Abstract
Introduction
The purpose of this study was to compare digital periapical and cone beam computed tomography (CBCT) images to determine the number of canals in the mesiobuccal root (MB) of maxillary molars and to compare these counts to micro CT (μCT), which was also used to determine canal configuration.
Methods
Digital periapical (RVG 6100), CBCT (9000 3D) and μCT images (the reference standard) were obtained of 18 hemi-maxillas. With periapical and CBCT images, 2 endodontists independently counted the number of canals in each molar and repeated counts 2 weeks later. Teeth were extracted, scanned with μCT, and 2 additional endodontists, by consensus, determined the number and configuration of canals. The Friedman test was used to test for differences.
Results
In mesiobuccal roots, 2 canals were present in 100% (13/13) of maxillary first and 57% (8/14) second molars, and 69% (9/13) and 100% (8/8) of these exited as two or more foramina. There was no difference in canal counts for original and repeat reads by the two observers with periapicals (P = 0.06) and with CBCT (P = 0.88) and no difference when CBCT counts were compared with μCT counts (P = 0.52); however, when periapical counts were compared with μCT counts there was a significant difference (P = 0.04).
Conclusions
For cadaver maxillary molars, μCT canal counts were significantly different from digital periapical radiograph counts but not different from Carestream 9000 3D CBCT counts.
Introduction
Knowledge and understanding of root-morphology is imperative for planning and performing endodontic therapy. Traditionally, prior to the initiation of root canal therapy multiple periapical films (PAs) at different angles are taken to identify the number of canals in teeth (1, 2). Multiple PAs are required because a PA is a 2-dimensional (2D) image of complex 3-dimensional (3D) anatomy. Superimposition of anatomical structures and image distortions, especially in the maxilla, often obscure canals (3). This makes diagnosis of the initial canal anatomy difficult, especially for the mesiobuccal (MB) root of maxillary molars.
The literature review by Cleghorn et al highlighted the difference between laboratory and clinical studies in identifying multiple canals in the MB roots of maxillary first molars (4). The weighted average of the referenced laboratory studies reported that 60.5% of the MB roots of the maxillary first molars had multiple canals, with an incidence of only 54.7% identified in clinical studies (4). Laboratory studies used various clearing techniques (5–14), tooth sectioning (15, 16), and radiographic methods (17–19). The methodologies of these studies have their strengths and weaknesses, and other than radiologic surveys, no laboratory study was able to reproduce relatively accurate canal anatomy without destroying the tooth.
In 1995, it was demonstrated that micro computed tomography (μCT) could document the internal and external morphologies of the tooth without its destruction (20). Micro CT was subsequently used to assess root canal system geometry before and after instrumentation (21, 22). With voxel sizes as small as 19 μm, 3D images were produced and analyzed to determine the number and configuration of canals and foramina (20–25). Some limitations of using μCT for research are expense of the unit and technician’s time, time required for scanning and reconstruction, and the cost of the software for manipulating images and making image measurements (25). In addition, because of size constraints, there is no μCT scanner that can be used to scan the head of a living human; therefore, in most μCT-studies of human teeth, only extracted teeth (or jaw segments containing teeth) are scanned. Micro CT can, however, be considered the reference (gold) standard for laboratory studies of canal anatomy (23).
Recently, small field of view cone beam computed tomography (CBCT) has been shown to have a high degree of accuracy in all spatial planes and is therefore, useful in identifying the number of canals (4, 26). CBCT systems have been demonstrated to have low effective radiation doses and produce undistorted 3D images of the teeth and surrounding structures (5). The Carestream Dental 9000 3D (Carestream Dental, Atlanta, GA) is a recently introduced CBCT system, which has a voxel size of 76 μm. This voxel size is important because histologically, the smallest canal size reported when the canal appeared radiographically obliterated was a diameter of 100 μm (27). With the 9000 3D, it should be possible to identify most canals with diameters of 100 μm although to image accurately (and avoid aliasing) all 100-μm canals, the Nyquiest sampling theorem requires that the voxel size should be 50-μm or less (28).
To date, CBCT ex vivo studies have been performed on human extracted teeth (15, 26, 29, 30). This eliminates image distortion and superimposition of anatomical structures that are often present with 2D images of in situ teeth; however, extrapolating laboratory findings to the clinic (in which teeth have overlying bone, soft tissue, and other anatomic features) is problematic in studies for which 3D CBCT images and 2D radiographic images are compared for determining the presence and configuration of root canals (31).
The purpose of this study was to use digital periapical and CBCT images to determine the number of canals in human maxillary molars and to compare these counts with counts determined with μCT.
Material and Methods
A convenience sample of eighteen human hemi-maxillas was obtained from a large collection of hemi-maxillas maintained by the Saint Louis University Medical School Department of Anatomy. The hemi-maxillas were from cadavers that were donated (with prior consent) for use in medical research and teaching. Ages and sexes for the hemi-maxillas were unknown. Under the requirements of the U.S. Department of Health and Human Services (HHS) regulations at 45 CFR part 46, research involving non-living individuals is not considered research involving human subjects and does not require institutional review board approval.
Each hemi-maxilla was stabilized to ensure consistent beam geometry and source to object distance. A Gendex 770 DC dental x-ray generator (KaVo Dental Gendex Imaging, Milanino, Italy) was used to acquire images with a #2-size RVG 6100 sensor (Carestream Dental, Atlanta, GA). Each image contained 2.76 megapixels. The pixel size was 1.08 microns, the grey-level dynamic range was 12 bits, and the spatial resolution was > 20 line pairs/mm. Exposures were made at 70 kVp and 7 mA, with a nominal focal spot size of 0.6 mm, a focal distance of 4 cm, and an exposure time of 0.18 seconds. Three periapical images were taken for each tooth. Teeth were excluded only if root canal therapy had been previously performed. Twenty-seven maxillary first and second molars met the criteria for the study; thirteen first molars and fourteen second molars. A protractor was used to confirm all angulations. The first image was taken perpendicular to the tooth and x-ray detector. The second image was taken with a 20-degree, distal horizontal angulation and the third image with a 20-degree, mesial horizontal angulation. TDO (San Diego, CA) software version (11.117a) was used with the RVG 6100.
The hemi-maxillas were re-positioned with the teeth resting on a bite mount, midline centered in the focal trough, and scanned with a 9000 3D CBCT (Carestream Dental, Atlanta, GA). A voxel size of 76 μm was used with a bit depth of 15 bits. Settings were 68 kVp, 6.3 mA, and 11 seconds. Volume-renderings and multiplanar volume reconstructions were performed using Carestream imaging software version (2.4.11). Teeth in the CBCT volume were viewed in axial and sagittal planes.
Two endodontists independently viewed the digital periapical images and CBCT volumes to determine the number of canals in each maxillary molar. Observation conditions were performed with dimmed lighting and a black background. Images were viewed with a 33.7 cm × 27.0 cm monitor (Dell 1704FPV, Dell, Austin, TX) that displayed 1.3 M pixels, with a pixel depth of 24 bits. The luminance of the monitor was 280 cd/m2. The ambient light level was <50 lux. After a minimum of two weeks, each observer performed repeat canal counts.
Teeth were extracted, cleaned in 3% NaOCl, and scanned with μCT (VivaCT 40, Scanco Medical, Brüttisellen, Switzerland): voxel size 20 μm, 70 kVp, 114 μA, and 20 minutes. Micro CT was the reference (gold) standard for the study. For each tooth, a μCT 3D model of the root canal system was constructed with 3D IPO image processing language (version 5.15, Scanco Medical, Brüttisellen, Switzerland). The reconstructed μCT 3D models of the root canal systems and 2D slices (n = 502/tooth) were viewed independently by two endodontists who by consensus, determined canal numbers and configurations.
As part of the variability/gauge (multivariate chart analysis) platform available in JMP statistical software (JMP (Release 9.0.0, SAS Institute, Inc., Cary, NC), a Bayesian variance components analysis was performed with the dependent variable being number of canals and the independent variables being: (1) modality, CBCT or digital; (2) tooth (maxillary right and left first and second molars) nested within modality; (3) observer (1 or 2) nested within tooth nested within modality; and (4) time 1 or 2 (original or repeat count) nested within observer, nested within tooth, nested within observer, nested within modality. A variance components analysis determines the percentage of variation that occurs at various levels [that is, between modalities, among teeth, between observers, and between times (original and repeat determinations)]. Data permutation was used to calculate exact P values for the Friedman test to determine whether or not there were differences in the number of canals among original and repeat canal counts made by both observers with: (1) periapical images (2) CBCT images, (3) periapical images plus counts with μCT, and (4) CBCT images plus counts with μCT. Statistical analyses were performed with JMP Statistical Software (Release 9.0.0, SAS Institute, Inc., Cary, NC) and StatXact 9 Statistical Software for Exact Nonparametric Inference (Cytel, Inc., Cambridge, MA). Alpha was set at 0.05.
Results
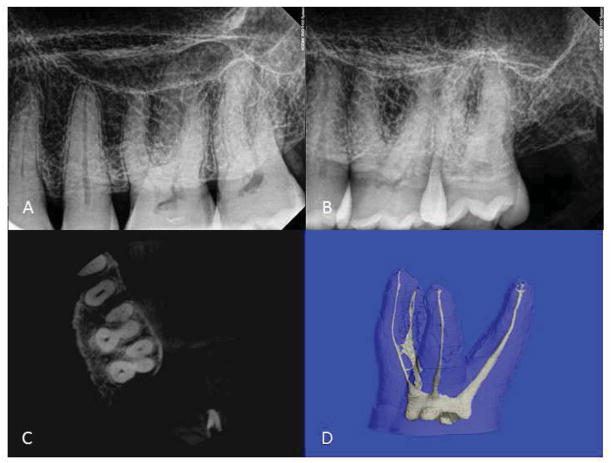
Twenty-seven teeth from 18 cadavers were examined; 13 first molars and 14 second molars. Figure 1 is an example of one samples’ images comparing periapical and CBCT images (axial slice) to μCT. The observers’ canal counts for periapical (digital) and CBCT and the number of canals identified with μCT in the MB roots of the maxillary first and second molars are summarized in Table 1. One hundred percent (13/13) of maxillary-first-molar MB roots had two canals of which 69% (9/13) exited as two or more foramina. Fifty seven percent (8/14) of maxillary-second-molar MB roots had two canals of which 100% (8/8) exited as two or more foramina The variance components analyses indicated that 1.8% of variation in counts was attributable to differences between readers (inter-rater) and less than 0.1% of variation in counts occurred within readers (intra-rater). The variance components attributable to the inter- and intra-observer agreement were not significant (P > 0.05). A Friedman test of digital radiographic counts by observers 1 and 2 at both time 1 and 2 (original and repeat counts) demonstrated no difference (P = 0.06).
FIGURE 1. Comparison images and micro CT of a maxillary left first molar.
(A) Periapical radiograph with the x-ray beam perpendicular to the x-ray detector. (B) With the x-ray beam at a 20-degree distal angle. (C) CBCT axial slice. (D) Micro CT 3D image.
TABLE 1.
Numbers of canals counted for 27 teeth by observers 1 and 2 for their first and second reads with periapical images (digital) and CBCT images. Micro CT counts are also listed.
| Observer 1 Digital 1st Read | Observer 1 Digital 2nd Read | Observer 1 CBCT 1st Read | Observer 1 CBCT 2nd Read | Observer 2 Digital 1st Read | Observer 2 Digital 2nd Read | Observer 2 CBCT 1st Read | Observer 2 CBCT 2nd Read | Micro CT | |
|---|---|---|---|---|---|---|---|---|---|
| Number Of canals | |||||||||
| 2 | 2 | 2 | 2 | 2 | 2 | ||||
| 3 | 15 | 13 | 1 | 1 | 9 | 9 | 1 | 2 | 3 |
| 4 | 12 | 14 | 23 | 22 | 18 | 18 | 22 | 21 | 21 |
| 5 | 2 | 1 | 2 | 1 | |||||
| 6 | 1 | 1 | |||||||
| Totals | 27 | 27 | 27 | 27 | 27 | 27 | 27 | 27 | 27 |
When the observers’ digital counts plus μCT counts were compared, there was a difference (P = 0.04). There was no difference among counts by observers 1 and 2 at times 1 and 2 for CBCT (P = 0.88), and no difference when the observers’ CBCT counts and μCT counts were combined (P = 0.52).
Interestingly, in one sample both observers on their first reads counted 6 canals; for their second reads, both observers counted 5 canals in this tooth. The number of canals counted with μCT for this tooth was 4. Upon comparison of the μCT and CBCT images, it was determined that the discrepancy was caused by pulpal calcifications visible with μCT. With CBCT images, the calcifications appeared to be splits in the canal while the μCT images indicated the presence of a single canal that contained calcifications.
Discussion
In this study, μCT images indicated that second MB canals were present in 100% (13/13) of maxillary first molars and 57% (8/14) of maxillary second molars. These percentages are higher than those found in a CBCT study of a Korean tooth sample for which additional canals were found in 63.59% of MB roots of maxillary first molars and 34.39% of maxillary second molars (32) but are in agreement with previous studies that used clearing and SEM techniques and found second MB canals in: 93.5% of maxillary first and 59% of maxillary second molars (7) and 90.5% of maxillary first and 70.3% of maxillary second molars (13). Reasons for the differences in percentages could be (1) the different populations represented in the studies and (2) the ages of the patients studied. Although the ages were unknown for the specimens used in this study, most of the medical school cadavers are older adults for whom the percentages of multiple canals increase with age (17, 18). A third reason for the differences in percentages could be attributable to the limited number of root canal anatomy studies that have used μCT (23, 24). It is important to know these percentages when treating maxillary molars. Of more importance (in this study), a significant difference in number of canals was detected when 2D counts were compared with reference-standard, μCT counts, but no difference was detected when CBCT counts were compared with μCT counts. This is important clinically with regard to the ability to make pre-intervention diagnoses of maxillary molar, root canal anatomies.
The root of the maxillary molar is relatively slender mesiodistally and is broad buccolingually (19). This presents a challenge in using pretreatment radiographs to visualize canal anatomy. Root canals have complex 3D anatomy and representations of this anatomy provided by 2D intraoral radiographs contain little information on the buccolingual dimension (3). Canals that are aligned in a buccolingual plane cannot be easily differentiated from each other. It takes chance (due to the rotation of a tooth or by intentional alignment of the x-ray beam) to make overlapping canals somewhat visible; however, in the maxillary molar MB root, the small width of MB2 and its close proximity to the main canal makes visualization of this canal difficult (17). In addition, superimposition of anatomical structures and image distortion, especially in the maxilla, often obscure canal anatomy (3).
Because CBCT images are created from a volume of data, they are relatively unaffected by skull orientation during image acquisitions (33). CBCT images can provide high-resolution images in multiple planes while eliminating superimposition of surrounding structures (15). Just as a 2D digital image is subdivided into pixels, a 3D CBCT image is composed of voxels (34). Essentially, a voxel is a 3D pixel. With the CBCT images that were used in this study, voxels are isotropic, which means that 3D objects can be measured in three dimensions with relatively good accuracy (33). The accuracy is such that Janner et al suggested that existing CBCT scans can be useful as an adjunct for determination of endodontic working length (35). When using appropriate tomographic techniques, it is possible to look at each root separately (36). Multiplanar reconstructions can be made so that coronal and sagittal images are parallel with the long axis of a root, with the axial images perpendicular to the long axis. These factors make CBCT superior to conventional 2D radiography (36).
It is, however, important to compare 2D radiographic and 3D CBCT images to understand the clinical importance of CBCT in determining root canal anatomy. For such testing, a reference is required to verify the results. Micro CT has become the reference standard for laboratory canal anatomy studies (23). Three-dimensional images can be produced and analyzed to record the numbers and configurations of canals, without destroying the tooth (20, 23, 24 and 25).
There are some weaknesses with this study. First, the sample size is small, which is attributable to the relatively high cost of using μCT as the reference standard. Second, discrepancies between observer canal counts with CBCT and μCT images were caused by intra-canal pulpal calcifications that were visible with μCT but appeared to be splits in the canals (that is, two canals) with CBCT. With additional technical improvements, this weakness may be overcome. Third, the results of this study may be better than what would occur with patients who may move and have more soft and hard tissue than did the hemi-maxillas that were used. Finally, only one dental CBCT system was studied. There are numerous manufacturers of dental CBCT systems, and the results of this study may not be representative of results using other systems.
Continued advances in CBCT technology enable clinicians to better understand tooth anatomy prior to endodontic therapy and thus improve treatment outcomes. In this study, it was determined that for cadaver maxillary molars the number of canals determined with μCT was significantly different from the number of canals determined with digital periapical radiographs but was not significantly different from the numbers of canals determined with Kodak 9000 3D CBCT.
Acknowledgments
The authors would like to sincerely thank Dr. Margaret Cooper, Saint Louis University Medical School Department of Anatomy for the use of the cadavers in this study. They would also like to thank Dr. Susan Adams and Dr. Adam Diliberto for their assistance with viewing the radiographs. For help with μCT scanning, the authors would like to thank Tarpit Patel in the Biomechanical Engineering Laboratory/Musculoskeletal Structure and Strength Core (supported by Grant # P30AR057235 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases) and Kirk Smith in the Department of Radiology at Washington University.
Footnotes
The authors deny any conflicts of interest related to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walton RE. Endodontic radiographic techniques. Dent Radiol Photog. 1973;46:51–9. [PubMed] [Google Scholar]
- 2.Fava LG, Dummer PH. Periapical radiographic techniques during endodontic diagnosis and treatment. Int Endod J. 1997;30:250–61. doi: 10.1046/j.1365-2591.1997.00078.x. [DOI] [PubMed] [Google Scholar]
- 3.Slowey RR. Radiographic aids in the detection of extra root canals. Oral Surg Oral Med Oral Pathol. 1974;37:762–72. doi: 10.1016/0030-4220(74)90142-x. [DOI] [PubMed] [Google Scholar]
- 4.Cleghorn BM, Christie WH, Dong CS. Root and root canal morphology of the human permanent maxillary first molar: a literature review. J Endod. 2006;32:813–21. doi: 10.1016/j.joen.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Hess W. The anatomy of the root canals of the teeth of the permanent dentition. London: John Bale, Sons and Danielsson Ltd; 1925. pp. 4–49. [Google Scholar]
- 6.Vertucci FJ. Root canal anatomy of the human permanent teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1984;58:589–99. doi: 10.1016/0030-4220(84)90085-9. [DOI] [PubMed] [Google Scholar]
- 7.Sert S, Bayirli GS. Evaluation of the root canal configurations of the mandibular and maxillary permanent teeth by gender in Turkish population. J Endod. 2004;30:391–98. doi: 10.1097/00004770-200406000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Alavi AM, Opasanon A, Ng YL, Gulabivala K. Root and canal morphology of Thai maxillary molars. Int Endod J. 2002;35:478–85. doi: 10.1046/j.1365-2591.2002.00511.x. [DOI] [PubMed] [Google Scholar]
- 9.Wasti F, Shearer AC, Wilson NHF. Root canal systems of the mandibular and maxillary first permanent molar teeth of south Asian Pakistanis. Int Endod J. 2001;34:263–66. doi: 10.1046/j.1365-2591.2001.00377.x. [DOI] [PubMed] [Google Scholar]
- 10.Al Shalabi RM, Omer OE, Glennon J, Jennings M, Claffey NM. Root canal anatomy of maxillary first and second permanent molars. Int Endod J. 2000;33:405–14. doi: 10.1046/j.1365-2591.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- 11.Caliskan MK, Pehlivan Y, Sepetcioglu F, Turkun M, Tuncer SS. Root canal morphology of human permanent teeth in a Turkish population. J Endod. 1995;21:200–04. doi: 10.1016/S0099-2399(06)80566-2. [DOI] [PubMed] [Google Scholar]
- 12.Pecora JD, Woelfel JB, Sousa Neto MD, Issa EP. Morphologic study of the maxillary molars Part II: Internal Anatomy. Braz Dent J. 1992;3:53–7. [PubMed] [Google Scholar]
- 13.Gilles J, Reader A. An SEM investigation of the mesiolingual canal of maxillary first and second molars. Oral Surg Oral Med Oral Pathol. 1990;70:638–43. doi: 10.1016/0030-4220(90)90415-o. [DOI] [PubMed] [Google Scholar]
- 14.Zurcher E. The anatomy of the root-canals of the teeth of the deciduous dentition and of the first permanent molar. Part 2. New York: William Wood and Co; 1925. [Google Scholar]
- 15.Blattner TC, George N, Lee CC, Kumar V, Yelton CD. Efficacy of cone-beam computed tomography as a modality to accurately identify the presence of second mesiobuccal canals in maxillary first and second molars: a pilot study. J Endod. 2010;36:867–70. doi: 10.1016/j.joen.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 16.Seidberg BH, Altman M, Guttuso J, Suson M. Frequency of two mesiobuccal root canals in maxillary permanent first molars. J Am Dent Assoc. 1973;87:852–56. doi: 10.14219/jada.archive.1973.0489. [DOI] [PubMed] [Google Scholar]
- 17.Pineda F, Kuttler Y. Mesiodistal and buccolingual roentgenographic investigation 7,275 root canals. Oral Surg Oral Med Oral Pathol. 1972;33:101–10. doi: 10.1016/0030-4220(72)90214-9. [DOI] [PubMed] [Google Scholar]
- 18.Thomas RP, Moule AJ, Bryant R. Root canal morphology of maxillary permanent first molar teeth at various ages. Int Endod J. 1993;26:257–67. doi: 10.1111/j.1365-2591.1993.tb00570.x. [DOI] [PubMed] [Google Scholar]
- 19.Weine FS, Healy HJ, Gerstein H, Evanson L. Canal configuration in the mesiobuccal root of the maxillary first molar and its endodontic significance. Oral Surg Oral Med Oral Pathol. 1969;28:419–25. doi: 10.1016/0030-4220(69)90237-0. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen BR, Alyassin A, Peters DD, Carnes DL, Lancaster J. Microcomputed tomography: an advanced system for detailed endodontic research. J Endod. 1995;21:561–68. doi: 10.1016/S0099-2399(06)80986-6. [DOI] [PubMed] [Google Scholar]
- 21.Peters OA, Schonenberger K, Laib A. Effects of four Ni-Ti preparation techniques on root canal geometry assessed by micro computed tomography. Int Endod J. 2001;34:221–30. doi: 10.1046/j.1365-2591.2001.00373.x. [DOI] [PubMed] [Google Scholar]
- 22.Bergmans A, Van Cleynenbreugel J, Wevers M, Lambrechts P. A methodology for quantitative evaluation of root canal instrumentation using microcomputed tomography. Int Endod J. 2001;34:390–98. doi: 10.1046/j.1365-2591.2001.00413.x. [DOI] [PubMed] [Google Scholar]
- 23.Park JW, Lee JK, Ha BH, Choi JH, Perinpanayagam H. Three-dimensional analysis of maxillary first molar mesiobuccal root canal configuration and curvature using microcomputed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:437–42. doi: 10.1016/j.tripleo.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 24.Bjorndal L, Carlsen O, Thuesen G, Darvann T, Kreiborg S. External and internal macromorphology in 3D-reconstcuted maxillary molars using computerized X-ray microtomography. Int Endod J. 1999;32:3–9. doi: 10.1046/j.1365-2591.1999.00172.x. [DOI] [PubMed] [Google Scholar]
- 25.Rhodes JS, Pitt Ford TR, Lynch JA, Liepins PJ, Curtis RV. Micro-computed tomography: a new tool for experimental endodontology. Int Endod J. 1999;32:165–70. doi: 10.1046/j.1365-2591.1999.00204.x. [DOI] [PubMed] [Google Scholar]
- 26.Michetti J, Maret D, Mallet JP, Diemer F. Validation of cone beam computed tomography as a tool to explore root canal anatomy. J Endod. 2010;36:1187–90. doi: 10.1016/j.joen.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 27.Kuyk JK, Walton RE. Comparison of the radiographic appearance of root canal size to its actual diameter. J Endod. 1990;16:528–33. doi: 10.1016/S0099-2399(07)80215-9. [DOI] [PubMed] [Google Scholar]
- 28.Kalender WA. Computed Tomography: Fundamentals, System Technology, Image Quality, Applications. Erlangen, Germany: Publicis Corporate Publishing; 2005. p. 294. [Google Scholar]
- 29.Neelakantan P, Subbarao C, Ahuja R, Subbarao CV, Gutman JL. Cone-beam computed tomography study of root and canal morphology of maxillary first and second molars in an Indian population. J Endod. 2010;36:1622–7. doi: 10.1016/j.joen.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Matherne RP, Angelopoulos C, Kulild JC, Tira D. Use of cone-beam computed tomography to identify root canal systems in vitro. J Endod. 2008;34:87–9. doi: 10.1016/j.joen.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 31.Neelakantan P, Subbarao C, Subbarao CV. Comparative Evaluation of modified canal staining and clearing technique, cone-beam computed tomography, peripheral quantitative computed tomography, spiral computed tomography, and plain and contrast medium-enhanced digital radiography in studying root canal morphology. J Endod. 2010;36:1547–51. doi: 10.1016/j.joen.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Kim Y, Lee S-J, Woo J. Morphology of maxillary first and second molars analyzed by cone-beam computed tomography in a Korean population: variations in the number or roots and canals and the incidence of fustion. J Endod. 2012;38:1063–68. doi: 10.1016/j.joen.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 33.Ludlow JB, Laster WS, See M, Bailey LT, Hershey HG. Accuracy of measurements of mandibular anatomy in cone beam computed tomography images. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:534–42. doi: 10.1016/j.tripleo.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cotton TP, Geisler TM, Holden DT, Schwartz SA, Schindler WG. Endodontic applications of cone beam volumetric tomography. J Endod. 2007;33:1121–32. doi: 10.1016/j.joen.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 35.Janner SFM, Jeger FB, Lussi A, Bornstein MM. Precision of endodontic working length measurements: a pilot investigation comparing cone-beam computed tomography scanning with standard measurement techniques. J Endod. 2011;37:1046–51. doi: 10.1016/j.joen.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Lofthag-Hansen S, Huumonen S, Grondahl K, Grondahl HG. Limited cone-beam CT and intraoral radiography for the diagnosis of periapical pathology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:114–9. doi: 10.1016/j.tripleo.2006.01.001. [DOI] [PubMed] [Google Scholar]