Abstract
Hypertension is a multifactorial condition which makes the development of treatment approaches difficult. The vast majority of patients are treated with lifestyle measures either alone or in combination with antihypertensive drugs, and this approach is largely successful in controlling blood pressure. However, for a subgroup of patients, control of blood pressure remains resistant to this approach and therefore the development of new strategies is imperative. The sympathetic nervous system has been known to be implicated in hypertension for many decades, and evidence from studies in the past has revealed the benefit of reducing sympathetic nerve activity in the control of blood pressure albeit with severe side effects. Recent technological advances have allowed for specific targeting of the renal sympathetic nerves by catheter ablation. The Symplicity HTN-1 and HTN-2 trials have provided strong evidence for renal denervation giving rise to considerable blood pressure reductions in treatment-resistant hypertensives and, due to the high incidence of hypertension worldwide, this carries the promise of further reducing the global burden of hypertension and its attendant complications. Here we review the evidence for renal denervation in the management of hypertension.
Keywords: hypertension, renal denervation, blood pressure
INTRODUCTION
Hypertension is a chronic condition which greatly increases the risk of developing cardiovascular disease, the leading cause of death worldwide as classified by the World Health Organisation (WHO)[1]. It is estimated that over a quarter of the world's adult population is hypertensive, and therefore therapeutic approaches that offer good control of blood pressure are of prime importance on a global scale[2].
Hypertension is a major modifiable risk factor for cardiovascular disease[3]. It has been shown that a systolic blood pressure (SBP) increase of over 20 mmHg doubles the cardiovascular mortality risk, and that even small reductions in blood pressure significantly reduce the risk of cardiovascular events such as stroke[4],[5]. First-line treatment involves advice about lifestyle changes, with subsequent pharmacological strategies being implemented if hypertension persists despite lifestyle measures. However, successful treatment of hypertension can be difficult to achieve in a significant minority of patients, who may fail to respond to antihypertensive medications of any class either alone or in combination.
Resistant hypertension is defined as blood pressure above 140/90 mmHg in spite of the use of three or more antihypertensive agents, including a diuretic[6],[7]. Essential hypertension has a multi-factorial aetiology which makes development of new therapeutic strategies a necessity, one novel approach being specific targeting of the sympathetic nervous system.
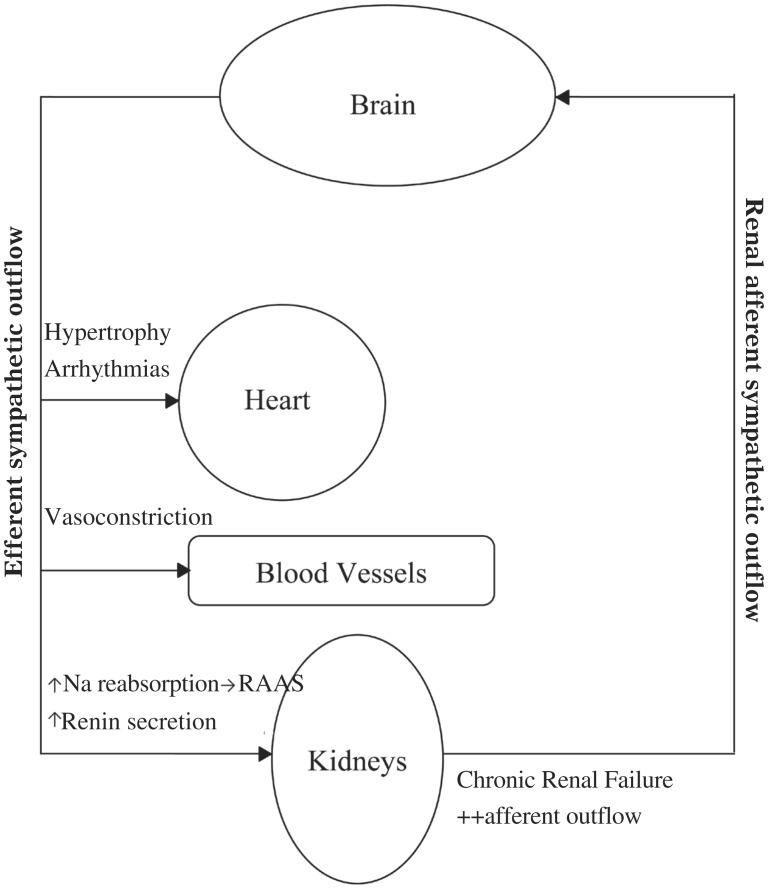
Sympathetic nerves innervate, amongst others, organs involved in cardiovascular regulation; of particular relevance to hypertension is sympathetic innervations of the kidney[8]. It is well established that sympathetic nerves are involved in the development and maintenance of hypertension through increased sympathetic activity to the kidneys. Renal efferent and afferent nerves are situated inside the renal artery wall and perivascular soft tissue respectively[9]. Efferent nerve stimulation to the kidneys acts to increase blood pressure firstly by increased sodium reabsorption from the tubules, and secondly by increased renin secretion thereby activating the renin-angiotensin-aldosterone axis[10]. Chronic activation of renal afferents is found in certain disease states such as renal failure, which results in the kidneys being a source of sympathetic drive in the hypertension associated with renal failure (Fig. 1).
Fig. 1. Renal afferent and efferent signalling from and to the kidney.
The involvement of sympathetic nerve activation in the hypertensive state makes these nerves a rational therapeutic target. Before the advent of antihypertensive drugs, non-specific surgical methods to decrease sympathetic nerve activity were used as early as the 1920s to reduce blood pressure in patients with severe hypertension[11]. These attempts were extremely successful at lowering blood pressure in treated patients, however, the lack of specificity with such extensive denervation meant that there was high periprocedural morbidity and mortality. Selective renal sympathetic denervation, therefore, represents a more attractive therapeutic approach.
Recent developments in device-based approaches to hypertension targeting the renal sympathetic system include a novel percutaneous catheter-based treatment. Here, radiofrequency energy is used to denervate sympathetic nerve fibres within the artery. The aim of this treatment is to selectively disrupt sympathetic nerve fibres without causing adverse effects in other organ systems[8]. Evidence from the Symplicity HTN-1 and HTN-2 trials in recent years has provided promising results which demonstrate significant and large decreases in blood pressure in patients with drug-resistant hypertension. At present, however, there is insufficient evidence of long term benefit and the procedure has only been trialled in relatively small numbers of patients[12].
PROCEDURE
A catheter-based approach to renal sympathetic denervation has been developed. Early approaches involving the sympathetic nervous system in hypertension, as mentioned before, have highlighted the need for targeting the renal sympathetic nerves more specifically. From the radical sympathectomies and splanchnicectomies of the 1920s, to the marginally more successful targeted removal of renal sympathetic ganglia by Grimson et al.[13], there have been several attempts at attenuating sympathetic nerve activity in the context of blood pressure[8]. These measures have demonstrated the effectiveness of therapies that reduce sympathetic nerve activity on long-term blood pressure control, but equally highlight the need for more specific methods without the side effects of previous approaches.
A minimally invasive, endovascular catheter-based procedure aims to disrupt neurogenic reflexes involved in the control of blood pressure using radiofrequency (RF) energy[12]. The rationale for this targeted treatment is based firstly on the fact that since the kidneys are retroperitoneal organs, direct access to the nerves is difficult, secondly, the fact that the renal efferent and afferent nerves are in close proximity to the renal artery wall, allowing for greater susceptibility to RF energy if approached by the endovascular route. In a human post-mortem histological study, Atherton et al.[9] used sections from human renal arteries and measured the distance of nerves from the lumen-intima interface. It was found that over 90% of renal sympathetic nerves resided within 2 mm of the renal artery lumen; considering the RF penetration depth of a cardiac ablation catheter is up to 9.5 mm[14], a catheter-based RF approach in specifically targeting renal sympathetic nerves is likely to be feasible.
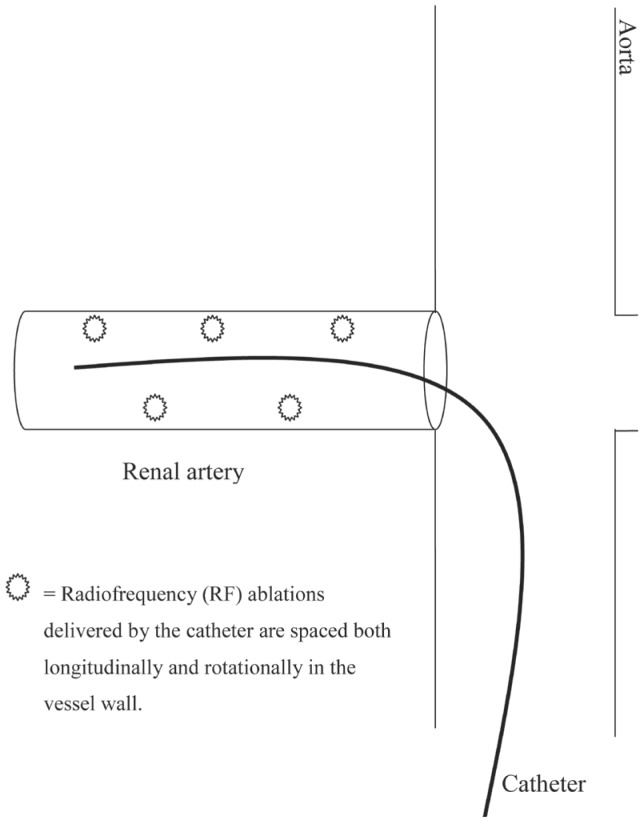
Before introducing the catheter, angiography is used to determine renal anatomic eligibility for the procedure, to exclude any renal artery stenosis or other abnormality of renal artery anatomy. The procedure is carried out under local anesthesia, whereby the catheter is inserted through the femoral artery and guided into each renal artery under fluoroscopic control. A generator delivers low-power RF ablations within each ‘renal artery at 4-6 points along its length, in a spiral pattern’[12]. Ablations last two minutes each and are spaced both longitudinally and rotationally along the course of the renal artery (Fig. 2). A predetermined algorithm allows for automatic generation of RF energy and careful monitoring throughout the procedure of temperature and impedance.
Fig. 2. Schematic representation of catheter placement and ablation sites.
PRECLINICAL EVIDENCE
Preclinical experiments that have used renal denervation therapeutically have used a variety of animal models in which to assess renal sympathetic nerve contribution to systemic organ function. One study by Kassab et al. assessed the effect of renal sympathetic nerve ablation on blood pressure using a canine model of obesity-induced hypertension[15]. Obesity is commonly observed in hypertensive patients and therefore is a good model to study the effect of a novel blood pressure-lowering strategy.
In this study, two groups were evaluated: a control group and a bilaterally renal-denervated group. Both groups were, after a control period, fed a high-fat diet, which resulted in a rise in body weight in the two groups by 50%. The study found that, with the high-fat diet, there was a significant increase in blood pressure in the control dogs (from 95 ± 2 to 109 ± 4 mmHg) but no significant change in blood pressure in the denervated dogs (87 ± 3 to 90 ± 4 mmHg). Another endpoint of this study looked at the effect of renal denervation on sodium retention, which is thought to be increased with efferent nerve stimulation. In the denervated dogs, cumulative sodium retention was approximately 50% less than in the controls (control: 445 ± 85 mmol, denervated: 252 ± 47 mmol). The findings from this study support the theory that renal sympathetic activity is involved in mediating hypertension and sodium retention associated with obesity. This is concordant with other renal denervation preclinical studies, that have similarly shown attenuation of hypertension in other animal models[16].
Of relevance to the catheter-based approach, a study by Rippy et al.[17] used a healthy swine model. Here they tested the vascular safety and healing response of RF ablations given via the Symplicity Catheter System. Angiography and histological analyses were carried out before the procedure and at 6 months post-ablation, assessing for any perfusion defects and vascular damage respectively. The study found that catheter-based denervation could selectively ablate the renal nerves without causing any clinically significant damage to the renal artery wall or other renal pathology, suggesting the catheter system to have a good safety profile and paving the way for proof-of-principle and randomized controlled trials in humans.
CLINICAL TRIALS
Proof-of-principle study
In recent years, several clinical trials have been published that provide good evidence for renal denervation having an antihypertensive effect. The first proof-of-principle cohort study was carried out by Krum and colleagues[18] with the aim of evaluating the safety and blood pressure-lowering efficacy of specific renal nerve denervation. In the Symplicity HTN-1 study, 45 patients with treatment-resistant hypertension underwent renal sympathetic nerve ablation using a catheter-based approach (Symplicity by Ardian Inc. Palo Alto, CA, USA). Treatment-resistant hypertension was defined as baseline SBP≥160 mmHg despite use of three or more antihypertensive drugs. Patients had a mean age of 58 ± 9 years, average blood pressure of 177/101 ± 20/15 mmHg and were using an average of 4.7 ± 1.5 antihypertensive medications. The procedure itself was noted to cause visceral abdominal pain that was present only during the procedure on application of RF energy; this was managed with intravenous narcotics and sedatives. The median procedure time was 38 min.
Short term follow-up angiography 14-30 days after the procedure and magnetic resonance (MR) angiography at 6 months showed a good vascular safety profile with no adverse clinical events related to the treatment, nor was any alteration in renal function noted. There was a significant decrease in blood pressure (systolic: 20-25 mmHg; diastolic: 10-15 mmHg) which persisted throughout the one year follow-up period (Table 1).
Table 1. Effect of renal denervation on blood pressure as a function of time.
| Follow up period | Change in Blood Pressure (mmHg) |
95% CI (Systolic/Diastolic) | n | |
| Systolic | Diastolic | |||
| 1 month | -14 | -10 | 4/3 | 41 |
| 3 months | -21 | -10 | 7/4 | 39 |
| 6 months | -22 | -11 | 10/5 | 26 |
| 9 months | -24 | -11 | 9/5 | 20 |
| 12 months | -27 | -17 | 16/11 | 9 |
Mean changes in blood pressure at 1, 3, 6, 9 and 12 months. From Krum et al.[18]
In agreement with the reductions in blood pressure seen after treatment, a decrease in efferent sympathetic activity was shown in terms of noradrenaline release from the kidneys (measured as spillover of noradrenaline into the circulation). In the subgroup of 10 patients who underwent this test, there was a mean decrease in noradrenaline spillover of 47% (95% CI, 28-65%). This demonstrates the efficacy of the catheter-based device in achieving efferent sympathetic denervation[18].
Independent review of angiograms and serious adverse events reduced the possibility of bias in data analysis. However, as the study was non-blinded and did not have a control group, due to the study being proof-of-concept, a placebo effect may have played a part. A serious limitation to this study is the selection of the trial population. Eligible patients were those who had resistant hypertension; however, resistant hypertension is a multifactorial condition which encompasses several causes including white coat hypertension, secondary hypertension and poor adherence to medication[6]. These are factors that were not evaluated and accounted for within the study. In addition, at the 9- and 12- month follow-up points, there was a considerable attrition of patients available for follow-up, as shown in Table 1. Overall, however, the study provides promising support for the effectiveness of renal sympathetic ablation as a strategy in the management of resistant hypertension.
Expanded cohort study
Two year follow-up evidence from the original study reaffirmed the notion that catheter-based renal denervation contributes to a long-term substantial decrease in blood pressure[19]. The expanded cohort study assessed the durability of the blood pressure-lowering effect, of importance due to sympathetic efferents having previously been shown to regrow after nerve injury[20]. In this expanded cohort study, 153 patients with the same inclusion criteria were treated by the same approach used in the initial cohort study[19]. It was found that the initial blood pressure reduction seen post-procedure in the initial study continued out to 24 months on follow-up. Over 90% of patients had an office blood pressure reduction of over 10 mmHg, averaging 32/14 mmHg at 24 months. This is of relevance when considering that the patient population was previously unresponsive to three or more hypertensive medications, further supporting the findings from the initial trial.
Symplicity HTN-2
The Symplicity HTN-2 randomized control trial[21] built upon the impressive findings from the proof-of-principle study. This study sought to further clarify the effectiveness and safety of catheter-based treatment of resistant hypertension. One hundred and six patients with treatment-resistant hypertension were enrolled. Similar inclusion criteria to the initial cohort study were used, with the additional inclusion of patients with type 2 diabetes who had a baseline SBP of ≥150 mmHg and fulfilled the additional criteria specified. Patients were then randomized to renal sympathetic denervation (n = 52) or control (n = 54) groups and followed up over a period of 6 months.
At 6 months post-procedure, office blood pressure measurements in the renal denervation group were decreased significantly, by 32/12 mmHg (SD 23/11, baseline mean blood pressure 178/96 mmHg; P < 0.0001), in comparison to no significant change in blood pressure observed in the control group. The difference in the primary endpoint of seated office blood pressure between the groups at 6 months was significant at 33/11 mmHg (P < 0.0001 for both SBP and DBP). Additionally, in a subgroup of patients where home and ambulatory blood pressure were measured, similar findings were observed. From baseline values, the renal denervation group (n = 20) had a decrease from baseline values for mean systolic and diastolic blood pressure of 11/7 mmHg (SD 15/11; P = 0.006 for SBP and P = 0.014 for DBP change) whereas there was no significant change in the control group (n = 25)[21]. Furthermore, no adverse clinical events related to the procedure were seen, which substantiates earlier trial evidence about the good safety profile of this technique.
Since this was a randomized controlled trial, this largely answers the potential criticisms above of a possible placebo effect and selection bias that could affect the validity of the results. Having said that, the control group did not undergo a sham operation; therefore, the trial was not double-blinded, so that some possibility of bias remains. Currently, a multicentre, double-blinded, randomized controlled trial (Symplicity HTN-3) is ongoing, evaluating the procedure in a larger group of patients.
The results of the Symplicity HTN-2 study provide strong evidence to support previous findings that renal denervation is a suitable and effective option in the management of treatment-resistant essential hypertension. The data are encouraging based on good efficacy in blood pressure control and good safety profile.
Future Prospects
Renal denervation by catheter ablation has been shown to have promising results in the Symplicity clinical trials. However, the exact pathophysiological mechanism by which denervation causes blood pressure reduction is yet to be elucidated precisely. Radiotracer dilution methods measuring noradrenaline spillover, as used in the proof-of-principle study above, have demonstrated reduced nordadrenaline spillover post-renal denervation, suggesting that the procedure may lower blood pressure by reducing efferent renal sympathetic nerve stimulation[8]. But whether a decrease in adrenergic activity occurs before blood pressure reduction remains to be determined.
Sympathetic reinnervation is important to consider in assessing the long-term durability of renal nerve denervation and its blood pressure-lowering effects. There is a possibility that sympathetic nerves can regrow after damage. The evidence for good long-term efficacy in the two year follow-up study suggests that, despite the possibility of regrowth and reinnervation, this appears not to affect the blood pressure-lowering effect of the procedure[19]. However, it would be useful to further clarify whether sympathetic reinnervation of the kidney occurs and whether this has an effect on blood pressure control[22]. This requires a longer follow-up period and an increased understanding of the process of reinnervation in the human kidney.
Importantly, clinical trials assessing renal denervation have been restricted to a specific group of patients with treatment-resistant essential hypertension. The clinical studies thus far have shown good efficacy and safety in these patients, but to further investigate the therapeutic benefit of renal denervation other subgroups of hypertensives need to be evaluated. These could include those that have milder forms of essential hypertension and in patients that have hypertension secondary to disease states such as renal impairment; in the future, it may be useful to examine whether renal denervation is appropriate in the earlier stages of hypertension as a preventative measure[23].
An additional consideration is that renal denervation has been examined thus far using RF catheter-based ablation. Other methods for achieving renal denervation using methods such as ultrasound, microwaves, lasers and robotic surgery may be useful to trial also.
CONCLUSION
Rrenal denervation is a potentially useful treatment modality in patients whose hypertension is refractory to conventional hypertension therapies. Targeting sympathetic nerves to reduce blood pressure is an old concept that has been brought to the fore of novel antihypertensive therapies with the development of sophisticated devices that have specificity in their application. The promising results from catheter-based renal sympathetic denervation have offered new prospects, specifically in the treatment of resistant essential hypertension. Both the HTN-1 and HTN-2 studies demonstrated the good safety profile and efficacy in reducing blood pressure using catheter nerve ablation. The UK National Institute for Health and Clinical Excellence has recently published guidelines for its use in suitably chosen patients with resistant hypertension[12].
Catheter-based treatment is still in the early stages of clinical research, and larger and longer-term follow-up trials will be needed to properly assess the durability of blood pressure reduction as well as long-term safety.
References
- 1.World Health Organisation The top 10 causes of death. 2011. Available at: http://www.who.int/mediacentre/factsheets/fs310/en/index.html. (accessed 12/05, 2012)
- 2.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 3.Poulter N. Global risk of cardiovascular disease. Heart. 2003;89(S2):ii2–ii5. doi: 10.1136/heart.89.suppl_2.ii2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vasan RS, Larson MG, Leip EP, Kannel WB, Levy D. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart Study: a cohort study. Lancet. 2001;358:1682–86. doi: 10.1016/S0140-6736(01)06710-1. [DOI] [PubMed] [Google Scholar]
- 5.Mancia G. Blood pressure reduction and cardiovascular outcomes: past, present, and future. Am J Cardiol. 2007;100(S):3J–9J. doi: 10.1016/j.amjcard.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–26. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 7.Sarafidis PA, Bakris GL. Resistant hypertension: an overview of evaluation and treatment. J Am Coll Cardiol. 2008;52:1749–57. doi: 10.1016/j.jacc.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 8.Schlaich MP, Krum H, Sobotka PA, Esler MD. Renal denervation and hypertension. Am J Hypertens. 2011;24:635–42. doi: 10.1038/ajh.2011.35. [DOI] [PubMed] [Google Scholar]
- 9.Atherton DS, Deep NL, Mendelsohn FO. Micro-anatomy of the renal sympathetic nervous system: a human postmortem histologic study. Clin Anat. 2012;25:628–33. doi: 10.1002/ca.21280. [DOI] [PubMed] [Google Scholar]
- 10.DiBona GF. Neural control of the kidney: past, present, and future. Hypertension. 2003;41:621–4. doi: 10.1161/01.HYP.0000047205.52509.8A. [DOI] [PubMed] [Google Scholar]
- 11.Morrissey DM, Brookes VS, Cooke WT. Sympathectomy in the treatment of hypertension; review of 122 cases. Lancet. 1953;1:403–8. doi: 10.1016/s0140-6736(53)91589-x. [DOI] [PubMed] [Google Scholar]
- 12.National Institute for Health and Clinical Excellence Percutaneous transluminal radiofrequency sympathetic denervation of the renal artery for resistant hypertension. 2012. Available at: http://guidance.nice.org.uk/ipg418. (accessed 11/19, 2012)
- 13.Grimson KS. Total Thoracic and partial to total lumbar sympathectomy and celiac ganglionectomy in the treatment of hypertension. Ann Surg. 1941;114:753–75. doi: 10.1097/00000658-194111440-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes D, Fish JM, Byrd IA, Dando JD, Fowler SJ, Cao H, et al. Contact sensing provides a highly accurate means to titrate radiofrequency ablation lesion depth. J Cardiovasc Electrophysiol. 2011;22:684–90. doi: 10.1111/j.1540-8167.2010.01963.x. [DOI] [PubMed] [Google Scholar]
- 15.Kassab S, Kato T, Wilkins FC, Chen R, Hall JE, Granger JP. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension. 1995;25:893–7. doi: 10.1161/01.hyp.25.4.893. [DOI] [PubMed] [Google Scholar]
- 16.Ye S, Zhong H, Yanamadala V, Campese VM. Renal injury caused by intrarenal injection of phenol increases afferent and efferent renal sympathetic nerve activity. Am J Hypertens. 2002;15:717–24. doi: 10.1016/s0895-7061(02)02959-x. [DOI] [PubMed] [Google Scholar]
- 17.Rippy MK, Zarins D, Barman NC, Wu A, Duncan KL, Zarins CK. Catheter-based renal sympathetic denervation: chronic preclinical evidence for renal artery safety. Clin Res Cardiol. 2011;100:1095–1101. doi: 10.1007/s00392-011-0346-8. [DOI] [PubMed] [Google Scholar]
- 18.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–81. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 19.Symplicity HTN-1 Investigators Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011;57:911–7. doi: 10.1161/HYPERTENSIONAHA.110.163014. [DOI] [PubMed] [Google Scholar]
- 20.Kaye DM, Esler M, Kingwell B, McPherson G, Esmore D, Jennings G. Functional and neurochemical evidence for partial cardiac sympathetic reinnervation after cardiac transplantation in humans. Circulation. 1993;88:1110–8. doi: 10.1161/01.cir.88.3.1110. [DOI] [PubMed] [Google Scholar]
- 21.Symplicity HTN-2 Investigators. Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–9. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 22.Grassi G, Mancia G. New therapeutic approaches for resistant hypertension. J Nephrol. 2012;25:276–81. doi: 10.5301/jn.5000097. [DOI] [PubMed] [Google Scholar]
- 23.Doumas M, Douma S. Renal sympathetic denervation: the jury is still out. Lancet. 2010;376:1878–80. doi: 10.1016/S0140-6736(10)62111-3. [DOI] [PubMed] [Google Scholar]