Abstract
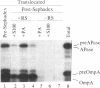
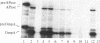
The energy requirement for translocation of alkaline phosphatase and the outer membrane protein OmpA into Escherichia coli membrane vesicles was studied under conditions that permit posttranslational translocation and, hence, prior removal of various components necessary for protein synthesis. Translocation could be supported by an ATP-generating system or, less well, by the protonmotive force generated by D-lactate oxidation; the latter might act by generating ATP from residual bound nucleotides. However, when protonmotive force inhibitors were used or when ATP was further depleted by E. coli glycerol kinase, D-lactate no longer supported the translocation. Furthermore, ATP could still support protein translocation in the presence of proton uncouplers or with membranes defective in the F1 fraction of the H+-ATPase. We conclude that ATP is required for protein translocation in this posttranslational system (and probably also in cotranslational translocation); the protonmotive force may contribute but does not appear to be essential.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker E. P., Randall L. L. The requirement for energy during export of beta-lactamase in Escherichia coli is fulfilled by the total protonmotive force. EMBO J. 1984 Apr;3(4):895–900. doi: 10.1002/j.1460-2075.1984.tb01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Rhoads D., Tai P. C. Alkaline phosphatase and OmpA protein can be translocated posttranslationally into membrane vesicles of Escherichia coli. J Bacteriol. 1985 Mar;161(3):973–980. doi: 10.1128/jb.161.3.973-980.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels C. J., Bole D. G., Quay S. C., Oxender D. L. Role for membrane potential in the secretion of protein into the periplasm of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5396–5400. doi: 10.1073/pnas.78.9.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. D., Tai P. C. The mechanism of protein secretion across membranes. Nature. 1980 Jan 31;283(5746):433–438. doi: 10.1038/283433a0. [DOI] [PubMed] [Google Scholar]
- Enequist H. G., Hirst T. R., Harayama S., Hardy S. J., Randall L. L. Energy is required for maturation of exported proteins in Escherichia coli. Eur J Biochem. 1981 May 15;116(2):227–233. doi: 10.1111/j.1432-1033.1981.tb05323.x. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A. The spontaneous insertion of proteins into and across membranes: the helical hairpin hypothesis. Cell. 1981 Feb;23(2):411–422. doi: 10.1016/0092-8674(81)90136-7. [DOI] [PubMed] [Google Scholar]
- Gasser S. M., Daum G., Schatz G. Import of proteins into mitochondria. Energy-dependent uptake of precursors by isolated mitochondria. J Biol Chem. 1982 Nov 10;257(21):13034–13041. [PubMed] [Google Scholar]
- Ginther C. L., Ingraham J. L. Nucleoside diphosphokinase of Salmonella typhimurium. J Biol Chem. 1974 Jun 10;249(11):3406–3411. [PubMed] [Google Scholar]
- Hayashi S. I., Lin E. C. Purification and properties of glycerol kinase from Escherichia coli. J Biol Chem. 1967 Mar 10;242(5):1030–1035. [PubMed] [Google Scholar]
- Hunt A. G., Simplaceanu V., Hong J. S., Ho C. Phosphorus-31 nuclear magnetic resonance investigation of membrane vesicles from Escherichia coli. Biochemistry. 1983 Dec 20;22(26):6130–6134. doi: 10.1021/bi00295a014. [DOI] [PubMed] [Google Scholar]
- Inouye H., Michaelis S., Wright A., Beckwith J. Cloning and restriction mapping of the alkaline phosphatase structural gene (phoA) of Escherichia coli and generation of deletion mutants in vitro. J Bacteriol. 1981 May;146(2):668–675. doi: 10.1128/jb.146.2.668-675.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson L. G., Randall L. L. Different exported proteins in E. coli show differences in the temporal mode of processing in vivo. Cell. 1981 Jul;25(1):151–157. doi: 10.1016/0092-8674(81)90239-7. [DOI] [PubMed] [Google Scholar]
- Kinoshita N., Unemoto T., Kobayashi H. Proton motive force is not obligatory for growth of Escherichia coli. J Bacteriol. 1984 Dec;160(3):1074–1077. doi: 10.1128/jb.160.3.1074-1077.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. J., Kanazawa H., Ozols J., Wu H. C. An Escherichia coli mutant with an amino acid alteration within the signal sequence of outer membrane prolipoprotein. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4891–4895. doi: 10.1073/pnas.75.10.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Blobel G. In vitro translocation of bacterial proteins across the plasma membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7421–7425. doi: 10.1073/pnas.81.23.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages J. M., Lazdunski C. Maturation of exported proteins in Escherichia coli. Requirement for energy, site and kinetics of processing. Eur J Biochem. 1982 Jun;124(3):561–566. [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Export of protein in bacteria. Microbiol Rev. 1984 Dec;48(4):290–298. doi: 10.1128/mr.48.4.290-298.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reenstra W. W., Patel L., Rottenberg H., Kaback H. R. Electrochemical proton gradient in inverted membrane vesicles from Escherichia coli. Biochemistry. 1980 Jan 8;19(1):1–9. doi: 10.1021/bi00542a001. [DOI] [PubMed] [Google Scholar]
- Rhoads D. B., Tai P. C., Davis B. D. Energy-requiring translocation of the OmpA protein and alkaline phosphatase of Escherichia coli into inner membrane vesicles. J Bacteriol. 1984 Jul;159(1):63–70. doi: 10.1128/jb.159.1.63-70.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleyer M., Schmidt B., Neupert W. Requirement of a membrane potential for the posttranslational transfer of proteins into mitochondria. Eur J Biochem. 1982 Jun 15;125(1):109–116. doi: 10.1111/j.1432-1033.1982.tb06657.x. [DOI] [PubMed] [Google Scholar]
- Silhavy T. J., Benson S. A., Emr S. D. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. doi: 10.1128/mr.47.3.313-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Davis B. D. Nascent peptide as sole attachment of polysomes to membranes in bacteria. Proc Natl Acad Sci U S A. 1978 Feb;75(2):814–817. doi: 10.1073/pnas.75.2.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. Assembly of proteins into membranes. Science. 1980 Nov 21;210(4472):861–868. doi: 10.1126/science.7001628. [DOI] [PubMed] [Google Scholar]
- Wickner W., Kornberg A. DNA polymerase 3 star requires ATP to start synthesis on a primed DNA. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3679–3683. doi: 10.1073/pnas.70.12.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. M., Alderette J. F., Maloney P. C., Wilson T. H. Protonmotive force as the source of energy for adenosine 5'-triphosphate synthesis in Escherichia coli. J Bacteriol. 1976 Apr;126(1):327–337. doi: 10.1128/jb.126.1.327-337.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R., Wickner W. Energetics and intermediates of the assembly of Protein OmpA into the outer membrane of Escherichia coli. J Biol Chem. 1983 Mar 25;258(6):3920–3925. [PubMed] [Google Scholar]
- von Heijne G., Blomberg C. Trans-membrane translocation of proteins. The direct transfer model. Eur J Biochem. 1979 Jun;97(1):175–181. doi: 10.1111/j.1432-1033.1979.tb13100.x. [DOI] [PubMed] [Google Scholar]