−.16, .96] | .04 | 2.5+ |
| Craving for cannabis | 376 | .08* | [.02,.15] | .04 | [−.01, .09] | −.05+ | [−.09, .00] | .03 | 2.8+ |
| Problems related to cannabis use | 376 | .07* | [.02, .12] | .02 | [−.01, .04] | −.03* | [−.05, −.01] | .05 | 4.6** |
| Current cannabis use | 376 | .47* | [.12, .82] | .10 | [−.05, .24] | −.23* | [−.40,−.05] | .03 | 3.0+ |
| Current alcohol use | 376 | .35* | [.08, .63] | .30* | [.08, .51] | −.19 | [−.39,.01] | .07 | 6.4** |
| Current cigarette use | 376 | .22 | [−.03, .48] | .17 | [−.03, .37] | −.17+ | [−.35,.01] | .02 | 1.6 |
Note. IN=Inattention; HI=Hyperactivity-Impulsivity. Gender and comorbid internalizing and (non-substance use related) externalizing symptom counts were included as covariates in all models testing current symptoms. Gender only included as a covariate in all models testing childhood symptoms.
Trend level p<.05;
p< 0.01 level (2-tailed);
p< 0.001 (2-tailed).
Associations with age of initiation were tested in the smaller sample of users only.
Table 3 (second block) summarizes multiple regression models for childhood IN and HI. These analyses were parallel to the analyses of current symptom with the exception that current symptoms of other psychopathology were not controlled. Childhood IN were independently associated with higher levels of cannabis craving, MPI, and use of cannabis and alcohol. Childhood HI was independently associated with earlier age cannabis initiation and higher levels of alcohol use. Neither childhood IN nor HI was independently associated with MDS.
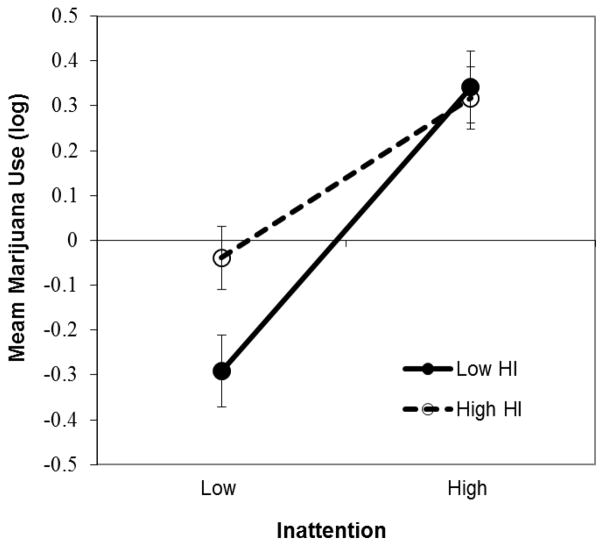
Significant IN x HI interactions were present in the models predicting MPI and level of cannabis use and were further examined using simple slope analyses (Aiken and West, 1991). Specifically, we estimated the slope of the association between IN and use at high and low levels of HI, defined as 1 SD above and below the sample mean. Each of the simple slope tests for the association between childhood IN and current use revealed a significant positive association (slope at high HI=.33, t=2.5, p<.01; slope at low HI=.61, t=2.7, p<.01) and these slopes differed significantly from each other (t=−2.6, p<.01). See Figure 1. An evaluation of means at high and low IN and high and low HI indicated that current use was similar for individuals with high IN regardless of HI symptoms (M=.34 for high IN/low HI and M=.32 for high IN/high HI) and that individuals with both low IN and low HI had the lowest levels of use (M=−0.29 for low IN/low HI and M=−0.03 for low IN/high HI). This pattern of significant slopes and means was the same for the associations among IN, HI, and MPI. Thus, for both current use and use-associated problems, higher IN was associated with more severe cannabis-related outcomes regardless of level of HI symptomatology, but the combination of low IN and HI was a protective factor.
Figure 1.
Days marijuana used in the past month (mean deviated and log transformed) as a function of high and low symptoms of inattention (IN), and high and low symptoms of hyperactivity-impulsivity (HI). High IN was associated with higher levels of use, regardless of level of HI symptomatology, but the combination of low IN and HI was a protective factor associated with lower levels of use.
3.3. Level of current cannabis use as a moderator of associations among ADHD and cannabis measures
The three cannabis outcomes (MDS, craving, and MPI) that showed significant associations with either ADHD symptom dimension were followed up with models that tested whether current level of cannabis use moderated the association. The critical terms in these models, the ADHD symptom dimension x use interaction terms, are presented in Table 4.
Table 4.
Multiple regressions of current cannabis use x ADHD symptom dimension interactions in predicting cannabis-related outcomes.
| Multiple regressions testing current symptoms
| |||||
|---|---|---|---|---|---|
| Use X IN | Use x HI | Use x IN x HI | Model | ||
|
| |||||
| B | B | B | R2 | F | |
| Cannabis Outcome | |||||
| Cannabis Dependence | 0.14* | −0.11 | 0.00 | 0.65 | 78.1** |
| Cannabis Craving | 0.03 | 0.04 | 0.01 | 0.64 | 70.5** |
| Problems related to cannabis use | 0.06** | −0.01 | −0.03 | 0.73 | 107.5** |
| Multiple regressions testing childhood symptoms
| |||||
|---|---|---|---|---|---|
| Use X IN | Use x HI | Use x IN x HI | Model | ||
|
| |||||
| B | B | B | R2 | F | |
| Cannabis Dependence | −0.01 | 0.00 | 0.02 | 0.63 | 92.8** |
| Cannabis Craving | 0.09 | 0.00 | −0.04 | 0.62 | 85.3** |
| Problems related to cannabis use | 0.05+ | 0.01 | −0.2 | 0.68 | 109.2** |
Note. IN=Inattention; HI=Hyperactivity-Impulsivity. Models were constructed to include current cannabis use, IN, HI, all possible two-way interactions, and the three-way interaction between current use, IN, and HI as predictors of the cannabis outcome. Gender and comorbid internalizing and (non-substance use related) externalizing symptom counts were included as covariates in all models testing current symptoms. Gender only included as a covariate in all models testing childhood symptoms.
Trend level p<.05;
p< 0.01 level (2-tailed);
p< 0.001 (2-tailed).
Significant cannabis use x current IN interactions emerged for MDS and MPI and were examined as above using simple slope analyses (Aiken and West, 1991). Each of the simple slope tests for the association between current use and cannabis dependence revealed a significant positive association between use and MDS (slope at high IN=.47, t=16.9, p<.001; slope at low IN=.35, t=12.4, p<.001; slope difference test: t=−2.8, p<.01); however, the slope was steepest for individuals with high levels of IN symptoms (see Figure 2). An evaluation of means at high and low use and high and low IN indicated that MDS was highest among individuals with a combination of high current use and high IN symptoms (M=.18 for high use/high IN; M=.08 for high use/low IN; M=−.38 for low use/high IN; M=−.34 for low use/low IN). This pattern of significant slopes and means was the same for the associations among IN, use, and MPI. Thus, for both dependence and use-associated problems, higher current use was associated with more severe cannabis-related outcomes and this relation was strongest among those with higher IN.
Figure 2.
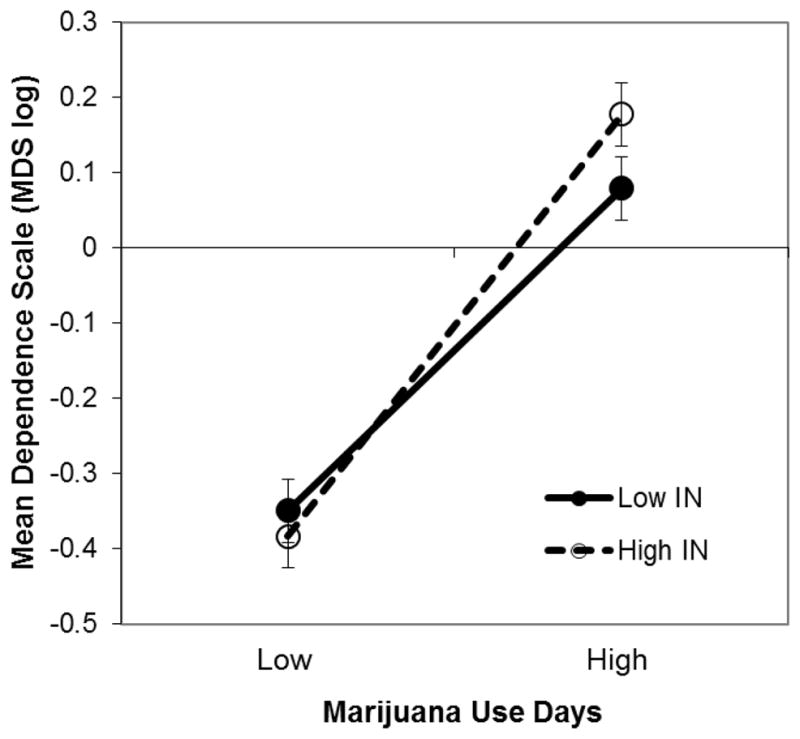
Marijuana dependence scale (MDS, mean deviated and log transformed scores) as a function of high and low marijuana use, and high and low symptoms of inattention (IN). Higher current use was associated with higher MDS scores and this relation was strongest among those with high IN.
4. DISCUSSION
Results from the current study indicate that both current and childhood ADHD symptoms are associated with a range of cannabis-related outcomes in a non-clinical sample of young adults and are consistent with prior meta-analytic work supporting a significant ADHD-CUD association (Lee et al., 2011). Our use of ADHD symptom dimensions (rather than nominal subtypes) and fine-grained measures related to cannabis use severity (rather than dichotomous CUD diagnoses) provides increased power to detect effects and allows for further disentangling specific facets of the CUD-ADHD comorbidity. In addition, our study extends previous research by demonstrating assocations with ADHD symptoms dimensions while statisitically controlling for current levels of comorbid internalizing and externalizing symptomology.
4.1. ADHD symptom dimensions
Current and childhood IN were independently associated with more severe cannabis outcomes in young adulthood including higher levels of current use, dependence (current IN only), craving, and use-related problems. In contrast, HI, specifically in childhood, was independently associated with earlier cannabis initiation, but no other cannabis-related outcome. These data suggest that the two symptom dimensions are not equivalent with regard to risk for cannabis outcomes and may show differential associations across stages of cannabis use. These findings are consistent with prior prospective (Molina and Pelham, 2003) and cross-sectional (Upadhyaya and Carpenter, 2008) work showing an independent association with IN and the frequency of marijuana use and may indicate that IN symptoms are particularly important in risk for cannabis-related outcomes in adulthood. In addition, findings from a large sample of twins followed from ages 11 to 18 (Elkins et al., 2007) indicated that childhood HI (prior to age 11), independently predicted onset of a CUD by age 18. These findings are broadly consistent with models that suggest that HI is associated with greater impairment in childhood with IN emerging as a more salient predictor of outcomes later in development (Barkley et al., 2008; Willcutt, 2012). Similarly, a prior study in nicotine dependence suggested that HI was associated with variables related to the progression from non-use to regular use, whereas both HI and IN were associated with levels of use in young adulthood (Fuemmeler et al., 2007). Thus, elevations of HI may be more strongly associated with initiation/early use of substances, whereas IN in young adulthood may be a greater liability once use is established.
4.2. Comorbidity with internalizing and externalizing symptoms
Despite strong associations among comorbid externalizing symptoms and substance measures, ADHD was independently associated with increased severity on the range of cannabis-related outcomes after controlling for concurrent internalizing and externalizing symptomatology. Although work in this area is limited, these findings are in contrast to prior studies that suggest that associations among ADHD and cannabis outcomes are largely attributable to comorbidity with oppositional defiant or conduct disorder symptoms (e.g., Pingault et al., 2013). However, a prior study that examined ADHD dimensionally reported that childhood ADHD symptoms, particularly HI, were significant risk factors for later CUD after controlling for the presence of a conduct disorder (Elkins et al., 2007). These studies suggest that comorbidity is a critical area for continued work and that there may be differential patterns of association with CUD-related outcomes when considering ADHD and comorbidities at the dimensional level and at various developmental stages.
4.3. Interactions with inattention and levels of current use
Significant IN x level of use interactions in association with cannabis outcomes indicated that IN further increased risk for dependence and problems among cannabis users. Although these results do not have any direct treatment implications, they suggest there may be merit in developing and refining targeted prevention and treatment efforts for problem users with high levels of ADHD IN symptoms. Considering these associations longitudinally and developmentally will be critical to teasing apart cause and effect relationships among these associations and determining which combinations of ADHD symptoms and cannabis use profiles are associated with treatment outcomes.
4.4. Associations with ADHD and other substances
We also report expected associations with ADHD symptoms and increased levels of alcohol and cigarette use. Although associations with ADHD and cigarette use were somewhat weaker than expected (only present for current IN and not for childhood), given that the ADHD-nicotine use association is highly robust (Charach et al., 2011; Kollins et al., 2005; Lee et al., 2011), it should be noted that cigarette use in this sample was quite low [M (SD)=0.4 (1.6) cig/day]. Despite this low smoking rate, significant ADHD-cigarette use associations were broadly supported in the current study and the consistency of these findings with prior work (Charach et al., 2011; Lee et al., 2011) is suggestive of the external validity of our CUD-related findings.
4.5. Limitations and Future Directions
We acknowledge the possibility that our findings of positive associations among self-report ratings of ADHD and more severe cannabis outcomes could be a result of neurocognitive changes that may occur with chronic cannabis use (Bolla et al., 2002). Thus, ADHD symptom ratings in users may have been exacerbated or biased by drug exposure and therefore did not contribute causally to the development of problem cannabis use, an explanation consistent with stronger associations with ratings of current symptoms. Buffering against this possibility are our findings of significant and independent associations with childhood ADHD symptoms and prior work showing a strong prospective associations with ADHD in childhood and risk for later CUD (Lee et al., 2011). However, prospective studies testing associations with dimensional measures of adult and childhood ADHD symptoms and cannabis outcomes are needed.
Many studies have demonstrated the reliability and validity of retrospective self-report measures of childhood and current ADHD symptoms in adults (Kooij et al., 2008; Willcutt et al., 2012) and several other studies of ADHD and substance use have used this approach (Bidwell et al., 2012; Fuemmeler et al., 2007; Upadhyaya and Carpenter, 2008). However, future studies could provide a useful extension of the current results by conducting a more comprehensive assessment of current and childhood ADHD through prospective designs or by obtaining diagnostic information from additional reporters such as parents and significant others.
Our results provide evidence that the relation between ADHD and cannabis use is not explained by concurrent internalizing and externalizing symptoms, but we did not have measures of these symptoms during childhood. Controlling for childhood oppositionality would have further substantiated our findings (Pingault et al., 2013).
An important methodological consideration for our study is the use of a non-clinical sample of college undergraduates. These students have advanced to the level of college, suggesting that they may have unique characteristics in comparison to the overall population of young adults with ADHD. Despite this potential conservative bias toward a higher functioning population, however, associations were significant between IN and cannabis use outcomes over and above HI and other comorbid symptomatology, underscoring the robust nature of the relation between ADHD and risk for problem substance use.
4.6. Conclusions
In summary, our findings extend work on the ADHD-CUD relation by using dimensional measures of ADHD, fine-grained measures of different aspects of cannabis use, and statistically controlling for comorbid psychopathology symptoms. Our findings indicate that IN and HI symptom dimensions may not be equivalent with regard to CUD risk and that gradations in ADHD symptom level that may not meet diagnostic thresholds associate with outcome severity. Further, our findings in a nonclinical sample suggest the robustness of these associations at subclinical levels and potentially generalize to a broader population of cannabis users beyond those with a clinical diagnosis of ADHD.
Acknowledgments
Role of Funding Source
This study was supported by DA024002 to Tiffany Ito and DA033302 to L. Cinnamon Bidwell.
We are grateful to Suzanne Taborsky-Barba, Jesse Kaye, Justin Busch, Luis Parra, Kevin Brown, Kismet Cordova, Holly Gerber, Kyle Healy, Loran Kelly, Caitlin Miner, and Nicholas Planet for their assistance with data collection.
Footnotes
Contributors
L. Cinnamon Bidwell was involved in the conceptualization of the analysis, data analysis and interpretation, drafting and editing the manuscript, and coordinating the contributions of all authors. Erika Henry was involved in data preparation and analysis, data interpretation, and drafting and editing the manuscript. Mikaela Kinnear was involved in the conceptualization of the analysis and drafting and editing the manuscript. Erik Willcutt and Tiffany Ito were each involved in the conceptualization of the overall project, oversight of data collection, conceptualization of these analyses, data interpretation, and drafting and editing the manuscript.
Conflict of Interest
The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA Adult Forms and Profiles. University of Vermont, Research Center for Children, Youth, and Families; Burlington, VT: 2003. [Google Scholar]
- Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Sage Publications; Newberry Park, CA: 1991. [Google Scholar]
- Barkley RA, Knouse LE, Murphy KR. Correspondence and disparity in the self- and other ratings of current and childhood ADHD symptoms and impairment in adults with ADHD. Psychol Assess. 2011;23:437–446. doi: 10.1037/a0022172. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Murphy K. Attention-deficit Hyperactivity Disorder: A Clinical Workbook. Vol. 2. Guilford Press; New York, NY: 1998. [Google Scholar]
- Barkley RA, Murphy KR, Fischer M. ADHD in Adults: What the Science Says. Guilford Press; New York, NY: 2008. [Google Scholar]
- Bidwell LC, Garrett ME, McClernon FJ, Fuemmeler BF, Williams RB, Ashley-Koch AE, Kollins SH. A preliminary analysis of interactions between genotype, retrospective ADHD symptoms, and initial reactions to smoking in a sample of young adults. Nicotine Tob Res. 2012;14:229–233. doi: 10.1093/ntr/ntr125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Wilens T, Mick E, Milberger S, Spencer TJ, Faraone SV. Psychoactive substance use disorders in adults with attention deficit hyperactivity disorder (ADHD): effects of ADHD and psychiatric comorbidity. Am J Psychiatry. 1995;152:1652–1658. doi: 10.1176/ajp.152.11.1652. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Charach A, Yeung E, Climans T, Lillie E. Childhood attention-deficit/hyperactivity disorder and future substance use disorders: comparative meta-analyses. J Am Acad Child Adolesc Psychiatry. 2011;50:9–21. doi: 10.1016/j.jaac.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Chung T, Martin C, Wood DS, Clark DB. Cannabis withdrawal is common among treatment-seeking adolescents with cannabis dependence and major depression, and is associated with rapid relapse to dependence. Addict Behav. 2008;33:1500–1505. doi: 10.1016/j.addbeh.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis ML, Funk R, Godley SH, Godley MD, Waldron H. Cross-validation of the alcohol and cannabis use measures in the Global Appraisal of Individual Needs (GAIN) and Timeline Followback (TLFB; Form 90) among adolescents in substance abuse treatment. Addiction. 2004;99(Suppl 2):120–128. doi: 10.1111/j.1360-0443.2004.00859.x. [DOI] [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Arch Gen Psychiatry. 2007;64:1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Youngstrom EA, Naugle RI. The latent structure of attention-deficit/hyperactivity disorder in a clinic-referred sample. Neuropsychology. 2007;21:45–64. doi: 10.1037/0894-4105.21.1.45. [DOI] [PubMed] [Google Scholar]
- Fuemmeler BF, Kollins SH, McClernon FJ. Attention deficit hyperactivity disorder symptoms predict nicotine dependence and progression to regular smoking from adolescence to young adulthood. J Pediatr Psychol. 2007;32:1203–1213. doi: 10.1093/jpepsy/jsm051. [DOI] [PubMed] [Google Scholar]
- Gudjonsson GH, Sigurdsson JF, Eyjolfsdottir GA, Smari J, Young S. The relationship between satisfaction with life, ADHD symptoms, and associated problems among university students. J Atten Disord. 2009;12:507–515. doi: 10.1177/1087054708323018. [DOI] [PubMed] [Google Scholar]
- Hayatbakhsh MR, Najman JM, Jamrozik K, Mamun AA, Alati R, Bor W. Cannabis and anxiety and depression in young adults: a large prospective study. J Am Acad Child Adolesc Psychiatry. 2007;46:408–417. doi: 10.1097/chi.0b013e31802dc54d. [DOI] [PubMed] [Google Scholar]
- Hjorthoj CR, Hjorthoj AR, Nordentoft M. Validity of Timeline Follow-Back for self-reported use of cannabis and other illicit substances--systematic review and meta-analysis. Addict Behav. 2012;37:225–233. doi: 10.1016/j.addbeh.2011.11.025. [DOI] [PubMed] [Google Scholar]
- Johnson V, White HR. An investigation of factors related to intoxicated driving behaviors among youth. J Stud Alcohol. 1989;50:320–330. doi: 10.15288/jsa.1989.50.320. [DOI] [PubMed] [Google Scholar]
- Kollins SH, McClernon FJ, Fuemmeler BF. Association between smoking and attention-deficit/hyperactivity disorder symptoms in a population-based sample of young adults. Arch Gen Psychiatry. 2005;62:1142–1147. doi: 10.1001/archpsyc.62.10.1142. [DOI] [PubMed] [Google Scholar]
- Kooij JJS, Boonstra AM, Swinkels SHN, Bekker EM, de Noord I, Buitelaar JK. Reliability, validity, and utility of instruments for self-report and information report concerning symptoms of ADHD in adult patients. J Atten Disord. 2008;11:445–458. doi: 10.1177/1087054707299367. [DOI] [PubMed] [Google Scholar]
- Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: a meta-analytic review. Clin Psychol Rev. 2011;31:328–341. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy F. Synaptic gating and ADHD: a biological theory of comorbidity of ADHD and anxiety. Neuropsychopharmacology. 2004;29:1589–1596. doi: 10.1038/sj.npp.1300469. [DOI] [PubMed] [Google Scholar]
- Looby A. Childhood attention deficit hyperactivity disorder and the development of substance use disorders: valid concern or exaggeration? Addict Behav. 2008;33:451–463. doi: 10.1016/j.addbeh.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Molina BS, Pelham WE., Jr Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. J Abnorm Psychol. 2003;112:497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- Pingault JB, Cote SM, Galera C, Genolini C, Falissard B, Vitaro F, Tremblay RE. Childhood trajectories of inattention, hyperactivity and oppositional behaviors and prediction of substance abuse/dependence: a 15-year longitudinal population-based study. Mol Psychiatry. 2013;18:806–812. doi: 10.1038/mp.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Association (SAMHSA) Results from the 2011 National Survey on Drug Use and Health: National Findings. U.S. Government Printing Office; Rockville, MD: 2011. [Google Scholar]
- Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. J Consult Clin Psychol. 2000;68:898–908. [PubMed] [Google Scholar]
- Upadhyaya HP, Carpenter MJ. Is attention deficit hyperactivity disorder (ADHD) symptom severity associated with tobacco use? Am J Addict. 2008;17:195–198. doi: 10.1080/10550490802021937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9:490–499. doi: 10.1007/s13311-012-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rohde LA, Tannock R, Loo SK, Carlson CL, McBurnett K, Lahey BB. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J Abnorm Psychol. 2012;121:991–1010. doi: 10.1037/a0027347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. J Abnorm Psychol. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]