Cutaneous neonatal lupus can occur with negative SSA/Ro or SSB/La but positive non-SSA/SSB ribonucleoprotein (RNP) maternal autoantibodies (Provost et al., 1987). No fetal-cardiac manifestations, however, have been reported in association with isolated maternal RNP autoantibodies. We report the first association of transient first-degree atrioventricular block (AVB) in a fetus exposed to maternal anti-RNP in the absence of SSA/Ro-SSB/La autoantibodies.
CASE REPORT
A 27-year-old mother with a history of systemic lupus erythematosus (SLE) underwent an obstetric ultrasound and the 22-week fetus was noted to have hypoplasia of the middle phalanx of the fifth digit and multiple cardiac echogenic foci. The mother was diagnosed with SLE at 20 years old and had received steroids for arthritis in the past. However, for the last 2 years before this pregnancy, she was in remission and on no medications. Because of the findings at 22 weeks of gestation, she was referred for a fetal echocardiogram that demonstrated a normal heart rate with regular rhythm and 1 : 1 atrioventricular conduction. As maternal antibodies were pending, we proceeded to measure a Doppler mechanical PR interval (MPRI) and found it to be normal at 132 ms (normal 120 ± 10 ms with 99th percentile at 150 ms) (Glickstein et al., 2004). Additionally, there were three echogenic intracardiac foci: two in the left ventricle and one in the right ventricle. Otherwise, there was no tricuspid regurgitation or any other intracardiac abnormalities, and there was no pericardial effusion or other fluid collections.
At 31 weeks of gestation, a second fetal echocardiogram demonstrated the MPRI to be 146 ms (+2 to +3 SD), and the other cardiac findings remained normal. The maternal antibodies were reported as negative for SSA/Ro and SSB/La.
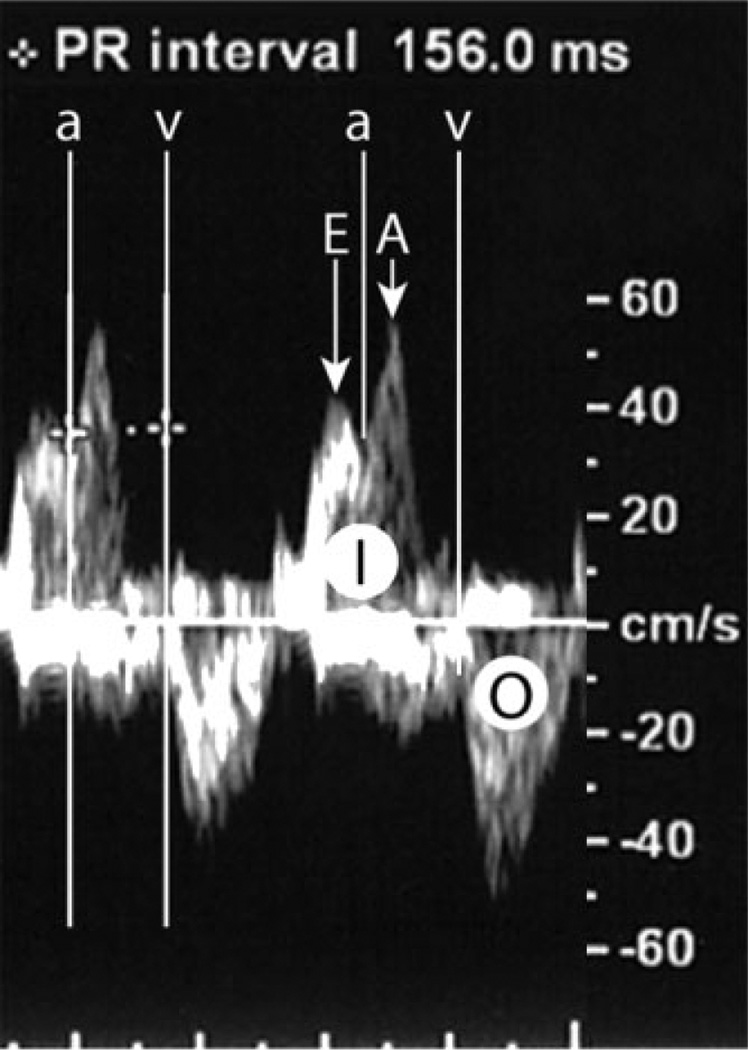
For confirmation, maternal sera were retested and again found to be absent in anti-SSA/Ro or anti-SSB/La reactivity. A third fetal echocardiogram at 32 weeks of gestation demonstrated prolongation of the MPRI at 156 ms (Figures 1 and 2), but otherwise normal echocardiographic findings. A third evaluation of the maternal sera was performed at the New York University School of Medicine’s CLIA-approved immunology laboratory and the research laboratory of one of the authors (JPB) using recombinant proteins La48, Ro52 and Ro60 as previously described (Clancy et al., 2005). Although antibodies to all components of the SSA/Ro–SSB/La complex were confirmed to be negative; however, antibodies to RNP were confirmed in high titer (14 848 EU with a negative value <19 EU).
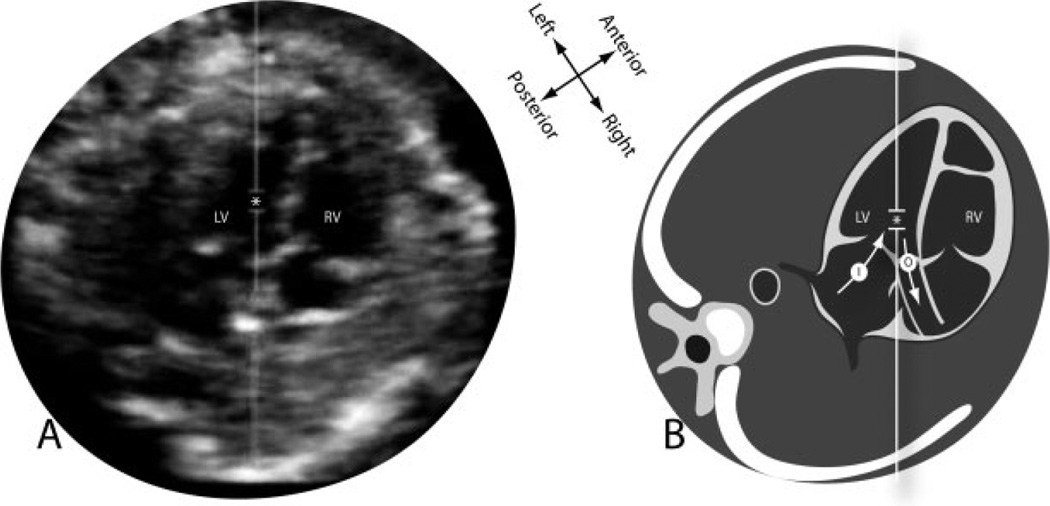
Figure 1.
Fetal echocardiogram in the five-chamber view (A) and a diagrammatic representation (B) showing the positioning of the Doppler sample (*) between the left ventricular inflow (I) and outflow (O). LV, left ventricle; RV, right ventricle
Figure 2.
Doppler MPRI. The left ventricular inflow (I) has an early flow wave (E) and a late flow wave during atrial contraction (A). The beginning of the mitral-valve A wave marks the beginning of the flow during atrial contraction (a). The left ventricular outflow (O) has a single wave and its beginning marks the beginning of the flow during ventricular contraction (v). The Doppler MPRI is measured from the beginning of the flow during atrial contraction (a) to the beginning of the flow during ventricular contraction (v)
Subsequent weekly fetal echocardiograms demonstrated that the MPRI was consistently >150 ms until 36 weeks of gestation when it normalized at 136 ms. The baby had a normal delivery. At 6 h of life, the physical exam, the electrocardiographic PR interval (104 ms), and echocardiogram were normal. The infant continues in good health at 1 month of age with a normal electrocardiographic PR interval at 94 ms.
DISCUSSION
In 1928, Aylward may have been the first to report fetal bradycardia between 40 and 60 bpm in a mother with Mikulicz’s disease [Sjögren’s syndrome (SS)] who had a previous child with congenital heart block (Aylward, 1928). It is now well established that fetal AVB-absent structural abnormalities are strongly associated with maternal autoantibodies to SSA/Ro and/or SSB/La RNPs, irrespective of whether the mother is asymptomatic or has clinical symptoms of a connective tissue disorder such as lupus erythematosus or SS (Buyon et al., 2009). The pathogenesis of maternal autoantibody-related fetal AVB likely represents a complex cascade initiated by transplacental anti-SSA/Ro–SSB/La antibodies binding with apoptotic fetal cardiocytes and driving subsequent inflammatory and fibrotic tissue responses (Clancy et al., 2006). The fetal Doppler MPRI is valuable for monitoring atrioventricular conduction and can detect the early stages of AVB. Fetal immune-related first-degree AVB may be an early indicator of developing second- and third-degree AVB and might herald the need for transplacental dexamethasone therapy in an attempt to prevent complete AVB (Friedman et al., 2008).
There are previous reports of transient and permanent second- and third-degree AVB in fetuses of mothers without clinical or laboratory evidence of connective tissue disorders and negative anti-SSA/Ro–SSB/La antibodies (Breur et al., 2005; Brucato et al., 2009). The authors speculated that viral infections may have played a role in some of these cases. However, this is the first description of a prolonged PR interval in a mother with SLE and positive anti-RNP antibodies but negative anti-SSA/Ro and anti-SSB/La antibodies, confirmed in separate laboratories and with different techniques.
The RNP antigen, similar to SSA/Ro and SSB/La antigens, is a RNP and thus a potential source of associated ssRNA within an immune complex on the surface of apoptotic fetal cardiocytes and may be capable of triggering Toll-like receptors, a class of proteins that are part of the innate immune system of infiltrating macrophages. To date, the RNP antigen has only been identified within but not on the surface of apoptotic blebs, suggesting these antigens are not accessible to extracellular antibodies. These data provided an explanation for the absence of an association with RNP antibodies in the sera of mothers and fetal AVB (Clancy et al., 2010). Our case suggests that perhaps this molecular paradigm needs to be reevaluated. It is noteworthy that the prolonged MPRI was identified later than 30 weeks of gestation (usual period of autoantibody vulnerability is between 16 and 24 weeks), spontaneously reversed and did not progress to advanced AVB or an emerging cardiomyopathy, suggesting a less or even nonpathogenic antibody. It thus remains uncertain whether isolated anti-RNP autoantibodies should be considered a risk factor for abnormal atrioventricular conduction. The prolonged MPRI may have also been secondary to other pathology not related to maternal antibodies. Thus, the clinical or pathological implication of the prolonged MPRI in our case remains uncertain. Additionally, if the prolonged MPRI in our case was not related to a pathological process, then an MPRI 3 standard deviations above the mean may still be normal. A clarification of whether a prolonged MPRI in cases like ours is pathological or normal is crucial, as progression of an MPRI of >150 ms to more advanced AVB has yet to be published.
Our case suggests the need for reevaluating the clinical implications of a prolonged MPRI and recommendations for maternal intervention. Furthermore, guidelines for which fetuses should undergo serial and detailed echocardiographic evaluation may also need refining. Consideration should be given to adding testing for anti-RNP in women with autoimmune diseases undergoing pregnancy counseling and in mothers of fetuses with signs of cardiac-conduction dysfunction.
ACKNOWLEDGEMENTS
The authors thank the outstanding support from the sonographers from the Desert Perinatal Associates.
REFERENCES
- Aylward RD. Congenital heart block. Br Med J. 1928;1:943. [Google Scholar]
- Breur JM, Oudijk MA, Stoutenbeck P, et al. Transient non-autoimmune fetal heart block. Fetal Diagn Ther. 2005;20:81–85. doi: 10.1159/000082427. [DOI] [PubMed] [Google Scholar]
- Brucato A, Grava C, Bortolati M, et al. Congenital heart block not associated with anti-Ro/La antibodies: comparison with anti-Ro/La-positive cases. J Rheumatol. 2009;36:1744–1748. doi: 10.3899/jrheum.080737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyon JP, Clancy RM, Friedman DM. Cardiac manifestations of neonatal lupus erythematosus: guidelines to management, integrating clues from the bench and bedside. Nat Clin Pract Rheumatol. 2009;5:139–148. doi: 10.1038/ncprheum1018. [DOI] [PubMed] [Google Scholar]
- Clancy RM, Alvarez D, Komissarova EV, et al. Ro60-associated single-stranded RNA links inflammation with fetal cardiac fibrosis via ligation of TLRs a novel pathway to autoimmune-associated heart block. J Immunol. 2010;184:2148–2155. doi: 10.4049/jimmunol.0902248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy RM, Buyon JP, Odeda K, et al. Maternal antibody responses to the 52-kd SSA/Ro p200 peptide and the development of fetal conduction defects. Arthritis Rheum. 2005;52:3079–3086. doi: 10.1002/art.21289. [DOI] [PubMed] [Google Scholar]
- Clancy RM, Neufing PJ, Zheng P, et al. Impaired clearance of apoptotic cardiocytes linked to anti-SSA/Ro-SSB/La antibodies in pathogenesis of congenital heart block. J Clin Invest. 2006;116:2413–2422. doi: 10.1172/JCI27803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DM, Kim MY, Copel JA, et al. Utility of cardiac monitoring in fetuses at risk for congenital heart block: the PR Interval and Dexamethasone Evaluation (PRIDE) prospective study. Circulation. 2008;117:485–493. doi: 10.1161/CIRCULATIONAHA.107.707661. [DOI] [PubMed] [Google Scholar]
- Glickstein J, Buyon J, Kim M, Friedman D. The fetal Doppler mechanical PR interval: a validation study. Fetal Diagn Ther. 2004;1:31–34. doi: 10.1159/000074256. [DOI] [PubMed] [Google Scholar]
- Provost TT, Watson R, Gammon WR, et al. The neonatal lupus syndrome associated with U1RNP (nRNP) antibodies. N Eng J Med. 1987;316:1135–1138. doi: 10.1056/NEJM198704303161807. [DOI] [PubMed] [Google Scholar]