Abstract
The sesquiterpene lactone parthenolide has recently attracted considerable attention owing to its promising antitumor properties, in particular in the context of stem-cell cancers including leukemia. Yet, the lack of viable synthetic routes for reelaborating this complex natural product have represented a fundamental obstacle toward further optimization of its pharmacological properties. Here, we demonstrate how this challenge could be addressed via selective, late-stage sp3C—H bond functionalization mediated by P450 catalysts with tailored site-selectivity. Taking advantage of our recently introduced tools for high-throughput P450 fingerprinting and fingerprint-driven P450 reactivity prediction, we evolved P450 variants useful for carrying out the highly regioselective hydroxylation of two aliphatic sites (C9and C14) in parthenolide carbocyclic backbone. By chemoenzymatic synthesis, a panel of novel C9- and C14-modified parthenolide analogs were generated in order to gain initial structure-activity insights on these previously inaccessible sites of the molecule. Notably, some of these compounds were found to possess significantly improved antileukemic potency against primary acute myeloid leukemia cells, while exhibiting low toxicity against normal mature and progenitor hematopoietic cells. By identifying two ‘hot spots’ for improving the anticancer properties of parthenolide, this study highlights the potential of P450-mediated C—H functionalization as an enabling, new strategy for the late stage manipulation of bioactive natural product scaffolds.
INTRODUCTION
The past decade has witnessed an increasing interest for the natural product class of sesquiterpene lactones, a group of plant-derived 15-carbon terpenoids arising from the biosynthetic assembly of three isoprene units and containing a lactone ring either cis- or trans- fused to the carbocyclic skeleton.1,2 Among them, parthenolide (1, PTL, Scheme 1) has attracted particular attention owing to its promising potential as an anticancer agent.3,4 In recent studies, PTL was found capable of inducing robust apoptosis in primary acute myelogenous leukemia (AML) cells, proving to be equally effective amongst all subpopulations within primary AML specimens, including the so-called leukemia stem cells (LSCs).5-7 LSCs are believed to play a crucial role not only in the genesis of AML8,9 but also in the clinical relapse of AML patients following traditional chemotherapy10 due to their reduced responsiveness to chemotherapeutic agents that kill actively cycling cells.11-13 Thus, the LSC-targeting ability of PTL makes this molecule particularly relevant toward the development of more effective treatments for AML and other hematologic malignancies. In addition, PTL was found to possess notable antiproliferative properties against many other types of human cancers, including breast,14, 15 lung,16 prostate,17 liver,18, 19 brain,20 pancreas,21 and bone22 cancer.
Scheme 1.
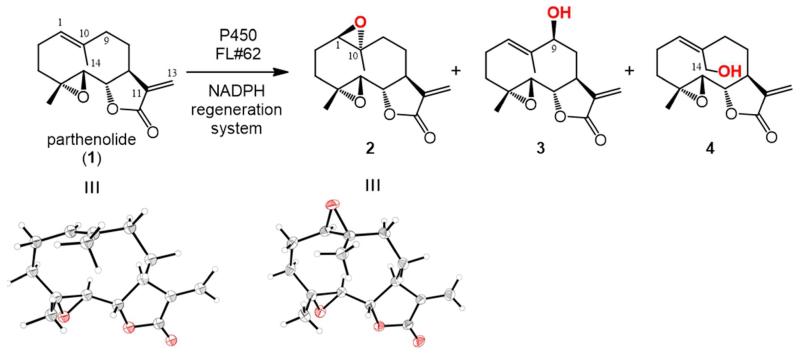
(a) Chemical structure of parthenolide (1, PTL) and of the oxidation products obtained from reaction with P450BM3 variant FL#62. Product distribution: 77% 2; 13% 3; 10% 4. X ray crystal structures of 163 and 2 (this study) are shown.
The anticancer properties of parthenolide has been primarily associated to its ability to inhibit the transcription factor NF-κB,23-25 which is known to control multiple tumor-related processes such as inflammation, proliferation, angiogenesis, and metastasis.4 However, additional mechanisms have been recently found to lie at the basis of the pharmacological effect of this molecule in cancer cells, which include activation of p535, 26 and proapoptotic Bcl-2 proteins27, induction of oxidative stress18, 28, 29, and alteration of epigenetic mechanisms30, 31.
Owing to the promising anticancer properties of PTL, and in particular to its ability to target cancer stem cells, there is currently a high interest in reelaborating this natural product scaffold to obtain derivatives with enhanced potency and improved drug-like properties. Previous efforts in this direction have taken advantage of the reactivity of the α-methylene-γ-lactone moiety, resulting in the preparation of various C13-modified PTL analogs.7, 32-36 However, the α-methylene-γ-lactone moiety is also critical for mediating PTL pharmacological effects, serving as an electrophilic center in Michael-type addition reactions with sulphydryl groups in the various cellular components (e.g. NF-κB, IκK, glutathione) targeted by the molecule.23, 24, 28, 37 As a result, the tolerance of this site to functionalization has been limited, with the corresponding C13-substituted derivatives often exhibiting a large decrease or complete loss of biological activity compared to PTL.7, 32-36 As an exception, a few C13-amino adducts, and in particular 11,13-dehydro-13-dimethylamino-parthenolide (DMAPT),7, 32 has been shown to retain comparable anticancer activity to PTL, while presenting improved oral bioavailability due to the increased water-solubility of the amine adduct.7 Notably, DMAPT has advanced to clinical trials for the treatment of AML and other hematologic malignancies. Despite this progress, improvements in PTL anticancer potency have not been achieved to date, posing a barrier to the development of more effective and selective parthenolide-based anticancer agents.
Based on our previous work,38, 39 we envisioned that P450-mediated C—H functionalization could provide a means to expand opportunities for the functional reelaboration of the PTL scaffold toward this goal. Cytochrome P450 enzymes constitute an attractive catalytic platform for the oxyfunctionalization of unactivated C—H bonds in organic molecules,40-48 complementing and often extending beyond the scope of chemical oxidation reagents and catalysts.49 In particular, methodologies for high-throughput P450 active-site mapping (“P450 fingerprinting”) and P450 reactivity prediction recently introduced by our group have provided a way to guide and accelerate the development of P450 catalysts with fine-tuned regio- and stereoselectivity,38, 39 a key requirement toward the application of these biological catalysts for synthetic applications.49 Here, we demonstrate how these tools proved useful in rapidly generating a panel of engineered P450 variants, derived from the bacterial, catalytically self-sufficient CYP102A1 (B. megaterium)50, for the highly regio- and stereoselective oxidative activation of two sp3 C—H sites (C9 and C14) as well as of the C1,C10 double bond in PTL carbocyclic skeleton. Using these catalysts, a series of novel, C9- and C14-substituted parthenolide derivatives were made available by chemoenzymatic synthesis for activity evaluation in assays with primary AML specimens. Importantly, these studies demonstrate that both the C9 and C14 positions constitute ‘hot spots’ for improving the antileukemic potency of parthenolide, while granting high selectivity against malignant cells over normal mature and progenitor hematopoietic cells.
RESULTS AND DISCUSSION
Parthenolide oxidation via a substrate-promiscuous P450BM3 variant
In previous studies,38 we found that a substrate-promiscuous variant of the fatty acid monooxygenase P450BM3 (B. megaterium),50 called FL#62, is able to oxidize a broad range of bulky compounds, including decaline-based terpenes. Accordingly, we expected that our target compound, parthenolide, could be equally well accepted by this P450 as a substrate for oxidation. Gratifyingly, FL#62 was found to be capable of efficiently oxidizing PTL, supporting more than 1,000 total turnovers (TTN) and producing a mixture of three monooxygenated products in 77:13:10 ratio as determined by GC-MS. Structural elucidation by NMR and X-ray crystallography revealed that the major product consisted of 1(R),10(R)-epoxy-parthenolide (2, 77%), while the two minor products consisted of 9(S)-hydroxy-parthenolide (3, 13%) and 14-hydroxy-parthenolide (4, 10%), respectively (Scheme 1). The stereochemical configuration of the newly formed oxirane ring in 2 was determined by X-ray crystallographic analysis (Scheme 1). On the other hand, the observation of NOEs between the 9(H) and 1(H) protons allowed for the unambiguous assignment of the S configuration to the C9 carbon in 3. In contrast to FL#62, wild-type P450BM3 showed minimal PTL-oxidation activity (30 TTN), producing the epoxide 2 as the only product.
Compounds 1 and 2 correspond to naturally occurring derivatives of parthenolide, 51, 52 whereas compound 4 has been previously obtained as a minor product (4%) from microbial metabolism of PTL53. The hydroxylation products 3 and 4 were of particular interest to us, as they can provide two valuable intermediates, not accessible via currently available synthetic methods, for reelaboration of parthenolide carbocyclic skeleton by chemoenzymatic means. Indeed, while chemical oxidation of the allylic site C14 in parthenolide has been reported (with SeO2 and t-BuOOH),54 this transformation is accompanied by a Z→E isomerization of the 1,10-double bond, leading to an important structural reorganization of the 10-membered ring of the molecule.55 Interestingly, the sites targeted by FL#62 (i.e. si face of 1,10 C=C bond, pro-S C(9)—H bond, and C(14)—H bond) are localized within the same region of the molecule, as evinced from inspection of the crystal structure of 2 (Scheme 1). While the expanded active-site of FL#62 is likely at the basis of the poor regio/stereocontrol in the oxidation of this and other substrates,38, 39 the preferential formation of 2 over 3 and 4 is likely to arise from the higher reactivity of the electron-rich olefinic group compared to the neighbouring allylic positions, C9 and C14, to P450-catalyzed oxidation. This electronic bias notwithstanding, our previous success in fine-tuning the site-selectivity of artemisinin-hydroxylating P450 variants suggested that P450 catalysts with improved site-selectivity toward each of the positions targeted by FL#62, and in particular the two, less activated aliphatic C—H sites, could be obtained via reelaboration of the enzyme active site in combination with our recently introduced P450 fingerprint-based tools38, 39 to expedite this process.
Prediction of PTL oxidation reactivity via fingerprint single component analysis (SCA)
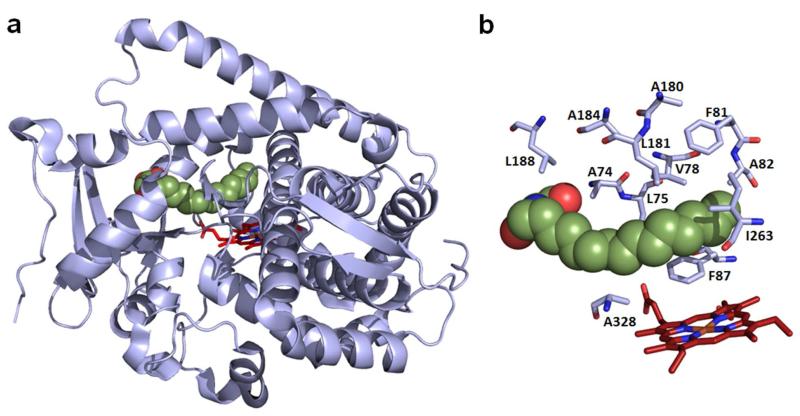
In the course of previous work, over 500 functionally diverse P450 catalysts derived from FL#62 were obtained via a two step process involving (a) simultaneous site-saturation mutagenesis of multiple ‘first-sphere’ active-site residues (i.e. 74, 78, 81, 82, 87, 181, and 184, Figure 1), followed by (b) high-throughput mapping of the active site configuration of the resulting engineered P450 variants by means of a panel of five structurally diverse chromogenic probes (compounds P1-P5, Figure S1). Through this process, a collection of 522 FL#62-derived P450s featuring a unique active site geometry and thus unique regio- and stereoselectivity properties (as derived from the uniqueness of their fingerprint profile)38 were thus made available for the search of more selective PTL-oxidizing P450 catalysts in the context of this work.
Figure 1.
(a) Crystal structure of P450BM3 heme domain in complex with N-palmitoylglycine (PDB code 1JPZ). The heme prosthetic group is displayed in red (stick model) and the enzyme-bound substrate in green (sphere model). (b) Close-up view of the enzyme active site, in which the amino acid residues targeted for mutagenesis in this study are highlighted.
We recently reported two complementary methods for predicting P450 reactivity based on the analysis of their fingerprints. A first one, suitable for probe-related target substrates, utilizes the fingerprint component corresponding to the probe most closely related, structurally, to the target substrate as a predictor of enzyme reactivity toward this molecule (referred to as fingerprint single component analysis, or SCA).38 More recently, we described a more general approach, applicable to probe-unrelated target molecules, that relies upon the multivariate analysis of fingerprint/substrate reactivity correlations using a randomly chosen training set of P450 variants (fingerprint multiple component analysis, or MCA).39 In the context of parthenolide, both approaches are feasible, which provided us with an interesting opportunity to compare side-by-side the performance and predictive capability of the two methods.
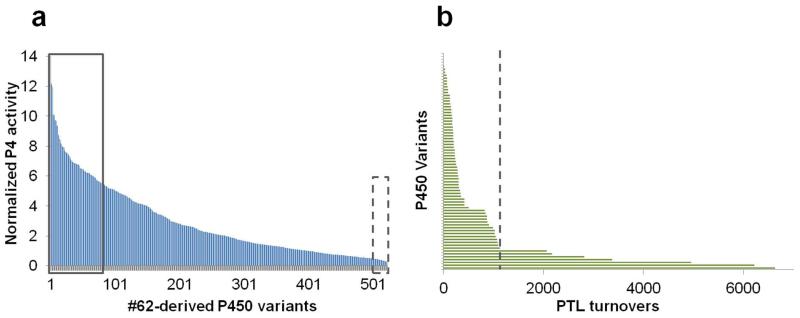
The Maximum Common Substructure (MCS) algorithm56, 57 is particularly well suited to assess the degree of structural similarity between core-related molecules.58 Based on this algorithm, the decaline-based probe P4 (Figure S1) was determined to represent the best predictor of parthenolide reactivity among the fingerprint probe set (SMCS = 0.68 vs. SMCS < 0.4 against P1, P2, P3, or P5, with SMCS ranging from 1 (structural identity) to 0 (max. structural dissimilarity)) via the SCA method. Accordingly, the collection of 522 FL#62-derived P450 catalysts were ranked based on their P4 probe activity in order to prioritize our search for PTL-oxidizing variants with improved regioselectivity (Figure 2a). Guided by these analyses, the 75 top ranking P450 variants were retrieved from the collection and tested for PTL-oxidation activity. Gratifyingly, we found that 81% of these variants (61/75) showed PTL oxidation activity at synthetically useful levels (>100 TTN; average = 695 TTN, Figure 2b). In addition, a good fraction (21%) exhibited higher PTL oxidation activity than the parent enzyme, with four of them supporting total turnover numbers in excess of 4,000 (Figure 2b). In contrast, 90% of the predicted inactive (i.e. bottom-ranking) variants (18/20) were found to be inactive on parthenolide, further supporting the reliability of the SCA predictions.
Figure 2.
Fingerprint-based prediction of parthenolide reactivity via fingerprint single component analysis (SCA). (a) Ranking of the 522 FL#62-derived P450 variants according to their normalized activity on the decaline-based probe P4 (Figure S1). The 75 top-ranking (solid box) and 20 bottom-ranking (dotted box) variants are highlighted. (b) Total turnovers in PTL oxidation for the 75 top-scoring P450 variants arranged from the least to the most active variant. TTN value for FL#62 is indicated for comparison (dotted line).
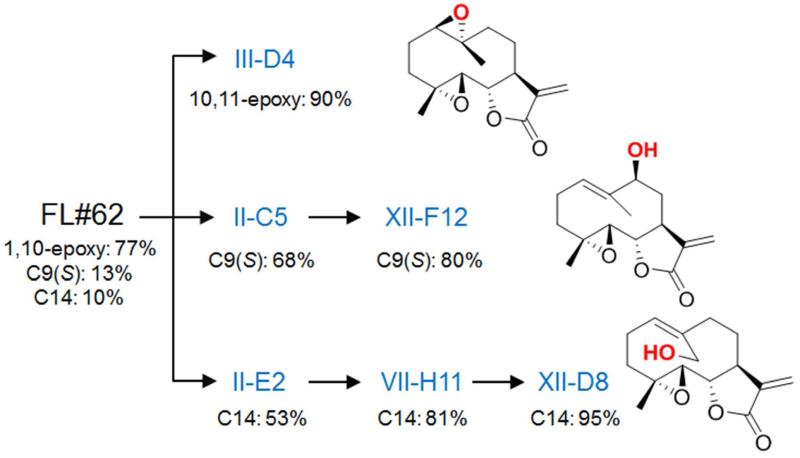
The parthenolide-oxidizing P450 variants identified in this manner exhibited in most cases diversified regioselectivity properties as expected from their different fingerprint profiles (Figure S2). Most importantly, P450 catalysts with improved regioselectivity toward each of three target oxidation sites were found. In particular, the triple mutant II-C5 displayed the largest improvement toward C9 hydroxylation (13% → 68%) while maintaining absolute S stereoselectivity and supporting more catalytic turnovers than FL#62 (Tables 1 and S2). On the other hand, the triple mutant II-E2 represented the best C14-hydroxylating catalyst (10% → 53%) within this generation of variants, supporting over 1,000 TTN in parthenolide oxidation (Tables 1 and S2). Finally, a P450 variant with improved regioselectivity and absolute stereoselectivity for production of 1(R),10(R)-epoxy-PTL (2), III-D4, was also found at this stage, this variant also exhibiting a nearly 5-fold increase in catalytic activity compared to the parent enzyme FL#62 (4,982 vs. 1,040 TTN).
Table 1.
Amino acid mutations and catalytic properties of parthenolide-oxidizing P450 variants.[a]
| Variant | Amino acid substitutions[b] | Product distribution (%) |
TTN | Product form. rate[c] |
Coupling efficiency (%)[d] |
Kd (μM) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 78 | 81 | 82 | 87 | 180 | 181 | 184 | 2 | 3 | 4 | |||||
| FL#62 | A | S | V | A | T | L | V | 77 | 13 | 10 | 1,042 ± 98 |
234 ± 23 |
55.8 | 243 ± 9 |
| III-D4 | F | V | A | 90 | 7 | 3 | 4,980 ± 430 |
214 ± 31 |
29.5 | 193 ± 67 |
||||
| II-C5 | T | I | A | 29 | 68 | 3 | 1,370 ± 153 |
59 ± 9 | 37.5 | 56 ± 12 |
||||
| XI-A11 | T | I | T | 22 | 77 | 1 | 1,710 ± 116 |
44 ± 2 | 60.8 | 340 ± 21 |
||||
| XII-F12 | T | I | T | A | 19 | 80 | 1 | 1,310 ± 71 |
27 ± 1 | 25.8 | 229 ± 38 |
|||
| II-E2 | N | F | A | 26 | 20 | 53 | 1,055 ± 59 |
176 ± 12 |
42.9 | 289 ± 116 |
||||
| VII-H11 | N | F | A | A | A | S | 17 | 2 | 81 | 420 ± 46 | 21 ± 3 | 31.1 | 478 ± 109 |
|
| XII-D8 | N | F | A | V | A | A | S | 4 | 0 | 95 | 60 ± 8 | 2 ± 0.6 | 1.4 | n.d. |
Mean values and standard deviations are calculated from triplicate experiments.
Compared to P450BM3, FL#62 carries the following mutations: V78A, F81S, A82V, F87A, P142S,T175I, A180T, A184V, A197V, F205C, S226R, H236Q, E252G, R255S, A290V, L353V.
Rates are measured over initial 30 seconds and expressed as mole product per mole P450 per minute.
Ratio between product formation rate and NADPH oxidation rate in the presence of parthenolide.
Parthenolide reactivity prediction via fingerprint multiple component analysis (MCA)
To compare the relative performance of MCA vs. SCA toward prediction of parthenolide reactivity, a training set consisting of 20 parthenolide-active P450 variants, including FL#62, was first assembled (Table S3). Then, a fingerprint-based predictive model of PTL reactivity was generated by correlating the fingerprints with the experimentally determined PTL oxidation activities (measured in TTNs) across the training set using multiple linear regression analysis (MLR) as described previously (Figure S2a).39 Using this model, the P450 catalysts in the 522-member collection were ranked according to their predicted PTL reactivity (Figure S2b). Experimental testing of the 75 top-ranking variants revealed that 62 of them (83%) were capable of PTL oxidation at synthetically useful levels (TTN > 100), supporting an average of 807 TTN (Figure S2c). To further probe the MCA-based predictions, the 20 bottom-ranking variants were also tested. Within this group, only six (30%) were found to be active (avg. TTN: 163). Interestingly, the group of 75 top-ranking P450s as defined by MCA largely overlapped with that based on SCA (64%) and included the best C9- and C14-hydroxylating variants (i.e. II-C5 and II-E2, respectively) previously identified by the latter method. None of the 27 variants uniquely found by MCA show further improved regioselectivity toward these positions. Based on these results, we conclude that both methods performed equally well in guiding the search for PTL oxidizing P450 catalysts.
Selective P450 catalysts for C9 and C14 hydroxylation
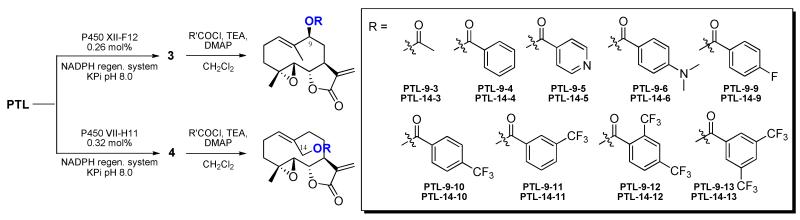
To achieve further improvements in regioselectivity for C9 hydroxylation, II-C5 was used as the parent enzyme for the construction of a series of single-mutant libraries via site-saturation mutagenesis (NNK degenerate codon) of positions 82, 87, 180, 181, 184, 263, and 328 (Figure 1). These positions correspond to ‘first-sphere’ active site residues (based on the available P450BM3 crystal structure59) that, with the exception of residue 82, have remained unmodified as compared to FL#62. High-throughput fingerprinting of these libraries (609 total recombinants screened) followed by fingerprint comparative analysis revealed the occurrence of 111 unique-fingerprint variants. Upon screening of these variants, three P450 catalysts with improved C9-selectivity (65-75%) were found, with the best one, XI-A11, also exhibiting higher catalytic activity than the parent enzyme II-C5 (1,710 vs. 1,040 TTN, Table 1). As expected, each of these variants carried a single active-site mutation, namely A82T (in XI-A11), A87S, and T180A. The latter two beneficial mutations were then introduced into XI-A11, alone and in combination. Among the resulting variants, XII-F12, which corresponds to XI A11(T180A), emerged as the best catalyst for C9-hydroxylation, being able to produce the desired product 3 with 80% regioselectivity, absolute S-stereoselectivity, and supporting over 1,300 TTN (Table 1).
Using a similar approach, the best C14-hydroxylating variant identified during the first step, II-E2, was selected as the starting point toward generating P450 catalysts that could provide selective access to the C14 aliphatic position. Conveniently, a panel of about 50 functionally diverse, II-E2 derived active-site variants were already available from our previous work.39 These variants were produced by triple site-saturation mutagenesis of active site residues 74, 181, and 184. Screening of this panel of P450 catalysts against parthenolide led to the discovery of a triple mutant variant, VII-H11, which showed significantly improved regioselectivity toward C14 hydroxylation compared to II-E2 (53% → 81%, Table 1), albeit at the expense of the catalytic activity (420 vs. 1,055 TTN). In a further round of directed evolution, additional first-sphere active residues left untouched in VII-H11 (i.e. positions 74, 75, 87, 263, and 328) were subjected to site-saturation mutagenesis. From these five single-mutant libraries, forty P450 variants were determined to be catalytically active and functionally diverse based on fingerprint analysis. Among these enzymes, XII-D8 represented the best catalyst for C14 hydroxylation, catalyzing the formation of 4 with excellent regioselectivity (95%, Table 1). Interestingly, also in this case, the improvement of C14-regioselectivity was accompanied by a reduction in the catalytic turnover number (Table 1).
Overall, the process outlined above enabled us to achieve our task of obtaining a panel of P450 catalysts with refined site-selectivity for each of the sites oxidized by the initial enzyme, FL#62, in the PTL carbocyclic scaffold (Figure 3). The fact that this goal could be accomplished by analyzing only a minimal fraction (1.8%) of the original engineered enzyme libraries (>16,500 members) highlights the peculiar advantage of the fingerprint-based analysis/prediction tools to guide this process.
Figure 3.
Overview of the directed evolution process leading to the selective parthenolide oxidizing P450 variants.
Characterization of the evolved P450 catalysts
Further experiments were conducted to characterize the best P450 variants for 1,10-epoxidation (III-D4), 9(S)-hydroxylation (XI-A11, XII-F12), and 14-hydroxylation (VII-H11, XII-D8), as well as their evolutionary intermediates (II-C5, II-E2), with respect to their kinetic parameters, substrate binding affinity, and coupling efficiency. Sequencing revealed that a small set of active site mutations (3-4), clustered within the back-end region of the heme pocket (i.e. positions 78, 81, 82, (180); Figure 1) were beneficial for steering the regioselectivity of the enzyme toward hydroxylation of the C9 site or toward epoxidation of the C1-C10 double bond (Table 1). In comparison, refinement of C14-regioselectivity required a larger number of amino acid substitutions (6-7), which include modification of the aforementioned positions as well as another cluster of spatially close active site residues (i.e. 180, 181, 184; Figure 1).
The effect of these mutations on the substrate binding affinity (KD) was assessed via substrate-induced heme spin shift experiments (Figures S3 S4). These analyses indicated that the parent enzyme FL#62 binds parthenolide with relatively moderate affinity (KD = 243 μM, Table 1), although this KD value remains within the range of those observed for wild-type P450BM3 and some fatty acid substrates (e.g. laurate; K = 270 μM).60 D Comparable equilibrium dissociation constants (190-290 μM) were measured for most of the improved variants, with the exception of VII-H11, which showed a roughly 2-fold higher KD, and XII-D8, which showed no signs of substrate-induced heme spin shift. Thus, these data suggest that, while being beneficial toward improving C14-selectivity, the mutations in the 180/181/184 cluster somewhat weaken the enzyme interaction with PTL.
Across the set of C9- and C14-hydroxylating variants, the progressive increase in site-selectivity was found to be generally accompanied by a decrease in the rate of substrate oxidation (Table 1). Interestingly, a similar trade-off was observed in the context of our previously reported artemisinin-hydroxylating P450s,39 suggesting that this trend may be a common feature within this class of the enzymes. This notwithstanding, all the improved variants, with the exception of VII-H11 and XII-D8, were able to support higher TTN compared to the initial enzyme FL#62, exhibiting coupling efficiencies (= ratio of product formation rate/NADPH oxidation rate) that range from 25% to 60% (Table 1). Interestingly, as noted above, the improvement of site-selectivity toward C14 hydroxylation was accompanied by a reduction in TTN, and in the case of XII-D8, also by a pronounced reduction in coupling efficiency, likely a result of the larger number of active site mutations accumulated by these variants. Nevertheless, the selectivity and catalytic efficiency of VII-H11 were well suited for synthetic purposes as demonstrated below.
Chemoenzymatic synthesis of PTL derivatives
With the strategy outlined above, three highly regio- and stereoselective P450 catalysts could thus be made available to explore the importance of the 1,10-double bond for parthenolide antileukemic activity (2) as well as provide two new points of entry (3, 4) for further functionalization of this molecule at the C9 and C14 sites via chemoenzymatic synthesis. To isolate sufficient quantities of 3 and 4 for these studies, preparative-scale reactions (100 mg PTL) were carried out using P450 variants XII-F12 (0.26 mol%) and VII-H11 (0.32 mol%), respectively, in the presence of a NADPH regeneration system consisting of a thermostable phosphite dehydrogenase61 and sodium phosphite as sacrificial reductant. VII-H11 was preferred over the more regioselective XII-D8 because of much higher total turnover numbers, making it a superior catalyst for synthetic purposes. From these reactions, about 75 mg of each of the desired oxidation products could be obtained in over 70% isolated yields.
The isolated 9(S)-hydroxy- (3) and 14-hydroxy-PTL (4) were then further processed to generate a panel of 9- and 14-substituted parthenolide derivatives (Scheme 2). To this end, direct acylation of 3 and 4 with acid chloride reagents was chosen as a rapid means to explore the accessibility of the C9 and C14 sites to substituents of varying size (e.g. acetyl vs. benzoyl group) and to gain initial structure activity insights on the impact of these modifications on PTL antileukemic activity. Based on the superior performance of the benzoylated analogs PTL-9/14-4 (vide infra), a second set of derivatives were prepared in which the benzoyl moiety was substituted with polar or lipophilic groups. Finally, the activity data collected with these compounds inspired the design and synthesis of a third set of parthenolide analogs in which two trifluoromethyl groups are installed at different positions of the benzoyl moiety.
Scheme 2.
Chemoenzymatic synthesis of the C9- and C14-substituted parthenolide derivatives (designated as PTL-9-(#) and PTL-14-(#), respectively).
Antileukemic activity of the PTL derivatives
To evaluate the antileukemic activity of the novel parthenolide derivatives, these compounds were tested against primary acute myelogenous leukemia (AML) cells obtained with informed consent from leukemia patients. In particular, two relapsed refractory AML specimens were utilized, which feature both a normal (AML100510) and a complex karyotype (AML123009), the latter exhibiting reduced sensitivity to PTL (LD50: 9.7 vs. 6.1 μM, Table 2). Dose-response curves were obtained by measuring the variation of cell viability at increasing compound concentration using a previously described assay based on cell staining with annexin-V and 7-amino-actinomycin (7-ADD) followed by flow cytometry analysis.62
Table 2.
LD50 values for parthenolide (PTL) and its chemoenzymatic derivatives against the two primary AML specimens and healthy bone marrow (BM) cells. The values in parenthesis indicate relative activities compared to PTL. n.d. = not determined.
| LD50
(μM) AML123009 |
LD50
(μM) AML100510 |
LD50 (μM) Bone Marrow |
|
|---|---|---|---|
|
| |||
| PTL | 9.7 (1) | 6.1 (1) | >80 |
|
| |||
| 2 | 13.5 (0.7) | 13.9 (0.4) | n.d. |
|
| |||
| 3 | 95 (0.1) | 17.4 (0.4) | n.d. |
| PTL-9-3 | > 100 | 24.2 (0.3) | n.d. |
| PTL-9-4 | 4.1 (2.4) | 6.2 (1.0) | >20 |
| PTL-9-5 | 6.1 (1.6) | 7.2 (0.8) | >50 |
| PTL-9-6 | 4.8 (2.0) | 3.1 (2.0) | >50 |
| PTL-9-9 | 6.3 (1.5) | 2.7 (2.2) | 25 |
| PTL-9-10 | 3.5 (2.7) | 4.3 (1.4) | 23 |
| PTL-9-11 | 4.6 (2.1) | 6.3 (1.0) | n.d. |
| PTL-9-12 | 2.3 (4.2) | 3.7 (1.6) | 44 |
| PTL-9-13 | 2.7 (3.6) | 5.1 (1.2) | 105 |
|
| |||
| 4 | >100 | > 100 | n.d. |
| PTL-14-3 | > 100 | 12.5 (0.5) | n.d. |
| PTL-14-4 | 6.4 (1.5) | 7.8 (0.8) | >80 |
| PTL-14-5 | 20.8 (0.5) | 8.7 (0.7) | n.d. |
| PTL-14-6 | 5.9 (1.7) | 5.4 (1.1) | n.d. |
| PTL-14-9 | 6.4 (1.5) | 6.2 (1.0) | n.d. |
| PTL-14-10 | 3.7 (2.6) | 3.1 (2.0) | 37 |
| PTL-14-11 | 7.8 (1.2) | 10 (0.6) | n.d. |
| PTL-14-12 | 5 (1.9) | 9.5 (0.6) | n.d. |
| PTL-14-13 | 2.5 (3.9) | 3.4 (1.8) | 25 |
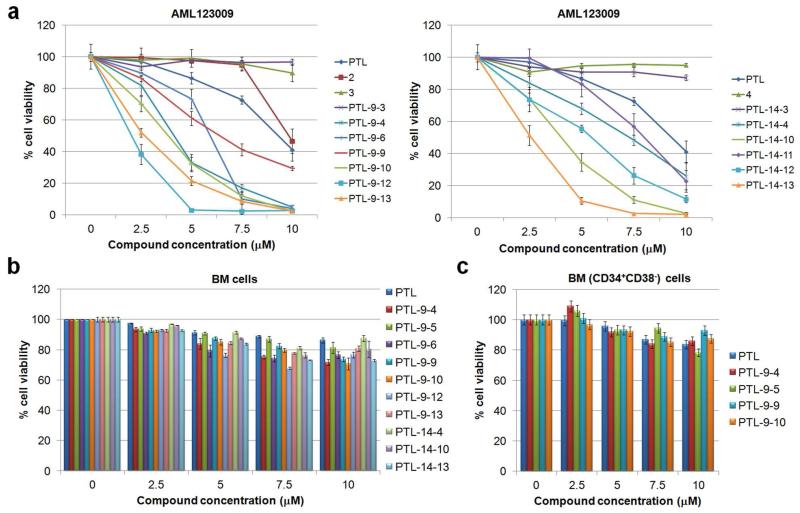
As illustrated in Figures 4a and S6, 1(R),10(R)-epoxy-PTL (2) was found to show a moderate (2-fold) reduction in potency compared to PTL in both AML specimens, possibly due to the slight structural change resulting from epoxidation of the 1,10-double bond as revealed by the crystallographic data (Scheme 1). Interestingly, a complete loss of activity was observed with the two hydroxylated derivatives 3 and 4, clearly pointing at the deleterious effect of introducing a polar, hydroxyl group at either the C9 or C14 site. Similarly, the acetylated derivatives PTL-9-3 and PTL-14-3, featured a dramatic reduction in antileukemic potency (Figures 4a, S6, and S7). In stark contrast, the benzoylated derivatives PTL-9-4 and PTL-14-4, were found to exhibit a significantly improved activity (compared to PTL) against the complex-karyotype AML cells (AML123009), as indicated by the two-fold lower LD50 values (Table 2). These results clearly showed the beneficial effect of larger, aromatic substituents at either the C9 or C14 sites toward potentiating PTL antileukemic activity. Accordingly, a set of compounds carrying variously substituted benzoyl groups at each of these positions. Notably, most of the resulting semisynthetic derivatives were found to be 2- to 3-fold more potent than parthenolide as illustrated by the dose-response curves in Figures 4a, S6, S7, and as summarized in Table 2. Within the C9-functionalized series, the largest increases in potency were achieved through substitution of the aryl moiety at the para position with fluorine (PTL-9-9), a dimethylamino (PTL-9-6), or trifluoromethyl group (PTL-9-10). A similar structure-activity trend was observed for the C14-functionalized series of compounds, although in this case the para-trifluoromethyl-benzoyl substituted derivative, PTL-14-10, emerged as the most potent derivative in the context of both AML specimens.
Figure 4.
Biological activity for representative parthenolide (PTL) analogs. (a) Dose response curves for C9- (left panel) and C14-modified (right panel) PTL derivatives tested against a complex-karyotype primary AML specimen (AML123009). Data for the remaining analogs with a second primary AML specimen (AML100510) cells are provided in Figure S6. (b-c) Cytotoxicity of the most potent PTL analogs against (b) total and (c) primitive (CD34+CD38−) normal bone marrow cells.
The beneficial effect of increasing the lipophilicity of the aryl moiety further suggested the design of compounds PTL-9/14-12 and PTL-9/14-13. Notably, the addition of a second trifluoromethyl group to the benzoyl moiety brought about a further increase in antileukemic potency for both the C9- and C14 modified analogs and in particular against AML123009 cells. Overall, the most promising compounds within each series, namely PTL-9-12 (LD50: 2.3 μM) and PTL-14-13 (LD50: 2.5 μM), were found to exhibit a 4.2- and 3.9-fold enhanced cytotoxicity, respectively, against primary AML cells compared to PTL (LD50: 9.7 μM, Table 2).
The most potent parthenolide derivatives identified in these studies were selected for further characterization to evaluate their selectivity against malignant over normal cells. For these studies, normal bone marrow cells (BM cells) obtained from healthy donors were utilized. Importantly, all these compounds, with the exception of PTL-9-6, did not significantly impart the viability of normal cells (Figure 4b), thus presenting the desired high selectivity against leukemic cells. Remarkably, at a concentration sufficient to kill 98% of primary AML cells (10 μM), compounds PTL-9-12 and PTL-9-13 were found to cause only less than 15% reduction in the viability of normal BM cells. For some of the most promising PTL analogs, it was also possible to test their cytotoxic effect on the progenitor (CD34+CD38−) cell sub-population of the bone marrow samples (Figure 4c). Notably, the compounds exhibited comparably low or even lower cytoxicity than in the context of mature BM cells.
Taken together, these results showed that functionalizations at the C9/C14 sites are not only beneficial in enhancing PTL cytotoxicity, but also that such effect is exerted with high selectivity in the context of leukemic cells. As a result, the LD50(BM)/LD50(AML) ratio of the original compound could be improved by several folds by means of these chemoenzymatic manipulations (e.g. 19 (PTL-9-12) and 39 (PTL-9-13) as compared to 8 for PTL with BM and AML123009 cells, Table 1). Given the relatively broad spectrum of cellular proteins/processes affected by PTL in cancer cells,5 it is possible that the functional modification at the C9/C14 sites may cause a shift in the protein-targeting profile of the molecule, in a way that affect preferentially the proliferation of malignant cells.
CONCLUSION
In conclusion, we have developed efficient, P450-based chemoenzymatic routes to access novel derivatives of the sesquiterpene lactone parthenolide with potent activity and high selectivity against AML cells. From a methodological point of view, this study demonstrates the efficiency of our P450 fingerprint-based tools as a way to accelerate the development of P450 oxidation catalysts with refined regio- and stereoselectivity, a current bottleneck toward the exploitation of these enzymes for synthetic applications. Since the aliphatic positions targeted by the engineered P450s developed here have so far remained inaccessible to chemical manipulation, this work also highlights the potential of P450-mediated C—H functionalization as a new, enabling strategy for the late-stage diversification and optimization of complex, bioactive molecules. A particularly relevant result from the present studies is the discovery that the C9 and C14 sites represent two ‘hot spots‘ for potentiating the antileukemic activity of parthenolide. Notably, the improvements in anticancer activity against AML cells could be achieved without increasing their cytotoxicity against normal hematopoietic cells, thereby effectively enhancing the therapeutic index of the molecule. These findings have important implications. On one hand, two parallel routes (i.e. via C9 or C14 functionalization) have now become available toward further optimization of parthenolide as a therapeutic agent. In this regard, the tolerance of each of these sites to variously substituted aryl substituents is very promising, as it suggests that a variety of functional groups may be explored at these positions toward this goal. Furthermore, our results raise intriguing questions regarding the biochemical mechanisms at the basis of the improved cytoxicity of the compounds in the context of malignant cells. Since parthenolide is known to target a variety of cellular components, it is plausible that the newly introduced functionalizations at the C9/C14 sites may shift the protein-targeting profile of the molecule in a way that affects preferentially the proliferation of malignant cells. We envision that future investigation of these aspects could provide valuable insights toward the development of effective pharmacological strategies for selective targeting of leukemia stem cells.
EXPERIMENTAL SECTION
For complete description of the materials and methods including reagents, cloning procedures, protein expression and purification, fingerprint-based analyses, and enzyme characterization, see Supporting Information.
Preparative-scale synthesis of 9(S)-, and 14-hydroxy-partenolide
To prepare 3, purified P450 variant XII-F12 (final conc: 2.5 μM; 0.26 mol%) was dissolved in 400 mL 50 mM phosphate buffer (pH 8.0) in the presence of parthenolide (100 mg, final conc.: 0.95 mM), PTDH (2 μM), NADP+ (150 μM), and sodium phosphite (50 mM). The reaction mixture was stirred for 12 hours at 4°C. The crude product was extracted with dichloromethane (3 × 80 mL). The collected organic layers were dried with sodium sulfate, concentrated under vacuum, and purified by flash chromatography (hexanes/ethyl acetate: 1/2) to afford 3 (75 mg, 70% (88% of theoretical maximum)) and 2 (16 mg, 15% (75% of theoretical maximum)). 9(S)-hydroxy-parthenolide (3): 1H NMR (500 MHz, CDCl3): δ 1.34 (s, 3 H), 1.76 (s, 3 H), 1.97-2.06 (m, 1 H), 2.15-2.27 (m, 4 H), 2.50 (dq, 1 H, J = 5.2, 12.4 Hz), 2.70 (d, 1 H, J = 8.7 Hz), 2.83-2.90 (m, 1 H), 3.86 (t , 1 H, J= 8.5 Hz), 4.27 (dd, 1 H, J = 2.2, 10.5 Hz), 5.42 (d, 1 H, J =11.3 Hz), 5.69 (d ,1 H, J = 3.2 Hz), 6.36 (d, 1 H, J = 3.6 Hz); 13C NMR (125 MHz, CDCl3): δ 10.9, 17.4, 23.9, 36.1, 38.0, 44.5, 61.4, 66.2, 79.7, 81.5, 121.6, 126.5, 136.6, 138.3, 168.8; HRMS (ESI) calcd for C15H20O4 [M+H]+ m/z: 265.1440; found: 265.1433; −83.9° (c: 0.43 g 100 mL−1, CH2Cl2). The 9(S) configuration of 3 was assigned based on the observed strong NOE signal (2.5%) between the 9(H) proton and 1(H) proton. 1(R),10(R)-epoxy-parthenolide (2): 1H NMR (500 MHz, CDCl3): δ 1.34 (s, 3 H), 1.36-1.42 (m, 4 H), 1.45-1.56 (m, 1 H), 1.56-1.65 (m, 1 H), 2.01-2.29 (m, 4 H), 2.47 (dd, 1 H, J= 8.1, 14.0 Hz), 2.70-2.76 (m, 1 H), 2.85 (d, 1 H, J= 12.2 Hz), 2.90 (d, 1 H, J= 8.9 Hz), 3.93 (t , 1 H, J= 8.9 Hz), 5.62 (d, 1 H, J= 3.0 Hz), 6.33 (d, 1 H, J= 3.0 Hz); 13C NMR (125 MHz, CDCl3): δ 17.0, 17.5, 24.0, 26.0, 35.1, 40.1, 47.6, 60.7, 63.7, 64.6, 81.8, 101.2, 121.4, 138.8, 168.9. HRMS (ESI) calcd for C15H20O4[M+H]+ m/z: 265.1440; found: 265.1441; = −71.0° (c: 0.24 g 100 mL−1, CH2Cl2).
To prepare 4, purified P450 variant VII-H11 (final conc: 3 μM; 0.32 mol%) was dissolved in 400 mL 50 mM phosphate buffer (pH 8.0) in the presence of parthenolide (100 mg, final conc.: 0.95 mM), PTDH (2 UM), NADP+ (150 UM), and sodium phosphite (50 mM). The reaction mixture was stirred for 12 hours at 4°C. The crude product was extracted with dichloromethane (3 × 80 mL). The collected organic layers were dried with sodium sulfate, concentrated under vacuum, and purified by flash chromatography (hexanes/ethyl acetate: 1/2) to afford 4 (77 mg, 72% (90% of theoretical maximum)) and 2 (17 mg, 16% (79% of theoretical maximum)). 14-hydroxy-parthenolide (4): 1H NMR (500 MHz, CDCl3): δ 1.31 (s, 3 H), 1.32-1.38 (m, 1 H), 1.82-1.1.92 (m, 1 H), 2.09-2.16 (m, 1 H), 2.20-2.32 (m, 3 H), 2.50 (dq, 1 H, J = 5.0, 13.4 Hz), 2.82-2.90 (m, 3 H), 3.92 (t , 1 H, J= 8.7 Hz), 4.16 (d, 1 H, J= 11.3 Hz), 4.46 (d, 1 H, J = 11.8 Hz), 5.43 (dd ,1 H, J= 4.1, 12.4 Hz), 5.68 (d, 1 H, J= 3.2 Hz), 6.39 (d, 1 H, J= 3.8 Hz). 13C NMR (125 MHz, CDCl3):δ 16.9, 23.7, 31.4, 36.3, 47.3, 59.8, 61.1, 66.2, 82.4, 121.4, 129.0, 137.8, 139.2, 169.3. HRMS (ESI) calcd for C15H20O [M+H]+ 4 m/z: 265.1440; found: 265.1440; =−61.7° (c: 0.13 g 100 mL 1, CH2Cl2).
General procedure for synthesis of parthenolide derivatives
To a solution of compound 3 (or 4) in 3 mL of anhydrous dichloromethane under argon atmosphere was added 4-dimethylaminopyridine (1 equiv.), triethylamine (5 equiv.), and the corresponding acid chloride (5 equiv.). Reaction was stirred at room temperature until complete disappearance of the starting material (ca. 2 hours). At this point, the reaction mixture was added with saturated sodium bicarbonate solution (5 mL) and extracted with dichloromethane (3 × 5 mL). The combined organic layers were dried over sodium sulfate, concentrated under reduced pressure, and the ester product was isolated by silica gel flash chromatography (5 to 40% ethyl acetate in hexanes). NMR and MS characterization data for these compounds are provided as Supporting Information.
Cell isolation and culture
Primary human AML and normal bone marrow (BM) cells were obtained from volunteer donors. Informed consent was obtained in accordance with the Declaration of Helsinki. All manipulation and analysis of human specimens was approved by the University of Rochester Institutional Review Board. In some cases, cells were cryopreserved in freezing medium of Iscove modified Dulbecco medium (IMDM), 40% fetal bovine serum (FBS), and 10% dimethylsulfoxide (DMSO) or in CryoStor CS-10 (VWR, West Chester, PA). Cells were cultured in serum-free medium (SFM)19 for 1 hour before the addition of parthenolide or its derivatives.
Cell viability assays
Apoptosis assays were performed as described.62 Briefly, after 24 hours of treatment, normal BM cells and AML specimens were stained for the surface antibodies CD38-allophycocyanin (APC), CD34-PECy7, and CD123-phycoerythin (Becton Dickinson, San Jose, CA) for 15 minutes. Cells were washed in cold PBS and resuspended in 200 μL of annexin-V–buffer (0.01 M HEPES/NaOH, 0.14 M NaCl, and 2.5 mM CaCl2). Annexin V-fluorescein isothiocyanate (FITC) and 7-aminoactinomycin (7-AAD; Molecular Probes, Eugene, OR) were added, and the tubes were incubated at room temperature for 15 minutes then analyzed on a BD LSRII flow cytometer (BD Biosciences, San Jose, CA). Analyses for phenotypically described stem cell subpopulations were performed by gating CD34+/CD38− populations. Viable cells were scored as Annexin-V negative/7-AAD negative. The percent viability data provided are normalized to untreated control specimens.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Health R01 grant GM098628 awarded to R.F.. C.T.J. was supported by a grant from the Leukemia and Lymphoma Society (TRP 6230-11). MS instrumentation was supported by the National Science Foundation grants CHE-0840410 and CHE-0946653. The authors are grateful to Bill Brennessel for assistance with the X-ray crystallographic analyses.
Footnotes
ASSOCIATED CONTENT
Supporting Information Chemical structures of probes P1-P5, oligonucleotide sequences, additional data corresponding to the fingerprint-based predictions and characterization of P450 variants, results from spin-shift experiments, characterization data for parthenolide derivatives. This material is available free of charge via the Internet at http://pubs.acs.org.
AUTHOR INFORMATION
Corresponding contributions R.F. and C.T.J conceived the project, J.N.K. conducted all the experiments concerning the engineering of the P450 catalysts and synthesis of the parthenolide derivatives, K.M.O. performed all the cellular and biochemical experiments concerning the biological activity of the compounds, R.F. wrote the manuscript with input from all the other co-authors.
REFERENCES
- (1).Ghantous A, Gali Muhtasib H, Vuorela H, Saliba NA, Darwiche N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov. Today. 2010;15:668–678. doi: 10.1016/j.drudis.2010.06.002. [DOI] [PubMed] [Google Scholar]
- (2).Janecka A, Wyrebska A, Gach K, Fichna J, Janecki T. Natural and synthetic alpha methylenelactones and alpha methylenelactams with anticancer potential. Drug Discov. Today. 2012;17:561–572. doi: 10.1016/j.drudis.2012.01.013. [DOI] [PubMed] [Google Scholar]
- (3).Guzman ML, Jordan CT. Feverfew: weeding out the root of leukaemia. Expert Opin. Biol. Th. 2005;5:1147–1152. doi: 10.1517/14712598.5.9.1147. [DOI] [PubMed] [Google Scholar]
- (4).Kreuger MR, Grootjans S, Biavatti MW, Vandenabeele P, D’Herde K. Sesquiterpene lactones as drugs with multiple targets in cancer treatment: focus on parthenolide. Anticancer Drugs. 2012;23:883–896. doi: 10.1097/CAD.0b013e328356cad9. [DOI] [PubMed] [Google Scholar]
- (5).Guzman ML, Rossi RM, Karnischky L, Li XJ, Peterson DR, Howard DS, Jordan CT. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;105:4163–4169. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Guzman ML, Karnischky L, Li XJ, Neering SJ, Rossi RM, Jordan CT. Selective induction of apoptosis in acute myelogenous leukemia stem cells by the novel agent parthenolide. Blood. 2004;104:697a–697a. [Google Scholar]
- (7).Guzman ML, Rossi RM, Li XJ, Corbett C, Hassane DC, Bushnell T, Carroll M, Sullivan E, Neelakantan S, Crooks PA, Jordan CT. A novel orally available parthenolide analog selectively eradicates AML stem and progenitor cells. Blood. 2006;108:74a–74a. doi: 10.1182/blood-2007-05-090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Cacerescortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A Cell Initiating Human Acute Myeloid Leukemia after Transplantation into Scid Mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- (9).Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- (10).van Rhenen A, Feller N, Kelder A, Westra AH, Rombouts E, Zweegman S, van der Pol MA, Waisfisz Q, Ossenkoppele GJ, Schuurhuis GJ. High stem cell frequency in acute myeloid leukemia at diagnosis predicts high minimal residual disease and poor survival. Clin. Cancer Res. 2005;11:6520–6527. doi: 10.1158/1078-0432.CCR-05-0468. [DOI] [PubMed] [Google Scholar]
- (11).Graham SM, Jorgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, Holyoake TL. Primitive, quiescent, Philadelphia positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–325. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- (12).Costello RT, Mallet F, Gaugler B, Sainty D, Arnoulet C, Gastaut JA, Olive D. Human acute myeloid leukemia CD34+/CD38 progenitor cells have decreased sensitivity to chemotherapy and Fas induced apoptosis, reduced immunogenicity, and impaired dendritic cell transformation capacities. Cancer Research. 2000;60:4403–4411. [PubMed] [Google Scholar]
- (13).Guzman ML, Swiderski CF, Howard DS, Grimes BA, Rossi RM, Szilvassy SJ, Jordan CT. Preferential induction of apoptosis for primary human leukemic stem cells. Proc. Natl. Acad. Sci. USA. 2002;99:16220–16225. doi: 10.1073/pnas.252462599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Sweeney CJ, Mehrotra S, Sadaria MR, Kumar S, Shortle NH, Roman Y, Sheridan C, Campbell RA, Murry DJ, Badve S, Nakshatri H. The sesquiterpene lactone parthenolide in combination with docetaxel reduces metastasis and improves survival in a xenograft model of breast cancer. Mol. Cancer Ther. 2005;4:1004–1012. doi: 10.1158/1535-7163.MCT-05-0030. [DOI] [PubMed] [Google Scholar]
- (15).Zhou J, Zhang H, Gu P, Bai J, Margolick JB, Zhang Y. NF-kappaB pathway inhibitors preferentially inhibit breast cancer stem like cells. Breast Cancer Res. Treat. 2008;111:419–427. doi: 10.1007/s10549-007-9798-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Zhang D, Qiu L, Jin X, Guo Z, Guo C. Nuclear factor kappaB inhibition by parthenolide potentiates the efficacy of Taxol in non-small cell lung cancer in vitro and in vivo. Mol. Cancer Res. 2009;7:1139–1149. doi: 10.1158/1541-7786.MCR-08-0410. [DOI] [PubMed] [Google Scholar]
- (17).Kawasaki BT, Hurt EM, Kalathur M, Duhagon MA, Milner JA, Kim YS, Farrar WL. Effects of the sesquiterpene lactone parthenolide on prostate tumor-initiating cells: An integrated molecular profiling approach. Prostate. 2009;69:827–837. doi: 10.1002/pros.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Wen J, You KR, Lee SY, Song CH, Kim DG. Oxidative-stress mediated apoptosis. The anticancer effect of the sesquiterpene lactone parthenolide. J. Biol. Chem. 2002;277:38954–38964. doi: 10.1074/jbc.M203842200. [DOI] [PubMed] [Google Scholar]
- (19).Park JH, Liu L, Kim IH, Kim JH, You KR, Kim DG. Identification of the genes involved in enhanced fenretinide-induced apoptosis by parthenolide in human hepatoma cells. Cancer Res. 2005;65:2804–2814. doi: 10.1158/0008-5472.CAN-04-2221. [DOI] [PubMed] [Google Scholar]
- (20).Zanotto Filho A, Braganhol E, Schroder R, de Souza LH, Dalmolin RJ, Pasquali MA, Gelain DP, Battastini AM, Moreira JC. NFkappaB inhibitors induce cell death in glioblastomas. Biochem. Pharmacol. 2011;81:412–424. doi: 10.1016/j.bcp.2010.10.014. [DOI] [PubMed] [Google Scholar]
- (21).Yip-Schneider MT, Nakshatri H, Sweeney CJ, Marshall MS, Wiebke EA, Schmidt CM. Parthenolide and sulindac cooperate to mediate growth suppression and inhibit the nuclear factor-kappa B pathway in pancreatic carcinoma cells. Mol. Cancer Ther. 2005;4:587–594. doi: 10.1158/1535-7163.MCT-04-0215. [DOI] [PubMed] [Google Scholar]
- (22).Zuch D, Giang AH, Shapovalov Y, Schwarz E, Rosier R, O’Keefe R, Eliseev RA. Targeting radioresistant osteosarcoma cells with parthenolide. J. Cell Biochem. 2012;113:1282–1291. doi: 10.1002/jcb.24002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Garcia Pineres AJ, Castro V, Mora G, Schmidt TJ, Strunck E, Pahl HL, Merfort I. Cysteine 38 in p65/NF-kappaB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J. Biol. Chem. 2001;276:39713–39720. doi: 10.1074/jbc.M101985200. [DOI] [PubMed] [Google Scholar]
- (24).Garcia Pineres AJ, Lindenmeyer MT, Merfort I. Role of cysteine residues of p65/NF-kappaB on the inhibition by the sesquiterpene lactone parthenolide and N-ethyl maleimide, and on its transactivating potential. Life Sci. 2004;75:841–856. doi: 10.1016/j.lfs.2004.01.024. [DOI] [PubMed] [Google Scholar]
- (25).Hehner SP, Heinrich M, Bork PM, Vogt M, Ratter F, Lehmann V, Schulze-Osthoff K, Droge W, Schmitz ML. Sesquiterpene lactones specifically inhibit activation of NF kappa B by preventing the degradation of I kappa B alpha and I kappa B beta. J. Biol. Chem. 1998;273:1288–1297. doi: 10.1074/jbc.273.3.1288. [DOI] [PubMed] [Google Scholar]
- (26).Gopal YN, Chanchorn E, Van Dyke MW. Parthenolide promotes the ubiquitination of MDM2 and activates p53 cellular functions. Mol Cancer Ther. 2009;8:552–562. doi: 10.1158/1535-7163.MCT-08-0661. [DOI] [PubMed] [Google Scholar]
- (27).Zhang S, Ong CN, Shen HM. Involvement of proapoptotic Bcl-2 family members in parthenolide induced mitochondrial dysfunction and apoptosis. Cancer Lett. 2004;211:175–188. doi: 10.1016/j.canlet.2004.03.033. [DOI] [PubMed] [Google Scholar]
- (28).Skalska J, Brookes PS, Nadtochiy SM, Hilchey SP, Jordan CT, Guzman ML, Maggirwar SB, Briehl MM, Bernstein SH. Modulation of cell surface protein free thiols: a potential novel mechanism of action of the sesquiterpene lactone parthenolide. PLoS One. 2009;4:e8115. doi: 10.1371/journal.pone.0008115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Wang W, Adachi M, Kawamura R, Sakamoto H, Hayashi T, Ishida T, Imai K, Shinomura Y. Parthenolide induced apoptosis in multiple myeloma cells involves reactive oxygen species generation and cell sensitivity depends on catalase activity. Apoptosis. 2006;11:2225–2235. doi: 10.1007/s10495-006-0287-2. [DOI] [PubMed] [Google Scholar]
- (30).Liu Z, Liu S, Xie Z, Pavlovicz RE, Wu J, Chen P, Aimiuwu J, Pang J, Bhasin D, Neviani P, Fuchs JR, Plass C, Li PK, Li C, Huang TH, Wu LC, Rush L, Wang H, Perrotti D, Marcucci G, Chan KK. Modulation of DNA methylation by a sesquiterpene lactone parthenolide. J. Pharmacol. Exp. Ther. 2009;329:505–514. doi: 10.1124/jpet.108.147934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Gopal YN, Arora TS, Van Dyke MW. Parthenolide specifically depletes histone deacetylase 1 protein and induces cell death through ataxia telangiectasia mutated. Chem. Biol. 2007;14:813–823. doi: 10.1016/j.chembiol.2007.06.007. [DOI] [PubMed] [Google Scholar]
- (32).Neelakantan S, Nasim S, Guzman ML, Jordan CT, Crooks PA. Aminoparthenolides as novel anti leukemic agents: Discovery of the NF kappaB inhibitor, DMAPT (LC-1) Bioorg. Med. Chem. Lett. 2009;19:4346–4349. doi: 10.1016/j.bmcl.2009.05.092. [DOI] [PubMed] [Google Scholar]
- (33).Nasim S, Crooks PA. Antileukemic activity of aminoparthenolide analogs. Bioorg Med. Chem. Lett. 2008;18:3870–3873. doi: 10.1016/j.bmcl.2008.06.050. [DOI] [PubMed] [Google Scholar]
- (34).Hwang DR, Chang CW, Lien TW, Chen WC, Tan UK, Hsu JTA, Hsieh HP. Synthesis and anti viral activity of a series of sesquiterpene lactones and analogues in the subgenomic HCV replicon system. Bioorgan. Med. Chem. 2006;14:83–91. doi: 10.1016/j.bmc.2005.07.055. [DOI] [PubMed] [Google Scholar]
- (35).Han C, Barrios FJ, Riofski MV, Colby DA. Semisynthetic derivatives of sesquiterpene lactones by palladium catalyzed arylation of the alpha-methylene-gamma-lactone substructure. J. Org. Chem. 2009;74:7176–7179. doi: 10.1021/jo901533e. [DOI] [PubMed] [Google Scholar]
- (36).Woods JR, Mo H, Bieberich AA, Alavanja T, Colby DA. Fluorinated amino-derivatives of the sesquiterpene lactone, parthenolide, as (19)f NMR probes in deuterium free environments. J. Med. Chem. 2011;54:7934–7941. doi: 10.1021/jm201114t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Kwok BH, Koh B, Ndubuisi MI, Elofsson M, Crews CM. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem. Biol. 2001;8:759–766. doi: 10.1016/s1074-5521(01)00049-7. [DOI] [PubMed] [Google Scholar]
- (38).Zhang K, El Damaty S, Fasan R. P450 Fingerprinting Method for Rapid Discovery of Terpene Hydroxylating P450 Catalysts with Diversified Regioselectivity. J. Am. Chem. Soc. 2011;133:3242–3245. doi: 10.1021/ja109590h. [DOI] [PubMed] [Google Scholar]
- (39).Zhang K, Shafer BM, Demars MD, 2nd, Stern HA, Fasan R. Controlled oxidation of remote sp3 C-H bonds in artemisinin via P450 catalysts with fine-tuned regio- and stereoselectivity. J. Am. Chem. Soc. 2012;134:18695–18704. doi: 10.1021/ja3073462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Glieder A, Farinas ET, Arnold FH. Laboratory evolution of a soluble, self-sufficient, highly active alkane hydroxylase. Nat. Biotechnol. 2002;20:1135–1139. doi: 10.1038/nbt744. [DOI] [PubMed] [Google Scholar]
- (41).Fasan R, Chen MM, Crook NC, Arnold FH. Engineered alkane-hydroxylating cytochrome P450(BM3) exhibiting native-like catalytic properties. Angew. Chem. Int. Ed. 2007;46 doi: 10.1002/anie.200702616. [DOI] [PubMed] [Google Scholar]
- (42).Rentmeister A, Arnold FH, Fasan R. Chemo-enzymatic fluorination of unactivated organic compounds. Nat. Chem. Biol. 2009;5 doi: 10.1038/nchembio.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Whitehouse CJ, Bell SG, Tufton HG, Kenny RJ, Ogilvie LC, Wong LL. Evolved CYP102A1 (P450BM3) variants oxidise a range of non-natural substrates and offer new selectivity options. Chem. Commun. 2008:966–968. doi: 10.1039/b718124h. [DOI] [PubMed] [Google Scholar]
- (44).Li S, Chaulagain MR, Knauff AR, Podust LM, Montgomery J, Sherman DH. Selective oxidation of carbolide C-H bonds by an engineered macrolide P450 mono-oxygenase. Proc. Natl. Acad. Sci. USA. 2009;106:18463–18468. doi: 10.1073/pnas.0907203106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Weber E, Seifert A, Antonovici M, Geinitz C, Pleiss J, Urlacher VB. Screening of a minimal enriched P450 BM3 mutant library for hydroxylation of cyclic and acyclic alkanes. Chem. Commun. 2011;47:944–946. doi: 10.1039/c0cc02924f. [DOI] [PubMed] [Google Scholar]
- (46).Robin A, Roberts GA, Kisch J, Sabbadin F, Grogan G, Bruce N, Turner NJ, Flitsch SL. Engineering and improvement of the efficiency of a chimeric [P450cam-RhFRed reductase domain] enzyme. Chem. Commun. 2009:2478–2480. doi: 10.1039/b901716j. [DOI] [PubMed] [Google Scholar]
- (47).Bordeaux M, Galarneau A, Fajula F, Drone J. A regioselective biocatalyst for alkane activation under mild conditions. Angew. Chem. Int. Ed. 2011;50:2075–2079. doi: 10.1002/anie.201005597. [DOI] [PubMed] [Google Scholar]
- (48).Rea V, Kolkman AJ, Vottero E, Stronks EJ, Ampt KA, Honing M, Vermeulen NP, Wijmenga SS, Commandeur JN. Active site substitution A82W improves the regioselectivity of steroid hydroxylation by cytochrome P450 BM3 mutants as rationalized by spin relaxation nuclear magnetic resonance studies. Biochemistry. 2012;51:750–760. doi: 10.1021/bi201433h. [DOI] [PubMed] [Google Scholar]
- (49).Fasan R. Tuning P450 Enzymes as Oxidation Catalysts. ACS Catal. 2012;2:647–666. [Google Scholar]
- (50).Narhi LO, Fulco AJ. Identification and characterization of two functional domains in cytochrome P-450BM-3, a catalytically self-sufficient monooxygenase induced by barbiturates in Bacillus megaterium. J. Biol. Chem. 1987;262:6683–6690. [PubMed] [Google Scholar]
- (51).Bloszyk E, Budesinsky M, Daniewski WM, Peskova E, Drozdz B, Holub M. Sesquiterpene lactones of Inula aschersoniana. Collect. Czech. Chem. Comm. 1990;55:1562–1567. [Google Scholar]
- (52).Abdel Sattar E, Galal AM, Mossa GS. Antitumor germacranolides from Anvillea garcinii. J. Nat. Prod. 1996;59:403–405. doi: 10.1021/np960064g. [DOI] [PubMed] [Google Scholar]
- (53).Galal AM, Ibrahim ARS, Mossa JS, El Feraly FS. Microbial transformation of parthenolide. Phytochemistry. 1999;51:761–765. [Google Scholar]
- (54).Nasim S, Pei S, Hagen FK, Jordan CT, Crooks PA. Melampomagnolide B: a new antileukemic sesquiterpene. Bioorg. Med. Chem. 2011;19:1515–1519. doi: 10.1016/j.bmc.2010.12.045. [DOI] [PubMed] [Google Scholar]
- (55).Gonzalez AG, Galindo A, Afonso MM, Mansilla H, Lopez M. The-Biomimetic Cyclization of Melampomagnolide B. Tetrahedron. 1988;44:4585–4589. [Google Scholar]
- (56).Cuissart B, Touffet F, Cremilleux B, Bureau R, Rault S. The maximum common substructure as a molecular depiction in a supervised classification context: experiments in quantitative structure/biodegradability relationships. J. Chem. Inf. Comput. Sci. 2002;42:1043–1052. doi: 10.1021/ci020017w. [DOI] [PubMed] [Google Scholar]
- (57).Cao Y, Jiang T, Girke T. A maximum common substructure-based algorithm for searching and predicting drug-like compounds. Bioinformatics. 2008;24:366–374. doi: 10.1093/bioinformatics/btn186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Rahman SA, Bashton M, Holliday GL, Schrader R, Thornton JM. Small Molecule Subgraph Detector (SMSD) toolkit. J. Cheminform. 2009;1:1–13. doi: 10.1186/1758-2946-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Sevrioukova IF, Li H, Zhang H, Peterson JA, Poulos TL. Structure of a cytochrome P450-redox partner electron-transfer complex. Proc. Natl. Acad. Sci. USA. 1999;96:1863–1868. doi: 10.1073/pnas.96.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Oliver CF, Modi S, Sutcliffe MJ, Primrose WU, Lian LY, Roberts GC. A single mutation in cytochrome P450 BM3 changes substrate orientation in a catalytic intermediate and the regiospecificity of hydroxylation. Biochemistry. 1997;36:1567–1572. doi: 10.1021/bi962826c. [DOI] [PubMed] [Google Scholar]
- (61).McLachlan MJ, Johannes TW, Zhao H. Further improvement of phosphite dehydrogenase thermostability by saturation mutagenesis. Biotechnol. Bioeng. 2008;99:268–274. doi: 10.1002/bit.21546. [DOI] [PubMed] [Google Scholar]
- (62).Guzman ML, Neering SJ, Upchurch D, Grimes B, Howard DS, Rizzieri DA, Luger SM, Jordan CT. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301–2307. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- (63).Quick A, Rogers D. Crystal and Molecular-Structure of Parthenolide [4,5-Epoxygermacra-1(10),11(13)-Dien-12,6-Olactone] J. Chem. Soc. Perkin Trans. 1976;2:465–469. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.