Abstract
Dengue viruses (DENV), a group of four serologically distinct but related flaviviruses, are responsible for one of the most important emerging viral diseases. This mosquito-borne disease has a great impact in tropical and subtropical areas of the world in terms of illness, mortality and economic costs, mainly due to the lack of approved vaccine or antiviral drugs. Infections with one of the four serotypes of DENV (DENV-1–4) result in symptoms ranging from an acute, self-limiting febrile illness, dengue fever, to severe dengue haemorrhagic fever or dengue shock syndrome. We reviewed the existing mouse models of infection, including the DENV-2-adapted strain P23085. The role of CC chemokines, interleukin-17 (IL-17), IL-22 and invariant natural killer T cells in mediating the exacerbation of disease in immune-competent mice is highlighted. Investigations in both immune-deficient and immune-competent mouse models of DENV infection may help to identify key host–pathogen factors and devise novel therapies to restrain the systemic and local inflammatory responses associated with severe DENV infection.
Keywords: chemokines, cytokines, dengue virus, invariant natural killer T cells, mouse model, T helper type 17
Introduction
Dengue is the most important arboviral infection transmitted by Aedes mosquitoes, leading to severe disease in 2·5 billion people, and represents a rapidly growing major public health concern. There are between 50 and 100 million infections each year in tropical and subtropical countries, with approximately 500 000 cases admitted to hospital with severe and potentially life-threatening disease1–2 (http://www.who.int/topics/dengue/en/). Bhatt et al.3 showed using updated cartographic approaches, that there are 390 million dengue infections per year, of which 96 million manifest some level of disease severity. In endemic countries, the burden of dengue is approximately 1300 disability-adjusted life-years per million population, which is similar to the disease burden of other tropical diseases, notably tuberculosis, in these regions.4–5 All four dengue virus serotypes (DENV-1–4) are now circulating in Asia, Africa and the Americas. The molecular epidemiology of these serotypes has been extensively studied in order to understand their evolutionary relationship.6 Treatment of dengue fever (DF) or dengue haemorrhagic fever/dengue shock syndrome (DHF/DSS) is largely supportive and the lack of clinical or laboratory markers for an efficient diagnostic, associated with the lack of a vaccine or specific treatment, puts a serious burden on the health systems of low-income countries.4
Basic virology
Dengue virus is a lipid-enveloped virus that contains a single-stranded, positive-sense RNA genome. The virus is a member of the Flaviviridae family and is related to the viruses that cause yellow fever and Japanese, St Louis and West Nile encephalitis. Similar to other flaviviruses, they are transmitted to the host by an infected vector, Aedes aegypti and Aedes albopictus mosquitoes. Flaviviruses enter target cells by receptor-mediated endocytosis and traffic to endosomes, where the acidic environment of the late endosome leads to important conformational changes in their envelope glycoprotein protein that is responsible for inducing fusion of the viral and host cell membranes.7–8 The released RNA encodes a polyprotein precursor of approximately 3400 amino acids. This polypeptide will be post-translationally processed by host cell signalases and the virus-encoded protease NS2B/NS3 to produce three structural and seven non-structural proteins. The structural proteins – core, pre-membrane and envelope – constitute the viral particle while the non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5) are involved in viral RNA replication, virus assembly and modulation of the host cell responses.7,9 The replication of flavivirus generally occurs on virus-induced host cell membranes. DENV requires autophagy for efficient replication, with recent studies showing that DENV infection induces autophagy, and the inhibition of autophagy reduces significantly DENV replication and release of viral particles.11,12 These structures may serve as a scaffold for anchoring the viral replication complexes, which consist of viral RNA, viral proteins and host cell factors.14
Clinical and immunological aspects of disease
Dengue is now considered an important neglected tropical disease. Although many studies have been carried out for almost a century, many aspects of disease remain unresolved. The great lack of knowledge on dengue pathogenesis is a major factor that contributes to a striking human and economic burden. Disease development is not fully understood, which has delayed the development of vaccines, treatments and effective methods for DENV detection.15
After infection of an immune-susceptible host, an acute, self-limiting febrile systemic syndrome starts to develop. Resolution of infection normally occurs within 4–7 days and is associated with a robust innate and adaptive immune response. The diagnosis is largely clinical, treatment is supportive and disease control is limited to the elimination of its vectors.1–2 Primary infection in older children and adults normally lead to DF, a febrile illness accompanied by a combination of non-specific symptoms that may include headache, retro-orbital pain, myalgia and occasionally haemorrhagic manifestations.1–16 Some patients, such as newborns and elderly people, occasionally develop DHF, the most severe form of dengue disease. The hallmark of DHF is the presence of plasma leakage and haemoconcentration, which can lead to the loss of intravascular volume and circulatory insufficiency.16 Significant bleeding is also a clinical feature associated with severe disease. Bleeding can be observed in both DF and DHF; more severe bleeding, such as bleeding from the gastrointestinal tract, is found more frequently in DHF than in DF. Increased liver enzymes [aspartate aminotransferase/alanine aminotransferase (AST/ALT)] and thrombocytopenia (platelet count < 100 000 cells/mm3) are commonly observed in both DF and DHF patients but are more severe in DHF.16–17 However, haematocrit readings can be affected by factors such as fever, dehydration and haemorrhage. Patients with DHF who have narrow pulse pressure (<20 mmHg) or who show signs of shock are classified as having DSS. Other severe clinical manifestations including hepatic failure and encephalopathy have been reported in dengue patients.16,17 Viral load is controlled by the host after a few days, when signs of systemic inflammation are still observed. Patients with DHF/DSS present a ‘cytokine storm’, with high levels of circulating pro-inflammatory cytokines leading to endothelial activation and vascular leak with haemorrhage and shock.1,4 T lymphocytes, monocytes, macrophages, hepatocytes and endothelial cells have been shown to contribute to a robust production of interferon-α (IFN-α), IFN-γ, tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-2, IL-6, IL-8, IL-10,CCL2, CCL3, CCL4, CCL5, CXCL-8, CXCL-10, CXCL-11, macrophage migration inhibitory factor and vascular endothelial growth factor in the plasma of DF and DHF patients.16–19 This cytokine storm is accompanied by activation of the coagulation system, acute-phase proteins, soluble receptors and other mediators of inflammation.2
Immunological features
There has been increasing interest in understanding the cellular mechanisms that DENV exploits to enter the host cell. Langerhans cells, dermal cells and interstitial dendritic cells have been proposed to be the initial targets for DENV infection at the site of the mosquito bite.2,10 Dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN)21 and the mannose receptor (CD206)22 have been described as potential host receptors for virus entry. These interactions allow clathrin-mediated or Rab5-mediated endocytosis and transport process, finally supporting viral replication.23–24 The mononuclear phagocyte lineage represents the primary target for DENV, but a variety of other host target cells have been identified so far25 and include hepatocytes, lymphocytes, endothelial cells, neuronal cells and muscle satellite cells.26 However, the mechanisms involved in cellular tropism and viral replication are not known. Regarding viral evasion, signal transducer and activator of transcription 2 (STAT2) appears to be a key component of the STAT1-independent mechanism of protection against DENV infection in mice. Perry et al.27 demonstrated that both STAT1 and STAT2 possess the ability to independently limit the severity of DENV pathogenesis. For many viruses, inhibition of STAT-mediated signalling is a major mechanism to evade antiviral responses. Their data suggest that DENV-mediated inactivation of STAT1 function alone is not sufficient to neutralize antiviral responses; emphasizing the importance of DENV mechanisms to specifically target host STAT2 function. Increasing evidence suggests that the relative ability of flaviviruses to subvert STAT signalling, including DENV, West Nile encephalitis virus, Japanese encephalitis virus and Kunjin virus, may be a contributing factor to their virulence.
The mechanisms underlying severe dengue disease are currently being investigated by several research groups, identifying components that are essential for dengue-induced immune enhancement. The imbalanced and deregulated cell-mediated immunity is a pivotal component.10–16 In this phenomenon, DENV infection of dendritic cells strongly activates CD4+ and CD8+ T cells. Activation of T lymphocytes leads to the production of pro-inflammatory cytokines (i.e. TNF-α, IFN-γ) that may be pathogenic in the context of excessive T-cell activation, which is commonly observed in severe dengue.28–29 However, another study showed that infants with DSS had more CD69+ natural killer (NK) cells and CD8+ and CD4+ T lymphocytes compared to those with DHF without shock syndrome.30 Hence, the use of CD4+ and CD8+ T-cell counts as predictors of severe dengue require further studies. Different cytokines are produced by DENV-specific T cells in response to the recognition of peptide–MHC complexes on target cells. The pattern of cytokine production follows a T helper type 1 (Th) or Th0 profile. These T cells may produce IFN-γ, TNF-α, IL-2 and CC chemokine ligand 4 [CCL4; also known as macrophage inflammatory protein-1β (MIP-1β)], whereas the production of Th2 type cytokines, such as IL-4 and IL-13, is less common and less investigated.31,32 Studies have shown that CD8+ T cells specific to the DENV serotype of a previous infection appear to be preferentially expanded during a secondary infection.34–35 Analysis of the functional phenotypes of CD8+ T cells in DHF cases have revealed that cross-recognition is associated with reduced cytolytic/cytotoxic activity without a significant effect on cytokine production.32–35 In addition, activation with peptide variants has been shown to induce different sets of cytokines when compared with stimulation with the original peptide in both CD4+ and CD8+ T cells.31–36 Cytokines and chemokines induced by suboptimal activation of T cells may augment vascular permeability leading to plasma leakage in DHF. Indeed, chemokines such as MIP-1β and monocyte chemoattractant protein 1 (MCP-1) are proteins that reduce tight junctions of vascular endothelium cells in different inflammatory diseases. High concentrations of these proteins have been reported in patients with DHF/DSS.37–38 Endothelium exposure to these chemokines can cause injury, amplification of the inflammatory response and finally lead to severe dengue disease.37
Approximately 90% of DHF/DSS cases are associated with secondary infection by a heterologous serotype, while the remaining 10% result from primary infection. In the context of a heterologous secondary infection, memory B cells generated against the primary infection will respond quickly, producing high titres of antibodies that will potentiate the current infection instead of neutralizing the virus. This response is another important component in immune enhancement, being defined as antibody-dependent enhancement (ADE). Heterologous non-neutralizing antibodies are able to recognize dengue viral epitopes and enhance infectivity in an Fc-dependent manner.2,5 Briefly, ADE potentiates infection by linking potentially infective virus to its target cells, essentially monocytes and macrophages. These cells express receptors for the Fc portion of antibodies, in this case FcγR, which binds IgG. Non-neutralizing virus/antibody complexes are captured via FcR and internalized. Since the uptake of virus–antibody complexes is more efficient than the entry of free virus through host cell receptor, DENV infection is enhanced.2–29 It is not well understood how a greater viral load emerges from ADE-infected cells and how a greater viral load would provoke severe disease, especially because increased viral load alone is not the direct cause of plasma leakage.1–16 The final interpretation of ADE in terms of mechanisms and real impact on disease remains to be further explored.
Mouse models of infection
There is no perfect animal model to the study of DENV infection pathogenesis. A great part of these studies was performed using patient samples (plasma or peripheral blood mononuclear cells). These studies were descriptive and a link between systemic and local immune responses in the course of infection is frequently not possible to address. Non-human primates have been extensively used to study ADE and to test the efficacy and safety of pre-clinical vaccines.39 However, there is an intense debate in terms of cost and accessibility of these models to answer precise questions about disease pathogenesis. In the face of those limitations, a genetically appropriate mouse model would be essential to determine how different immune system components regulate a protective immune response, and to investigate how T cells and other leucocytes, endothelial cells and cytokines contribute to severe disease during primary and secondary heterologous infection. Initial attempts to develop a mouse model for dengue in immunocompetent mice with high titre viral infection were unable to recapitulate several important aspects of human DENV infection, including replication in peripheral tissues and development of the hallmark symptoms of DENV disease.19–40
Immune-competent mouse models have been shown to be resistant to DENV infections, because of the ability of their innate immune system to respond efficiently with total viral clearance, though success has been seen with mouse-adapted viruses and/or artificial infection routes such as intracranial and intraperitoneal injection. In vivo studies have shown that DENV inhibits type I IFN production and that lack of type I IFN response renders mice susceptible, indicating that this mechanism of immune response subversion is critical for DENV success and so affects transmission.41–42 In addition, others have shown that downstream protein expression induced by type I IFN and the Janus kinase/STAT pathway play important roles in DENV infection control.42–43
Sabin and Schlesinger showed in 1945 that DENV can be propagated by intracerebral inoculation in mice.44 Even if the initial adaptation to the mouse seemed to be a tedious and difficult process, 16 consecutive passages have been achieved and the virus propagated in mice produced dengue in human volunteers, but was not pathogenic for cotton rats, hamsters, guinea-pigs or rabbits. Intracerebral infection with neurotropic DENV strains has been performed to adapt human isolates to mice. DENV isolates passed serially from brain to brain led to increased neurovirulence and neurotropism in mice44 and a clear attenuation in human volunteers.45 However, viral encephalitis is not a major clinical symptom in human dengue disease, as nervous system involvement in DENV infections is rare and few cases are reported.46 The IFN system is critical to the host antiviral response, which led to the use of AG129 mice, which are type I and II IFN-R-deficient 129 mice, immune deficient and highly susceptible.47 Intraperitoneal infection with the mouse-adapted neurotropic DENV-2 strain, New Guinea C, led to 100% lethality in AG129 mice, all of them presenting paralysis.48 The neuroinflammatory changes led to alterations in motor behaviour and muscle tone and strength in DENV-3-infected mice. The neuroinflammatory process was marked by up-regulation of the chemokines CCL2, CCL5, CXCL1 and CXCL2, and of the cytokines TNF-α and IFN-γ, which occurs in parallel with increased leucocyte rolling and adhesion in meningeal vessels and infiltration of immune cells into the brain.49 In summary, even if these models were used to study antiviral compounds or behaviour, the major limitation involving immune-compromised mice is that paralysis is not a major clinical observation in DENV infection.
Initial tropism studies using the AG129 (IFN type I and II receptor-deficient) model demonstrated that clinical isolates from all four DENV serotypes replicate efficiently in spleen, lymph node, bone marrow and muscle.50 Negative-strand viral RNA was detected in dendritic cells and macrophages of the lymph node and spleen.50 To develop an experimental model where viral encephalitis was not the major clinical observation, Shresta et al.47 infected AG129 mice intravenously with the DENV-2 strain PL046. Infected AG129 mice succumbed to DENV infection, presenting increased levels of TNF-α and vascular leakage syndrome. AG129 mice are able develop cross-reactive and long-lasting antibody responses to DENV.51 Sequential DENV infection in AG129 mice results in decreased viral load of the second serotype and full protection against lethal infection. AG129 and other mouse strains have been used to study ADE by passive transfer of anti-DENV monoclonal antibodies, cross-reactive immune serum, or diluted homotypic serum before infection.52–53 Mortality was associated with vascular leakage syndrome, high levels of TNF-α and thrombocytopenia, similar to the clinical findings observed in DHF/DSS in humans. No memory response was observed in mice receiving passive transfer of serum or antibody. Hence, models of sequential DENV infection may be useful to study ADE in the presence of a cellular memory immune response. The AG129 mouse model might therefore be useful to examine the role of antibody repertoire and function in mediating DENV protection and enhancement, and to unravel the contribution of B and T memory cells in modulating secondary DENV infection. As IFN signalling is essential to the protective immune response against DENV, an obvious limitation of models using AG129, IFN-α/βR−/− and STAT1−/− mice is the difficulty in studying the cell-mediated immune response against DENV as a whole in mice that lack important components of the host antiviral system.47–54
Humanized mice provide a controlled animal model that allows in vivo infection of human cells with DENV and elicits human DENV-specific immune responses. Using cord blood haematopoietic stem cell-engrafted NOD-scid IL2rγnull (NSG) mice, Jaiswal et al.55 showed that the engrafted mice support DENV infection. Human T cells from infected NSG mice expressing the HLA-A2 transgene produced IFN-γ and TNF-α upon stimulation with DENV peptides. These mice also developed moderate levels of IgM antibodies directed against the DENV envelope protein.55 Humanized NSG mice xenografted with human CD34+ cells from cord blood and infected with DENV-2 clinical strains showed signs of DF disease (fever, viraemia, erythema and thrombocytopenia).56 The NOD/SCID strain of mice lacks T and B cells and has defects in NK cell function and antigen-presenting cell development and function and genetically lacks C5, resulting in a deficiency in haemolytic complement; it therefore provides an excellent environment for reconstitution with human haematopoietic cells and tissues.57 The same research group demonstrated that the virus can infect human cells in the bone marrow, spleen and blood, with efficient secretion of cytokines and chemokines by human cells in humanized mice.58 Finally, upon virus transmission with A. aegypti exposure the authors showed DHF/DSS (higher viraemia, erythema and thrombocytopenia, production of IFN-γ, TNF-α, IL-4 and IL-10). This is the first animal model that allows an evaluation of human immunity to DENV infection after mosquito inoculation.59
Immunocompetent models
Wild-type mice (BALB/c or C57BL/6) are resistant to DENV infection, but they have been increasingly used to investigate details of DENV pathogenesis. Intradermal infection of C57BL/6 mice with a non-mouse adapted DENV-2 strain, 16681, resulted in systemic haemorrhage and death with a high inoculum.60 These mice also presented severe thrombocytopenia, high viraemia, TNF-α production, macrophage infiltration and endothelial cell apoptosis. The same group showed that intravenous infection of C57BL/6 mice with a high inoculum of DENV-2 16681 led to hepatic injury/dysfunction, an important feature of DENV infection in humans.61 One of the limitations of the latter model is the fact that disease is observed 3 days after infection using a high viral inoculum, which is inconsistent with clinical disease. BALB/c mice infected intraperitoneally with DENV-2 also showed hepatic damage and high levels of AST/ALT that peaked at day 7 post-infection.62 These studies revealed that wild-type (WT) mice can exhibit clinical signs of DENV infection, including thrombocytopenia, haemorrhage and liver damage; a close resemblance to DHF/DSS as observed in humans.
The DENV-2 P23085 adapted strain
A few research groups have adapted clinical DENV isolates to the murine host to obtain adapted strains that are able to induce disease resembling human infection. Atrasheuskaya et al.63 showed that young BALB/c mice (4-weeks old) were found to be sensitive to the challenge with a mouse-adapted DENV-2 (strain P23085, GenBank: AY927231.1). They developed clinical manifestations such as arching of the back, ruffling of the fur and slowing of activity. The presence of DENV-2 virus in the blood was confirmed by RT-PCR and mice showed severe weight loss ending in limb paralysis and 100% mortality. The most important changes in production of pro-inflammatory markers were seen in TNF-α, which quickly increased 24 hr before death. This model supports the notion that activation of the innate immune response is partially responsible for mortality in DENV-2 virus infection. In line with this hypothesis, anti-TNF-α treatment significantly reduced the mortality rates.63 Similarly, BALB/c mice-infected intraperitoneally with a DENV-2 isolate demonstrated liver damage, as determined by high AST and ALT levels that peaked at day 7 post-infection.64
Our group described a DENV infection model in adult BALB/c or C57BL/6 mice (≥ 8 weeks old), using the mouse-adapted DENV-2 strain (P23085), from Atrasheuskaya et al.63 The adapted virus given systemically (intraperitoneally) induced inoculum-dependent lethality that was preceded by major manifestations of severe DENV infection in humans such as mechanical hypernociception (an index of pain), thrombocytopenia, haemoconcentration, increased vascular permeability, hypotension, increased levels of cytokines and chemokines, tissue haemorrhage, viraemia and recovery of viral load in target organs of infection.65–71 Viral replication and lethality were abolished after in vitro or in vivo neutralization using the anti-DENV-2 monoclonal antibody 4G2.68 Moreover, the adapted DENV-2 strain was not found in the brain of intraperitoneally infected mice.71 This model of DENV-2 infection in immune competent mice provides an important tool to study host–virus interactions and mechanisms associated with severe disease manifestation, so contributing to the elucidation of DENV pathogenesis.65–70 However, a possible drawback of the model is that it uses a single strain that was adapted by multiple passages in mice. All eventual modifications of the virus to the murine host are currently under investigation because they may cause a disease that is significantly different to that of the original virus in humans.19 Table 1 summarizes the most studied mouse models of dengue infection available in the literature.
Table 1.
Different mouse models of dengue virus infection as reviewed in Costa et al.19 Fagundes et al.142, Rothman2, Zompi and Harris40, and Yauch and Shresta54
| Mice strains | Virus/inoculum | Outcome/symptoms | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|
| Humanized mice | Low or moderate viraemia, fever, rash, haemoconcentration, thrombocytopenia and minor production of TNF-α. | Mice develop functional human immune system, including adaptive immunity; infection of human cells lineages; study of ‘human’ response to infection. Study of DF pathogenesis, human cell versus virus tropism; screening of virulence factors among different genotypes and vaccine studies in vivo. | Mild disease manifestation (DF) with no cytokine storm or shock as observed in severe human disease (DHF/DSS). | Wu et al.134 | |
| SCID-hu-PBL | DENV-1 9 × 104–5 PFU i.p | ||||
| SCID-K562 | DENV-2 107 PFU i.t. | Lin et al.135 | |||
| SCID-HepG2 | DENV-2 106 PFU i.p | An et al.136 | |||
| SCID-HuH-7 | DENV-4 104 PFU i.t. | Blaney et al.137 | |||
| NOD/SCID-human CD34+ | DENV-2 104–7 PFU s.c | Bente et al.57 | |||
| RAG2−/−γc−/−-human CD34+ | DENV-2 106 PFU i.p./s.c | Kuruvilla et al.138 | |||
| Immune-deficient mice | |||||
| AG129 | DENV-2 (PL046) 107 PFU i.v, DENV-2 (D2S10) 107 PFU i.v, DENV-1 (strain 98J), DENV-2 (S221) | Low viraemia or high viraemia associated with late lethal encephalitis and elevated haematocrit; early plasma-leakage syndrome (TNF-α-mediated). | Replication of relevant dengue virus clinical isolates; development of marked viraemia; antibody-dependent enhancement model. Allows the investigation of tissue and cellular tropism of dengue virus; ideal for antiviral drugs screening. | Absence of important components of host antiviral system; inappropriate to study DENV immunopathogenesis; occurrence of paralysis and lethal encephalitis. | Shresta et al.47, Balsitis et al.52, Zellweger et al.53 |
| A/J | DENV-2 108 PFU i.v | Shresta et al.139, Huang et al.140 | |||
| STAT1−/− | DENV-2 105 PFU i.p./i.c. | Chen et al.141 | |||
| Immune competent mice | |||||
| C57BL/6 | DENV-2 4–8 × 107 PFU i.d.; DENV-2 (16681) 1–3 × 109 PFU i.d., DENV-2 108 PFU i.v.,DENV-2 (P23085) 10-100LD50, DENV-3 (Genotype I) | Haemorrhage; cytokine storm; shock; liver damage; haemoconcentration; thrombocytopenia. No dengue virus-specific antibody and T-cell responses. | Mice develop the major clinical manifestations of DHF/DSS (pain, thrombocytopenia, haemoconcentration, increased vascular permeability, hypotension, increased levels of cytokines, and chemokines, tissue haemorrhage and viraemia). | Dengue virus strains that were adapted by multiple passages in mice; modification of the virus to the murine host may involve pathological mechanisms different to that of the original virus in humans; severe disease occurs in primary infection. | Chen et al.61, Chen et al.60, Renessom et al.70, Guabiraba et al.68–69, Costa et al.66, Fagundes et al.67 |
| BALB/c | DENV-2 104 TCID 50 i.p, DENV-2 (P23085) 10-100LD50 | Paes et al.64, Souza et al.71, Assunção-Miranda et al.65 | |||
DENV, dengue virus; DF, dengue fever; DHF, dengue haemorrhagic fever; DSS, dengue shock syndrome; i.c., intra-cerebral; i.p., intra peritoneal; i.t., intrathechal; i.v., intravenous; PFU, plaque forming units; s.c., subcutaneous; TNF, tumour necrosis factor.
We discuss here important pro-inflammatory molecules and leucocyte populations that were identified as key players in the murine model of DENV-2 infection using the mouse-adapted strain P23085. The inflammatory response triggered by this model of DENV infection frequently leads to tissue damage and death. However, it is possible in this model to assess and distinguish mechanisms necessary for the host response to deal with infection from those that cause unwanted, misplaced and uncontrolled inflammation and drive disease. By understanding where/how host–pathogen interactions lead to disease, we may be able to suggest novel strategies to restrain severe systemic and local inflammatory responses.
Key players in experimental dengue infection
CC chemokines
Chemokines are members of a structurally related family of cytokines involved in leucocyte traffic during infection and inflammation. They are classified according to the relative position of conserved N-terminal cysteine residues, in which CC chemokines represent the most abundant family and have the first two cysteines placed adjacently.72 Chemokine receptors are expressed on the surface of leucocytes and are G protein-coupled receptors containing seven transmembrane domains.73
Experimental and epidemiological evidence suggests an important role for chemokines, especially those from the CC family, and their receptors in infectious diseases such as HIV and herpes simplex virus 1.74–75 The expression of CC chemokines dominates over the expression of CXC chemokines during viral infections, although this observation does not represent a general rule.75 Among the CC chemokines, CCL3/MIP-1α and CCL5/regulated upon activation, normal T cell-expressed and secreted (RANTES) are widely associated with viral infections 74–76 During intranasal influenza virus infection in mice, CCL2/monocyte chemotactic protein-1 (MCP-1) is detected in the lungs at various time-points post-infection, whereas other chemokines, including CCL3 and CCL5, are not expressed.77 On the other hand, respiratory syncytial virus-infected mice display high levels of expression of numerous chemokines in the lungs, including CCL3 and CCL5.78
Among flaviviruses, CC chemokine receptors play an important role in leucocyte recruitment to the central nervous system.79 Besides a deleterious pro-inflammatory role that CC chemokines could play in central nervous system, a well-studied example involves acute infection by West Nile virus in mice, in which the lack of CCR2 and CCR5 leads to decreased leucocyte recruitment, increased viral load in the central nervous system and enhanced mortality. West Nile virus infection induces high and continuous levels of CCL2 and CCL5, which are required for the local accumulation of NK cells, macrophages and T lymphocytes to control infection.79–80
Recent clinical studies in areas where DENV is endemic describe a correlation between DHF/DSS and levels of CC chemokines, including CCL4/MIP-1β and CCL3/MIP-1α, both ligands for the CCR1 receptor, and for CCL2/MCP-1, the ligand for CCR2.37,81 Bozza et al. showed that CCL4 might be associated with a protective pathway for its chemoattractive and activating effect on NK cells (CD56+), which in turn are efficient cells in early virus clearance. CCL2 would be associated with thrombocytopenia and vascular permeability, which leads to plasma leakage and haemoconcentration.37 In addition, both chemokines are able to induce the recruitment of monocytes, lymphocytes, dendritic cells among other types of leucocytes in infection and inflammation.76 Sierra et al. showed that heterologous ex vivo re-challenge using peripheral blood mononuclear cells from patients induces high production of CCL2 and CCL3 in DENV-1- and DENV-3-immune subjects, which coincides with an induction of heterologous inflammatory IFN-γ and TNF-α and with weak expression of the regulatory cytokine IL-10. These findings indicate the critical importance of previous serotype-specific immunity as an initial event linked to expression of these chemokines.81 Both chemokines markedly activate macrophages to secrete TNF-α, IL-1 and IL-6,35 all involved in dengue pathogenesis.1,2 CCL2 also causes endothelial cell tight junction openings in vitro83 and its induced expression in vascular endothelial cells increases endothelial permeability changes,32 finally contributing to the characteristic plasma leakage of DHF. A link between CCL5, a CCR1/CCR5 ligand, and hepatic dysfunction had already been shown.84–85 In fact, the chemokine system appears to have a dual ‘protective versus pathological’ role during experimental DENV infection.
We have recently described the putative role of CC chemokine receptors CCR1, CCR2 and CCR4 in the experimental DENV-2 infection model using the adapted P23085 strain.69 We observed that CCR1 does not seem to have a major role in DENV pathogenesis. Levels of CCL3 were increased in spleen and liver of infected mice at day 6 post-infection. However, we found that the course of infection in CCR1−/− mice was similar to that in WT mice. Levels of CCL3 were greater in spleen and liver of infected CCR1−/− compared with infected WT animals, which is in agreement with the idea that chemokine receptors work as important negative modulators or scavengers of their own ligands.86 Elevated levels of CCL3 could eventually activate the other CCL3 receptor, CCR5. We have not investigated the role of CCR5 in DENV-2 infection outcome but it is clear that CCR1−/− mice had no major phenotype when infected with an inoculum that causes severe disease in mice. CCL2 was increased in liver and spleen of WT mice, which is a finding consistent with the literature.37,81 In CCR2−/− infected mice, levels of IL-6 and IFN-γ, but not TNF-α, were decreased systemically. There was also decreased activation of major cell types involved in DENV infection, including CD4+ and CD8+ T cells, natural killer T (NKT) cells and macrophages. Therefore, decreased leucocyte activation in infected CCR2−/− mice may explain the decreased cytokine storm and decreased tissue damage observed in these animals.
The CCR4 receptor shown to be relevant for virus-induced liver damage and the associated systemic inflammation in the present model. We also found that CCL17/TARC, one of the ligands for CCR4, was detectable at high levels in the spleen of infected mice. Viral load was not altered in CCR4−/− when compared with WT animals, which suggest that that CCR4 does not play a major role in the control of viral entry and replication, but contribute mostly to the cascade of events that lead to tissue and systemic damage. Interestingly, CCR4 deficiency is associated with attenuated severity of murine polymicrobial sepsis and lipopolysaccharide-induced endotoxic shock, implicating this receptor in the pathogenesis of acute conditions.88–89 Other experiments, however, have found a protective role for CCL22/MDC, a CCR4 ligand, in a caecal ligation and puncture model of sepsis in mice.90 It is difficult to suggest the cellular and molecular mechanisms by which CCR4 may contribute to the pathogenesis of dengue. However, CCR4 may be important for the trafficking and activation of NKT/invariant NKT (iNKT) cells and naive CD8+ cells by at least two independent chemokine pathways, including CCL17/TARC and CCL22.91–92 Moreover, pulmonary localization of iNKT cells is critical for the induction of airway hyperreactivity and requires CCR4 expression by iNKT cells.93 In fact, excessive NKT/iNKT activation contributes to the pathogenesis of severe disease in our model.70 Our studies suggest that the chemokine storm that follows severe primary DENV infection is associated with the development of inflammation rather than protection against severe disease. Hence, blockade of the chemokine system may be beneficial as co-adjuvant treatment for severe DENV infection and might be further explored. A summary of the role of CC chemokines and their receptors in DENV infection is shown in Table 2.
Table 2.
CC chemokines and their receptors during dengue virus infection
| Chemokines/Receptors | Experimental system | Pathological observations | References |
|---|---|---|---|
| CCL2 (MCP-1, JE), CCR2 | Human blood, Human PBMC, PBMC/HUVEC, mice (DENV-2 P23085) | Increased permeability and disrupted tight junctions of human vascular endothelium cells; hypotension; association with DF and DHF; liver damage and shock. | Lee et al.87, Bozza et al.37, Sierra et al.81, Guabiraba et al.69, Tolfvenstam et al.143 |
| CCL3 (MIP-1α) | Human PBMC, Human monocytes/macrophages, mice (DENV-2 P23085) | Replication-competent virus was required to induce MIP-1α; association with DF and DHF; strong influence on the early immune response after dengue re-infection; liver damage and shock. | Spain-Santana et al.82, Chen et al.144, Sierra et al.81, Guabiraba et al.69 |
| CCL4 (MIP-1β) | Human blood, Human PBMC, KU812 or HMC-1 human mast cell-basophil lines | Association with severity of secondary heterologous infection; role of mast cells in the initiation of chemokine-dependent host responses to dengue virus infection; levels were observed in patients with mild dengue. Also associated to CD56+ NK cell circulating rates. | King et al.145, Bashyam et al.146, Bozza et al.37 |
| CCL5 (RANTES), CCR5 | Human blood, Human endothelial cells, Hepatocytes/HepG2, KU812 or HMC-1 human mast cell-basophil lines, mice (DENV-2 P23085) | Association with complement activation, chemokine induction, apoptotic cell death and short-lived vascular leakage; liver damage and ADE, T-cell activation. | Avirutnan et al.147, Lin et al.84, King et al.145, Brown et al.148, Conceição et al.149, Guabiraba et al.68–69 |
| CCL17/22, CCR4 | Human PMBC, Human liver biopsies, mice (DENV-2 P23085) | Association with tissue injury and shock in experimental infection; influence on CD8+ T cells effector function and migration into the liver. | de-Oliveira-Pinto et al.150, Guabiraba et al.69 |
ADE, antibody-dependent enhancement; DENV, dengue virus; DF, dengue fever; DHF, dengue haemorrhagic fever; HUVEC, human umbilical vein endothelial cells; MIP-1α, macrophage inflammatory protein-1α; NK, natural killer; PBMC, peripheral blood mononuclear cells.
Invariant NKT cells
The NKT cells constitute a heterogeneous population of non-conventional αβ T lymphocytes that recognize self and foreign (glyco) lipid antigens through their T-cell receptors (TCRs). NKT TCR-mediated responses are restricted by CD1d, a member of the non-polymorphic CD1 antigen-presenting protein family that promotes the presentation of endogenous and pathogen-derived lipid antigens to the TCR.94,95 CD1d-restricted NKT cells are divided into invariant (iNKT cells, or type I NKT cells), the predominant subset which express an invariant TCR-α chain (Vα14Jα18 in mice), and variant (vNKT cells, or non-invariant or type II NKT cells), which express more diverse TCRs.94–95 Invariant NKT cells have regulatory functions in autoimmune and inflammatory diseases, cancer and infection.97–98 The activation of iNKT cells by molecules such as the high-affinity lipid α-galactosylceramide (α-GalCer) induces the robust production of Th1 (IFN-γ), Th2 (IL-4) and Th17 (IL-17, IL-22) -associated cytokines that may influence the ongoing immune responses during infection.99–100 Murine NKT cells are present in large amounts in the liver (10–30% of intra-hepatic T cells),101 and mouse models have shown a pivotal role for NKT/iNKT cell activation in liver pathology during virus-induced and concanavalin A-induced hepatitis.102–103 In a closely related manner, liver function is frequently affected in patients during DHF/DSS.1,16 As the studies on the impact of iNKT activity on viral immunity continues to develop, iNKT cells will probably be found to contribute to the host response in different viral infections.96 Their role during hepatitis C virus, a hepacivirus of the Flaviviridae family, has been investigated in humans, although conflicting data about the frequency and function of iNKT cells in both liver and blood have been reported.102 Some evidence also suggests the mast cell-mediated recruitment of NKT cells to sites of DENV infection.104
In our experimental model of DENV-2 infection using the adapted P23085 strain, we consistently observed that mice lacking iNKT cells (Jα18−/− mice) were resistant to severe DENV infection.70 Haemoconcentration and plasma leakage were strongly reduced in DENV-infected Jα18−/− mice compared with infected WT mice. In parallel, histopathological analysis of liver sections revealed that infected Jα18−/− mice developed less hepatic damage. Hence, in agreement with other studies that demonstrated a detrimental role of iNKT cells in liver disease,101–106 our data strongly suggest that iNKT cells contribute to hepatic injury during DENV infection. The viral load was significantly reduced in spleen and liver of Jα18−/− mice compared with WT animals. Previous findings have suggested that pro-inflammatory mediators favour DENV replication in vivo and in vitro,107 so it is likely that in our experimental setting, iNKT cells indirectly favour virus replication by promoting inflammation. As the inflammatory response is strongly reduced in Jα18−/− mice, this positive feedback for viral replication would be down-regulated. Importantly, Jα18−/− mice reconstituted with purified iNKT cells from naive intra-hepatic leucocytes presented 80% lethality. The incomplete restoration of the WT phenotype could be due to an interfering effect of vNKT cells. In some disease conditions, vNKT cells and iNKT cells exert opposing functions in immune regulation.96–100
The exact mechanisms by which iNKT cells contribute to DENV pathogenesis are yet to be defined. It is possible that they act through an early production of inflammatory cytokines that are able to directly and/or indirectly promote injury. As described in concanavalin A-induced hepatic injury it is also possible that, through Fas or Fas ligand interaction, iNKT cells mediate cytotoxic effects on hepatocytes, so contributing to liver disease.108 In this system, the Fas/Fas ligand pathway may also participate in vascular leakage by promoting apoptosis of endothelial cells. Furthermore, iNKT cells express chemokines receptors such as CCR2 and CCR4, that were earlier mentioned in the present review to play important roles in disease progression during experimental DENV-2 infection.93–109
We aim to investigate further the potential role of iNKT cells during acute DENV infection in the human system, which would include the activation status of iNKT cells in patients infected with DENV in endemic areas. A better understanding of the in vivo iNKT cell activation, the chemokines involved in their recruitment to target organs and their precise functions in DENV infection may pave the way to development of novel therapeutic approaches such as targeting the development and expansion of the iNKT population.
Th17-like cytokines
Th17 cells are induced upon TCR activation in the presence of cytokines that activate STAT3, including IL-6, IL-21 and IL-23.110 They are an important subset of lymphocytes involved in the immune response to extracellular pathogens including bacteria and virus, and participate actively in inflammation and autoimmune diseases.110,111 Th17 polarization is characterized by the expression of chemokine receptor CCR6 and its ligand CCL20, and by production of IL-17A, IL-17F, IL-21 and IL-22.111–112 Some research groups also identified the Th22 subset, which is a human T helper subset described by Trifari et al. in 2009,113 that is characterized by the production of IL-22 and TNF-α, but not IL-17 or IFN-γ.110,113 Interleukin-22 is a member of the IL-10 cytokine family and has been described as playing key roles in inflammation and tissue homeostasis.115–116 The IL-22 receptor complex (IL-22R) is expressed in non-haematopoietic cells in the skin, kidney, liver, lungs and gut, which allows an important IL-22-mediated regulation of local tissue responses during infection and/or inflammation.111–117 The IL-22 is produced not only by Th17 or Th22 cells but also by NK cells, NKT cells, γδT cells and lymphoid tissue-inducer-like cells.114,118
Both IL-17 and IL-22 can induce an innate immune response in epithelial cells, but their functional properties are distinct. Whereas IL-17 induces an inflammatory tissue response, IL-22 is believed to be mainly protective and/or regenerative.114–118 In human and experimental viral infections, IL-22 seems to a play a protective role in primary respiratory infection by influenza A virus, not contributing to viral clearance, whereas the IL-17 pathway contributes to acute lung injury caused by the flu.120–121 Interleukin-22 also appears to be an important mediator of the inflammatory response following recognition of hepatitis B virus by T cells in the liver.122 Importantly, the role of the IL-22 and IL-17 pathway in infections caused by flaviviruses is poorly known. Hepatic IL-22 expression is up-regulated in viral hepatitis (caused by hepatitis C virus) without any effect on viral replication or control.123–124 Therefore, IL-22 is likely to be an important factor in the pathogenesis and clinical outcome of hepatitis B virus and hepatitis C virus infections, where the liver is a major target organ such as in DHF/DSS.64–125
Recently, it has been shown that acute DENV-2 infection elicited high levels of IL-17 in patients with severe disease (DHF).126 However, other studies found a correlation between IL-17 levels and mild infection (DF).127 Malavige et al.128 found no differences for IL-17 levels in patients with DHF who developed shock and those who did not. Furthermore, Talarico et al.33 demonstrated age-related differences in the primary response to DENV, characterized by an immature Th2 polarization and Th17 suppression in infants. Hence, the ultimate role of Th17 cytokines in the pathogenesis of dengue is yet to be unveiled.
In the experimental model of DENV-2 infection, using the P23085 adapted strain, we showed that mice deficient for the cytokine IL-22 were more susceptible to experimental DENV infection, presenting increased inflammation and severe tissue injury, especially in the hepatic parenchyma.68 This was associated with increased mortality, levels of AST/ALT in serum, greater neutrophil accumulation and/or activation and a small increase in viral load in the liver. DENV-2-infected HepG2 cells treated with recombinant human IL-22 showed reduced cell death and IL-6 production. These data clearly suggest that IL-22 appears to play a key role in liver homeostasis in the course of DENV infection.
Regarding the main leucocyte subsets that participate in our experimental system, γδ T cells and NK cells were the major sources of IL-17A and IL-22, respectively. Although we had observed a minor production of IL-17 by CD4+ Th17 cells in the spleens of infected WT mice, these populations do not appear to represent the real key players in this experimental setting. Recently, γδ T cells (but not Th17 cells) have been shown to be the primary source of IL-17A production in the early phase of Escherichia coli infection, which is related to an early infiltration of neutrophils such as in our model of DENV-2 in mice.129 Moreover, γδ T-cell-derived IL-17A is critical for the optimal induction of cytotoxic T lymphocyte responses and protection against primary intracellular Listeria monocytogenes infection in the liver.130 Interleukin-17A production during experimental DENV-2 infection was strongly correlated with disease severity, which was confirmed by the fact that infected IL-17RA-deficient mice were less susceptible than WT mice.68 Immature or mature NK cells (CD3− NKp46+) have been identified in the mucosa and found to be capable of producing IL-22 in different models of infection.121–131 We have shown here that NK cells (CD3− NK1.1+) are the major producers of IL-22 in the present model. Although the mechanisms of activation and local function of these populations in host defence and tissue homeostasis during DENV-2 infection require further investigation, we described for the first time that γδ T cells and NK cells participate in the production of IL-17A and IL-22 in experimental DENV (or in any other flavivirus) infection.
One of the striking observations in the IL-17/IL-22 axis in our experimental model of DENV-2 infection is the fact that infected IL-22−/− mice presented increased production of IL-17A in the spleen and liver, and neutralization of IL-17A in these mice reverted the worsened phenotype observed in mice lacking IL-22. Other studies have addressed the cross-talk between IL-17A and IL-22 production. Besnard et al.132 showed that IL-22 may regulate the expression and pro-inflammatory properties of IL-17A in allergic lung inflammation. Sonnenberg et al.112 described that IL-17A could suppress IL-22 expression in Th17 cells after bleomycin-induced lung inflammation and fibrosis. Although a reciprocal regulation of IL-17A and IL-22 is observed in vivo, the underlying cellular and molecular mechanisms that may affect the functional properties of these cytokines in distinct peripheral tissues are yet to be described. Therefore, IL-22 seems to counterbalance the production of IL-17A in experimental severe dengue infection. Pro-inflammatory mediators produced by epithelial cells in response to IL-17A are neutrophil- and granulocyte-attracting chemokines (i.e. CXCL1, CXCL2), IL-6 and several growth factors.13,14 Neutrophil accumulation and activation are increased in DENV infection, so this could be an important function for IL-17A in this disease. In addition, IL-17A expression is markedly reduced in the spleens of iNKT-cell-deficient mice (Jα18−/−) during infection (R. Guabiraba, J. Renneson, and F. Trottein, unpublished data). The close association of iNKT cells and the production of IL-17 or IL-22 in experimental DENV infection might require further investigation.
Role of platelets, inflammasome and IL-1
Although thrombocytopenia is observed in mild and severe forms of DENV infection, the role of platelet activation in dengue pathogenesis has not been fully elucidated. Hottz et al.133 hypothesize that platelets have major roles in inflammatory amplification and increased vascular permeability during severe forms of dengue. They reported an increased expression of IL-1β in platelets and platelet-derived microparticles from patients with dengue or after platelet exposure to dengue virus in vitro. Further, DENV infection led to microparticle release through mechanisms dependent on NLRP3 inflammasome activation and caspase-1-dependent IL-1β secretion by platelets. Inflammasome activation and platelet shedding of IL-1β-rich microparticles correlated with signs of increased vascular permeability. Moreover, microparticles from DENV-stimulated platelets induced enhanced permeability in vitro in an IL-1-dependent manner.133 These data provide strong evidence that platelets contribute to increased vascular permeability in dengue virus infection by inflammasome-dependent release of IL-1β. Additional studies on the role of platelets and IL-1 family members may be important to fully understand their roles in DENV pathogenesis.
In summary, strategies that may limit IL-1 and IL-17 production at local sites of inflammation and viral replication during DENV might represent a step forward in the attenuation of severe manifestations of the disease such as DHF/DSS. In addition, any eventual strategy that allows local release of IL-22 or enhances IL-22 production to counterbalance the up-regulation of IL-17 would also bring a beneficial impact to limit tissue damage and hepatic dysfunction during DHF/DSS. However, further experimental studies are necessary to understand the complex interactions of the virus with the host cells and the regulation of cytokines, chemokines and other mediators of inflammation including complement, tissue homeostasis and metabolism at large.
Concluding remarks
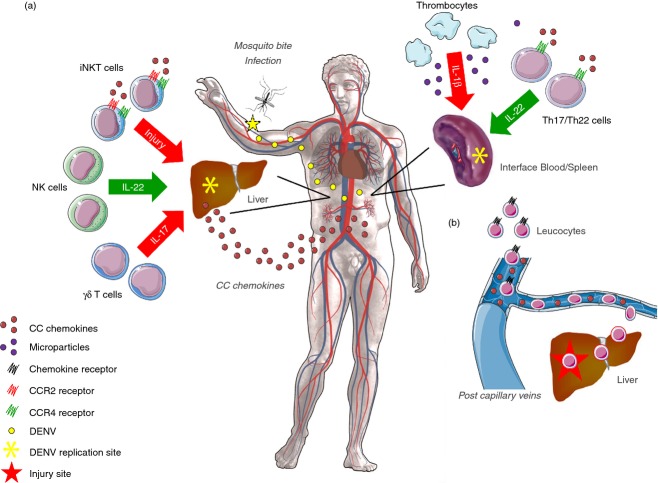
This is a comprehensive review of DENV biology and research, especially of the different mouse models used to study the pathogenesis of DENV infection. Overall, each mouse model has its advantages and disadvantages and the researcher must carefully select the optimal model to investigate dengue immunopathogenesis and pre-clinical testing of antiviral drugs and vaccines. With a focus on the immune competent mouse model of DENV-2 infection, we described important molecular and cellular mechanisms underlying the exacerbated inflammatory response triggered by uncontrolled viral replication in mice (Fig. 1). These studies will help to define new potential targets to attenuate disease severity and outcome in patients. Although the P23085 adapted strain represents progress, further studies are required to define how the altered sequence by this adapted strain influence host–pathogen interactions and to scrutinize the phenotype against the known clinical aspects of DHF/DSS in humans.
Figure 1.
Schematics showing the intricate role of chemokines, cytokines and inflammatory leucocytes in the pathogenesis of dengue virus (DENV) infection. After mosquito bite and infection of host skin and bystander immune cells, certain organs such as liver and spleen will become important sites of viral replication and inflammation. The disseminated inflammatory response observed in dengue haemorrhagic fever/dengue shock syndrome (DHF/DSS) will allow a great production of CC chemokines in blood, liver and spleen. This elevated production of chemokines will contribute to local tissue damage and to the recruitment of different leucocyte populations from the capillary veins into the target organs through the activation of chemokine receptors. The spleen and blood appear to be primary and privileged sites of leucocyte activation, especially for T helper type 17 (Th17)/Th22 lymphocytes and thrombocytes, which can express the CCR4 receptor and produce interleukin-22 (IL-22), or produce IL-1β and microparticles, respectively. Once leucocytes are activated they come into contact with CC chemokines (b) and are recruited into the liver. (a) Invariant natural killer T (iNKT) cells can be recruited trough the CCR2 and CCR4 receptors and play a deleterious role in the liver, greatly contributing to tissue injury. Natural killer (NK) cells are the main sources of IL-22 in the hepatic parenchyma, and appear to play a pro-homeostatic role during DENV infection. γδ T cells are major sources of IL-17 in the liver, that together with CC chemokines contribute to the massive inflammatory response observed during DHF/DSS. Targeting the CC chemokines, IL-17A and IL-1 family of cytokines may represent an effective adjunct therapy to attenuate the severity of disease manifestations observed in DHF/DSS.
Acknowledgments
We acknowledge Dr Mauro M. Teixeira (UFMG, Brazil) and Dr François Trottein (INSERM, Lille, France) for their mentorship and support. Our work was supported by research grants from The Centre National de la Recherche Scientifique (CNRS), Institut National de la Santé et de la Recherche Médicale (INSERM), the French National Research Agency (ANR), Fondation pour la Recherche Médicale (FRM), Fond Européen de Développement Régional (FEDER) and the Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq, Brazil). The research on DENV-2 experimental infection was developed and performed under the auspices of the programme INCT em Dengue (Brazil).
Glossary
- ADE
antibody-dependent enhancement
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- DENV
dengue virus
- DF
dengue fever
- DHF
dengue haemorrhagic fever
- DSS
dengue shock syndrome
- IFN-α
interferon-α
- IL
interleukin
- MCP
monocyte chemotactic protein
- MIP
macrophage inflammatory protein
- NSG
NOD-scid IL2rγnull
- STAT
signal transducer and activator of transcription
- Th1
T helper type 1
- TNF-α
tumour necrosis factor-α
- vNKT
variant NKT
- WT
wild-type
Disclosures
The authors declare that they have no financial or commercial conflict of interests.
References
- Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–96. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol. 2011;11:532–43. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496:504–7. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman MG, Kouri G. Dengue: an update. Lancet Infect Dis. 2002;2:33–42. doi: 10.1016/s1473-3099(01)00171-2. [DOI] [PubMed] [Google Scholar]
- Halstead SB. Dengue. Lancet. 2007;370:1644–52. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- Gubler DJ, Meltzer M. Impact of dengue/dengue hemorrhagic fever on the developing world. Adv Virus Res. 1999;53:35–70. doi: 10.1016/s0065-3527(08)60342-5. [DOI] [PubMed] [Google Scholar]
- Bressanelli S, Stiasny K, Allison SL, Stura EA, Duquerroy S, Lescar J, Heinz FX, Rey FA. Structure of a flavivirus envelope glycoprotein in its low-pH-induced membrane fusion conformation. EMBO J. 2004;23:728–38. doi: 10.1038/sj.emboj.7600064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modis Y, Ogata S, Clements D, Harrison SC. Structure of the dengue virus envelope protein after membrane fusion. Nature. 2004;427:313–9. doi: 10.1038/nature02165. [DOI] [PubMed] [Google Scholar]
- Martina BE, Koraka P, Osterhaus AD. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev. 2009;22:564–81. doi: 10.1128/CMR.00035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Sanchez E, Despres P, Cedillo-Barron L. Innate immune responses to dengue virus. Arch Med Res. 2005;36:425–35. doi: 10.1016/j.arcmed.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Heaton NS, Randall G. Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microbe. 2010;8:422–32. doi: 10.1016/j.chom.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakpoor A, Panyasrivanit M, Wikan N, Smith DR. A role for autophagolysosomes in dengue virus 3 production in HepG2 cells. J Gen Virol. 2009;90(Pt 5):1093–103. doi: 10.1099/vir.0.007914-0. [DOI] [PubMed] [Google Scholar]
- Panyasrivanit M, Khakpoor A, Wikan N, Smith DR. Linking dengue virus entry and translation/replication through amphisomes. Autophagy. 2009;5:434–5. doi: 10.4161/auto.5.3.7925. [DOI] [PubMed] [Google Scholar]
- Mackenzie J. Wrapping things up about virus RNA replication. Traffic. 2005;6:967–77. doi: 10.1111/j.1600-0854.2005.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead SB, Suaya JA, Shepard DS. The burden of dengue infection. Lancet. 2007;369:1410–1. doi: 10.1016/S0140-6736(07)60645-X. [DOI] [PubMed] [Google Scholar]
- Green S, Rothman A. Immunopathological mechanisms in dengue and dengue hemorrhagic fever. Curr Opin Infect Dis. 2006;19:429–36. doi: 10.1097/01.qco.0000244047.31135.fa. [DOI] [PubMed] [Google Scholar]
- Kalayanarooj S, Vaughn DW, Nimmannitya S, et al. Early clinical and laboratory indicators of acute dengue illness. J Infect Dis. 1997;176:313–21. doi: 10.1086/514047. [DOI] [PubMed] [Google Scholar]
- Schnittler HJ, Feldmann H. Viral hemorrhagic fever – a vascular disease? Thromb Haemost. 2003;89:967–72. [PubMed] [Google Scholar]
- Costa VV, Fagundes CT, Souza DG, Teixeira MM. Inflammatory and innate immune responses in dengue infection: protection versus disease induction. Am J Pathol. 2013;182:1950–61. doi: 10.1016/j.ajpath.2013.02.027. [DOI] [PubMed] [Google Scholar]
- Wu SJ, Grouard-Vogel G, Sun W, et al. Human skin Langerhans cells are targets of dengue virus infection. Nat Med. 2000;6:816–20. doi: 10.1038/77553. [DOI] [PubMed] [Google Scholar]
- Navarro-Sanchez E, Altmeyer R, Amara A, Schwartz O, Fieschi F, Virelizier JL, Arenzana-Seisdedos F, Despres P. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 2003;4:723–8. doi: 10.1038/sj.embor.embor866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JL, de Wet BJ, Martinez-Pomares L, Radcliffe CM, Dwek RA, Rudd PM, Gordon S. The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathog. 2008;4:e17. doi: 10.1371/journal.ppat.0040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan MN, Sukumaran B, Pal U, Agaisse H, Murray JL, Hodge TW, Fikrig E. Rab 5 is required for the cellular entry of dengue and West Nile viruses. J Virol. 2007;81:4881–5. doi: 10.1128/JVI.02210-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schaar HM, Rust MJ, Waarts BL, van der Ende-Metselaar H, Kuhn RJ, Wilschut J, Zhuang X, Smit JM. Characterization of the early events in dengue virus cell entry by biochemical assays and single-virus tracking. J Virol. 2007;81:12019–28. doi: 10.1128/JVI.00300-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyde K, Kyle JL, Harris E. Recent advances in deciphering viral and host determinants of dengue virus replication and pathogenesis. J Virol. 2006;80:11418–31. doi: 10.1128/JVI.01257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warke RV, Becerra A, Zawadzka A, et al. Efficient dengue virus (DENV) infection of human muscle satellite cells upregulates type I interferon response genes and differentially modulates MHC I expression on bystander and DENV-infected cells. J Gen Virol. 2008;89(Pt 7):1605–15. doi: 10.1099/vir.0.2008/000968-0. [DOI] [PubMed] [Google Scholar]
- Perry ST, Buck MD, Lada SM, Schindler C, Shresta S. STAT2 mediates innate immunity to Dengue virus in the absence of STAT1 via the type I interferon receptor. PLoS Pathog. 2011;7:e1001297. doi: 10.1371/journal.ppat.1001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer DR, Sun P, Celluzzi C, Bisbing J, Pang S, Sun W, Marovich MA, Burgess T. Differential effects of dengue virus on infected and bystander dendritic cells. J Virol. 2005;79:2432–9. doi: 10.1128/JVI.79.4.2432-2439.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DG. The relationship of interacting immunological components in dengue pathogenesis. Virol J. 2009;6:211. doi: 10.1186/1743-422X-6-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau TN, Hieu NT, Anders KL, et al. Dengue virus infections and maternal antibody decay in a prospective birth cohort study of Vietnamese infants. J Infect Dis. 2009;200:1893–900. doi: 10.1086/648407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangada MM, Rothman AL. Altered cytokine responses of dengue-specific CD4+ T cells to heterologous serotypes. J Immunol. 2005;175:2676–83. doi: 10.4049/jimmunol.175.4.2676. [DOI] [PubMed] [Google Scholar]
- Dong T, Moran E, Vinh Chau N, et al. High pro-inflammatory cytokine secretion and loss of high avidity cross-reactive cytotoxic T-cells during the course of secondary dengue virus infection. PLoS One. 2007;2:e1192. doi: 10.1371/journal.pone.0001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarico LB, Bugna J, Wimmenauer V, et al. T helper type 2 bias and type 17 suppression in primary dengue virus infection in infants and young children. Trans R Soc Trop Med Hyg. 2013;107:411–9. doi: 10.1093/trstmh/trt044. [DOI] [PubMed] [Google Scholar]
- Liu CC, Huang KJ, Lin YS, Yeh TM, Liu HS, Lei HY. Transient CD4/CD8 ratio inversion and aberrant immune activation during dengue virus infection. J Med Virol. 2002;68:241–52. doi: 10.1002/jmv.10198. [DOI] [PubMed] [Google Scholar]
- Mongkolsapaya J, Dejnirattisai W, Xu XN, et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med. 2003;9:921–7. doi: 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- Beaumier CM, Mathew A, Bashyam HS, Rothman AL. Cross-reactive memory CD8+ T cells alter the immune response to heterologous secondary dengue virus infections in mice in a sequence-specific manner. J Infect Dis. 2008;197:608–17. doi: 10.1086/526790. [DOI] [PubMed] [Google Scholar]
- Bozza FA, Cruz OG, Zagne SM, Azeredo EL, Nogueira RM, Assis EF, Bozza PT, Kubelka CF. Multiplex cytokine profile from dengue patients: MIP-1β and IFN-γ as predictive factors for severity. BMC Infect Dis. 2008;8:86. doi: 10.1186/1471-2334-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi UC, Agarwal R, Elbishbishi EA, Mustafa AS. Cytokine cascade in dengue hemorrhagic fever: implications for pathogenesis. FEMS Immunol Med Microbiol. 2000;28:183–8. doi: 10.1111/j.1574-695X.2000.tb01474.x. [DOI] [PubMed] [Google Scholar]
- Halstead SB. In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J Infect Dis. 1979;140:527–33. doi: 10.1093/infdis/140.4.527. [DOI] [PubMed] [Google Scholar]
- Zompi S, Harris E. Animal models of dengue virus infection. Viruses. 2012;4:62–82. doi: 10.3390/v4010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Madoz JR, Bernal-Rubio D, Kaminski D, Boyd K, Fernandez-Sesma A. Dengue virus inhibits the production of type I interferon in primary human dendritic cells. J Virol. 2010;84:4845–50. doi: 10.1128/JVI.02514-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofferson RC, McCracken MK, Johnson AM, Chisenhall DM, Mores CN. Development of a transmission model for dengue virus. Virol J. 2013;10:127. doi: 10.1186/1743-422X-10-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Jordan JL. Subversion of interferon by dengue virus. Curr Top Microbiol Immunol. 2010;338:35–44. doi: 10.1007/978-3-642-02215-9_3. [DOI] [PubMed] [Google Scholar]
- Sabin AB, Schlesinger RW. Production of immunity to dengue with virus modified by propagation in mice. Science. 1945;101:640–2. doi: 10.1126/science.101.2634.640. [DOI] [PubMed] [Google Scholar]
- Sabin AB. Research on dengue during World War II. Am J Trop Med Hyg. 1952;1:30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- Patey O, Ollivaud L, Breuil J, Lafaix C. Unusual neurologic manifestations occurring during dengue fever infection. Am J Trop Med Hyg. 1993;48:793–802. doi: 10.4269/ajtmh.1993.48.793. [DOI] [PubMed] [Google Scholar]
- Shresta S, Sharar KL, Prigozhin DM, Beatty PR, Harris E. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J Virol. 2006;80:10208–17. doi: 10.1128/JVI.00062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AJ, Roehrig JT. New mouse model for dengue virus vaccine testing. J Virol. 1999;73:783–6. doi: 10.1128/jvi.73.1.783-786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DC, Rachid MA, Vilela MC, et al. Intracerebral infection with dengue-3 virus induces meningoencephalitis and behavioral changes that precede lethality in mice. J Neuroinflammation. 2011;8:23. doi: 10.1186/1742-2094-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle JL, Beatty PR, Harris E. Dengue virus infects macrophages and dendritic cells in a mouse model of infection. J Infect Dis. 2007;195:1808–17. doi: 10.1086/518007. [DOI] [PubMed] [Google Scholar]
- Kyle JL, Balsitis SJ, Zhang L, Beatty PR, Harris E. Antibodies play a greater role than immune cells in heterologous protection against secondary dengue virus infection in a mouse model. Virology. 2008;380:296–303. doi: 10.1016/j.virol.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsitis SJ, Williams KL, Lachica R, et al. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathog. 2010;6:e1000790. doi: 10.1371/journal.ppat.1000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger RM, Prestwood TR, Shresta S. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell Host Microbe. 2010;7:128–39. doi: 10.1016/j.chom.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch LE, Shresta S. Mouse models of dengue virus infection and disease. Antiviral Res. 2008;80:87–93. doi: 10.1016/j.antiviral.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Pearson T, Friberg H, Shultz LD, Greiner DL, Rothman AL, Mathew A. Dengue virus infection and virus-specific HLA-A2 restricted immune responses in humanized NOD-scid IL2rγnull mice. PLoS One. 2009;4:e7251. doi: 10.1371/journal.pone.0007251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota J, Rico-Hesse R. Humanized mice show clinical signs of dengue fever according to infecting virus genotype. J Virol. 2009;83:8638–45. doi: 10.1128/JVI.00581-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bente DA, Melkus MW, Garcia JV, Rico-Hesse R. Dengue fever in humanized NOD/SCID mice. J Virol. 2005;79:13797–9. doi: 10.1128/JVI.79.21.13797-13799.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota J, Rico-Hesse R. Dengue virus tropism in humanized mice recapitulates human dengue fever. PLoS One. 2011;6:e20762. doi: 10.1371/journal.pone.0020762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J, Mota J, Sukupolvi-Petty S, Diamond MS, Rico-Hesse R. Mosquito bite delivery of dengue virus enhances immunogenicity and pathogenesis in humanized mice. J Virol. 2012;86:7637–49. doi: 10.1128/JVI.00534-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Hofman FM, Kung JT, Lin YD, Wu-Hsieh BA. Both virus and tumor necrosis factor α are critical for endothelium damage in a mouse model of dengue virus-induced hemorrhage. J Virol. 2007;81:5518–26. doi: 10.1128/JVI.02575-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Lai SY, Sung JM, et al. Lymphocyte activation and hepatic cellular infiltration in immunocompetent mice infected by dengue virus. J Med Virol. 2004;73:419–31. doi: 10.1002/jmv.20108. [DOI] [PubMed] [Google Scholar]
- Paes MV, Pinhao AT, Barreto DF, et al. Liver injury and viremia in mice infected with dengue-2 virus. Virology. 2005;338:236–46. doi: 10.1016/j.virol.2005.04.042. [DOI] [PubMed] [Google Scholar]
- Atrasheuskaya A, Petzelbauer P, Fredeking TM, Ignatyev G. Anti-TNF antibody treatment reduces mortality in experimental dengue virus infection. FEMS Immunol Med Microbiol. 2003;35:33–42. doi: 10.1111/j.1574-695X.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- Paes MV, Lenzi HL, Nogueira AC, et al. Hepatic damage associated with dengue-2 virus replication in liver cells of BALB/c mice. Lab Invest. 2009;89:1140–51. doi: 10.1038/labinvest.2009.83. [DOI] [PubMed] [Google Scholar]
- Assuncao-Miranda I, Amaral FA, Bozza FA, et al. Contribution of macrophage migration inhibitory factor to the pathogenesis of dengue virus infection. FASEB J. 2010;24:218–28. doi: 10.1096/fj.09-139469. [DOI] [PubMed] [Google Scholar]
- Costa VV, Fagundes CT, Valadao DF, et al. A model of DENV-3 infection that recapitulates severe disease and highlights the importance of IFN-γ in host resistance to infection. PLoS Negl Trop Dis. 2012;6:e1663. doi: 10.1371/journal.pntd.0001663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CT, Costa VV, Cisalpino D, et al. IFN-γ production depends on IL-12 and IL-18 combined action and mediates host resistance to dengue virus infection in a nitric oxide-dependent manner. PLoS Negl Trop Dis. 2011;5:e1449. doi: 10.1371/journal.pntd.0001449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guabiraba R, Besnard AG, Marques RE, et al. IL-22 modulates IL-17A production and controls inflammation and tissue damage in experimental dengue infection. Eur J Immunol. 2013;43:1529–44. doi: 10.1002/eji.201243229. [DOI] [PubMed] [Google Scholar]
- Guabiraba R, Marques RE, Besnard AG, Fagundes CT, Souza DG, Ryffel B, Teixeira MM. Role of the chemokine receptors CCR1, CCR2 and CCR4 in the pathogenesis of experimental dengue infection in mice. PLoS One. 2010;5:e15680. doi: 10.1371/journal.pone.0015680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renneson J, Guabiraba R, Maillet I, et al. A detrimental role for invariant natural killer T cells in the pathogenesis of experimental dengue virus infection. Am J Pathol. 2011;179:1872–83. doi: 10.1016/j.ajpath.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza DG, Fagundes CT, Sousa LP, et al. Essential role of platelet-activating factor receptor in the pathogenesis of Dengue virus infection. Proc Natl Acad Sci U S A. 2009;106:14138–43. doi: 10.1073/pnas.0906467106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- Mantovani A. Chemokines. Introduction and overview. Chem Immunol. 1999;72:1–6. [PubMed] [Google Scholar]
- Glass WG, Rosenberg HF, Murphy PM. Chemokine regulation of inflammation during acute viral infection. Curr Opin Allergy Clin Immunol. 2003;3:467–73. doi: 10.1097/00130832-200312000-00008. [DOI] [PubMed] [Google Scholar]
- Melchjorsen J, Sorensen LN, Paludan SR. Expression and function of chemokines during viral infections: from molecular mechanisms to in vivo function. J Leukoc Biol. 2003;74:331–43. doi: 10.1189/jlb.1102577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locati M, Murphy PM. Chemokines and chemokine receptors: biology and clinical relevance in inflammation and AIDS. Annu Rev Med. 1999;50:425–40. doi: 10.1146/annurev.med.50.1.425. [DOI] [PubMed] [Google Scholar]
- Dawson TC, Beck MA, Kuziel WA, Henderson F, Maeda N. Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus. Am J Pathol. 2000;156:1951–9. doi: 10.1016/S0002-9440(10)65068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeberle HA, Kuziel WA, Dieterich HJ, Casola A, Gatalica Z, Garofalo RP. Inducible expression of inflammatory chemokines in respiratory syncytial virus-infected mice: role of MIP-1α in lung pathology. J Virol. 2001;75:878–90. doi: 10.1128/JVI.75.2.878-890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JK, Murphy PM. Chemokine control of West Nile virus infection. Exp Cell Res. 2011;317:569–74. doi: 10.1016/j.yexcr.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass WG, Lim JK, Cholera R, Pletnev AG, Gao JL, Murphy PM. Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J Exp Med. 2005;202:1087–98. doi: 10.1084/jem.20042530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra B, Perez AB, Vogt K, et al. MCP-1 and MIP-1α expression in a model resembling early immune response to dengue. Cytokine. 2010;52:175–83. doi: 10.1016/j.cyto.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Spain-Santana TA, Marglin S, Ennis FA, Rothman AL. MIP-1α and MIP-1β induction by dengue virus. J Med Virol. 2001;65:324–30. doi: 10.1002/jmv.2037. [DOI] [PubMed] [Google Scholar]
- Stamatovic SM, Keep RF, Kunkel SL, Andjelkovic AV. Potential role of MCP-1 in endothelial cell tight junction ‘opening’: signaling via Rho and Rho kinase. J Cell Sci. 2003;116(Pt 22):4615–28. doi: 10.1242/jcs.00755. [DOI] [PubMed] [Google Scholar]
- Lin YL, Liu CC, Chuang JI, Lei HY, Yeh TM, Lin YS, Huang YH, Liu HS. Involvement of oxidative stress, NF-IL-6, and RANTES expression in dengue-2-virus-infected human liver cells. Virology. 2000;276:114–26. doi: 10.1006/viro.2000.0524. [DOI] [PubMed] [Google Scholar]
- Suksanpaisan L, Cabrera-Hernandez A, Smith DR. Infection of human primary hepatocytes with dengue virus serotype 2. J Med Virol. 2007;79:300–7. doi: 10.1002/jmv.20798. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Sasse ME, Liu L, Cardona SM, Mizutani M, Savarin C, Hu T, Ransohoff RM. Scavenging roles of chemokine receptors: chemokine receptor deficiency is associated with increased levels of ligand in circulation and tissues. Blood. 2008;112:256–63. doi: 10.1182/blood-2007-10-118497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YR, Liu MT, Lei HY, et al. MCP-1, a highly expressed chemokine in dengue haemorrhagic fever/dengue shock syndrome patients, may cause permeability change, possibly through reduced tight junctions of vascular endothelium cells. J Gen Virol. 2006;87(Pt 12):3623–30. doi: 10.1099/vir.0.82093-0. [DOI] [PubMed] [Google Scholar]
- Di Stasi A, De Angelis B, Rooney CM, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113:6392–402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traeger T, Kessler W, Assfalg V, et al. Detrimental role of CC chemokine receptor 4 in murine polymicrobial sepsis. Infect Immun. 2008;76:5285–93. doi: 10.1128/IAI.00310-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa A, Hogaboam CM, Lukacs NW, Lincoln PM, Evanoff HL, Kunkel SL. Pivotal role of the CC chemokine, macrophage-derived chemokine, in the innate immune response. J Immunol. 2000;164:5362–8. doi: 10.4049/jimmunol.164.10.5362. [DOI] [PubMed] [Google Scholar]
- Kim CH, Johnston B, Butcher EC. Trafficking machinery of NKT cells: shared and differential chemokine receptor expression among Vα24+Vβ11+ NKT cell subsets with distinct cytokine-producing capacity. Blood. 2002;100:11–6. doi: 10.1182/blood-2001-12-0196. [DOI] [PubMed] [Google Scholar]
- Semmling V, Lukacs-Kornek V, Thaiss CA, et al. Alternative cross-priming through CCL17-CCR4-mediated attraction of CTLs toward NKT cell-licensed DCs. Nat Immunol. 2010;11:313–20. doi: 10.1038/ni.1848. [DOI] [PubMed] [Google Scholar]
- Meyer EH, Wurbel MA, Staton TL, et al. iNKT cells require CCR4 to localize to the airways and to induce airway hyperreactivity. J Immunol. 2007;179:4661–71. doi: 10.4049/jimmunol.179.7.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Van Kaer L, Joyce S. Innate immunity: NKT cells in the spotlight. Curr Biol. 2005;15:R429–31. doi: 10.1016/j.cub.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Juno JA, Keynan Y, Fowke KR. Invariant NKT cells: regulation and function during viral infection. PLoS Pathog. 2012;8:e1002838. doi: 10.1371/journal.ppat.1002838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NR, Garg S, Brenner MB. Antigen presentation by CD1 lipids, T cells, and NKT cells in microbial immunity. Adv Immunol. 2009;102:1–94. doi: 10.1016/S0065-2776(09)01201-2. [DOI] [PubMed] [Google Scholar]
- Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol. 2007;5:405–17. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009;9:28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr Opin Immunol. 2008;20:358–68. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajuebor MN. Role of NKT cells in the digestive system. I. Invariant NKT cells and liver diseases: is there strength in numbers? Am J Physiol Gastrointest Liver Physiol. 2007;293:G651–6. doi: 10.1152/ajpgi.00298.2007. [DOI] [PubMed] [Google Scholar]
- Durante-Mangoni E, Wang R, Shaulov A, He Q, Nasser I, Afdhal N, Koziel MJ, Exley MA. Hepatic CD1d expression in hepatitis C virus infection and recognition by resident proinflammatory CD1d-reactive T cells. J Immunol. 2004;173:2159–66. doi: 10.4049/jimmunol.173.3.2159. [DOI] [PubMed] [Google Scholar]
- Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest. 2007;117:2302–12. doi: 10.1172/JCI31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John AL, Rathore AP, Yap H, Ng ML, Metcalfe DD, Vasudevan SG, Abraham SN. Immune surveillance by mast cells during dengue infection promotes natural killer (NK) and NKT-cell recruitment and viral clearance. Proc Natl Acad Sci U S A. 2011;108:9190–5. doi: 10.1073/pnas.1105079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltman AM, Ter Borg MJ, Binda RS, et al. α-galactosylceramide in chronic hepatitis B infection: results from a randomized placebo-controlled Phase I/II trial. Antivir Ther. 2009;14:809–18. doi: 10.3851/IMP1295. [DOI] [PubMed] [Google Scholar]
- Wondimu Z, Santodomingo-Garzon T, Le T, Swain MG. Protective role of interleukin-17 in murine NKT cell-driven acute experimental hepatitis. Am J Pathol. 2010;177:2334–46. doi: 10.2353/ajpath.2010.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamau E, Takhampunya R, Li T, Kelly E, Peachman KK, Lynch JA, Sun P, Palmer DR. Dengue virus infection promotes translocation of high mobility group box 1 protein from the nucleus to the cytosol in dendritic cells, upregulates cytokine production and modulates virus replication. J Gen Virol. 2009;90(Pt 8):1827–35. doi: 10.1099/vir.0.009027-0. [DOI] [PubMed] [Google Scholar]
- Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci U S A. 2000;97:5498–503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metelitsa LS, Wu HW, Wang H, et al. Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. J Exp Med. 2004;199:1213–21. doi: 10.1084/jem.20031462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranski P, Restifo NP. Essentials of Th17 cell commitment and plasticity. Blood. 2013;121:2402–14. doi: 10.1182/blood-2012-09-378653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyerich S, Eyerich K, Cavani A, Schmidt-Weber C. IL-17 and IL-22: siblings, not twins. Trends Immunol. 2010;31:354–61. doi: 10.1016/j.it.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med. 2010;207:1293–305. doi: 10.1084/jem.20092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH17, TH1 and TH2 cells. Nat Immunol. 2009;10:864–71. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- Eyerich S, Eyerich K, Pennino D, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–85. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–90. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- Zenewicz LA, Flavell RA. IL-22 and inflammation: leukin’ through a glass onion. Eur J Immunol. 2008;38:3265–8. doi: 10.1002/eji.200838655. [DOI] [PubMed] [Google Scholar]
- Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–54. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Cella M, Fuchs A, Vermi W, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–5. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M, Murakawa M, Kadoshima-Yamaoka K, Tanaka Y, Nagahira K, Fukuda Y, Nishimura T. Murine NKT cells produce Th17 cytokine interleukin-22. Cell Immunol. 2009;254:81–4. doi: 10.1016/j.cellimm.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Crowe CR, Chen K, Pociask DA, et al. Critical role of IL-17RA in immunopathology of influenza infection. J Immunol. 2009;183:5301–10. doi: 10.4049/jimmunol.0900995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Topham DJ. Interleukin-22 (IL-22) production by pulmonary Natural Killer cells and the potential role of IL-22 during primary influenza virus infection. J Virol. 2010;84:7750–9. doi: 10.1128/JVI.00187-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Cobleigh MA, Lian JQ, Huang CX, Booth CJ, Bai XF, Robek MD. A proinflammatory role for interleukin-22 in the immune response to hepatitis B virus. Gastroenterology. 2011;141:1897–906. doi: 10.1053/j.gastro.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambacher J, Beigel F, Zitzmann K, Heeg MH, Goke B, Diepolder HM, Auernhammer CJ, Brand S. The role of interleukin-22 in hepatitis C virus infection. Cytokine. 2008;41:209–16. doi: 10.1016/j.cyto.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Cobleigh MA, Robek MD. Protective and pathological properties of IL-22 in liver disease: implications for viral hepatitis. Am J Pathol. 2013;182:21–8. doi: 10.1016/j.ajpath.2012.08.043. [DOI] [PubMed] [Google Scholar]
- Couvelard A, Marianneau P, Bedel C, Drouet MT, Vachon F, Henin D, Deubel V. Report of a fatal case of dengue infection with hepatitis: demonstration of dengue antigens in hepatocytes and liver apoptosis. Hum Pathol. 1999;30:1106–10. doi: 10.1016/s0046-8177(99)90230-7. [DOI] [PubMed] [Google Scholar]
- Jain A, Pandey N, Garg RK, Kumar R. IL-17 level in patients with Dengue virus infection and its association with severity of illness. J Clin Immunol. 2013;33:613–8. doi: 10.1007/s10875-012-9855-0. [DOI] [PubMed] [Google Scholar]
- Becquart P, Wauquier N, Nkoghe D, Ndjoyi-Mbiguino A, Padilla C, Souris M, Leroy EM. Acute dengue virus 2 infection in Gabonese patients is associated with an early innate immune response, including strong interferon α production. BMC Infect Dis. 2010;10:356. doi: 10.1186/1471-2334-10-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavige GN, Huang LC, Salimi M, Gomes L, Jayaratne SD, Ogg GS. Cellular and cytokine correlates of severe dengue infection. PLoS One. 2012;7:e50387. doi: 10.1371/journal.pone.0050387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vδ1 + γδ T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. 2007;178:4466–72. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- Hamada S, Umemura M, Shiono T, et al. IL-17A produced by γδ T cells plays a critical role in innate immunity against Listeria monocytogenes infection in the liver. J Immunol. 2008;181:3456–63. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31:15–23. doi: 10.1016/j.immuni.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Besnard AG, Sabat R, Dumoutier L, et al. Dual role of IL-22 in allergic airway inflammation and its cross-talk with IL-17A. Am J Respir Crit Care Med. 2011;183:1153–63. doi: 10.1164/rccm.201008-1383OC. [DOI] [PubMed] [Google Scholar]
- Hottz ED, Lopes JF, Freitas C, et al. Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation. Blood. 2013 doi: 10.1182/blood-2013-05-504449. doi: 10.1182/blood-2013-05-504449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SJ, Hayes CG, Dubois DR, Windheuser MG, Kang YH, Watts DM, Sieckmann DG. Evaluation of the severe combined immunodeficient (SCID) mouse as an animal model for dengue viral infection. Am J Trop Med Hyg. 1995;52:468–76. doi: 10.4269/ajtmh.1995.52.468. [DOI] [PubMed] [Google Scholar]
- Lin YL, Liao CL, Chen LK, et al. Study of dengue virus infection in SCID mice engrafted with human K562 cells. J Virol. 1998;72:9729–37. doi: 10.1128/jvi.72.12.9729-9737.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J, Kimura-Kuroda J, Hirabayashi Y, Yasui K. Development of a novel mouse model for dengue virus infection. Virology. 1999;263:70–7. doi: 10.1006/viro.1999.9887. [DOI] [PubMed] [Google Scholar]
- Blaney JE, Jr, Johnson DH, Manipon GG, Firestone CY, Hanson CT, Murphy BR, Whitehead SS. Genetic basis of attenuation of dengue virus type 4 small plaque mutants with restricted replication in suckling mice and in SCID mice transplanted with human liver cells. Virology. 2002;300:125–39. doi: 10.1006/viro.2002.1528. [DOI] [PubMed] [Google Scholar]
- Kuruvilla JG, Troyer RM, Devi S, Akkina R. Dengue virus infection and immune response in humanized RAG2–/–γc–/– (RAG-hu) mice. Virology. 2007;369:143–52. doi: 10.1016/j.virol.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Shresta S, Kyle JL, Robert Beatty P, Harris E. Early activation of natural killer and B cells in response to primary dengue virus infection in A/J mice. Virology. 2004;319:262–73. doi: 10.1016/j.virol.2003.09.048. [DOI] [PubMed] [Google Scholar]
- Huang KJ, Li SY, Chen SC, Liu HS, Lin YS, Yeh TM, Liu CC, Lei HY. Manifestation of thrombocytopenia in dengue-2-virus-infected mice. J Gen Virol. 2000;81(Pt 9):2177–82. doi: 10.1099/0022-1317-81-9-2177. [DOI] [PubMed] [Google Scholar]
- Chen ST, Lin YL, Huang MT, et al. CLEC5A is critical for dengue-virus-induced lethal disease. Nature. 2008;453:672–6. doi: 10.1038/nature07013. [DOI] [PubMed] [Google Scholar]
- Fagundes CT, Costa VV, Cisalpino D, Souza DG, Teixeira MM. Therapeutic opportunities in dengue infection. Drug Dev Res. 2011;72:480–500. [Google Scholar]
- Tolfvenstam T, Lindblom A, Schreiber MJ, Ling L, Chow A, Ooi EE, Hibberd ML. Characterization of early host responses in adults with dengue disease. BMC Infect Dis. 2011;11:209. doi: 10.1186/1471-2334-11-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Wang SY. Activation of terminally differentiated human monocytes/macrophages by dengue virus: productive infection, hierarchical production of innate cytokines and chemokines, and the synergistic effect of lipopolysaccharide. J Virol. 2002;76:9877–87. doi: 10.1128/JVI.76.19.9877-9887.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CA, Anderson R, Marshall JS. Dengue virus selectively induces human mast cell chemokine production. J Virol. 2002;76:8408–19. doi: 10.1128/JVI.76.16.8408-8419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashyam HS, Green S, Rothman AL. Dengue virus-reactive CD8+ T cells display quantitative and qualitative differences in their response to variant epitopes of heterologous viral serotypes. J Immunol. 2006;176:2817–24. doi: 10.4049/jimmunol.176.5.2817. [DOI] [PubMed] [Google Scholar]
- Avirutnan P, Malasit P, Seliger B, Bhakdi S, Husmann M. Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J Immunol. 1998;161:6338–46. [PubMed] [Google Scholar]
- Brown MG, King CA, Sherren C, Marshall JS, Anderson R. A dominant role for FcγRII in antibody-enhanced dengue virus infection of human mast cells and associated CCL5 release. J Leukoc Biol. 2006;80:1242–50. doi: 10.1189/jlb.0805441. [DOI] [PubMed] [Google Scholar]
- Conceicao TM, El-Bacha T, Villas-Boas CS, Coello G, Ramirez J, Montero-Lomeli M, Da Poian AT. Gene expression analysis during dengue virus infection in HepG2 cells reveals virus control of innate immune response. J Infect. 2010;60:65–75. doi: 10.1016/j.jinf.2009.10.003. [DOI] [PubMed] [Google Scholar]
- de-Oliveira-Pinto LM, Marinho CF, Povoa TF, et al. Regulation of inflammatory chemokine receptors on blood T cells associated to the circulating versus liver chemokines in dengue fever. PLoS One. 2012;7:e38527. doi: 10.1371/journal.pone.0038527. [DOI] [PMC free article] [PubMed] [Google Scholar]