Abstract
Introduction
MTA Plus (MTAP; Avalon Biomed Inc., Bradenton, FL) is a new calcium silicate cement with unknown cytotoxicity characteristics. The objectives of this study were to examine the effect of MTA Plus on the viability, apoptosis/necrosis profile and oxidative stress levels of rat odontoblast-like cells.
Methods
MDPC-23 cells were exposed to gray and white MTA Plus (GMTAP, WMTAP), gray and white ProRoot® MTA (GMTA, WMTA; Dentsply Tulsa Dental Specialties, Tulsa, OK) cements or their eluents. The cells were evaluated for: i) cell viability using XTT assay, ii) apoptosis/necrosis using flow cytometry and confocal laser scanning microscopy, and iii) oxidative stress by measuring reactive oxygen species.
Results
XTT assay showed that all test cements exhibited marked initial cytotoxicity that decreased with time. By the end of the third week, GMTAP and GMTA were comparable to untreated cells (negative control) in terms of cell viability, while WMTAP and WMTP were significantly lower than the untreated cells. Apoptosis/necrosis profiles of cells exposed to WMTAP and GMTAP were not significantly different from untreated cells, while cells exposed to WMTA and GMTA showed significantly less viable cells. All experimental groups exhibited reduction of intracellular ROS formation compared to untreated cells, although cells exposed to WMTA was not significantly different from untreated cells.
Conclusions
Both the gray and white versions of MTA Plus possess negligible in-vitro cytotoxic risks that are time and dilution dependent. They enrich the spectrum of hydraulic calcium silicate cements currently available to clinicians for endodontic applications.
Keywords: apoptosis, cell viability, hydraulic calcium silicate cements, necrosis, reactive oxygen species
INTRODUCTION
Hydraulic calcium silicate cements (HCSCs) have become an integral component of endodontists’ armamentarium due to their bioactivity [1], biocompatibility [2] and osteogenicity [3]. These cements have successfully replaced traditional dental cements in endodontic surgeries, apexification, vital pulp therapy, perforation repair, and in regenerative endodontic procedures [4]. Despite the favorable biological properties of current HCSCs, high operational cost, suboptimal handling properties, relatively long setting times [5], initial cytotoxicity [6], incompatibility with other restorative materials [7] and potential staining of tooth structure [8] are some of the drawbacks of contemporary HCSCs that hinder their use in endodontics. Although some of those hurdles have been addressed in more recent formulations [9,10], none of the currently available HSCSs addresses all of the above challenges.
MTA Plus (Avalon Biomed Inc., Bradenton, FL, gray and white versions) is a HCSC with finer particle size that mixes with a proprietary water-based gel for enhanced handling and application, as well as improved washout resistance [11]. Due to its variable stoichiometric properties [12], the powder/gel ratio of MTA Plus may be adjusted to enable more diverse applications, ranging from perforation repair, root-end filling, direct pulp capping (thicker consistency) to sealing of the cleaned-and-shaped root canal space (thinner consistency).
Because HCSCs are usually applied in intimate contact with pulpal or periapical tissues, in-vitro testing of biocompatibility and cellular responses is an important preliminary step in assessing the overall biocompatibility of such cements. Thus, the objective of the present study was to evaluate the cellular viability, apoptosis and necrosis profiles, as well as oxidative stress levels exhibited by a rat odontoblast-like cell line after their exposure to the gray and white versions of MTA Plus.
MATERIALS AND METHODS
The main constituents and primary phases of HCSCs included in the present study are summarized in the Table. White and gray MTA Plus (WMTAP, GMTAP) were mixed with the proprietary hydrogel, using a liquid/powder ratio of 0.3. For comparison, white and gray ProRoot® MTA (WMTA, GMTA; Dentsply Tulsa Dental Specialties, Tulsa, OK) were also examined; these HSCSs were mixed with deionized water using the same liquid/powder ratio. The mixed materials were placed in pre-sterilized Teflon molds (5-mm diameter and 3-mm thick), covered with pre-sterilized Mylar sheets, and allowed to set in a 100% humidity chamber for 24 hours. Untreated cells were used as the negative control. Discs of similar dimensions to the test cements and prepared from a zinc oxide–eugenol cement (Intermediate Restorative Material (IRM), Dentsply Caulk, Milford, DE) were assigned as the positive control. All set materials were sterilized with ultraviolet light for 4 hours before testing.
Table.
Comparison of gray and white versions of ProRoot MTA® and MTA Plus™
| Characteristic | White ProRoot® MTA |
Gray ProRoot® MTA |
MTA Plus™ | Grey MTA Plus™ |
|---|---|---|---|---|
| Liquid | Water | Water | Water-based gel with water-soluble thickening agents & polymers* | Water-based gel with water-soluble thickening agents & polymers* |
| Powder:Liquid ratio (by weight) | 3:1 | 3:1 | Variable from 1:1 to 4:1 depending on indication | Variable from 1:1 to 4:1 depending on indication |
| Primary Phases | 3CaO·SiO2, 2CaO·SiO2, Bi2O3,, 3CaO·Al2O3 CaSO4 | 3CaO·SiO2, 2CaO·SiO2, Bi2O3,, 3CaO·Al2O3 CaSO4, Ca2(Al,Fe)2O5 | 3CaO·SiO2, 2CaO·SiO2, Bi2O3,, 3CaO·Al2O3 CaSO4 | 3CaO·SiO2, 2CaO·SiO2, Bi2O3,, & 3CaO·Al2O3 CaSO4 Ca2(Al,Fe)2O5 |
Contents are GRAS (generally regarded as safe)
Note: Calcium aluminoferrite (Ca2(Al,Fe)2O5) is only present in the gray version of both cements. After setting, hydrated calcium silicates form with calcium hydroxide.
Cell Culture
Rat odontoblast-like cells derived from the apical papilla (MDPC-23) were employed [13]. The cells were plated in complete growth medium and incubated at 37°C in a humidified 5% CO2 atmosphere for 24 hours until fully established. The growth medium consisted of Dulbecco modified Eagle medium (Lonza, Wakersville, MD) and 10% fetal bovine serum (Invitrogen Corp., Carlsbad, CA) supplemented with 2 mmol/L L-glutamine and 100 U/mL penicillin/streptomycin.
Cell Viability
An XTT Cell Viability Assay Kit (Biotium Inc., Hayward, CA) was used to determine cell viability based on the cleavage of the yellow tetrazolium salt 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) by mitochondrial enzymes in metabolically-active cells to form a soluble orange formazan product. Production of formazan is directly proportional to the number of vital cells and is quantified by measuring its absorbance at 490 nm. The viability of untreated cells was used as control and absorbance of the control was adjusted to 100%, with which the relative dehydrogenase activities of the other groups were compared (N=12). The XTT assay was performed on cells that have been directly exposed to test cements and on eluents derived from those materials.
Direct evaluation of the test materials was performed on a weekly basis according to a cycling regime [14,15]. A weekly cycle consisted of direct evaluation of the toxicity of the cement disks over the plated cells for 3 days and indirect evaluation of the effect of eluents derived from the set cements on the plated cells. The latter was achieved by immersion of the disks in complete growth medium for 4 days to collect eluents. Accordingly, during the first part of each weekly cycle, cement and control disks were placed individually in transwell inserts with a 3-mm pore size (BD Falcon, Franklin Lakes, NJ) to prevent direct contact of the cells by the specimen. After the inserts were placed over the plated cells, an additional 2 mL of growth medium was added to each well to ensure that the level of the culture medium was above the sides of the transwell insert. The disks were exposed to the plated cells for 3 days, without further change in culture medium, before testing for mitochondrial dehydrogenase activity. During the second part of each weekly cycle, the disks were retrieved and incubated at 37°C with complete growth medium (1 disk/2 mL) for 4 days to collect the eluents from the set cement before using the same disks for the next cycle. For each disk, the same growth medium was used for eluent collection throughout the entire testing period. This cycling regimen was repeated weekly for 3 weeks (i.e. 3 cycles) until the material disks were rendered noncytotoxic (i.e. >85% of the mean dehydrogenase activity exhibited by the untreated control).
For indirect evaluation of the eluents, each eluent concentrate collected after the 2-week aging period was diluted with fresh growth medium to 1:1, 1:5, and 1:10 of its original concentration to achieve a final volume of 2 mL (N=12). Each diluted, eluent-containing growth medium was then used as the respective culture medium for freshly plated rat dental papilla–derived odontoblast-like cell line (MPDC-23) cells for testing cell viability.
Apoptosis/Necrosis
Flow Cytometry
After MDPC-23 cells were exposed to test materials for 3 days, the cells were detached, centrifuged and re-suspended at 1×104 cells/mL in 1× binding buffer (Biotium Inc). The cells were stained with fluorescein isothiocyanate (FITC)–annexin V (AnV; labs/lem= 492/514 nm, green fluorescence) and ethidium homodimer-III (Etd; labs/lem = 528/617 nm, red fluorescence) and incubated for 15 min in the dark. The stained cells were subjected to fluorescence-activated cell sorting (FACS) using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) to determine the percentage distribution of vital (AnV/Etd negative), early apoptotic (AnV positive, Etd negative), late apoptotic (secondary necrosis; AnV/Etd positive), and necrotic (AnV negative, Etd positive) cell populations. Experiments were performed in triplicates.
Confocal Laser Scanning Microscopy
Cells were plated at 2500 cells/cm2 onto coverslips in 6-well plates and exposed to the test materials for 3 days. The cells were triple-stained with Hoechst 33342 (labs/lem = 350/461 nm, blue fluorescence), Etd (red fluorescence), and FITC-AnV (green fluorescence). The coverslips were mounted on slides for qualitative evaluation of cell death (apoptosis vs necrosis) after exposure to the materials. A 2-photon confocal laser scanning microscope (CLSM; LSM 510 META, Carl Zeiss Microscopy, Thornwood, NY) coupled to an MIRA 900 Ti:Sapphire laser (Coherent Inc, Santa Clara, CA) was used for imaging.
Oxidative Stress
Detection of oxidative stress in MDPC-23 cells was performed by measuring intracellular reactive oxygen species formation (ROS), using the CellROX® Orange Oxidative Stress Reagent (Invitrogen, Carlsbad, CA). After the cells were exposed to test materials for 3 days, they were detached, centrifuged and re-suspended in 1% phosphate-buffered saline. CellROX® Orange (a fluorescent redox cytoplasmic stain; labs/lem = 545/565 nm) was added to the cells at a final concentration of 5 µM and incubated at 37°C for 30 min. The FACSCalibur flow cytometer was used to detect the percentage of ROS-positive cells in each group (N=6). The experiment was run in triplicates; untreated cells were used for comparisons with the results derived from cells exposed to different materials. Additional cells were plated on coverslips, double-stained with CellROX® Orange and Hoechst 33342 and examined with a fluorescent microscope (Axioplan 2 Imaging, Carl Zeiss) for qualitative evaluation of intracellular ROS distribution.
Statistical Analyses
The IRM positive control group was excluded from all statistical analyses to increase the robustness of the respective test. For XTT assay of the effect of materials on cell viability, results derived from each weekly cycle were analyzed separately using one-factor ANOVA and post-hoc Tukey test, or their non-parametric equivalents. Similar tests were employed for analyzing the effect of dilution of the eluents derived from the test materials on cell viability. Parametric versions of these tests were used after evaluation of the normality (Shapiro-Wilk test) and equal variance assumptions (modified Levene test) of the individual data tests. If those assumptions were violated, the data was non-linearly transformed to satisfy those assumptions prior to using parametric testing methods. If those assumptions remained violated after non-linear transformation, the original data set was analyzed using Kruskal-Wallis ANOVA and Dunn’s multiple comparison tests. For apoptosis/necrosis, the numbers of vital, non-apoptotic, non-necrotic cells in each group were analyzed using one-factor ANOVA and post-hoc Tukey test. For oxidative stress evaluation, the numbers of ROS-positive cells in each group were also analyzed using one-factor ANOVA and post-hoc Tukey test. Statistical significances for all analyses were set at α=0.05.
RESULTS
Cell Viability
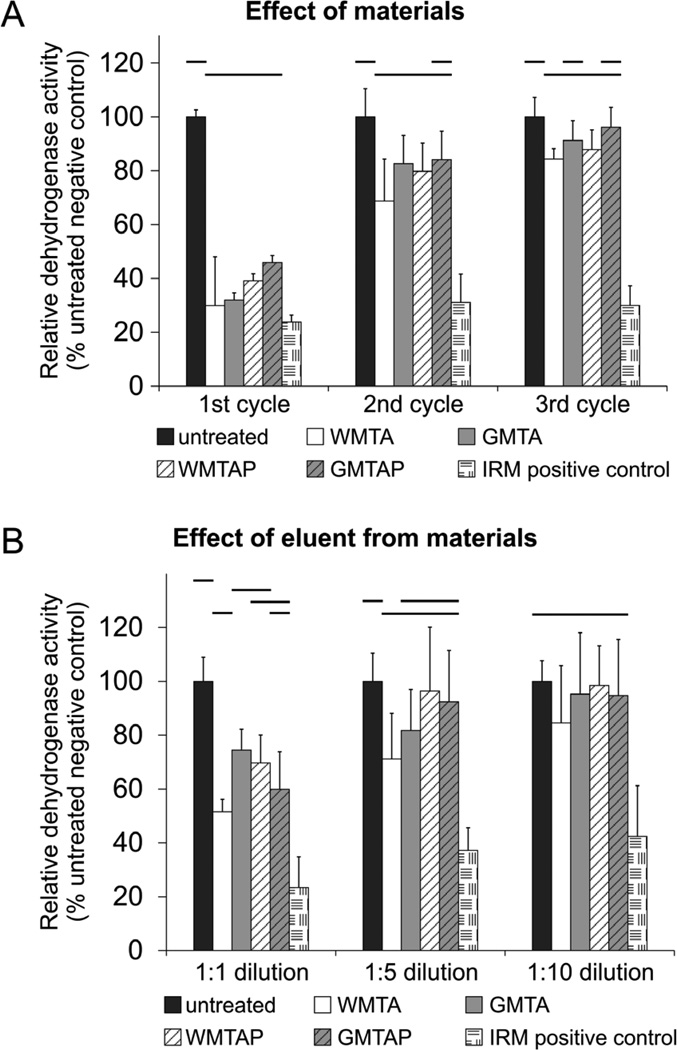
Relative mitochondrial dehydrogenase activities of MPDC-23 cells after their direct exposure to the test cements are delineated in 3 weekly cycles and summarized in Figure 1A. For the first cycle, all cements were significantly cytotoxic (p<0.001). For the second cycle, all cements still exhibited significant cytotoxicity except for GMTAP; the latter caused the cells to produce a similar level of dehydrogenase activity as the untreated cells (p=0.07). For the third cycle: GMTAP and GMTA were not significantly different (p=0.762, p=0.128, respectively), while WMTAP and WMTA were significantly different (p=0.009, p<0.001, respectively) from the activities of the untreated cells. Nevertheless, the four HSCS groups were not significantly different from each other at each weekly cycle.
Figure 1.
(A) Direct evaluation of the effect of test cements (N = 12) on viability of MDPC-23 cells using XTT assay at different time points (first, second and third weeks after retrieval from growth medium). (B) Indirect evaluation of the effect of eluents (N = 12) extracted from the test cements using XTT assay at varying eluent dilutions (1:1, 1:5, and 1:10). For each chart, groups connected with a horizontal bar are not significantly different (P > 0.05). Values are expressed as percentages of the dehydrogenase activities of the untreated cells (negative control).
Indirect evaluation of the effect of eluent concentrations on dehydrogenase activities of MPDC-23 cells is depicted in Figure 1B. At 1:1 dilution, cells cultured with growth medium containing eluents derived from different HSCSs were significantly more cytotoxic than cells cultured in the eluent-free medium (p<0.001). At 1:5 dilution, only WMTA was significantly more cytotoxic than the untreated control (p<0.05). At 1:10 dilution, there was no difference among the HSCSs and untreated control (p=0.565).
Apoptosis/Necrosis
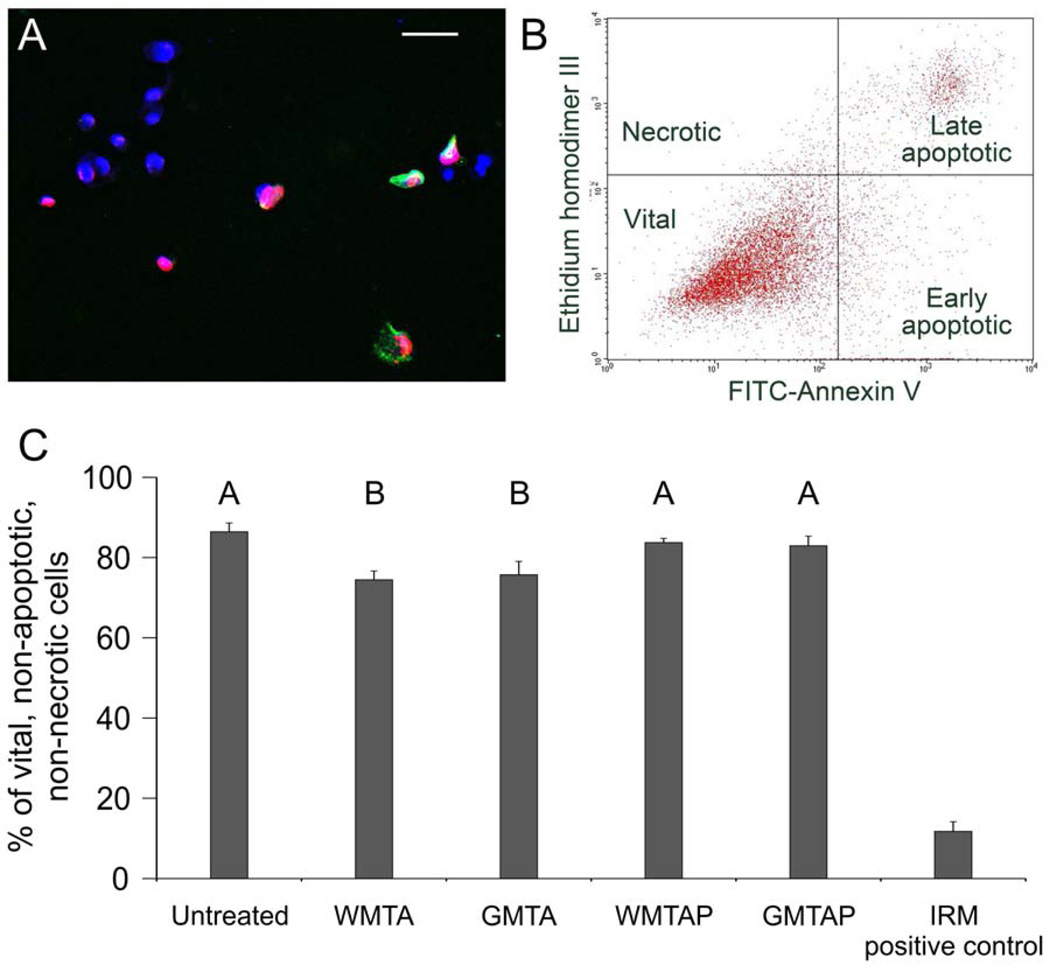
A representative CLSM image of vital, apoptotic and necrotic cells is illustrated in Figure 2A. A 2D plot of the distribution of vital, early apoptotic, late apoptotic and necrotic cells in a typical cell-sorting procedure is shown in Figure 2B. Cells exposed to WMTAP and GMTAP were not significantly different from unexposed (untreated) cells in the percentage of vital cells, while cells exposed to WMTA and GMTA had a significantly lower percentage of vital cells (p<0.001, p=0.002, respectively). There was no difference between WMTAP and GMTAP (p=0.992), as well as between WMTA and GMTA (p=0.962) (Figure 2C). The flow cytometry results were qualitatively confirmed by CLSM imaging. Cells exposed to the HSCSs were mostly vital and exhibited blue-fluorescent nuclei with minimal signs of apoptosis/necrosis. These cells exhibited comparable fluorescence characteristics as the untreated cells. By contrast, cells exposed to IRM were mostly apoptotic, with prevalence of green-fluorescent cytoplasm and occasional necrotic pink nuclei (merging of blue and red fluorescence) that are characteristic of late apoptosis and necrosis.
Figure 2.
(A) A representative CLSM image of MPDC-23 cells that were triple-stained with ethidium homodimer III (Etd; red-fluorescent non-vital DNA dye), Heoschst 33342 (blue-fluorescent nuclear counter-stain), and fluorescein isothiocyanate-annexin V (FITC-AnV; green-fluorescent phosphatidylserine-binding cytoplasmic dye) after their exposure to the test cements. Healthy cell nuclei are stained blue, apoptotic cells show green cytoplasm, necrotic cells show red or pink nuclei. (B) A representative 2-dimensional flow cytometry dot plot of the data derived from FITC-AnV and Etd-stained MDPC-23 cells after their exposure to test cements for 3 days. (C) A bar graph comparing the percentage of vital cells (lower left quadrant of Figure 2B) after exposure to the test cements (N = 3). Groups labeled with the same letter designators are not significantly different (P > 0.05).
Oxidative Stress
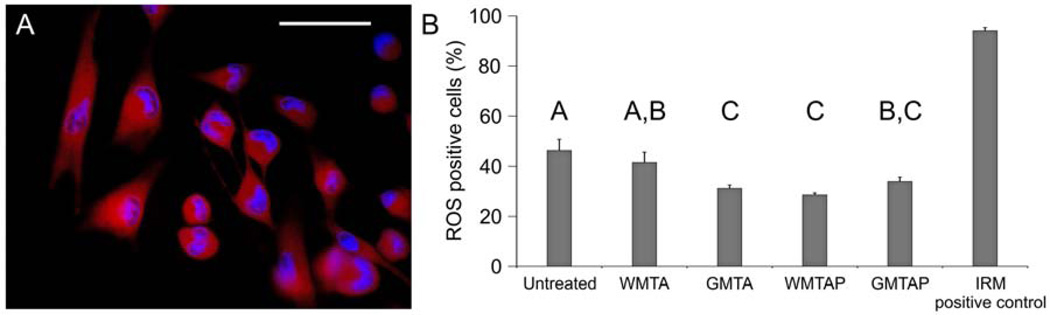
A fluorescent microscopic image of MDPC-23 cells with Hoechst 33342-stained, blue fluorescent nuclei and diffuse orange cytoplasmic fluorescence that is indicative of the production of ROS is shown in Figure 3A. Cells exposed to IRM had very high levels of ROS (Figure 3A). Untreated cells exhibited significantly higher ROS levels compared to cells that were exposed to GMTA, GMTAP and WMTAP (p<0.001), while there was no difference between the oxidative stress levels of untreated cells and cells that were exposed to WMTA (p=0.315).
Figure 3.
(A) A representative fluorescent microscopy image of MPDC-23 cells that were double-stained with Heoschst 33342 and CellROX® orange (orange-fluorescent redox cytoplasmic dye) showing intracellular ROS distribution. (B) Bar chart showing the percentage of ROS-positive MDPC-23 cells after 3 days of exposure to the test cements (N = 3). Groups labeled with the same letter designators are not significantly different (P > 0.05).
DISCUSSION
The use of MDPC-23 cells for the present work was based on their potential clinical significance with the use of HSCSs for direct pulp capping, and for their superior sensitivity, while maintaining the same cytotoxicity ranking, when compared to transformed fibroblast and osteoblast cell lines [16,17]. The XTT assay evaluates cell viability through colorimetric quantification of formazan produced via reduction of tetrazolium salts by mitochondrial dehydrogenases [18]. These enzymes are expressed only in vital cells and are inactivated shortly after cell death. Accordingly, formation of highly colored formazan dyes is indicative of a metabolically-active cell population. In the present work, a modified cycling regime [14,15] was used to test the direct effects of test materials on cell viability at different time points, as well as the indirect cytotoxic effects through eluents derived from the HSCSs [19]. Results from direct and indirect evaluations were in agreement and suggest that, under the experimental conditions, gray versions of both cements appear to be less cytotoxic than the white versions. The results further support previously-reported findings that gray MTA is more biocompatible than white MTA [20,21].
Analysis of the mode of cell death (apoptosis/necrosis) via flow cytometry and fluorescent microscopy is helpful in better understanding the cytotoxic effects of the test materials on cell permeability. Exposure to MTA Plus (both gray and white versions) resulted in more vital cells than ProRoot® MTA (both gray and white versions). Judging by the apoptosis/necrosis profile of all test cements, it seems that the initial cytotoxicity of MTA Plus and ProRoot® MTA is more likely to be attributed to apoptosis rather than necrosis, as evidenced by detection of phosphatidylserine expression on the cell surfaces via the use of annexin V [22]. This suggests that the initial sites of irreversible damage by the cytotoxic agents are extracellular, while the nuclear membranes of those cells still remain intact.
Evaluation of intracellular ROS formation provides another perspective toward understanding of cellular responses to the test materials. Reactive oxygen species is a natural byproduct of normal oxygen metabolism and have been found to play important roles in cell signaling, proliferation and survival [23]. Increase in ROS levels occurs during stress, results in significant damages to cell structures, and has been implicated in diseases such as cancer, aging, neurodegenerative diseases, and diabetes [24–27]. Interestingly, exposure to HCSCs resulted in decreasing the ROS levels in GMTA, WMTAP and GMTAP when compared to levels identified from unexposed cells. A plausible explanation is that the pH elevation caused by release of calcium hydroxide from HCSCs may decrease ROS formation. These findings support earlier reports relating the production of ROS to decreases in extracellular pH [28–30]. It is of interest to see if the decreased level of oxidative stress when MDPC-23 cells are exposed to MTA Plus may enhance the ability of these cells to differentiate and deposit mineralized matrix when they are cultured in osteogenic differentiation medium. Research in this direction is in order.
Within the limits of the present study, it may be concluded that the cytotoxic effects imposed by MTA Plus on MDPC-23 cells are both time and concentration dependent, and that they possess negligible cytotoxic risks after the elution of their cytotoxic components. Because these risks are significantly lower than those imposed by a zinc oxide-eugenol-based cement, a favorable in vivo tissue response is likely to occur. It may also be concluded that MTA Plus helps lower oxidative stress levels in MDPC-23 cells. Finally, GMTAP may favor more cell growth and viability compared to WMTAP.
Acknowledgments
This study was supported by grant R44 DE20204-02 from the National Institute of Dental & Craniofacial Research. The authors report that a financial affiliation exists for this paper: Dr. Primus is the inventor of MTA Plus. The MDPC-23 cell line utilized in the present study was a generous gift from Professor Jacques Nör, University of Michigan Dental School, Ann Arbor, MI. The authors thank Mrs. Petra Lockwood for her assistance with cell culture.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod. 2005;31:97–100. doi: 10.1097/01.don.0000133155.04468.41. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell PJ, Pitt Ford TR, Torabinejad M, McDonald F. Osteoblast biocompatibility of mineral trioxide aggregate. Biomaterials. 1999;20:167–173. doi: 10.1016/s0142-9612(98)00157-4. [DOI] [PubMed] [Google Scholar]
- 3.Shabahang S, Torabinejad M, Boyne PP, Abedi H, McMillan P. A comparative study of root-end induction using osteogenic protein-1, calcium hydroxide, and mineral trioxide aggregate in dogs. J Endod. 1999;25:1–5. doi: 10.1016/S0099-2399(99)80388-4. [DOI] [PubMed] [Google Scholar]
- 4.Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review--part III: clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36:400–413. doi: 10.1016/j.joen.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Kogan P, He J, Glickman GN, Watanabe I. The effects of various additives on setting properties of MTA. J Endod. 2006;32:569–572. doi: 10.1016/j.joen.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Wei W, Qi YP, Nikonov SY, Niu LN, Messer RL, Mao J, et al. Effects of an experimental calcium aluminosilicate cement on the viability of murine odontoblast-like cells. J Endod. 2012;38:936–942. doi: 10.1016/j.joen.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Eid AA, Komabayashi T, Watanabe E, Shiraishi T, Watanabe I. Characterization of the mineral trioxide aggregate-resin modified glass ionomer cement interface in different setting conditions. J Endod. 2012;38:1126–1129. doi: 10.1016/j.joen.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bortoluzzi EA, Araujo GS, Guerreiro Tanomaru JM, Tanomaru-Filho M. Marginal gingiva discoloration by gray MTA: a case report. J Endod. 2007;33:325–327. doi: 10.1016/j.joen.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Modareszadeh MR, Di Fiore PM, Tipton DA, Salamat N. Cytotoxicity and alkaline phosphatase activity evaluation of endosequence root repair material. J Endod. 2012;38:1101–1105. doi: 10.1016/j.joen.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Abdullah D, Ford TR, Papaioannou S, Nicholson J, McDonald F. An evaluation of accelerated Portland cement as a restorative material. Biomaterials. 2002;23:4001–4010. doi: 10.1016/s0142-9612(02)00147-3. [DOI] [PubMed] [Google Scholar]
- 11.Formosa LM, Mallia B, Camilleri J. A quantitative method for determining the antiwashout characteristics of cement-based dental materials including mineral trioxide aggregate. Int Endod J. 2013;46:179–186. doi: 10.1111/j.1365-2591.2012.02108.x. [DOI] [PubMed] [Google Scholar]
- 12.Alizadeh R, Beaudoin JJ, Raki L. Mechanical properties of calcium silicate hydrates. Mater Struct. 2011;44:13–28. [Google Scholar]
- 13.Hanks CT, Sun ZL, Fang DN, Edwards CA, Wataha JC, Ritchie HH, et al. Cloned 3T6 cell line from CD-1 mouse fetal molar dental papillae. Connect Tissue Res. 1998;37:233–249. doi: 10.3109/03008209809002442. [DOI] [PubMed] [Google Scholar]
- 14.Bryan TE, Khechen K, Brackett MG, Messer RL, El-Awady A, Primus CM, et al. In vitro osteogenic potential of an experimental calcium silicate-based root canal sealer. J Endod. 2010;36:1163–1169. doi: 10.1016/j.joen.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 15.Eid AA, Nikonov SY, Looney SW, Didato A, Niu LN, Levin MD, et al. In vitro biocompatibility evaluation of a root canal filling material that expands on water sorption. J Endod. 2013;39:883–888. doi: 10.1016/j.joen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Lee DH, Lim BS, Lee YK, Yang HC. Effects of hydrogen peroxide (H2O2) on alkaline phosphatase activity and matrix mineralization of odontoblast and osteoblast cell lines. Cell Biol Toxicol. 2006;22:39–46. doi: 10.1007/s10565-006-0018-z. [DOI] [PubMed] [Google Scholar]
- 17.Mantellini MG, Botero T, Yaman P, Dennison JB, Hanks CT, Nör JE. Adhesive resin and the hydrophilic monomer HEMA induce VEGF expression on dental pulp cells and macrophages. Dent Mater. 2006;22:434–440. doi: 10.1016/j.dental.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 18.Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, et al. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- 19.Camps J, About I. Cytotoxicity testing of endodontic sealers: a new method. J Endod. 2003;29:583–586. doi: 10.1097/00004770-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Perez AL, Spears R, Gutmann JL, Opperman LA. Osteoblasts and MG-63 osteosarcoma cells behave differently when in contact with ProRoot MTA and White MTA. Int Endod J. 2003;36:564–570. doi: 10.1046/j.1365-2591.2003.00691.x. [DOI] [PubMed] [Google Scholar]
- 21.Oviir T, Pagoria D, Ibarra G, Geurtsen W. Effects of gray and white mineral trioxide aggregate on the proliferation of oral keratinocytes and cementoblasts. J Endod. 2006;32:210–213. doi: 10.1016/j.joen.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 22.Verhoven B, Schlegel RA, Williamson P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med. 1995;182:1597–1601. doi: 10.1084/jem.182.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Floyd RA. Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB J. 1990;4:2587–2597. [PubMed] [Google Scholar]
- 25.Ames BN, Shigenaga MK. Oxidants are a major contributor to aging. Ann N Y Acad Sci. 1992;663:85–96. doi: 10.1111/j.1749-6632.1992.tb38652.x. [DOI] [PubMed] [Google Scholar]
- 26.Jenner P. Oxidative damage in neurodegenerative disease. Lancet. 1994;344:796–798. doi: 10.1016/s0140-6736(94)92347-7. [DOI] [PubMed] [Google Scholar]
- 27.Dandona P, Thusu K, Cook S, Snyder B, Makowski J, Armstrong D, et al. Oxidative damage to DNA in diabetes mellitus. Lancet. 1996;347:444–445. doi: 10.1016/s0140-6736(96)90013-6. [DOI] [PubMed] [Google Scholar]
- 28.Shu Z, Jung M, Beger HG, Marzinzig M, Han F, Butzer U, et al. pH-dependent changes of nitric oxide, peroxynitrite, and reactive oxygen species in hepatocellular damage. Am J Physiol. 1997;273:G1118–G1126. doi: 10.1152/ajpgi.1997.273.5.G1118. [DOI] [PubMed] [Google Scholar]
- 29.Yi AK, Tuetken R, Redford T, Waldschmidt M, Kirsch J, Krieg AM. CpG motifs in bacterial DNA activate leukocytes through the pH-dependent generation of reactive oxygen species. J Immunol. 1998;160:4755–4761. [PubMed] [Google Scholar]
- 30.Baldini PM, De Vito P, Martino A, Fraziano M, Grimaldi C, Luly P, et al. Differential sensitivity of human monocytes and macrophages to ANP: a role of intracellular pH on reactive oxygen species production through the phospholipase involvement. J Leukoc Biol. 2003;73:502–510. doi: 10.1189/jlb.0702377. [DOI] [PubMed] [Google Scholar]