Abstract
Background
Intra-dialytic hypotension is common and is associated with increased morbidity and mortality in chronic hemodialysis patients. Higher dialysis ‘dose’ may generate transient intra-dialytic osmotic gradients, predisposing to intracellular fluid shifts and resulting in hypotension.
Study Design
We performed a post-hoc analysis of the HEMO Study, a multi-center trial that randomized chronic hemodialysis patients to high vs. standard Kt/V and higher vs. lower membrane flux. In order to achieve dose targets, per protocol, adjustments were made in membrane efficiency, blood flow, or dialysate flow before changing session length. Detailed hemodynamic and urea kinetic modeling data were abstracted from 1825 individuals. The primary outcome was the occurrence of hypotensive events necessitating clinical intervention (saline infusion, lowering of ultrafiltration rate or reduced blood flow).
Results
Intra-dialytic hypotensive events occurred more frequently in the higher Kt/V group (18.3 vs. 16.8%; p<0.001). Participants randomized to higher target Kt/V had a greater adjusted risk of intra-dialytic hypotension than those randomized to standard Kt/V (OR 1.12; 95%CI 1.01–1.25). Higher KoA and rate of urea removal was associated with greater adjusted odds of intra-dialytic hypotension (OR 1.05; 95%CI 1.04–1.06 per mg/dL/hr).
Conclusions
Higher dialysis dose, over relatively constrained treatment times, may associate with an increased risk of intra-dialytic hypotension. These findings raise the possibility that rapidity of intra-dialytic reductions in plasma osmolality may have an important role in mediating hemodynamic instability.
Keywords: intra-dialytic hypotension, dose, urea reduction, osmolality, hemodialysis, HEMO Study
Introduction
Intra-dialytic hypotensive events are a common complication of maintenance hemodialysis, affecting up to one-third of chronic dialysis treatment sessions [1–3]. Intra-dialytic hypotension (IDH) can be defined as an abrupt decline in blood pressure that causes symptoms and/or requires an intervention [4,5]. IDH has been associated with many adverse clinical events, including myocardial stunning [6], cerebral atrophy [7] and increased mortality [8]. Predisposing factors include intrinsic patient-related factors – such as the presence of autonomic neuropathy [9], abnormal cardiac reserve [10] and reduced venous compliance [11]; and potentially modifiable treatment-related parameters – such as ultrafiltration profiling [12] and changes in serum calcium concentration [13].
The rapidity of removal of urea, sodium and other osmotically active substances from the intravascular compartment, in conjunction with delayed re-equilibration from intracellular compartments, may result in a transient decline in plasma osmolality and intra-vascular volume depletion secondary to trans-cellular movement of water. This may predispose to the development of IDH [14]. In support of this concept, investigators have reported that administration of hypertonic glucose or hypertonic saline ameliorate intra-dialytic blood pressure decline and other dialysis-associated symptoms [15]. In addition, the use of sodium modeling algorithms has been associated with improved hemodynamic parameters in patients with a prior history of IDH [4].
In the HEMO Study [16], a multi-center randomized trial in maintenance hemodialysis, patients were randomized to higher vs. standard Kt/V and higher vs. lower membrane flux. HEMO Study investigators were instructed to increase blood flow, dialysate flow and filter surface area before increasing session length in order to achieve higher Kt/V targets. Therefore, we used the randomized dose assignment to examine the effect of rapid osmotic shifts during hemodialysis and tested the hypothesis that higher dialysis dose associates with a greater risk of IDH.
Methods
Study Design and Population
The study protocol was deemed exempt by the Partners Healthcare Institutional Review Board. All data were abstracted from the HEMO Study with the permission of the National Institute of Diabetic and Digestive and Kidney diseases. The design of the HEMO Study has been previously reported [16,17]. Briefly, HEMO was a prospective, multicenter, randomized clinical trial of low vs. high flux membranes and high vs. standard dialysis dose (target single-pool Kt/V 1.65 vs. 1.25; approximately equivalent to urea reduction ratios (URR) of 75% vs. 65%) among prevalent adult subjects receiving thrice-weekly in-center hemodialysis. Exclusion criteria included a baseline serum albumin <2.6g/dL, residual urea clearance of ≥1.5ml/min/35L of urea distribution volume, inability to consistently achieve an equilibrated Kt/V of >1.3, or the presence of end-stage co-morbid conditions other than kidney failure. Of the 1846 HEMO Study participants, we excluded those who did not have available hemodynamic and kinetic modeling data after randomization (n=20); our final cohort consisted of 1825 individuals and 62,095 hemodialysis treatment sessions.
Exposures and Outcomes
The primary analysis examined the HEMO Study randomized Kt/V assignment (higher vs. lower) as the exposure of interest. In secondary analyses the association of individual components of dialysis ‘dose’ with IDH was examined (blood flow [Qb], dialysate flow [Qd], session length, KoAurea and rate of decline in plasma BUN). In vitro KoAurea values were obtained for 22 HEMO Study approved membranes at dialysate flows of 800mL/min [18]. The rate of decline in plasma BUN was calculated using blood urea nitrogen (BUN) measurements as: (pre-dialysis BUN – post-dialysis BUN)/session length, after exclusion of the top and bottom 1% of individual BUN measurements as outliers. Data for all urea-based exposure calculations were abstracted from monthly two-sample BUN modeling sessions, where post-BUN was taken 15 seconds (from line disconnect) or 20 seconds (from sampling port) post dialysis inlet slowing.
The primary outcome was the occurrence of an intra-dialytic hypotensive event, defined as a hypotensive episode requiring either saline infusion, lowering of the ultrafiltration rate (UF) or reduction in blood flow. This was a pre-specified outcome of the HEMO Study that was assessed and recorded on a monthly basis during monitored hemodialysis sessions by trained study personnel. In sensitivity analyses, an alternative definition of IDH was examined: decline in SBP of ≥50mmHg with headache, cramps or vomiting and requirement for an intervention (lowering of Qb, UF or saline infusion).
Study data
All study data in the HEMO Study were obtained by subject interview, chart review and self-reported questionnaires. Demographic data including sex, race, and age were recorded at baseline (taken as the time of the first kinetic modeling session after randomization for the purposes of this study; median time after randomization of 20 days [IQR 13–28]). Pre-randomization kinetic modeling sessions were used to assess the baseline incidence of IDH. Other variables of interest included co-morbidities (diabetes, ischemic heart disease, peripheral vascular disease, congestive heart failure and arrhythmia; recorded annually); dialysis treatment and hemodynamic parameters (monthly); and laboratory parameters (6 monthly). Co-morbidities were graded on the Index of Co-existing Disease (ICED) scale. Analytically these were dichotomized (0 if ICED score=0; 1 if ICED score≥1) except for congestive heart failure where more granular categorization was considered (0 if ICED score=0; 1 if ICED score=1; 2 if ICED score≥2)[19].
Statistical analysis
Continuous variables were examined graphically and recorded as means (± standard deviations) for normally distributed data, or medians (with inter-quartile ranges) for non-normally distributed data. Comparisons were made using t-tests, Wilcoxon rank sum tests, analysis of variance or Kruskal-Wallis tests as appropriate. Categorical variables were examined by frequency distribution, recorded as proportions and comparisons made using the χ2 test. Initially, unadjusted generalized linear regression models (using a binomial distribution and logit link function) were fit [20] to assess the relationship between randomized Kt/V group and hypotensive events (yes or no). These models used clustered variance estimates to account for non-independence of covariates within subject. Subsequently adjustment was made for randomized HEMO Study flux assignment and baseline pre-specified covariates as done in the original published HEMO analyses (age, sex, race, vintage, diabetes, index of co-existent disease score and serum albumin) [16]; in our analyses ICED score was considered as a categorical variable (0, 1, 2 or 3). In secondary analyses the associations of categories of Qb (≤250, 251–3530, 351–540, >450 mL/min), Qd (≤500, 501–799, ≥800 mL/min), session length (≤180, 181–209, 210–239, ≥240 mins), KoAurea (<1000, ≥1000mL/min) and rate of decline in plasma BUN (mg/dL/hr) with IDH were each individually examined using unadjusted and adjusted generalized linear models. For these analyses, exposure variables and covariates were considered as the most proximate value preceding each kinetic modeling session. Multivariable models (Model 1) included terms for HEMO Study flux assignment, age, race, sex, post-dialysis weight, height, access, pre-dialysis SBP, ischemic heart disease, congestive heart failure, diabetes, peripheral vascular disease, arrhythmia, pre-dialysis serum sodium, UF requirement, serum albumin, creatinine, phosphorus and bicarbonate. As the prognostic significance of body size may differ according to gender, two-way sex-by-post-dialysis-weight cross product terms were included. In Model 2, the association of KoAurea [Model 2a] and rate of decline in plasma BUN [Model 2b] with IDH were each individually examined after adjustment for categories of Qb, Qd and session length, in addition to the same covariates as Model 1 (categories of session length were removed from the list of covariates in Model 2b due to collinearity). Covariates for all models were selected on the basis of clinical and biological plausibility, without use of probabilistic selection criteria. Effect modification of randomized Kt/V group on the basis of flux (high vs. low) assignment and pre-dialysis SBP was examined for (and excluded) via the inclusion of two-way cross product terms.
Nominal two-sided p-values of <0.05 were considered statistically significant. Analyses were performed using STATA 10.0MP (College Station, TX).
Results
The primary cohort consisted of 1825 individuals and 62,095 unique hemodialysis sessions. Mean age was 57.8 ± 14.1 years; 62.6% were black and 44.7% were diabetic. Consistent with other reports from the HEMO Study, there was a slightly greater proportion of patients with peripheral vascular disease in those randomized to the higher Kt/V group [21]; otherwise no significant baseline differences between randomized groups were evident (Table 1). Those in the higher Kt/V group had significantly longer session length, higher blood flows and higher dialysate flows compared with those in the lower Kt/V group (Table 2). There were no significant differences in pre-intervention rates of IDH between randomized Kt/V groups.
Table 1.
Baseline characteristics of the total study cohort according to target Kt/V assignment.
| Kt/V assignment | ||||
|---|---|---|---|---|
| Total Cohort (n=1825) | Standard (n=915) | Higher (n=910) | pa | |
| Male (%) | 43.8 | 44.0 | 43.6 | 0.86 |
| Age (yrs) | 57.8 ± 14.1 | 58.0 ± 14.0 | 57.6 ± 14.1 | 0.51 |
| Black (%) | 62.6 | 64.2 | 61.1 | 0.18 |
| Vintage (yrs) | 2.3 (1.1–4.8) | 2.4 (1.2–5.0) | 2.2 (1.1–4.6) | 0.16 |
| Diabetes (%) | 44.7 | 44.7 | 44.6 | >0.9 |
| Ischemic heart disease (%) | 39.3 | 39.9 | 38.7 | 0.60 |
| Congestive Heart Failure (%) | 0.88 | |||
| Mild | 27.9 | 27.5 | 28.3 | |
| Moderate/Severe | 11.5 | 11.8 | 11.2 | |
| Peripheral Vascular Disease (%) | 25.6 | 23.5 | 27.7 | 0.04 |
| Arrhythmia (%) | 30.8 | 32.4 | 29.3 | 0.16 |
| ICED Score | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 0.41 |
| Pre-dialysis SBP (mmHg) | 152.1 ± 26.3 | 152.6 ± 26.6 | 151.6 ± 26.0 | 0.42 |
| Post-dialysis weight (kg) | 69.4 ± 14.8 | 69.9 ± 14.9 | 68.9 ± 14.7 | 0.15 |
| UF requirement (L) | 2.9 ± 1.3 | 2.9 ± 1.3 | 2.9 ± 1.3 | 0.32 |
| UFR (mL/kg/hr) | 12.2 ± 5.6 | 13.1 ± 5.8 | 11.3 ± 5.3 | <0.001 |
| Access (%) | 0.86 | |||
| AVG | 59.9 | 59.8 | 60.1 | |
| AVF | 33.7 | 33.5 | 33.9 | |
| Catheter | 6.4 | 6.7 | 6.0 | |
| Serum sodium (mmol/L) | 138.2 ± 3.9 | 138.3 ± 4.0 | 138.1 ± 3.9 | 0.34 |
| Serum albumin (g/dL) | 3.8 ± 0.4 | 3.8 ± 0.4 | 3.8 ± 0.4 | >0.9 |
| Serum creatinine (mg/dL) | 10.3 ± 2.9 | 10.3 ± 2.8 | 10.3 ± 3.0 | 0.89 |
| Serum phosphorus (mg/dL) | 5.8 ± 1.9 | 5.8 ± 1.9 | 5.8 ± 1.9 | 0.81 |
| Serum bicarbonate (mmol/L) | 21.4 ± 3.6 | 21.4 ± 3.7 | 21.5 ± 3.4 | 0.67 |
P value for difference; significance testing was by t-test or Wilcoxon Rank Sum tests for continuous variables or χ2 test for categorical variables.
ICED, index of co-existent disease; SBP, systolic blood pressure; AVF, arteriovenous fistula; AVG, arteriovenous graft; UF, ultrafiltration; UFR; ultrafiltration rate.
Table 2.
Baseline dialysis session characteristics of the total study cohort according to target Kt/V assignment.
| Kt/V assignment | ||||
|---|---|---|---|---|
| Total Cohort (n=1825) | Standard (n=915) | Higher (n=910) | pa | |
| Session length (mins) | 210 (180–225) | 185 (175–210) | 215 (200–240) | <0.001 |
| Prescribed Qb (mL/min) | 400 (300–450) | 350 (300–400) | 400 (400–450) | <0.001 |
| Prescribed Qd (mL/min) | 700 (500–800) | 600 (500–800) | 800 (600–800) | <0.001 |
| KoAurea ≥1000 mL/min (%) | 43.6 | 36.3 | 51.1 | <0.001 |
P value for difference; significance testing was by Wilcoxon Rank Sum tests.
Qb, blood flow; Qd, dialysate flow; KoAurea, dialyzer mass transfer coefficient for urea.
At baseline, those with greater rate of decline in plasma BUN were more likely to be female, non-black, of lesser post-dialysis weight, have less peripheral vascular disease, but have greater serum albumin and creatinine. In addition they were more likely to have greater ultrafiltration volume, shorter session length, lower blood flows and higher dialysate flows (Supplementary Tables A and B).
HEMO Study target Kt/V assignment
In the higher Kt/V group, 18.3% of sessions were complicated by hypotensive events, compared with 16.8% of the lower Kt/V group (p<0.001). The mean intra-dialytic decline in SBP in sessions with IDH was 52.9 ± 27.5 mmHg, compared with 29.3 ± 22.0 mmHg in those without (p-difference <0.001).
In patient-level analyses, using generalized linear models, the unadjusted and adjusted odds of experiencing a hypotensive event were 11% higher (OR 1.11; 95%CI 0.99–1.24; p=0.07) and 12% higher (OR 1.12; 95%CI 1.01–1.25; p=0.04) respectively for the higher vs. lower Kt/V group. There was no evidence for effect modification according to randomized flux target (p-interaction=0.19).
In sensitivity analyses a more restrictive definition of IDH was considered (decline in SBP of 50mmHg, with symptoms or need for intervention). In this model, higher Kt/V remained associated with greater odds for IDH (OR 1.10; 95%CI 1.00–1.21; p=0.049).
Session length, Qb and Qd
Unadjusted and adjusted models were fit (individually for session length, Qb, Qd and KoAurea) to examine which of these contributors to dialysis dose were independently associated with IDH (Table 4). Compared with the reference for each exposure, there was no evidence for an association of greater session length, Qb, Qd or KoAurea with a greater risk for IDH. In fact, greater Qb appeared to be associated with less risk of IDH in adjusted models. However, upon additional adjustment for Qb, Qd and session length in Model 2a, higher (vs. lower) KoAurea became significantly associated with greater risk of IDH (OR 1.15; 95%CI 1.04–1.27).
Table 4.
Association of individual components of dialysis dose with IDH
| Odds Ratio for IDH (95%CI) | |||
|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | |
| Session Length (mins) | |||
| ≤180 (ref) | 1.00 | 1.00 | - |
| 181–209 | 1.06 (0.93–1.21) | 1.15 (1.01–1.33) | - |
| 210–239 | 1.00 (0.87–1.15) | 1.08 (0.94–1.24) | - |
| ≥240 | 0.85 (0.73–1.00) | 0.95 (0.80–1.12) | - |
| Unadjusted | Model 1 | ||
| Qb (mL/min) | |||
| ≤250 (ref) | 1.00 | 1.00 | - |
| 251–350 | 0.84 (0.75–0.95) | 0.89 (0.78–1.01) | - |
| 351–450 | 0.77 (0.68–0.88) | 0.87 (0.75–0.99) | - |
| >450 | 0.61 (0.49–0.76) | 0.71 (0.56–0.90) | - |
| Unadjusted | Model 1 | ||
| Qd (mL/min) | |||
| ≤500 (ref) | 1.00 | 1.00 | - |
| 501–799 | 0.56 (0.49–0.64) | 0.58 (0.50–0.66) | - |
| ≥800 | 0.98 (0.88–1.09) | 0.98 (0.88–1.09) | - |
| Unadjusted | Model 1 | Model 2a | |
| KoAurea (mL/min) | |||
| <1000 (ref) | 1.00 | 1.00 | 1.00 |
| >=1000 | 0.98 (0.89–1.08) | 1.05 (0.95–1.16) | 1.15 (1.04–1.27) |
| Unadjusted | Model 1 | Model 2b | |
| Rate of decline in plasma BUN (mg/dL/hour) | 1.04 (1.03–1.06) | 1.05 (1.04–1.06) | 1.05 (1.04–1.06) |
Associations of exposures of interest with intra-dialytic hypotension are presented as odds ratios (95% confidence intervals). Estimates were calculated using generalized linear models. Model 1 presents the effect estimates for each exposure of interest after adjustment for HEMO study flux assignment (higher vs lower), age, race (black, non-black), sex, post-dialysis weight, sex-by-weight cross-product terms, access, pre-dialysis SBP, height, ischemic heart disease, congestive heart failure (none, mild, moderate/severe), peripheral vascular disease, diabetes mellitus, arrhythmia, serum sodium, creatinine, albumin, phosphorus, bicarbonate and ultrafiltration requirement. Model 2a adjusts for the same covariates in Model 1, in addition to Qb, Qd and session length. Model 2b adjusts for the same covariates as Model 1, in addition to Qb and Qd.
Qb, blood flow; Qd, dialysate flow; KoAurea, dialyzer mass transfer coefficient for urea.
Rate of decline in plasma BUN
In light of the observation that higher KoAurea associates with greater risk of IDH in fully adjusted models, we revisited our original hypothesis that rapid solute removal may generate temporary osmolar gradients and predispose to IDH. Therefore we considered the rate of decline in plasma BUN as an alternative metric to be examined. In patient-level analyses, using generalized linear models, the unadjusted and adjusted odds (Model 1) of experiencing IDH were 4% higher (OR 1.04; 95%CI 1.03–1.06) and 5% higher (OR 1.05; 95%CI 1.04–1.06) respectively per unit increase in the rate of decline in plasma BUN (mg/dL/hour). Of note, this association was independent of Qb, Qd and UF volume (the latter also being significantly associated with IDH [1.05; 95%CI 1.02–1.09; Model 2; Table 4]).
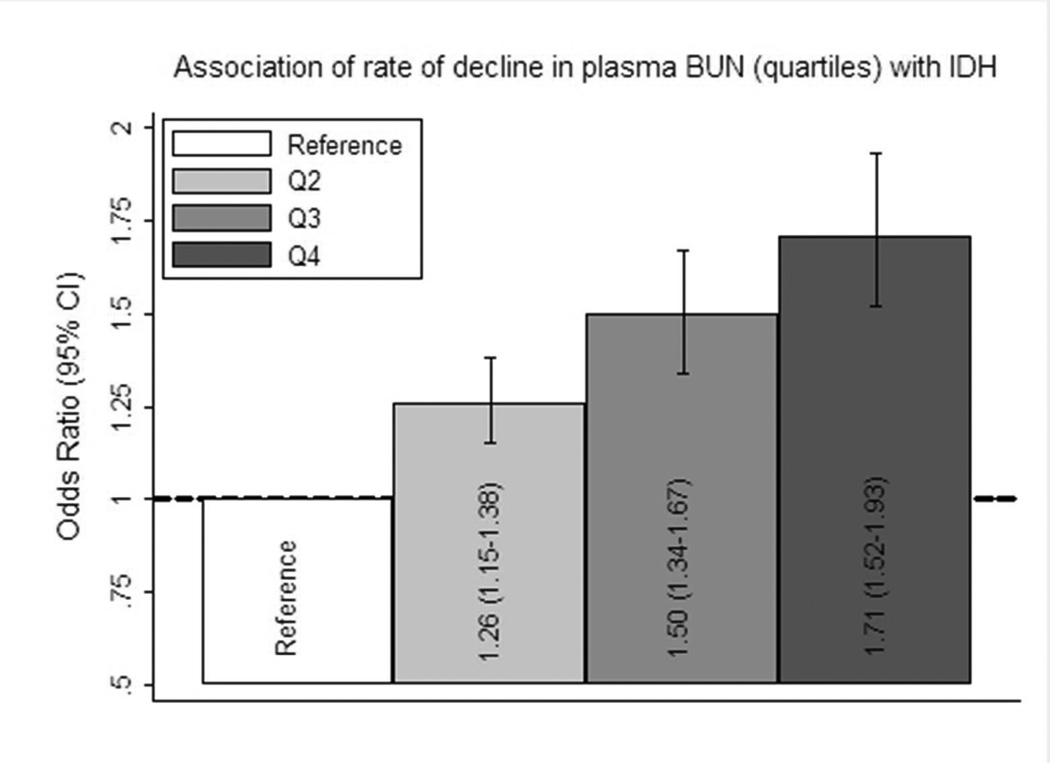
In order to assess for possible non-linear relationships, rate of decline in plasma BUN was then considered as quartiles. A progressive increase in the proportion of sessions complicated by IDH was noted for increasing quartiles of rate of decline in plasma BUN (Table 3). Of note, this pattern was also present in analyses of the pre-interventional period. In adjusted analyses a similar pattern was noted, with increasing odds for IDH associated with increasing quartiles of rate of decline in plasma BUN (Figure 1).
Table 3.
Percentage of hemodialysis sessions complicated by IDH according to categories of exposure (unadjusted).
| Randomized Kt/V group |
Quartile of rate of decline in plasma BUN |
|||||||
|---|---|---|---|---|---|---|---|---|
| % of sessions complicated by IDH |
Standard | Higher | pa | Q1 | Q2 | Q3 | Q4 | pa |
| Before Intervention | 18.4 | 18.5 | 0.93 | 14.0 | 17.5 | 20.2 | 21.4 | <0.001 |
| During Intervention | 16.8 | 18.3 | <0.001 | 14.1 | 16.4 | 18.6 | 20.6 | <0.001 |
P value for difference; significance testing was by χ2 tests.
Q, quartile.
Figure 1. The association between quartiles of rate of decline in plasma BUN and intra-dialytic hypotension.
Associations between quartiles (Q; referent Q1) of rate of decline in plasma BUN and intra-dialytic hypotension (IDH) are presented as odds ratios (95% confidence intervals). Estimates were calculated using generalized linear models and adjusted for HEMO study flux assignment (higher vs lower), session length (≤180, >180, ≥210, ≥240 mins), blood flow (≤250, 250–349, 350–449, ≥450 mL/min), dialysate flow (0–500, 501–800, >800 mL/min), age, race (black, non-black), sex, post-dialysis weight, sex-by-weight cross-product terms, access, pre-dialysis SBP, height, ischemic heart disease, congestive heart failure (none, mild, moderate/severe), peripheral vascular disease, diabetes mellitus, arrhythmia, serum sodium, creatinine, albumin, phosphorus, bicarbonate and ultrafiltration requirement.
Discussion
Using data from the HEMO Study, the largest study of dialysis dose to date, we found that patients randomized to higher dose (target single-pool Kt/V 1.65 vs. 1.25) were more likely to experience IDH during hemodialysis. Notably these findings occurred despite lower baseline ultrafiltration rates (11.3 vs. 13.1 mL/kg/hour) in the higher Kt/V group, suggesting that other factors must be involved in the genesis of IDH.
Potential risk factors for IDH may include the presence of autonomic neuropathy [22] and diminished cardiac reserve [10]; however we found no differences in the proportion of individuals with diabetes or heart failure according to the randomized Kt/V groups in our study. We did note a higher proportion of individuals with peripheral vascular disease, as previously reported in the HEMO Study, but found no difference in effect estimates after additionally adjusting for peripheral vascular disease in multivariable models. Other possibilities include impaired rate of plasma refilling [23], inflammatory cytokine release [24] and reduced venous compliance [11]. In our secondary analyses, we attempted to further dissect the relationship between ‘dose’ and IDH by examining the individual associations of session length, Qb, Qd and KoAurea. These analyses were informative, in that we found no significant association of greater session length or Qd with IDH, and actually found lower odds of IDH with greater Qb in adjusted models. A potential explanation for this observation may be confounding by patients who are able to achieve higher Qb being less likely to have vascular disease or diabetes, and thus able to tolerate higher flows. However, in line with our original hypothesis, we did note an association of greater KoAurea and greater rate of decline in plasma BUN with greater odds for hypotension (independent of Qb and Qd; Model 2), suggesting that this factor is an important determinant in the relationship between dialysis ‘dose’ and IDH.
Experimental data in animals [25,26] and humans [27] support a role for rapid urea removal by hemodialysis in the generation of temporary osmotic gradients between body compartments. Others argue that the induction of temporary gradients for other molecules, including sodium, are more important [28], but there appears to be a consensus that these gradients contribute to many of the features of dialysis disequilibrium, including cerebral edema, mental status changes, seizures and even death [29]. Several investigators have also noted an association between intra-dialytic generation of trans-cellular osmotic gradients and IDH [28,30,31]. In order to achieve the higher randomized Kt/V target, HEMO Study investigators were encouraged to increase membrane size, dialysate flow or blood flow before increasing treatment time. Therefore, in effect, dialysis session lengths were relatively constrained, which likely increased the rapidity of temporary osmotic gradient generation between the intra- and extra-vascular compartments. Our findings, based on the original HEMO targeted Kt/V randomization groups provide evidence for an association between the rapidity of intra-dialytic plasma osmolality decline and development of hypotensive events. The absence of differences in the percentage of hypotensive events among pre-intervention dialysis sessions, according to randomized Kt/V groups, provides supportive evidence that the higher dose intervention is truly associated with greater risk of IDH. In the secondary analyses, the association of increasing quartiles of rate of decline in plasma BUN (which are non-randomized categories) with greater IDH was present for both pre-intervention and post-intervention sessions. These analyses support the presence of a dose-response relationship between the achieved rate of decline in plasma BUN and risk of IDH, irrespective of the randomized trial intervention.
There are numerous reports detailing interventions aimed at minimizing the rate of osmolality decline that may be useful in the treatment of hypotensive-prone patients. Dialysis physicians initially increased the dialysate sodium concentration, finding that this reduced the frequency of dialysis-related symptoms [32], with subsequent studies demonstrating improved hemodynamic stability [33,34]. Prior reports of increased thirst and inter-dialytic weight gain (IDWG) tempered the initial enthusiasm of these findings [12,34], but more recently the use of biofeedback-controlled sodium profiling appears to have less associated IDWG [35]. Other methods include the administration of hypertonic mannitol and other hypertonic solutions [15,30,36,37]. In addition to trans-cellular fluid shifts due to osmotic changes, rapid decline of intra-vascular osmolality may suppress the release of vasopressin, which has pressor-like effects on the vasculature; in support of this concept, infusion of vasopressin during hemodialysis has been associated with improved hemodynamic stability [38]. Given the association of IDH with numerous adverse outcomes (including access failure [39], cerebral atrophy [7], myocardial stunning [40] and death [8]), and the promise shown by previous small interventional studies, future research effort focusing on manipulation of osmolality changes, without compromising clearance, may be fruitful in addressing the unacceptably high morbidity and mortality of our patients.
The major strength of this study is the utilization of the original randomized assignments from the HEMO Study, which should minimize the presence of residual confounding in the primary analyses of targeted Kt/V. In this regard, although the HEMO Study did not record potentially confounding variables such as dialysate sodium or dialysate temperature, it is likely that randomization produced minimal imbalance according to these parameters between the targeted Kt/V groups. Furthermore, the HEMO Study was performed at a time when the move away from lower sodium dialysate concentrations had already occurred [41]. Additional strengths include the large and detailed collection of exposure and outcomes data over the complete duration of follow-up in the setting of a randomized controlled trial. Without the primacy of randomization in secondary analyses, multiple covariates had to be considered in the model-building process, leading to the possibility of residual confounding. There are theoretical concerns of dose-targeting bias in relation to the analysis of rate of decline in plasma BUN as the exposure of interest. For example, in the dose-mortality analyses of the HEMO Study, significant associations between higher achieved dose and reduced mortality have been reported (at odds with the original intention-to-treat analyses demonstrating no advantage to higher vs. standard Kt/V) [42]. It is possible that individuals who were able to attain a higher achieved Kt/V were healthier, more compliant, and had other beneficial characteristics that could potentially confound the relationship between achieved dose and mortality. The associations we present are less likely to suffer from this bias, as we found that higher rates of urea removal (a metric of clearance, in which higher values may be interpreted as beneficial) were in fact associated with greater odds for hypotension. This is opposite to what we would expect if beneficial dose-targeting bias were an issue. However, on the other hand, a very high rate of decline in plasma BUN can also be achieved in malnourished individuals with low muscle mass, or those with shorter session lengths, who may be at risk for adverse outcomes. This may have contributed to the increased association with hypotensive events which we observed in these individuals. The HEMO Study did not record the timing of hypotensive events, prohibiting a more granular assessment of the competing effects of solute removal rate and ultrafiltration rates, which may be expected to predispose to hypotension at different time points during the dialysis procedure. However, we were able to confirm that both the rate of decline in plasma BUN and ultrafiltration volume were independently associated with greater risk of IDH. Finally, participants in a randomized controlled setting may not be comparable to the wider chronic hemodialysis population, limiting the generalizability of our results.
In conclusion, higher dialysis dose appears to increase the risk for intra-dialytic hypotensive events requiring a clinical intervention. In patients at risk of IDH, targeted strategies to reduce the rapidity of decline in plasma osmolality during hemodialysis may be beneficial. Potential interventions may include selection of lower efficiency membranes, extending the session length, selected use of higher dialysate sodium or infusion of osmotically active substances. While ensuring preservation of hemodialysis adequacy, future studies should assess the potential benefit of these interventions in prospective clinical trials, particularly in patients who suffer from repeated episodes of IDH.
Acknowledgements
We thank the HEMO Study investigators and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) data repository for the data used in this study. The HEMO Study was performed by the HEMO Study investigators and supported by the NIDDK. This paper was not prepared in collaboration with the investigators of the HEMO Study and does not necessarily reflect the opinions or views of the HEMO Study or the NIDDK.
Disclosures:
Dr. Mc Causland was supported by a Clinical Fellowship Grant from the National Kidney Foundation (2011–13).
Dr. Brunelli has served as an advisor to Amgen, C.B. Fleet Company and Proctor & Gamble. Since completing work on this study, Dr. Brunelli has become a full time employee of DaVita Clinical Research. He has received speaking honoria from Fresenius Medical Care North America. His spouse is employed by Astra Zeneca. Dr. Brunelli is supported by DK079056.
Dr. Waikar is supported by DK093574, DK075941 and U01DK085660.
Footnotes
Portions of this work were presented in abstract form at the American Society of Nephrology annual meeting in San Diego, 2012.
References
- 1.Bos WJ, Bruin S, van Olden RW, Keur I, Wesseling KH, Westerhof N, Krediet RT, Arisz LA. Cardiac and hemodynamic effects of hemodialysis and ultrafiltration. Am J Kidney Dis. 2000;35:819–826. doi: 10.1016/s0272-6386(00)70250-2. [DOI] [PubMed] [Google Scholar]
- 2.Boon D, van Montfrans GA, Koopman MG, Krediet RT, Bos WJ. Blood pressure response to uncomplicated hemodialysis: The importance of changes in stroke volume. Nephron Clin Pract. 2004;96:c82–c87. doi: 10.1159/000076745. [DOI] [PubMed] [Google Scholar]
- 3.Palmer BF, Henrich WL. Recent advances in the prevention and management of intradialytic hypotension. J Am Soc Nephrol. 2008;19:8–11. doi: 10.1681/ASN.2007091006. [DOI] [PubMed] [Google Scholar]
- 4.Dheenan S, Henrich WL. Preventing dialysis hypotension: A comparison of usual protective maneuvers. Kidney Int. 2001;59:1175–1181. doi: 10.1046/j.1523-1755.2001.0590031175.x. [DOI] [PubMed] [Google Scholar]
- 5.KDOQI: K/doqi clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45:S1–S153. [PubMed] [Google Scholar]
- 6.Owen PJ, Priestman WS, Sigrist MK, Lambie SH, John SG, Chesterton LJ, McIntyre CW. Myocardial contractile function and intradialytic hypotension. Hemodial Int. 2009;13:293–300. doi: 10.1111/j.1542-4758.2009.00365.x. [DOI] [PubMed] [Google Scholar]
- 7.Mizumasa T, Hirakata H, Yoshimitsu T, Hirakata E, Kubo M, Kashiwagi M, Tanaka H, Kanai H, Fujimi S, Iida M. Dialysis-related hypotension as a cause of progressive frontal lobe atrophy in chronic hemodialysis patients: A 3-year prospective study. Nephron Clin Pract. 2004;97:c23–c30. doi: 10.1159/000077592. [DOI] [PubMed] [Google Scholar]
- 8.Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004;66:1212–1220. doi: 10.1111/j.1523-1755.2004.00812.x. [DOI] [PubMed] [Google Scholar]
- 9.Kersh ES, Kronfield SJ, Unger A, Popper RW, Cantor S, Cohn K. Autonomic insufficiency in uremia as a cause of hemodialysis-induced hypotension. N Engl J Med. 1974;290:650–653. doi: 10.1056/NEJM197403212901203. [DOI] [PubMed] [Google Scholar]
- 10.Poldermans D, Man in 't Veld AJ, Rambaldi R, Van Den Meiracker AH, Van Den Dorpel MA, Rocchi G, Boersma E, Bax JJ, Weimar W, Roelandt JR, Zietse R. Cardiac evaluation in hypotension-prone and hypotension-resistant hemodialysis patients. Kidney Int. 1999;56:1905–1911. doi: 10.1046/j.1523-1755.1999.00737.x. [DOI] [PubMed] [Google Scholar]
- 11.Kooman JP, Gladziwa U, Bocker G, van Bortel LM, van Hooff JP, Leunissen KM. Role of the venous system in hemodynamics during ultrafiltration and bicarbonate dialysis. Kidney Int. 1992;42:718–726. doi: 10.1038/ki.1992.339. [DOI] [PubMed] [Google Scholar]
- 12.Song JH, Park GH, Lee SY, Lee SW, Kim MJ. Effect of sodium balance and the combination of ultrafiltration profile during sodium profiling hemodialysis on the maintenance of the quality of dialysis and sodium and fluid balances. J Am Soc Nephrol. 2005;16:237–246. doi: 10.1681/ASN.2004070581. [DOI] [PubMed] [Google Scholar]
- 13.Maynard JC, Cruz C, Kleerekoper M, Levin NW. Blood pressure response to changes in serum ionized calcium during hemodialysis. Ann Intern Med. 1986;104:358–361. doi: 10.7326/0003-4819-104-3-358. [DOI] [PubMed] [Google Scholar]
- 14.Depner TA. Assessing adequacy of hemodialysis: Urea modeling. Kidney Int. 1994;45:1522–1535. doi: 10.1038/ki.1994.199. [DOI] [PubMed] [Google Scholar]
- 15.Canzanello VJ, Hylander-Rossner B, Sands RE, Morgan TM, Jordan J, Burkart JM. Comparison of 50% dextrose water, 25% mannitol, and 23.5% saline for the treatment of hemodialysis-associated muscle cramps. ASAIO Trans. 1991;37:649–652. [PubMed] [Google Scholar]
- 16.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, Ornt DB, Rocco MV, Schulman G, Schwab SJ, Teehan BP, Toto R. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347:2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 17.Greene T, Beck GJ, Gassman JJ, Gotch FA, Kusek JW, Levey AS, Levin NW, Schulman G, Eknoyan G. Design and statistical issues of the hemodialysis (hemo) study. Control Clin Trials. 2000;21:502–525. doi: 10.1016/s0197-2456(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 18.Leypoldt JK, Cheung AK, Agodoa LY, Daugirdas JT, Greene T, Keshaviah PR. Hemodialyzer mass transfer-area coefficients for urea increase at high dialysate flow rates. The hemodialysis (hemo) study. Kidney Int. 1997;51:2013–2017. doi: 10.1038/ki.1997.274. [DOI] [PubMed] [Google Scholar]
- 19.Miskulin DC, Athienites NV, Yan G, Martin AA, Ornt DB, Kusek JW, Meyer KB, Levey AS. Comorbidity assessment using the index of coexistent diseases in a multicenter clinical trial. Kidney Int. 2001;60:1498–1510. doi: 10.1046/j.1523-1755.2001.00954.x. [DOI] [PubMed] [Google Scholar]
- 20.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 21.Liu T, Liang KV, Rosenbaum A, Stephenson R, Pike F, Weissfeld L, Unruh ML. Peripheral vascular disease severity impacts health outcomes and health-related quality of life in maintenance hemodialysis patients in the hemo study. Nephrol Dial Transplant. 2012;27:2929–2936. doi: 10.1093/ndt/gfr760. [DOI] [PubMed] [Google Scholar]
- 22.Lilley JJ, Golden J, Stone RA. Adrenergic regulation of blood pressure in chronic renal failure. J Clin Invest. 1976;57:1190–1200. doi: 10.1172/JCI108387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schroeder KL, Sallustio JE, Ross EA. Continuous haematocrit monitoring during intradialytic hypotension: Precipitous decline in plasma refill rates. Nephrol Dial Transplant. 2004;19:652–656. doi: 10.1093/ndt/gfg590. [DOI] [PubMed] [Google Scholar]
- 24.Tomita M, Malhotra D, Dheenan S, Shapiro JI, Henrich WL, Santoro TJ. A potential role for immune activation in hemodialysis hypotension. Ren Fail. 2001;23:637–649. doi: 10.1081/jdi-100107360. [DOI] [PubMed] [Google Scholar]
- 25.Arieff AI, Massry SG, Barrientos A, Kleeman CR. Brain water and electrolyte metabolism in uremia: Effects of slow and rapid hemodialysis. Kidney Int. 1973;4:177–187. doi: 10.1038/ki.1973.100. [DOI] [PubMed] [Google Scholar]
- 26.Pappius HM, Oh JH, Dossetor JB. The effects of rapid hemodialysis on brain tissues and cerebrospinal fluid of dogs. Can J Physiol Pharmacol. 1967;45:129–147. doi: 10.1139/y67-014. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy AC, Linton AL, Eaton JC. Urea levels in cerebrospinal fluid after haemodialysis. Lancet. 1962;1:410–411. doi: 10.1016/s0140-6736(62)91365-x. [DOI] [PubMed] [Google Scholar]
- 28.Mann H, Stiller S. Urea, sodium, and water changes in profiling dialysis. Nephrol Dial Transplant. 1996;11(Suppl 8):10–15. doi: 10.1093/ndt/11.supp8.10. [DOI] [PubMed] [Google Scholar]
- 29.Patel N, Dalal P, Panesar M. Dialysis disequilibrium syndrome: A narrative review. Semin Dial. 2008;21:493–498. doi: 10.1111/j.1525-139X.2008.00474.x. [DOI] [PubMed] [Google Scholar]
- 30.Henrich WL, Woodard TD, Blachley JD, Gomez-Sanchez C, Pettinger W, Cronin RE. Role of osmolality in blood pressure stability after dialysis and ultrafiltration. Kidney Int. 1980;18:480–488. doi: 10.1038/ki.1980.161. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigo F, Shideman J, McHugh R, Buselmeier T, Kjellstrand C. Osmolality changes during hemodialysis. Natural history, clinical correlations, and influence of dialysate glucose and intravenous mannitol. Ann Intern Med. 1977;86:554–561. doi: 10.7326/0003-4819-86-5-554. [DOI] [PubMed] [Google Scholar]
- 32.Port FK, Johnson WJ, Klass DW. Prevention of dialysis disequilibrium syndrome by use of high sodium concentration in the dialysate. Kidney Int. 1973;3:327–333. doi: 10.1038/ki.1973.51. [DOI] [PubMed] [Google Scholar]
- 33.Locatelli F, Covic A, Chazot C, Leunissen K, Luno J, Yaqoob M. Optimal composition of the dialysate, with emphasis on its influence on blood pressure. Nephrol Dial Transplant. 2004;19:785–796. doi: 10.1093/ndt/gfh102. [DOI] [PubMed] [Google Scholar]
- 34.Sang GL, Kovithavongs C, Ulan R, Kjellstrand CM. Sodium ramping in hemodialysis: A study of beneficial and adverse effects. Am J Kidney Dis. 1997;29:669–677. doi: 10.1016/s0272-6386(97)90118-9. [DOI] [PubMed] [Google Scholar]
- 35.Locatelli F, Stefoni S, Petitclerc T, Coli L, Di Filippo S, Andrulli S, Fumeron C, Frasca GM, Sagripanti S, Savoldi S, Serra A, Stallone C, Aucella F, Gesuete A, Scarlatella A, Quarello F, Mesiano P, Ahrenholz P, Winkler R, Mandart L, Fort J, Tielemans C, Navino C. Effect of a plasma sodium biofeedback system applied to hfr on the intradialytic cardiovascular stability. Results from a randomized controlled study. Nephrol Dial Transplant. 2012;2012:4. doi: 10.1093/ndt/gfs091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimizu K, Kurosawa T, Sanjo T. Effect of hyperosmolality on vasopressin secretion in intradialytic hypotension: A mechanistic study. Am J Kidney Dis. 2008;52:294–304. doi: 10.1053/j.ajkd.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 37.Mc Causland FR, Prior LM, Heher E, Waikar SS. Preservation of blood pressure stability with hypertonic mannitol during hemodialysis initiation. Am J Nephrol. 2012;36:168–174. doi: 10.1159/000341273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Zee S, Thompson A, Zimmerman R, Lin J, Huan Y, Braskett M, Sciacca RR, Landry DW, Oliver JA. Vasopressin administration facilitates fluid removal during hemodialysis. Kidney Int. 2007;71:318–324. doi: 10.1038/sj.ki.5001885. [DOI] [PubMed] [Google Scholar]
- 39.Chang TI, Paik J, Greene T, Desai M, Bech F, Cheung AK, Chertow GM. Intradialytic hypotension and vascular access thrombosis. J Am Soc Nephrol. 2011;22:1526–1533. doi: 10.1681/ASN.2010101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McIntyre CW. Haemodialysis-induced myocardial stunning in chronic kidney disease - a new aspect of cardiovascular disease. Blood Purif. 2010;29:105–110. doi: 10.1159/000245634. [DOI] [PubMed] [Google Scholar]
- 41.Flanigan M. Dialysate composition and hemodialysis hypertension. Semin Dial. 2004;17:279–283. doi: 10.1111/j.0894-0959.2004.17327.x. [DOI] [PubMed] [Google Scholar]
- 42.Greene T, Daugirdas J, Depner T, Allon M, Beck G, Chumlea C, Delmez J, Gotch F, Kusek JW, Levin N, Owen W, Schulman G, Star R, Toto R, Eknoyan G. Association of achieved dialysis dose with mortality in the hemodialysis study: An example of "Dose-targeting bias". J Am Soc Nephrol. 2005;16:3371–3380. doi: 10.1681/ASN.2005030321. [DOI] [PubMed] [Google Scholar]