Abstract
Background
Adjuvant radiation therapy (A-RT) for resected pancreatic adenocarcinoma (PAC) is controversial. We aim to determine if there is an association between overall survival (OS) and A-RT dose.
Methods
National Cancer Data Base (NCDB) data were obtained for all patients who underwent A-RT for resected PAC from 1998-2002. Univariate (UV) and multivariable (MV) survival analysis were performed along with Kaplan-Meier (KM) estimates for A-RT levels < 40 Gy, 40 to < 50 Gy, 50 to < 55 Gy, and ≥ 55 Gy.
Results
1,385 patients met inclusion criteria. Median age was 64 (29-87); all patients underwent surgical resection and A-RT +/- chemotherapy. 231 patients were AJCC 5th edition stage I, 273 stage II, 734 stage III, and 126 stage IVA; 21 were unknown. Median A-RT dose was 45 Gy (1.63 Gy-69 Gy). Median OS was 21 months (95% CI 19 - 23). On MV analysis A-RT dose < 40 Gy (HR, 1.30 [95% CI 1.03-1.66]; p = 0.031), A-RT dose 40 to < 50 Gy (HR, 1.17 [95% CI 1.00-1.37]; p = 0.05), and A-RT dose ≥ 55 Gy (HR, 1.44 [95% CI 1.08-1.93]; p = 0.013) predicted worse OS when compared with the reference category of 50 to < 55 Gy.
Conclusions
A-RT doses of less than 40 Gy, 40 to < 50 Gy, and ≥ 55 Gy were associated with inferior OS. The dose of A-RT delivered appears to influence OS and a prospective study evaluating the addition of optimally delivered A-RT for resected PAC is needed.
Keywords: Pancreatic Adenocarcinoma adjuvant Radiation Therapy, Resected Pancreatic Adenocarcinoma, Post-operative management in resected pancreatic adenocarcinoma, Adjuvant radiation dose in resected pancreatic adenocarcinoma
Introduction
Pancreatic Adenocarcinoma (PAC) is a devastating malignancy and the outcomes for this disease remain dismal.1 The only opportunity for cure from PAC is surgical resection, however 5 year overall survival (OS) persists at less than 20%.2-7 Furthermore, the majority of patients are not surgical candidates due to locally advanced or metastatic disease at presentation.6-8
The primary justification for A-RT use in the United States comes from a trial conducted nearly three decades ago by the Gastrointestinal Tumor Study Group (GITSG). The GITSG study demonstrated an improvement in the median OS in resected PAC with the addition of a 40 Gy split course of A-RT, followed by adjuvant chemotherapy.9, 10 This dose of A-RT is considered inferior to the modern dosing schedule of approximately 50 Gy delivered over 5-6 weeks. Results supporting the use of this modern A-RT dose have been presented by single institution trials 11 as well as large retrospective reviews.12-15
The European Organization for Research and Treatment of Cancer (EORTC) was unable to reproduce the findings of GITSG again using a 40 Gy split course of A-RT.16 Furthermore, the European Study Group for Pancreatic Cancer-1 (ESPAC-1) trial demonstrated a detrimental effect of A-RT using a range of doses from 40-60 Gy.17, 18 However, conclusions from ESPAC-1 remain controversial.19, 20
While chemotherapeutic variations have been examined in prospective clinical trials21, few studies have measured the impact of A-RT dose on patient outcomes. One early phase clinical trial examined escalated A-RT dose, finding no benefit, however there remains a paucity of such studies.22 Given the heterogeneity of the prospective trial conclusions, the role of A-RT and optimal dose range remain controversial.12-15 The aim of the current study was to determine if the dose of A-RT influences OS in patients with resected PAC, and to explore whether an optimal A-RT dose range exists.
Patients and Methods
Our patient population was obtained from the pancreatic Participant Use Data File (PUF) from the NCDB, which is the one of the worlds largest clinical cancer registries.23 The NCDB is supported by the American College of Surgeons and the American Cancer Society23 and includes more than 1,440 hospitals in the United States. Data available includes patient demographics, pathologic characteristics, detailed staging, A-RT dose information, chemotherapy data, and OS data.
Emory University was granted alpha-test user site status for the PUF, which includes all incident cases of pancreatic cancer reported to the NCDB for the 5-year period 1998-2002. PUF’s are entirely de-identified data files available to selected investigators at Commission on Cancer (CoC) approved institutions for the advancement of patient care. Results reported are in compliance with the privacy requirements of the Health Insurance Portability and Accountability Act of 1996 as described in the Standards for Privacy of Individually Identifiable Health Information; Final Rule (45 CFR Parts 160 and 164). The use and publication of these data have been previously subject to peer review and approval by the NCDB.
There were 94,385 incident cases in the Pancreatic PUF for the 1998-2002 period. Of these, we initially selected 13,580 patients with a primary tumor site in the pancreas who had a definitive surgery on the primary site. From this group we selected those patients who had reported OS data of any duration, which left 12,674 patients. We then selected patients that received external beam A-RT, leaving 5,623 patients. We then selected patients for whom the radiation dose was not missing, leaving a total of 1,489. Patients with inaccurately coded A-RT doses (defined as inconceivable doses either greater than 400 Gy or less than 1 Gy) were eliminated; this resulted in 11 patients (0.7% of the patients) being eliminated for inconceivable A-RT doses, leaving a total of 1,478. The non-metastatic patients were then selected, leaving a total of 1,452 patients. Finally, neoadjuvant patients were excluded, resulting in our total of 1,385 patients (Figure 1).
Figure 1.
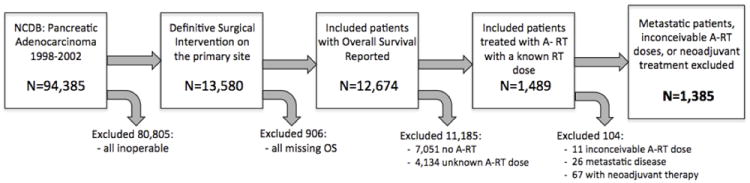
Patient Selection Schematic
A frequency table for each categorical variable and summary statistics for each continuous variable were calculated to describe patient-related and disease-specific variables calculated. Univariate (UV) survival analysis was carried out by assessing the relationship between each variable on OS using both the Kaplan-Meier log-rank test and a hazard ratio (with 95% confidence intervals) derived through Cox proportional hazards modeling.
Martingale residual plots were used to identify potential non-linear effects of all continuous covariates on OS. A non-linear relationship was observed for A-RT dose and we further categorized A-RT dose into the 4 different levels based on the non-linear relationship. The individual association between categorized A-RT dose and each of the other covariates was analyzed by Chi-square test for categorical covariates and ANOVA for continuous covariates.
Multivariable (MV) survival analysis started with all potential confounding variables from the UV analysis and followed backward elimination steps in a Cox proportional hazards model with an alpha = 0.05 removal criteria. In both UV and MV analysis, patients receiving A-RT dose between 50 - 55 Gy were treated as the reference group. Facility volume was measured as the total number of resected cases in a given facility regardless of facility type with 10 as the unit of incremental increase. Facility types were Community Cancer Programs (CCP), Comprehensive Community Cancer Programs (CCCP), and Academic/Research Cancer Program (ARCP), which includes NCI-designated Comprehensive Cancer Centers (NCI).
The analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC).
Results
A total of 1,385 patients were included. Median age was 64 years (range: 29-87 years), 53.1% were male, and 89.8% were Caucasian. All patients underwent surgical resection and A-RT with or without chemotherapy. The majority of the patients, 92.1% (1265/1385), received A-RT with concurrent chemo and 7.9% (108/1385) received A-RT alone. The use of chemotherapy was unknown in 0.9% (12/1385). The staging was 5th edition American Joint Committee on Cancer (AJCC) in which stage I and II included node negative (N0) T1-T3 patients, stage III patients included node positive (N1) but still resectable (T1-T3), and stage IVA included both resectable patients with tumor extension into adjacent organs and unresectable patients. Staging groups for the included patients consisted of 231 stage I, 273 stage II, 734 stage III, 126 stage IVA, and 21 patients with missing stage. Median A-RT dose was 45 Gy (range 1.63 Gy-69 Gy), and median treatment duration was 39 days (range 5-100 days). One hundred sixty-four patients (11.8%) received < 40 Gy, 634 (45.8%) received ≥ 40 to < 50 Gy, 498 (36.0%) received ≥ 50 Gy to < 55 Gy, and 89 (6.4%) received ≥ 55 Gy. A detailed summary of patient characteristics is found in Table 1.
Table 1.
All Patients Baseline Characteristics
| Demographic: | N=1385 | |||||
|---|---|---|---|---|---|---|
| Age | ||||||
| Mean | 63.18 | |||||
| Median (Range) | 64 (29-87) | |||||
|
| ||||||
| Gender | ||||||
| Male no. (%) | 735 (53.1) | |||||
|
| ||||||
| Race | ||||||
| White | 1231 (89.8) | |||||
| Other | 140 (10.2) | |||||
| Missing | 14 | |||||
|
| ||||||
| Treatment Characteristics: | ||||||
|
| ||||||
| Facility Type | ||||||
| CCP | 192 (13.9) | |||||
| CCCP | 697 (50.3) | |||||
| ARCP | 496 (35.8) | |||||
|
| ||||||
| Radiation Dose (Gy) | Radiation Dose Category (Gy) | |||||
| Mean | 45.21 | < 40 | 164 (11.8) | |||
| Median | 45 | ≥ 40 - < 50 | 634 (45.8) | |||
| Range | 1.63 - 69 | ≥ 50 - < 55 | 498 (36.0) | |||
| ≥ 55 | 89 (6.4) | |||||
|
| ||||||
| Radiation Duration (days) | ||||||
| Mean | 40.18 | |||||
| Median (Range) | 39 (1-100) | |||||
| Missing | 695 | |||||
|
| ||||||
| Concurrent Chemotherapy | ||||||
| Yes | 1265 (92.1) | |||||
| No | 108 (7.9) | |||||
| Missing | 12 | |||||
|
| ||||||
|
Tumor Characteristics:
|
||||||
| Stage (AJCC 5th) | ||||||
| I | 231 (16.9) | |||||
| II | 273 (20.0) | |||||
| III | 734 (53.8) | |||||
| IVA | 126 (9.3) | |||||
| Missing | 21 | |||||
|
| ||||||
| Tumor Size (mm) | Size Groupings (mm) | |||||
| Mean | 35.88 | ≤20 | 269 (21.6) | |||
| Median (Range) | 30.0 (1-750) | >20 - ≤ 30 | 399 (32.0) | |||
| >30 - ≤ 40 | 304 (24.4) | |||||
| >40 | 274 (22.0) | |||||
| Missing | 139 | |||||
|
| ||||||
| Number of LN’s Examined | LN Positive | |||||
| Mean | 9.75 | Yes | 800 (61.7) | |||
| Median (Range) | 8 (0-60) | No | 497 (38.3) | |||
| Missing | 100 | Missing | 88 | |||
|
| ||||||
| Histologic Grade | ||||||
| Unspecified | 113 (8.2) | |||||
| I | 165 (11.9) | |||||
| II | 655 (47.3) | |||||
| III/IV | 452 (32.6) | |||||
|
| ||||||
| Margin | ||||||
| Negative | 899 (71.3) | |||||
| Positive | 361 (28.7) | |||||
| Missing | 125 | |||||
Gy- Gray, LN- Lymph node, CCP-Community Cancer Program, CCCP- Comprehensive Community Cancer Programs, ARCP- Academic Research Cancer Program, LN- Lymph nodes AJCC-American Joint Committee on Cancer, no.- Number
At a median follow up of 60 months the median OS for all patients was 20 months (95% CI 19 - 22). The median OS for patients receiving less than 40 Gy was 15 months (95% CI 11-20); for those patients receiving between 40-50 Gy was 20 months (95% CI 19-22); and for those receiving greater than 55 Gy was 16 months (95% CI 14-21). Patients receiving between 50-55 Gy had the longest median OS of 23 months (95% CI 19-25). The KM OS analysis for the entire cohort is seen in Figure 2 and for each dose level is shown in Figure 3.
Figure 2.
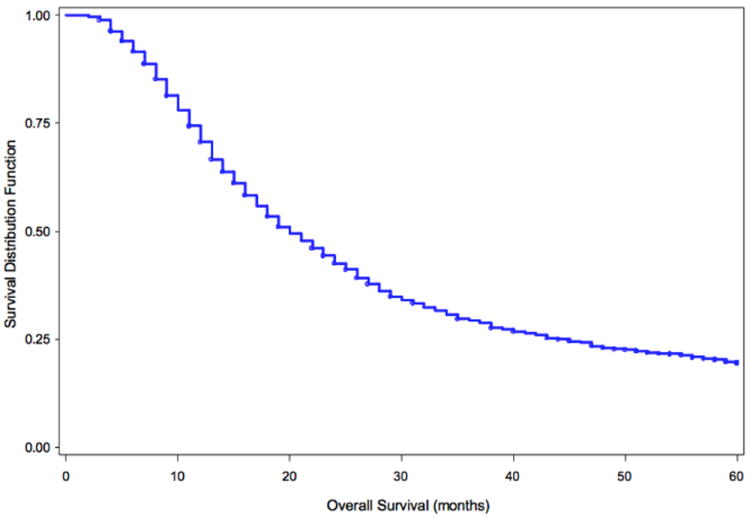
Overall Survival (OS) of all 1,385 patients included in the analysis, median OS for all patients is 20 months (95% CI 19 - 22).
Figure 3.
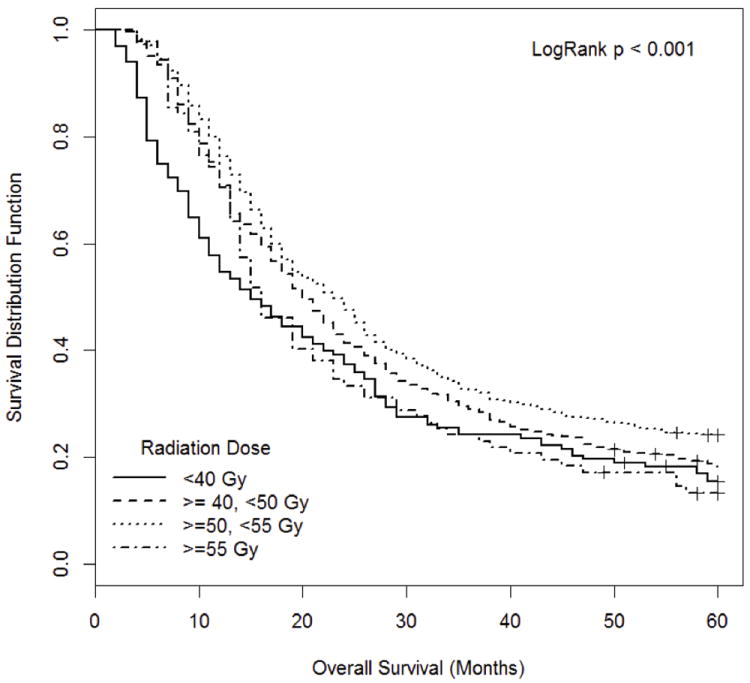
Overall Survival (OS) of patients receiving different adjuvant radiation therapy doses is reported. Patients are grouped as receiving less than 40 Gy (median OS 15 months (95% CI 11-20)), greater than or equal to 40 Gy and less than 50 Gy (median OS 20 months (95% CI 19-22)), greater than or equal to 50 Gy and less than 55 Gy (median OS 23 months (95% CI 19-25)), or greater than 55 Gy (median OS 16 months (95% CI 14-21)).
In the UV survival analysis parameters associated with higher risk of death including A-RT dose < 40 Gy, A-RT dose ≥ 40 to < 50 Gy, and A-RT dose ≥ 55 Gy. Factors significantly associated with lower risk of death included facility volume, negative surgical margin, LN negativity, smaller tumor size, lower stage, lower grade, and younger age. The results of the UV analysis can be found in Table 2. The UV association analysis is summarized in Table 3. It can be seen that the margin status, tumor size, and stage were independent of the A-RT dose.
Table 2.
Univariate Survival Analysis
| N = 1385 | HR (95% CI) | P-Value | |
|---|---|---|---|
| Age (years) | |||
| < 50 | 174 | 0.662 (0.519-0.845) | <0.001 |
| 50- < 65 | 579 | 0.749 (0.617-0.910) | 0.004 |
| 65- < 75 | 479 | 0.802 (0.658-0.978) | 0.029 |
| ≥ 75 | 153 | 1.0 | -- |
|
| |||
| Gender | |||
| Female | 650 | 0.905 (0.803-1.020) | 0.102 |
| Male | 735 | 1.0 | -- |
|
| |||
| Race | |||
| White | 1231 | 1.0 | -- |
| Other | 140 | 0.965 (0.790-1.178) | 0.726 |
|
| |||
| Radiation Dose (Gy) | |||
| < 40 | 164 | 1.456 (1.194-1.776) | <0.001 |
| 40 - < 50 | 634 | 1.167 (1.020-1.336) | 0.025 |
| 50 - < 55 | 498 | 1.0 | -- |
| ≥ 55 | 8 | 1.383 (1.080-1.770) | 0.010 |
|
| |||
| Concurrent Chemotherapy | |||
| No | 108 | 1.019 (0.810-1.281) | 0.872 |
| Yes | 1265 | 1.0 | -- |
|
| |||
| Radiation Duration (days) | |||
| ≤ 35 | 105 | 1.201 (0.904-1.595) | 0.206 |
| > 35 - ≤ 40 | 258 | 0.934 (0.739-1.182) | 0.571 |
| > 40 - ≤ 45 | 194 | 0.972 (0.760-1.245) | 0.824 |
| > 45 | 133 | 1.0 | -- |
|
| |||
| Surgical Margin | |||
| Negative | 899 | 0.739 (0.645-0.846) | <0.001 |
| Positive | 361 | 1.0 | -- |
|
| |||
| LN Positive | |||
| No | 497 | 0.679 (0.596-0.773) | <0.001 |
| Yes | 800 | 1.0 | -- |
|
| |||
| Number of Nodes Examined | |||
| ≤ 12 | 913 | 1.093 (0.952-1.254) | 0.207 |
| > 12 | 372 | 1.0 | -- |
|
| |||
| Tumor Size (mm) | |||
| ≤ 20 | 269 | 0.601 (0.494-0.731) | <0.001 |
| > 20 - ≤ 30 | 399 | 0.741 (0.624-0.881) | <0.001 |
| > 30 - ≤ 40 | 304 | 0.859 (0.717-1.029) | 0.099 |
| >40 | 274 | 1.0 | -- |
|
| |||
| Stage (AJCC 5th) | |||
| I | 231 | 0.475 (0.372-0.606) | <0.001 |
| II | 273 | 0.544 (0.431-0.687) | <0.001 |
| III | 734 | 0.789 (0.646-0.964) | 0.02 |
| IVA | 126 | 1.0 | -- |
|
| |||
| Histologic Grade | |||
| Unspecified | 113 | 0.592 (0.461-0.759) | <0.001 |
| I | 165 | 0.594 (0.482-0.732) | <0.001 |
| II | 655 | 0.810 (0.709-0.926) | 0.002 |
| III/IV | 452 | 1.0 | -- |
|
| |||
| Facility Type | |||
| CCP | 192 | 1.167 (0.968-1.407) | 0.105 |
| CCCP | 697 | 1.053 (0.923-1.201) | 0.440 |
| ARCP | 496 | 1.0 | -- |
|
| |||
| Facility Volume (Unit = 10) | 1385 | 0.975 (0.961-0.989) | <0.001 |
HR- Hazard Ratio, CCP- Community Cancer Program, CCCP- Comprehensive Community Cancer Programs, ARCP- Academic Research Cancer Program, Gy- Gray, AJCC-American Joint Committee on Cancer, Facility Volume (Unit = 10)- total number of resected cases in a given facility regardless of facility type, unit of incremental increase = 10
Table 3.
Variable Association with RT Dose Levels
| <40 Gy N=164 |
≥40 - <50 Gy N=634 |
≥ 50 - < 55 Gy N=498 |
≥ 55 Gy N=89 |
Parametric P-Value* | |
|---|---|---|---|---|---|
| Age (years) N(%) | 0.282 | ||||
| ≤ 50 | 21(12.8) | 66 (10.41) | 69 (13.86) | 18 (20.22) | |
| > 50 - ≤ 65 | 69 (42.07) | 263 (41.48) | 216 (43.37) | 31 (34.83) | |
| > 65 - ≤ 75 | 56 (34.15) | 229 (36.12) | 165 (33.13) | 29 (32.58) | |
| > 75 | 18(10.98) | 76 (11.99) | 48 (9.64) | 11 (12.36) | |
|
| |||||
| Surgical Margin | 0.135 | ||||
| Negative | 104(71.23) | 425(73.53) | 323 (70.37) | 47(61.04) | |
| Positive | 42(28.77) | 153 (26.47) | 136 (29.63) | 30 (38.96) | |
|
| |||||
| LN Positive | 0.529 | ||||
| No | 51 (34) | 240 (40) | 177 (38.06) | 29 (35.37) | |
| Yes | 99 (66) | 360 (60) | 288 (61.94) | 53 (64.63) | |
|
| |||||
| Tumor Size(mm) | 0.508 | ||||
| ≤ 20 | 29 (21.48) | 123 (21.39) | 100 (21.83) | 17 (21.79) | |
| > 20 - ≤ 30 | 42 (31.11) | 195 (33.91) | 143 (31.22) | 19 (24.36) | |
| > 30 - ≤ 40 | 26 (19.26) | 143 (24.87) | 113 (24.67) | 22 (28.21) | |
| > 40 | 38 (28.15) | 114 (19.83) | 102 (22.27) | 20 (25.64)) | |
|
| |||||
| Concurrent Chemotherapy | 0.032 | ||||
| No | 22 (13.66) | 42 (6.67) | 37 (7.51) | 7 (7.87) | |
| Yes | 139 (86.34) | 588 (93.33) | 456 (92.49) | 82 (92.13) | |
|
| |||||
| Stage (AJCC 5th) | 0.826 | ||||
| I | 24 (14.91) | 101 (16.06) | 94 (19.22) | 12 (14.12) | |
| II | 29 (18.01) | 131 (20.83) | 96 (19.63) | 17 (20) | |
| III | 90 (55.9) | 337 (53.58) | 260 (53.17) | 47 (55.29) | |
| IVA | 18 (11.18) | 60 (9.54) | 39 (7.98) | 9 (10.59) | |
|
| |||||
| Histologic Grade | 0.031 | ||||
| Unspecified | 19 (11.59) | 40 (6.31) | 45 (9.04) | 9 (10.11) | |
| I | 20 (12.2) | 66 (16.72) | 61(12.25) | 18 (20.22) | |
| II | 65 (39.63) | 317 (50) | 239(47.99) | 34 (38.2) | |
| III/IV | 60 (36.59) | 211 (33.28) | 153 (30.72) | 28 (31.46) | |
|
| |||||
| Facility Type | 0.016 | ||||
| CCP | 25 (15.24) | 86 (13.56) | 68 (13.65) | 13 (14.61) | |
| CCCP | 97 (59.15) | 310 (48.9) | 236 (47.39) | 54 (60.67) | |
| ARCP | 42 (25.61) | 238 (37.54) | 194 (38.96) | 22 (24.72) | |
LN- Lymph node, mm- millimeters, CCP-Community Cancer Program, CCCP- Comprehensive Community Cancer Programs, ARCP- Academic Research Cancer Program, Gy- Gray,
The parametric p-value is calculated using ANOVA for numerical covariates and chi-squared for categorical covariates, AJCC-American Joint Committee on Cancer
In the MV survival analysis, A-RT dose < 40 Gy (HR, 1.30 [95% CI 1.03-1.66]; p = 0.031), A-RT dose ≥ 40 Gy and < 50 Gy (HR, 1.17 [95% CI 1.00-1.37]; p = 0.05), and A-RT dose ≥ 55 Gy (HR, 1.44 [95% CI 1.08-1.93]; p = 0.013) were all significantly associated with worse OS. In addition to radiation dose level age, surgical margin status, stage, tumor size, grade, and facility volume were all found to be significant on MV analysis (Table 4).
Table 4.
Multivariate Survival Analysis
| HR (95% CI) | P-Value | |
|---|---|---|
| Age (years) | ||
| < 50 | 0.62(0.47-0.82) | <0.001 |
| 50 - ≤ 65 | 0.73(0.58-0.91) | 0.005 |
| 65 - ≤ 75 | 0.78(0.62-0.97) | 0.028 |
| > 75 | 1.0 | --- |
|
| ||
| Radiation Dose (Gy) | ||
| < 40 | 1.30(1.03-1.66) | 0.031 |
| ≥ 40 - < 50 | 1.17(1.00-1.37) | 0.05 |
| ≥ 50 - < 55 | 1.0 | --- |
| ≥ 55 | 1.44(1.08-1.93) | 0.013 |
|
| ||
| Surgical Margin | ||
| Negative | 0.73(0.63-0.86) | <0.001 |
| Positive | 1.0 | --- |
|
| ||
| Stage (AJCC 5th) | ||
| I | 0.61(0.45-0.82) | <0.001 |
| II | 0.64(0.48-0.85) | 0.002 |
| III | 0.96(0.75-1.23) | 0.743 |
| IVA | 1.0 | --- |
|
| ||
| Tumor Size (mm) | ||
| ≤ 20 | 0.55(0.44-0.69) | <0.001 |
| > 20 - ≤ 30 | 0.73(0.60-0.89) | 0.002 |
| > 30 - ≤ 40 | 0.75(0.62-0.92) | 0.006 |
| > 40 | 1.0 | --- |
|
| ||
| Histologic Grade | ||
| Unspecified | 0.52(0.37-0.73) | <0.001 |
| I | 0.58(0.45-0.69) | <0.001 |
| II | 0.83(0.71-0.97) | 0.017 |
| III/IV | 1.0 | --- |
|
| ||
| Facility Volume: Unit = 10 | 0.98(0.97-1.00) | 0.014 |
CI – confidence interval, HR- Hazard Ratio, CCP-Community Cancer Program, CCCP- Comprehensive Community Cancer Programs, ARCP- Academic Research Cancer Program, Gy- Gray, AJCC-American Joint Committee on Cancer, Facility Volume (Unit = 10)- total number of resected cases in a given facility regardless of facility type, unit of incremental increase = 10, mm- millimeters
The duration of time over which each of the respective A-RT doses was delivered is summarized in Table 5.
Table 5.
Duration of Radiation Therapy Administration
| Radiation Duration (Days)(%) | <40 Gy N=81 |
40 - <50 Gy N=342 |
≥ 50 - <55 Gy N=236 |
≥ 55 Gy N=31 |
Parametric P-Value* |
|---|---|---|---|---|---|
| <10 | 4 (5) | 0 (0) | 0 (0) | 0 (0) | <0.001 |
|
| |||||
| 10-20 | 18 (22.2) | 1 (0.2) | 0 (0) | 0 (0) | |
|
| |||||
| 21-30 | 19 (23.5) | 6 (1.7) | 1(.4) | 0 (0) | |
|
| |||||
| 31-40 | 19 (23.5) | 218 (63.7) | 77(32.6) | 0 (0) | |
|
| |||||
| 41-50 | 16 (19.8) | 96 (28.1) | 132 (55.9) | 22 (70.9) | |
|
| |||||
| 51-60 | 2 (2.4) | 11 (3.2) | 17 (7.7) | 4 (12.9) | |
|
| |||||
| >60 | 3 (3.7) | 10 (2.9) | 9 (3.8) | 5 (16.1) | |
|
| |||||
| Mean Duration | 30.62 | 39.62 | 42.84 | 51.1 | |
The parametric p-value is calculated using ANOVA for numerical covariates and chi-square for categorical covariates, Gy-Gray
Discussion
Despite three prospective randomized trials the role of A-RT in resected PAC remains controversial. Most of the recent series examining A-RT in resected PAC support doses of approximately 50-55 Gy, which differs from that used in past prospective trials.14, 21 The purpose of this study was to analyze the impact of A-RT dose on OS in patients with resected PAC and explore whether an optimal A-RT dose exists.
Much of the current rationale for A-RT comes from the landmark GITSG 91-73 analysis.10 The A-RT dose in GITSG was 40 Gy delivered over 20 fractions as a split course and resulted in a median OS of 20 months.9, 10 The role of A-RT in PAC was again examined in EORTC 40891 which also used a 40 Gy split course. The median OS between the two arms was not statistically different in this EORTC study, which led to the conclusion that the routine use of A-RT was not warranted.16, 24 Furthermore, ESPAC-1 showed a survival detriment when using a 40-60 Gy split course of A-RT.17,18 The ESPAC-1 study design has drawn substantial criticism since its publication and the quality of the A-RT delivery is unknown.19
More recently the Radiation Therapy Oncology Group (RTOG) 9704 trial examined the addition of gemcitabine chemotherapy to 5-FU.21 High quality A-RT was delivered as 50.4 Gy at 1.8 Gy per fraction with continuous infusion 5-FU in both arms. This was the first large scale trial to use a more contemporary A-RT dosing and fractionation schedule.21 The outcomes were similar to the current series and GITSG with a median OS of 20.5 months. On quality assurance review nearly 50% of the A-RT in 9704 deviated from protocol guidelines. Abrams et al. conducted a secondary analysis of 9704 and demonstrated that A-RT not delivered per-protocol was a negative predictor of OS on MV analysis.25 Abrams et al. was the first series to demonstrate that A-RT quality and variation could impact OS.
In addition to prospective trials, several large retrospective analyses have been conducted. The recent Mayo Clinic and Johns Hopkins collaborative retrospective case series by Hsu et al. examined 1,045 patients with resected PAC, with 530 (50.7%) receiving 5-FU/XRT.14 The patients in this series also received high quality A-RT of 50.4 Gy at 1.8 Gy per fraction. Investigators demonstrated that A-RT was associated with an improved OS among all patients and in all sub-groups regardless of age, tumor size, margin status, node status, and tumor differentiation.14
The current series supports the hypothesis that the dose of A-RT in resected PAC appears to influence OS. Furthermore, it can be seen in our analysis that patients treated to doses between 50-55 Gy had the longest median OS. The current series, along with the secondary analysis of RTOG 9704 by Abrams et al., both provide supportive evidence that A-RT parameters impact OS.25 These data support the hypothesis that the lack of A-RT benefit shown in past trials may have been secondary to sub-optimal A-RT delivery. Additionally it should be noted that facility volume did appear to influence OS, reflecting the complexity of pancreatic cancer management and the importance of facility experience and treatment quality.
The current series demonstrates a significant association between patients treated with A-RT doses less than 40 Gy and inferior OS. It is likely these patients did not complete a full course of A-RT due to disease progression, medical comorbidities, or a combination of these factors. Patients treated to A-RT doses greater than 55 Gy also demonstrated inferior OS compared to the reference 50-55 Gy cohort. This could potentially be due to increased toxicity, or adverse imaging features on CT simulation that motivated doses greater than 55 Gy. Patients treated to doses of 40 to < 50 Gy also demonstrated an inferior OS when compared to the reference cohort of 50 to < 55 Gy. These two groups both had the largest patient number, comparable patient characteristics and similar chemotherapy use. This difference remained significant on MV analysis and was independent of tumor size, stage, grade, surgical margin status, and facility volume. This OS difference is supportive evidence that A-RT dose appears to influence OS.
The results of this analysis should be interpreted with caution due to some important limitations beyond the retrospective design of the study. First, while the number of patients in our analysis was large, given the total patients in the NCDB this was a relatively small fraction. A portion of the patients in the database did have missing, incomplete, or inaccurately coded A-RT information and were consequently eliminated. Excluding this large number of cases could have introduced a source of selection bias into the analysis. We applied an extensive array of statistical tools in an attempt to offset this bias, including a propensity score weighted analysis and analysis of all characteristics of eliminated patients, with no significant impact on the overall conclusions. Additionally every attempt to minimize the practice of eliminating patients based on perceived coding errors was made, which explains the rather unusual A-RT dose range from 1.63-69 Gy. While those patients with perceived unusual doses could have been excluded, including any conceivable dose was our attempt to present the data as purely as possible.
An additional limitation of the current series is the lack of information on the precise use of chemotherapy. It should be noted that approximately 8% of the patients are coded as having received no concurrent chemotherapy with the A-RT, which is likely secondary to prohibitive medical comorbidities, patient refusal, or inaccurate coding. Additionally a lack of statistical difference in OS was demonstrated on the UV analysis when comparing patients that did not received chemotherapy with those that did. The authors attributed this finding primarily to the large discrepancy in patient numbers present in these two cohorts, thus making a reliable statistical comparison difficult. Additionally it should be noted that patients treated in different facility types received differing A-RT doses. This likely reflects differences in institutional adoption of novel A-RT dose recommendations or the experience of the attending radiation oncologist. The lack of other known prognostic factors in the NCDB, such as CA-19-9 level and performance status, is also a limitation. Additionally it should be noted that certain variables have large numbers of missing values including radiation duration, number of lymph nodes, size, and margin status. Finally, specific information obtained from the CT simulation scan for the purpose of A-RT planning is unknown. Those patients receiving over 55 Gy could have received this due to residual disease on planning CT scan, potentially influencing OS, despite no reported difference in margin status.
While the limitations of this study are essential to consider, it is also important to take note of the strengths and novelty of the analysis. The current series includes a large number of patients, with an array of A-RT doses, delivered in a variety of facility types. General chemotherapy, A-RT, pathologic parameters, and facility volume differences are known and accounted for. Such a comparison of A-RT dose levels would be difficult to complete with a single institutional database, given the probable absence of a wide range of A-RT doses. Furthermore it is highly unlikely that the impact of A-RT dose variation on OS would be addressed in a prospective randomized trial.
In the most general sense the current series demonstrates that the manner in which A-RT is delivered in resected PAC appears to influence OS, which is also supported by the secondary analysis of RTOG 9704.25 Our series specifically shows A-RT dose impacts OS, which is a relatively easily adoptable and verifiable A-RT component. These data bring into further question previously conducted prospective trials examining the addition of A-RT in resected PAC. Finally, this series supports the significant importance of the current prospective randomized trial, RTOG 0848, which applies 50.4 Gy delivered at 1.8 Gy per fraction of high quality A-RT to select patients following resection and adjuvant chemotherapy.
Conclusions
We have presented a large outcomes based analysis for patients treated with A-RT in resected PAC. Based on these data the optimal dose of A-RT appears to fall between 50 and 55 Gy. These data support the hypothesis that the characteristics of A-RT delivery influence OS. Additionally these data support the most common and currently used A-RT dose fractionation schedule and underscore the importance of prospective investigation into the role of A-RT in resected PAC using modern A-RT delivery. Ongoing prospective trials (such as RTOG 0848) will define the true role of high quality A-RT in resected PAC.
Footnotes
Disclaimers:
No financial disclosures from any authors.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: a cancer journal for clinicians. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Cameron JL, Crist DW, Sitzmann JV, et al. Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. American journal of surgery. 1991;161:120–124. doi: 10.1016/0002-9610(91)90371-j. discussion 124-125. [DOI] [PubMed] [Google Scholar]
- 3.Piorkowski RJ, Blievernicht SW, Lawrence W, Jr, et al. Pancreatic and periampullary carcinoma. Experience with 200 patients over a 12 year period. American journal of surgery. 1982;143:189–193. doi: 10.1016/0002-9610(82)90064-2. [DOI] [PubMed] [Google Scholar]
- 4.Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Annals of surgery. 1995;221:59–66. doi: 10.1097/00000658-199501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gudjonsson B. Cancer of the pancreas. 50 years of surgery. Cancer. 1987;60:2284–2303. doi: 10.1002/1097-0142(19871101)60:9<2284::aid-cncr2820600930>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Annals of surgery. 1990;211:447–458. doi: 10.1097/00000658-199004000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. American journal of surgery. 1993;165:68–72. doi: 10.1016/s0002-9610(05)80406-4. discussion 72-63. [DOI] [PubMed] [Google Scholar]
- 8.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Annals of surgery. 2003;237:74–85. doi: 10.1097/00000658-200301000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Archives of surgery. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 10.Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Gastrointestinal Tumor Study Group. Cancer. 1987;59:2006–2010. doi: 10.1002/1097-0142(19870615)59:12<2006::aid-cncr2820591206>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.Yeo CJ, Abrams RA, Grochow LB, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Annals of surgery. 1997;225:621–633. doi: 10.1097/00000658-199705000-00018. discussion 633-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corsini MM, Miller RC, Haddock MG, et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: the Mayo Clinic experience (1975-2005) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:3511–3516. doi: 10.1200/JCO.2007.15.8782. [DOI] [PubMed] [Google Scholar]
- 13.Herman JM, Swartz MJ, Hsu CC, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:3503–3510. doi: 10.1200/JCO.2007.15.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu CC, Herman JM, Corsini MM, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Annals of surgical oncology. 2010;17:981–990. doi: 10.1245/s10434-009-0743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hazard L, Tward JD, Szabo A, Shrieve DC. Radiation therapy is associated with improved survival in patients with pancreatic adenocarcinoma: results of a study from the Surveillance, Epidemiology, and End Results (SEER) registry data. Cancer. 2007;110:2191–2201. doi: 10.1002/cncr.23047. [DOI] [PubMed] [Google Scholar]
- 16.Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Annals of surgery. 1999;230:776–782. doi: 10.1097/00000658-199912000-00006. discussion 782-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576–1585. doi: 10.1016/s0140-6736(01)06651-x. [DOI] [PubMed] [Google Scholar]
- 18.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. The New England journal of medicine. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 19.Abrams RA, Lillemoe KD, Piantadosi S. Continuing controversy over adjuvant therapy of pancreatic cancer. Lancet. 2001;358:1565–1566. doi: 10.1016/S0140-6736(01)06666-1. [DOI] [PubMed] [Google Scholar]
- 20.Koshy MC, Landry JC, Cavanaugh SX, et al. A challenge to the therapeutic nihilism of ESPAC-1. International journal of radiation oncology, biology, physics. 2005;61:965–966. doi: 10.1016/j.ijrobp.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 22.Abrams RA, Grochow LB, Chakravarthy A, et al. Intensified adjuvant therapy for pancreatic and periampullary adenocarcinoma: survival results and observations regarding patterns of failure, radiotherapy dose and CA19-9 levels. International journal of radiation oncology, biology, physics. 1999;44:1039–1046. doi: 10.1016/s0360-3016(99)00107-8. [DOI] [PubMed] [Google Scholar]
- 23.Winchester DP, Stewart AK, Bura C, Jones RS. The National Cancer Data Base: a clinical surveillance and quality improvement tool. Journal of surgical oncology. 2004;85:1–3. doi: 10.1002/jso.10320. [DOI] [PubMed] [Google Scholar]
- 24.Smeenk HG, van Eijck CH, Hop WC, et al. Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: long-term results of EORTC trial 40891. Annals of surgery. 2007;246:734–740. doi: 10.1097/SLA.0b013e318156eef3. [DOI] [PubMed] [Google Scholar]
- 25.Abrams RA, Winter KA, Regine WF, et al. Failure to adhere to protocol specified radiation therapy guidelines was associated with decreased survival in RTOG 9704--a phase III trial of adjuvant chemotherapy and chemoradiotherapy for patients with resected adenocarcinoma of the pancreas. International journal of radiation oncology, biology, physics. 2012;82:809–816. doi: 10.1016/j.ijrobp.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]


