Abstract
Anti-citrullinated peptide/protein antibodies (ACPAs) are highly sensitive and specific markers of rheumatoid arthritis (RA). Identification of peptide epitopes that may detect different subgroups of RA patients might have diagnostic and prognostic significance. We have investigated citrulline- and arginine-containing peptide pairs derived from filaggrin, collagen or vimentin, and compared this citrulline-peptide panel with the serological assays conventionally used to detect ACPAs. Furthermore, we studied if the same citrulline-peptides identify antibody-secreting cells in in vitro cultures of RA B cells. Recognition of citrulline- and arginine-containing filaggrin, vimentin and collagen peptide epitopes were tested by Multipin ELISA system, by indirect ELISA and by a peptide-specific microarray. B cells were purified from blood by negative selection; antibody-producing cells were enumerated by ELISPOT assay. The panel composed of citrulline-peptide epitopes of filaggrin, collagen and vimentin was recognized by RA sera with a sensitivity and specificity comparable with the currently used tests. Moreover, the combined citrulline-peptide panel including the new short epitope peptide of filaggrin, fil311-315, also identified nearly one-third of RA cases that were negative for antibodies against cyclic citrullinated peptides, mutated citrullinated vimentin or for rheumatoid factor. The results with the peptide-specific microarray have shown that although most ACPAs recognizing the four citrulline peptides are IgG, some of them specifically recognizing citrulline-containing filaggrin peptides (fil311–315 and fil306–326) are IgM, and so may be produced either by newly formed activated B cells or by unswitched B memory cells. Furthermore, the citrulline-peptides of filaggrin and vimentin detect ACPA-producing cells, and so could also be applied to study the B cells of RA patients.
Keywords: anti-citrullinated peptide antibodies, B cell, citrulline-peptide, diagnosis, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease affecting about 0·5–1% of the world’s population. It is distinguished by chronic inflammation of synovial joints, which often results in progressive joint destruction and disability. Several autoantigens have been identified in RA, including collagen, filaggrin, vimentin and fibrinogen.1,2 The heterogeneity of disease manifestations, clinical course and treatment responses suggests that subtypes of RA exist. The presence or absence of antibodies to citrullinated protein/peptide antigens (ACPAs) has been suggested to be an informative biomarker for subdivision of RA.4 ACPAs are considered as highly specific markers of RA (94–99%), but only about 70% of RA patients appear to be ACPA-positive. ACPAs recognize proteins or peptides with arginine residues converted to citrulline by the enzyme peptidylarginine deiminase.1–5 The high RA specificity of ACPAs appears to be a result of an abnormal antibody response to citrullinated proteins, and most probably depends on the patient’s genetic background and environmental risk factors6–7 Citrullinated proteins and ACPAs may form immune complexes in vivo that may have an important pro-inflammatory role in the pathogenesis of RA. In addition, a direct link between the ACPA and osteoclast differentiation was recently described.9
Citrullinated proteins that may trigger ACPA production could be used as diagnostic tools for RA.10–11 Filaggrin extracted from human epidermis12 and in vitro citrullinated recombinant filaggrin13 were primarily used as antigens in ELISA, but these proteins did not provide adequate standardization because of their structural heterogeneity. Schellekens et al. designed a synthetic cyclic citrullinated peptide (CCP) based on a sequence from filaggrin, and used it as a new antigenic substrate in anti-CCP1 ELISA to detect ACPAs.14
Vimentin is a member of the intermediate filament family of proteins, and is present in the joint both intra- and extracellularly. Vimentin is a target of various post-translational modifications including citrullination. Tumour necrosis factor α (TNF-α) induces the secretion of citrullinated vimentin from activated macrophages in the synovium.3–15 Citrullinated vimentin appears to be one of the synovial deiminated autoantigens generated during apoptosis.16 The anti-mutated citrullinated vimentin (anti-MCV) ELISA has been developed to improve detection of the antibody against citrullinated vimentin.17
Antibodies against collagen type II (CII) are present in about 30% of RA patients and they all have HLA-DR4.18 Further, anti-CII-producing B cells were shown to be present in the joints of the majority of these patients.19 However, it is not clear whether the occurrence of anti-collagen autoantibodies plays a role in the pathogenesis of RA. In contrast to fibrinogen, α-enolase and vimentin, reactivity to CII apparently is not restricted to the citrullinated form.2–11 An evolutionary conserved, triple helical conformational epitope of CII (between 359 and 369 amino acids) was found be recognized by antibodies of RA patients’ sera as well as the pathogenic antibodies in the murine model, collagen-induced arthritis.20
Our aim was to develop new molecular tools for the detection of ACPA in sera and to investigate ACPA-producing B cells of RA patients. We have synthesized peptide pairs corresponding to citrulline/arginine-containing short sequences derived from filaggrin (fil306–326 and fil311–315), vimentin (vim65–77) and CII (coll359–369) and compared the reactivity of RA and healthy sera samples by using ELISA and peptide microarray methods. We have found that this citrulline-peptide panel identifies approximately one-third of RA sera that were ACPA negative by the conventional diagnostic tests, furthermore, filaggrin and vimentin peptides can be applied to detect ACPA-producing B cells.
Materials and methods
Patients
For this cross-sectional study, sera samples were collected from 263 RA patients with established disease, 46 CCP-negative, non-RA patients with other autoimmune diseases, 18 patients with systemic lupus erythematosus and 152 age-matched healthy controls. Sera from the healthy group were obtained from the 3rd Department of Medicine Semmelweis University, the samples were kindly provided by Dr Zoltán Prohászka.
The diagnosis of the disease was established on the basis of the revised ACR/EULAR classification criteria.21 Blood samples were taken with ethical permission and after the patients had signed a written consent. The baseline data of RA patients were as follows: 32 men/176 women; age: 58·4 ± 14·3 years; rheumatoid factor (RF) +/–: 127/30; CCP2 +/–: 157/27; MCV +/–: 164/25; disease duration: 9·8 ± 9·4 years.
Antibodies
Goat anti-human IgG + IgM (H+L) horseradish peroxidase conjugate was from Jackson Immuno Research Europe Ltd (Suffolk, UK). Rabbit anti-human IgG horseradish peroxidase conjugate was purchased from Dako Agilent Technologies Company (Glostrup, Denmark).
Peptide synthesis
First, we used conventional solid-phase peptide synthesis, carried out on Multipin NCP non-cleavable kit (Mimotopes Pty Ltd, Clayton, Australia). Citrulline-containing epitope peptides of filaggrin 306SHQESTXGXSXGRSGRSGS326, 311TXGRS315, 311TXGXS315 and 311TRGXS315; vimentin 65SAVRAXSSVPGVR77; and collagen 359AXGLTGXPGDA369 (where X stands for citrulline), as well as the respective unmodified counterparts, were synthesized on the pins.22 The N-terminus of peptides was acetylated. Selected peptides were also synthesized manually by solid-phase peptide synthesis, according to Fmoc/tBu strategy.
The C-terminally biotinylated forms were made using biotinyl-6-amino-hexanoic acid (LC-biotin) and 4,7,10-trioxa-1,13-tridecanediamino succinic acid linker (Ttds) and biotin.23 Lysine was built into the C-terminus of the peptide to conjugate the biotin derivatives to its side chain (ε-amino group). The crude products were purified by HPLC. The structure of the peptides was proved by ESI mass spectrometry. The list of synthesized peptides is shown in Table 1.
Table 1.
Summary of specificity and sensitivity values obtained with biotin conjugated peptide epitopes1
| Peptides | Per definition | Optimalized | ||||||
|---|---|---|---|---|---|---|---|---|
| Specificity (%) | Sensitivity (%) | Specificity (%) | Sensitivity (%) | |||||
| Filaggrin306–326 SHQESTXGXSXGRSGRSGS 19mer C-terminal LC- biotin | 97 | 56 | 95 | 62 | ||||
| Filaggrin311–315 TXGRS C-terminal LC- biotin | 98 | 36 | 95 | 40 | ||||
| Filaggrin311–315 TXGRS C-terminal Ttds-biotin | 98 | 58 | 95 | 60 | ||||
| Vimentin65–77 SAVRAXSSVPGVR C-terminal LC-biotin | 96 | 39 | 95 | 44 | ||||
| Collagen359–369 AXGLTGXPGDA C-terminal LC-biotin | 98 | 38 | 95 | 41 | ||||
Sensitivity: percentages of rheumatoid arthritis (RA) patients giving an ELISA ratio higher than the cut-off value, compared with the total number of patients; specificity: percentages of healthy samples having an ELISA ratio below cut-off compared with total number of samples.
CCP2 assay
Sera of patients were tested for anti-CCP positivity using an Immunoscan CCPlus® kit (Euro-Diagnostica, Malmö, Sweden)14 according to the manufacturer’s instructions. Samples with results < 25 U/ml are defined as negative; samples with results ≥ 25 U/ml are defined as positive.
MCV assay
Autoantibodies recognizing mutated citrullinated vimentin were measured using the Orgentec anti-MCV ELISA kit (ORGENTEC Diagnostika GmbH, Mainz, Germany.). Cut-off value was 20 U/ml.
Multipin ELISA
To detect peptide-specific antibodies, pins were blocked (2% BSA, 0,1% Tween in PBS) for an hour. Serum samples were diluted 1 : 1000 in the same buffer and pins were incubated in the diluted sera overnight, rinsed, then incubated with goat anti-human IgG + IgM conjugated to horseradish peroxidase for an hour, rinsed again, and the signal was developed by tetramethyl benzidine. Absorbance was measured at 450 nm. ELISA ratios were calculated [optical density (OD) with citrulline-containing/OD with arginine-containing peptide] and compared between groups. We have used ELISA ratios because these values are not influenced by the individual variations between plates prepared at different time-points. In the case of arginine-peptides the range of OD values varied from 0·02 (minimum value) to 1·3 (maximum value), whereas in the case of citrulline-peptides these were between 0·02 and 2·9.
ELISA
Biotinylated peptides (1 μg/ml in PBS) were bound to a NeutrAvidin (5 μg/ml in PBS) pre-coated plate. Plates were blocked (150 mm NaCl, 2% BSA in PBS), then sera samples were added (1 : 100). After overnight incubation and rinsing the plate, rabbit anti-human IgG conjugated to horseradish peroxidase was added for 1 hr, and the signal was developed as above. The ELISA reaction was stopped in its linear phase and ELISA ratio was calculated as described above. Cut-off values for each peptide pair were calculated from ELISA ratios of 138 healthy samples (the means of ELISA ratios + 2*SD). These were for fil311–315: 1·352, for coll359–369: 1·377, for fil306–326: 1·253, and for vim65–77 peptide: 1·6. All sera that have shown a higher ELISA ratio than these values were considered to be positive for the given peptide.
Microarray production and measurements
Biotinylated citrulline and arginine-containing peptides were mixed with Neutravidin (Thermo Scientific, Rockford, IL) at different ratios that were initially set between 2 : 1 and 8 : 1 for each peptide to minimize the presence of unbound peptides or empty neutravidin-binding sites. Complexes were printed in triplicates onto 16-pad nitrocellulose-covered FAST Slides (Whatman) using the BioOdyssey Calligrapher miniarrayer (BioRad, Hercules, CA). Arrays were developed as described previously.24 Briefly, arrays were treated with sera diluted 1 : 100, washed and then reacted with anti-human IgG-DyL649 (Jackson, diluted 1 : 2500) and anti-human IgM-Cy3 (Jackson, diluted 1 : 3500). Microarrays were scanned with the Axon GenePix 4300A (Molecular Devices, Sunnyvale, CA) equipped with GenePix Pro 7·0. Fluorescence intensity was calculated for each spot as the median fluorescence of the feature minus the fluorescence of the local background.
B-cell purification and ELISPOT
B cells were isolated from the blood of healthy donors and RA patients by negative selection by using RosetteSep antibody cocktail (Stem Cell Techologies, Vancouver, BC, Canada), according to the manufacturer’s instructions. The purity of the resulting B-cell population was 80–85%. Between 2 × 106 and 5 × 106 cells/ml were cultured in RPMI-1640 containing 10% fetal calf serum in the presence of 10 ng/ml recombinant human interleukin-2 and 1 μg/ml R848 polyclonal activator provided with the ELISpot kit (Mabtech, Stockholm, Sweden). The cells were harvested at the 3rd day, and transferred into the wells of ELISpot plates pre-coated with neutravidin and the biotinylated citrulline- or arginine-containing peptides, and with anti-IgG, respectively. The spots were developed after 18 hr. The frequency of total and peptide-specific IgG-producing cells was determined using a C.T.L. Immunospot analyzer (CTL-Europe Gmbh, Bonn, Germany).
Statistics
The ELISA ratio indicates the strength of the sample OD compared with that of a negative control on the same ELISA plate (OD citrulline/OD arginine control). To analyse the results, ELISA ratios were compared between various groups using Kruskal–Wallis test. Cut-off values were obtained from ELISA ratios of 138 healthy samples (mean + 2 SD). To compare the peptides data with clinical parameters, Spearman correlation was used. Dunn’s multiple comparison test was used to compare antibody levels between groups. We used GraphPad Prism 4 software for statistical analysis (GraphPad Software, La Jolla, CA). In all tests the P-values < 0·05 were considered significant.
Results
Comparison of citrulline-peptide epitopes of filaggrin, collagen or vimentin by Multipin ELISA
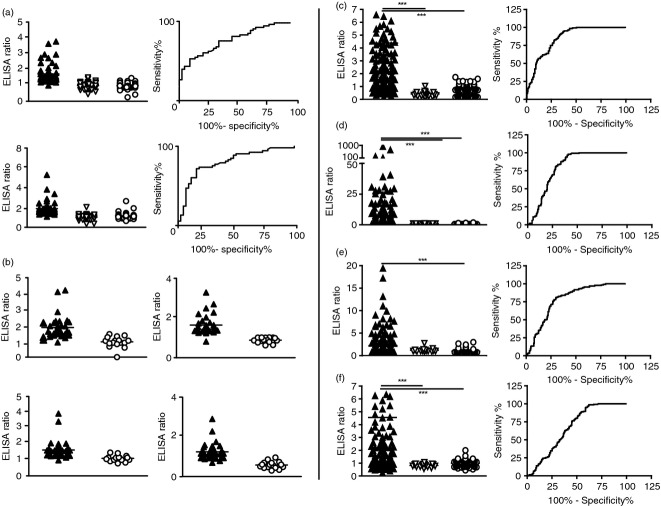
To find the optimal combination of peptide epitopes detecting most RA patients, we compared the reactivity of sera samples against various epitopes of filaggrin, vimentin and collagen. Based on an arginine-rich sequence of human filaggrin14 a set of filaggrin peptides, fil306–326, containing three citrulline residues (306SHQESTXGXSXGRSGRSGS326, X stands for citrulline), its partial sequence fil311–315 (311TXGRS315), and the respective arginine counterparts, were synthesized using a parallel solid phase strategy. In the first set of experiments the recognition of these pin-attached oligopeptides by sera of RA patients (n = 53), patients with other autoimmune diseases, (n = 46) and healthy volunteers (n = 45) was investigated. There was a significant difference in the recognition of both the fil306–326 and the fil311–315 epitopes (TXGRS), when RA, CCP-negative non-RA patients and healthy sera samples were compared. RA sera recognized the fil306–326 peptide with 42% sensitivity at 100% specificity, and the fil311–315 peptide with 72% sensitivity at 82% specificity; that significantly differed (P < 0.001) from the CCP-negative non-RA patients and the healthy group (Fig. 1a).
Figure 1.
Recognition of citrulline-containing peptide (CCP) epitopes by Anti-citrullinated peptide/protein antibodies (ACPA) as detected by multipin and conventional ELISA. (a), (b) Multipin ELISA. (a) Reactivity of 53 sera from rheumatoid arthritis (RA) patients (black triangle), 46 sera from CCP-negative non-RA patients patients (white triangle) and 45 sera from healthy volunteers (circle) with the fil306–326 (upper panel) and the fil 311TXGRS315 (lower panel) epitopes synthethized on the pin. The calculated area under the curve of ROC (AUC) was 0·7748 for the fil306–326, and 0·8034 for the 5-mer peptide, respectively. (b) Comparison of RA (n = 33) and healthy (n = 16) sera’s reactivity against the fil306–326 epitope (upper left), the double citrulline-containing fil311–315 (upper right), vim65–77 (lower left) and coll359–369 (lower right). Differences between RA and healthy groups were significant, P < 0·0002, P < 0·0001, P < 0·0258, and P < 0·001, respectively. (c–f) Conventional ELISA. Reactivities of RA (n = 198), CCP-negative systemic lupus erythematosus patients (n = 18) or healthy (n = 138) sera samples with: (c) C-terminally biotinylated fil306–326, (d) fil 311TXGRS315 with Ttds linker, (e) vim65–77, and (f) coll359–369 peptides bound to neutravidin pre-coated plates. The sensitivity values were 62%, 60%, 44% and 41%, respectively. ROC curve analysis has shown AUC values 0·7991 for fil306–326, 0·7593 for fil311–315-Ttds-biotin, 0·787 for vim65–77, and 0·6258 for coll359–369.
In the next set of pins we compared the fil306–326 with the fil311–315 peptides containing two citrulline residues (TXGXS) and with the one containing citrulline residue only at position 314 (TRGXS). We also included in this set of experiments previously identified epitopes of collagen (coll359–369, AXGLTGXPGDA) and vimentin (vim65–77, SAVRAXSSVPGVR) together with the corresponding arginine-containing counterparts (Fig. 1b). A significantly higher number of RA sera recognized the citrulline-containing peptides, compared with the healthy controls.
Validation of the multipin ELISA results using conventional ELISA
Selected pin-bound peptides were tested also by conventional ELISA. As the peptides were attached to the pin at their C-terminal amino acid residue, we supposed that biotinylation of a pin-free peptide at the C-terminal end will not influence markedly the antibody binding, hence epitope recognition. Therefore, citrulline- as well as arginine-containing peptides (fil311–315 TXGRS and the fil306–326), and also the vim65–77 and coll359–369 peptides were produced with biotin at the C-terminal end, using spacers with different length (biotinhexanoyl- or Ttds-biotin derivatives), then tested in solution by conventional ELISA on neutravidin-coated plates (Fig. 1c–f). We have compared the reactivity of sera from 198 RA patients, 18 CCP-negative systemic lupus erythematosus patients and 138 normal healthy donors on the peptide panel. We could verify the earlier findings obtained with the multipin ELISA system and determined the specificity and sensitivity values of the assays. At a 95% specificity level RA sera recognized the peptides fil311–315 TXGRS-K-(Ttds-biotin), TXGRS-K-(LC-biotin) and fil306–326 SHQESTXGXSXGRSGRSGS-K-(LC-biotin) with 60%, 40% and 62% sensitivity, while the citrulline-containing vimentin and collagen epitopes were recognized with 44% and 41% sensitivity, respectively. Table 1 summarizes the sensitivity and specificity values of the test.
When the sensitivity of the assay was calculated from the cumulated data derived from the four individual peptides: the fil306–326 with three citrulline residues, the five-mer filaggrin peptide TXGRS-K(Ttds-biotin), the vim65-77 and coll359-369 epitope peptides, the sensitivity of the assay was 81%, comparable with the conventional anti-CCP2 test.
Investigation of cross-reactivity of anti-citrullinated peptide antibodies by competitive ELISA
Next we tested whether the fil311–315 (TXGRS-K(Ttds-biotin)) and the citrulline-containing vimentin peptide could compete for ACPAs. When the filaggrin peptide was present in the assay in soluble form, it significantly reduced binding of serum antibodies to the same peptide coat, whereas the recognition of the vimentin peptide remained the same. Vice versa, the presence of vimentin peptide in solution did not inhibit the binding of ACPA to the fil311–315, but significantly reduced the recognition of the vimentin peptide coat (Fig. 2). We have seen a 20–80% reduction in all individual cases, when peptide pre-treated and untreated samples were compared using the coat-identical peptides for competition.
Figure 2.
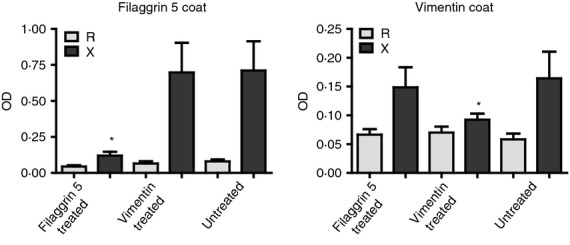
Filaggrin and vimentin peptide-specific anti-citrullinated peptide/protein antibodies (ACPAs) are not cross-reactive. Cross-reactivity between sera that recognized both vim65–77 and the fil311–315 epitopes was tested by ELISA. OD values obtained on citrulline (X) versus arginine (R) -containing peptide coats are compared. Sera were pre-incubated with soluble fil311–315 or with vim65–77 epitopes or left untreated, and tested on vim65–77 and fil311–315 peptide-coated plates, respectively. Mean values of eight patients are shown.
Association between the recognition of citrulline-peptide panel and the clinical data
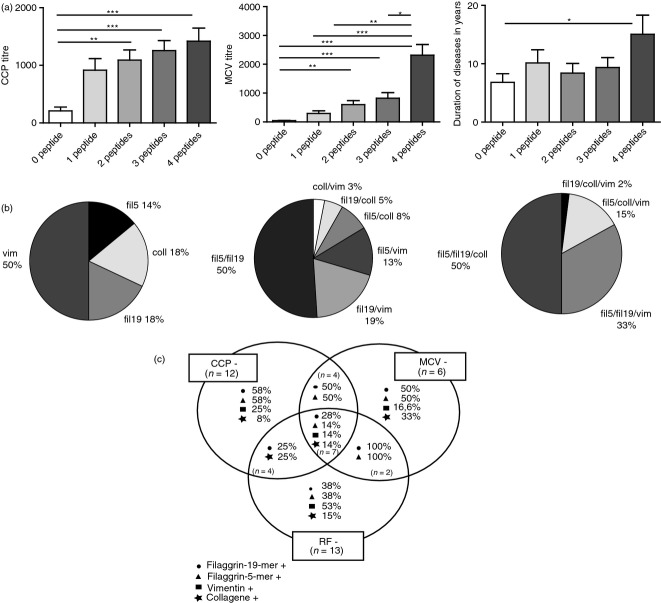
To test whether the recognition of these citrulline epitopes by RA sera show any association with the anti-CCP and anti-MCV titres obtained with commercially available tests, the titres were compared with the number of peptides specifically recognized by any individual sera. The number of recognized epitopes significantly increased with the titres, RA sera reacting with all four citrulline-peptides had the highest anti-CCP and anti-MCV titres (Fig. 3a). However, we did not find an association between the number of peptide epitopes recognized and the CRP values or the DAS28 index of the patients (data not shown). Furthermore, we have seen an association trend between duration of disease and number of peptides reacted with individual sera; the duration of the disease was significantly longer (P < 0·05) in the group recognizing four citrulline peptides compared with the group recognizing none (Fig. 3a).
Figure 3.
Association between citrulline-containing peptide 2 (CCP2) or mutated citrullinated vimentin (MCV) titres of rheumatoid arthritis (RA) sera and the number of recognized citrulline-peptides; the distribution of peptide specificities. RA sera recognizing more citrulline-containing peptides had significantly higher anti-CCP2 (Fig. 3a, left) and anti-MCV titres (Fig. 3a, middle) compared with sera recognizing less peptide. Duration of the disease was significantly longer (P < 0·05) in the group recognizing four citrulline peptides than in the group recognizing none (Fig. 3a, right). Distribution of fine specificities within subsets of RA sera that recognize one, two or three citrulline-containing peptides (Fig. 3b). Percentages of single/double/triple-negative RA sera showing positive reaction with the individual peptides of the peptide panel. The absolute number of CCP, MCV and rheumatoid factor (RF) single-negative patients were 12, 6 and 13, respectively, from those patients nine, five and nine sera were identified as anti-citrullinated peptide/protein antibody (ACPA) positive by the peptide panel (Fig. 3c).
When individual citrulline peptides were tested with RA sera, we calculated the Spearman correlation between the strength of binding (ELISA ratio) and the anti-CCP2 and anti-MCV titres. The recognition of filaggrin or collagen epitopes and the anti-CCP2 titre has shown a weak but significant correlation (r = 0·34, P < 0·0001), whereas a stronger and significant correlation (r = 0·45, P < 0·0001) between peptide recognition and the MCV titres was observed.
The distribution of fine specificities of sera recognizing one, two or three peptides was analysed on a group of 184 diagnosed RA patients, and the results are shown on Fig. 3(b). Within the first group recognizing only one peptide 50% of sera reacted with the vimentin epitope, 18% bound to fil306–326 and 14% to fil311–315 peptides, while 18% bound to the collagen epitope. In the group recognizing two peptides half of the RA sera reacted with the two filaggrin epitopes, while among the sera binding to three peptides half of them reacted with the two filaggrins and the collagen epitope. Nineteen per cent of RA patients’ sera did not recognize any of the citrulline peptides.
Most importantly, comparing sera reactivities by Venn diagrams made it clear that about one-third of CCP-, MCV- and RF-negative RA sera (triple negatives) were detected as positives by using the peptide panel. We identified five of the six MCV single negative, nine of the 12 CCP2 single negative, and nine of the 13 RF seronegative RA patients by the peptide panel. The percentages of the single, double and triple negative RA sera exerting a positive reaction with the individual citrulline-peptides of the panel are shown in Fig. 3(c). These data indicate that the combined peptide assay is suitable to detect ACPA-positive RA cases that appeared to be CCP2-, MCV- and RF-negative in conventional tests.
Citrulline-peptide specific IgG but not IgM identifies RA subjects
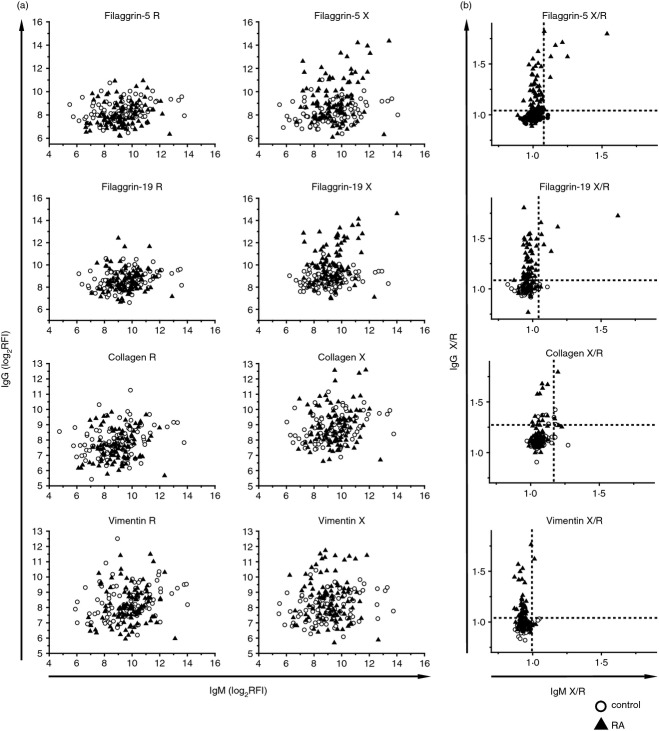
To examine the nature of the immune response against the peptides, peptide microarrays with the arginine and citrulline forms of the peptides were reacted with serum samples from non-autoimmune control subjects (n = 93) and RA subjects (n = 98). Fig. 4. shows IgG reactivity as a function of IgM reactivity in individual samples. These patterns indicate that while increased IgM reactivity against both the arginine and citrulline forms of the peptides is observed in control subjects, the selective increase in IgG reactivity against the citrulline forms is specific for RA. Nevertheless, a few RA samples showed IgM reactivity against the citrulline-containing filaggrin peptides (Fig. 4b).
Figure 4.
Comparison of peptide reactivity of IgM and IgG in control and rheumatoid arthritis (RA) subjects. Microarrays displaying the native and citrullinated forms of the peptides were reacted with sera of control and RA subjects, followed by simultaneous detection of bound IgM and IgG. Scatterplots show individual reactivity patterns for arginine and citrulline (X) -containing peptides (a) and for the X/R ratio (b). Dashed lines stand for mean + 2SD of X/R ratio of the control group.
In vitro synthesis of citrulline-peptide-specific IgG by RA B cells
To detect if B cells triggered in vitro by non-specific stimuli are able to produce citrulline-peptide-specific antibodies, purified B cells from RA patients and healthy individuals were stimulated by R848 polyclonal B-cell activator and IL-2 according to the manufacturers’ instructions (Mabtech). The frequencies of total and peptide-specific IgG-secreting cells were determined by ELISpot assay. While a comparable number of B cells synthesized IgG from healthy individuals and RA patients, only B cells from RA patients produced citrulline-peptide-specific IgG (Fig. 5). No remarkable number of arginine- or citrulline-containing peptide-specific IgG-producing B cells was observed in cultures from healthy subjects (spot number did not exceed 10). On the contrary, we detected a significantly higher frequency of cells producing IgG specific for the citrulline-containing fil306–326 and for the vimentin epitope compared with their arginine-containing counterparts (Fig. 5a). In the case of the fil311–315 peptide we observed a small but not significant elevation in the number of citrulline-peptide-specific IgG-producing cells compared with the arginine-peptide-specific control, whereas there was no difference in the number of cells synthesizing collagen peptide-specific IgG whether or not the peptide contained citrulline (Fig. 5b). A representative picture of the ELISPOT assay is shown in Fig. 5(c).
Figure 5.
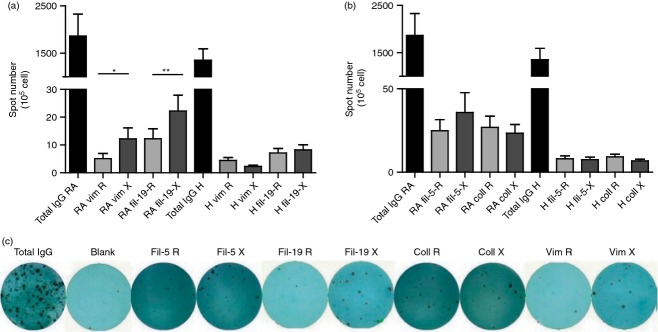
In vitro activated B cells of rheumatoid arthritis (RA) patients produce IgG specific for citrulline-containing peptide epitopes of filaggrin and vimentin. Purified B cells (2 × 106 to 5 × 106/ml) from 11 RA patients and eight healthy individuals were cultured for 3 days with polyclonal B-cell activators. The frequency of peptide-specific and total IgG-producing cells was determined by ELISpot assay, spot numbers were normalized to 105 cells. A representative picture of the ELISpot assay is also shown (Fig. 5c).
Discussion
Detection of ACPAs is the most specific and most sensitive early laboratory diagnostic test for RA; however, approximately 30% of patients were found to be ACPA negative. Identification of new peptide epitopes is essential to detect the false-negative cases among this group. The first cyclic citrullinated peptide used to develop an ELISA-based diagnostic kit, anti-CCP1, derived from filaggrin.25 The epitope peptide contained the sequence 306SHQESTXGRSRGRSGRSGS324 (X = citrulline). We modified this peptide by substituting the arginine residues with citrulline at positions 314, 316, 318 and identified the minimal epitope of the fil306–326 peptide, TXGRS, which is still specifically recognized by RA sera.26 Here we report our findings on the antibody recognition of these peptide epitopes in a larger cohort, and compare their antibody binding with that of other citrulline-peptide epitopes derived from collagen and vimentin.
First we chemically prepared the citrulline- or arginine-containing peptides attached at the C-terminal to a solid support of multiple pins and screened on a cohort of 53 RA patients, 46 CCP-negative non-RA patients (disease controls) and 45 healthy controls. The data obtained with the multipin ELISA system indicated that both the fil306–326 and its minimal epitope, fil311–315 peptide are recognized by RA sera with 42% and 72% sensitivity and high specificity. The vimentin and the collagen epitope peptides were also recognized with high specificity, but lower sensitivity. Our data indicate that the design for using multipin ELISA to screen sera reactivity has to consider the position of citrulline and its distance from the solid surface of the pin.
To confirm these data we turned to conventional ELISA. To assure the even binding of various peptides of different lengths to ELISA plates all peptides were biotinylated and applied on neutravidin-precoated plates. Biotinylation, especially if the peptide has a short sequence, may alter the availability of the citrulline residues within the epitope responsible for the specific autoantibody binding. Therefore we first compared antibody binding to the short TXGRS peptide biotinylated either at the N-terminal or C-terminal end. Based on these results27 we used long-chain biotin and labelled the peptides at the C-terminal end. In addition, we used different spacers to increase the distance between the epitope and the biotin moiety. Based on previous experiments,23 biotin-6-amino hexanoyl acid derivative as well as a longer spacer (Ttds) was used in the case of the fil311–315 peptide. The longer Ttds biotin-labelled TXGRS peptide appeared to be better recognized (60% sensitivity) compared with the LC-biotin hexanoyl labelled one with shorter spacer (40% sensitivity). RA sera recognized the fil306–326, the vimentin and the collagen epitope peptides with 62%, 44% and 41% sensitivity, respectively, at 95% specificity. As filaggrin is not present in synovia, it was suggested that cross-reacting auto-antibodies may recognize this protein.28 We have observed previously cross-reaction of affinity-purified MCV autoantibodies with the citrulline-containing fil306–326 peptides of fillaggrin.9 To study whether cross-reactivity exists between fil311–315 and vim65–77 peptides, competitive ELISA was used. The results show that there is competition between the soluble and solid phase bound peptides if their sequences are identical, but there is no cross-competition, that is that the citrulline-containing fil311–315 peptide does not inhibit the recognition of the vim65–77 peptide and vice versa. However, the competition was not complete, possibly because the avidity of ACPA is generally considered to be low, higher avidity ACPA was seen only in patients with symptoms.29 These data suggest that the ACPA response is polyclonal and that autoantibodies recognizing citrulline-containing filaggrin peptides might be cross-reactive with some other citrullinated epitope of vimentin or other protein.
A highly diverse ACPA response was observed by others,2–33 suggesting that several citrullinated proteins can be targeted by various ACPA clones in patients with established RA, and only a limited cross-reactivity is observed. Lundberg et al.34 detected 17 distinct RA subsets based on the ACPA fine specificity profile, and reported that the HLA-DRB1 shared epitope allele, PTPN22 gene and smoking are associated with the presence of antibodies recognizing citrullinated α-enolase and vimentin. These data indicate that specific ACPA reactivity may identify distinct RA subsets. Others found that the number of epitopes recognized by RA sera correlates with anti-CCP level but does not associate with clinical parameters of the patients.31 Epitope spreading in RA has been shown to occur before disease onset30–35 and it is questionable whether recognition of different epitope patterns is associated with distinct clinical phenotypes in established disease.
We have analysed the four-peptide panel composed of citrulline-containing 5-mer and 19-mer sequences of filaggrin, collagen and vimentin for correlation with clinical parameters of the patients. As different antibody populations may recognize various peptides, we have combined the data of multiple tests and calculated the sensitivity values. The sensitivity of the four-peptide panel in ELISA appears to be 81% at 95% specificity, which is comparable with commercially available tests. Furthermore, we found a correlation between the number of epitopes recognized by RA sera and the anti-CCP or anti-MCV titres. Distribution of peptide positivity has shown that in the group of sera recognizing only one peptide, half of the RA samples reacted with vim65–77, whereas in the group recognizing two peptides half of them bound to the two filaggrin peptides, indicating that these might be dominant and non-cross-reactive autoantigen epitopes.
Most importantly, seven out of twelve CCP2-negative, five out of six MCV-negative sera, two out of the double-negative sera and also two cases out of the seven triple-negative sera (CCP2, MCV and RF negatives) were recognized by the peptide panel, indicating that this combined assay is able to detect some of the false ACPA-negative RA samples as well. There are differences in the pathomechanisms of ACPA-positive and ACPA-negative RA,9–36 and the early detection of all ACPAs has a significant advantage from both diagnostic and therapeutic aspects. Although in line with others31–34 we observed no significant correlation between the ACPA fine-specificity profile and the clinical patient parameters such as CRP and DAS28, one cannot exclude the possibility that the detection of peptide-specific distinct ACPA profiles might be useful to develop individually targeted therapy. We could not detect a significant correlation between the number of peptides recognized and the duration of the disease, though in the group recognizing four citrulline-peptides the time-span of established disease was significantly longer compared with the group that does not react with any peptides, possibly reflecting epitope spreading.
ACPAs belonging to different immunoglobulin classes have been observed.35,37 Although rheumatoid factors are mainly of the IgM class, ACPAs are dominantly IgG antibodies. These observations strengthen the possibility that reactivity against the identified peptide epitopes develops during the disease and the involved B-cell clones go through isotype switching. However, results with the peptide-specific microarray have shown that although most ACPA recognizing the four citrulline peptides are IgG, some ACPA specifically recognizing the citrulline-containing fil311–315 and fil306–326 epitopes are IgM, so may be produced either by newly formed activated B cells as an indication of the ongoing autoimmune response or by unswitched B memory cells.
Secretion of anti-CCP antibodies by in vitro activated B cells of RA patients has been reported.39–40 In contrast with the previous reports, we observed no remarkable citrulline-peptide-specific antibody production in B-cell cultures of healthy individuals. This might be explained by the different culture conditions and by the different citrulline-peptides we applied. The peptide-specific ELISpot assays have shown that a significantly higher number of activated memory B cells from RA patients produce IgG specific for the citrulline-containing fil306–326 and vim65–77 peptides as compared to the arginine-containing counterparts.
Together these data suggest that the citrulline-peptide panel composed of filaggrin, vimentin and collagen sequences detects more ACPA-positive RA patients compared with conventional tests, and the individual citrulline-peptides are also suitable for the detection and study of the function of ACPA-producing autoreactive B cells.
Acknowledgments
This work was supported by the National Office of Research and Development (Pázmány grant RET-06/2006); by the Hungarian National Science Fund (NFÜ-OTKA CK80689 to GS); and the European Union and the European Social Fund have provided financial support to the project under the grant agreement no. TÁMOP 4.2.1./B-09/1/KMR-2010-0003.
Glossary
- ACPA
anti-citrullinated protein antibodies
- AUC
area under curve
- BSA
bovine serum albumin
- CCP
citrulline-containing protein/peptide
- CII
type II collagen
- Fmoc
fluorenylmethoxycarbonyl
- LC-biotin
biotinyl-6-aminohexanoic acid (long-chain biotin)
- MCV
mutated citrullinated vimentin
- RA
Rheumatoid arthritis
- RF
rheumatoid factor
- ROC
receiver operating characteristic
- TNF-α
tumour necrosis factor-α
- tBu
terc-butiloxicarbonyl
- Ttds
4,7,10-trioxa-1,13-tridecanediamino succinic acid
Disclosures
The authors declare no conflict of interest.
References
- Yamada R, Suzuki A, Chang X, Yamamoto K. Citrullinated proteins in rheumatoid arthritis. Front Biosci. 2005;10:54–64. doi: 10.2741/1506. [DOI] [PubMed] [Google Scholar]
- Snir O, Widhe M, von Spee C, et al. Multiple antibody reactivities to citrullinated antigens in sera from patients with rheumatoid arthritis: association with HLA-DRB1 alleles. Ann Rheum Dis. 2009;68:736–43. doi: 10.1136/ard.2008.091355. [DOI] [PubMed] [Google Scholar]
- Van Steendam K, Tilleman K, Deforce D. The relevance of citrullinated vimentin in the production of antibodies against citrullinated proteins and the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 2011;50:830–7. doi: 10.1093/rheumatology/keq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klareskog L, Malmström V, Lundberg K, Padyukov L, Alfredsson L. Smoking, citrullination and genetic variability in the immunopathogenesis of rheumatoid arthritis. Semin Immunol. 2011;23:92–8. doi: 10.1016/j.smim.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Yamada R, Suzuki A, Chang X, Yamamoto K. Peptidylarginine deiminase type 4: identification of a rheumatoid arthritis-susceptible gene. Trends Mol Med. 2003;9:503–8. doi: 10.1016/j.molmed.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Puszczewicz M, Iwaszkiewicz C. Role of anti-citrullinated protein antibodies in diagnosis and prognosis of rheumatoid arthritis. Arch Med Sci. 2011;7:189–94. doi: 10.5114/aoms.2011.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossenaar ER, Smeets TJ, Kraan MC, Raats JM, van Venrooij WJ, Tak PP. The presence of citrullinated proteins is not specific for rheumatoid synovial tissue. Arthritis Rheum. 2004;50:3485–94. doi: 10.1002/art.20584. [DOI] [PubMed] [Google Scholar]
- Makrygiannakis D, af Klint E, Lundberg IE, Löfberg R, Ulfgren AK, Klareskog L, Catrina AI. Citrullination is an inflammation-dependent process. Ann Rheum Dis. 2006;65:1219–22. doi: 10.1136/ard.2005.049403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harre U, Georgess D, Bang H, et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest. 2012;122:1791–802. doi: 10.1172/JCI60975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner G. Auto-antibodies and autoreactive T-cells in rheumatoid arthritis: pathogenetic players and diagnostic tools. Clin Rev Allergy Immunol. 2007;32:23–36. doi: 10.1007/BF02686079. [DOI] [PubMed] [Google Scholar]
- Wegner N, Lundberg K, Kinloch A, Fisher B, Malmstrom V, Feldmann M, Venables PJ. Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunol Rev. 2010;233:34–54. doi: 10.1111/j.0105-2896.2009.00850.x. [DOI] [PubMed] [Google Scholar]
- Palosuo T, Lukka M, Alenius H, et al. Purification of filaggrin from human epidermis and measurement of antifilaggrin autoantibodies in sera from patients with rheumatoid arthritis by an enzyme-linked immunosorbent assay. Int Arch Allergy Immunol. 1998;115:294–302. doi: 10.1159/000069460. [DOI] [PubMed] [Google Scholar]
- Girbal-Neuhauser E, Durieux JJ, Arnaud M, et al. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues. J Immunol. 1999;162:585–94. [PubMed] [Google Scholar]
- Schellekens GA, Visser H, de Jong BA, van den Hoogen FH, Hazes JM, Breedveld FC, van Venrooij WJ. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000;43:155–63. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Mor-Vaknin N, Punturieri A, Sitwala K, Markovitz DM. Vimentin is secreted by activated macrophages. Nat Cell Biol. 2003;5:59–63. doi: 10.1038/ncb898. [DOI] [PubMed] [Google Scholar]
- Vossenaar ER, Nijenhuis S, Helsen MM, van der Heijden A, Senshu T, van den Berg WB, van Venrooij WJ, Joosten LA. Citrullination of synovial proteins in murine models of rheumatoid arthritis. Arthritis Rheum. 2003;48:2489–500. doi: 10.1002/art.11229. [DOI] [PubMed] [Google Scholar]
- Bang H, Egerer K, Gauliard A, et al. Mutation and citrullination modifies vimentin to a novel autoantigen for rheumatoid arthritis. Arthritis Rheum. 2007;56:2503–11. doi: 10.1002/art.22817. [DOI] [PubMed] [Google Scholar]
- Ronnelid J, Lysholm J, Engstrom-Laurent A, Klareskog L, Heyman B. Local anti-type II collagen antibody production in rheumatoid arthritis synovial fluid. Evidence for an HLA-DR4-restricted IgG response. Arthritis Rheum. 1994;37:1023–9. doi: 10.1002/art.1780370707. [DOI] [PubMed] [Google Scholar]
- Tarkowski A, Klareskog L, Carlsten H, Herberts P, Koopman WJ. Secretion of antibodies to types I and II collagen by synovial tissue cells in patients with rheumatoid arthritis. Arthritis Rheum. 1989;32:1087–92. doi: 10.1002/anr.1780320906. [DOI] [PubMed] [Google Scholar]
- Burkhardt H, Koller T, Engstrom A, Nandakumar KS, Turnay J, Kraetsch HG, Kalden JR, Holmdahl R. Epitope-specific recognition of type II collagen by rheumatoid arthritis antibodies is shared with recognition by antibodies that are arthritogenic in collagen-induced arthritis in the mouse. Arthritis Rheum. 2002;46:2339–48. doi: 10.1002/art.10472. [DOI] [PubMed] [Google Scholar]
- Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–8. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- Geysen HM, Meloen RH, Barteling SJ. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc Natl Acad Sci USA. 1984;81:3998–4002. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos A, Uray K, Hudecz F. New biotin derivatives for labeling and solubilizing IgG peptides. Biopolymers. 2009;92:110–5. doi: 10.1002/bip.21141. [DOI] [PubMed] [Google Scholar]
- Papp K, Vegh P, Hobor R, Szittner Z, Voko Z, Podani J, Czirjak L, Prechl J. Immune complex signatures of patients with active and inactive SLE revealed by multiplex protein binding analysis on antigen microarrays. PLoS ONE. 2012;7:e44824. doi: 10.1371/journal.pone.0044824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101:273–81. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar A, Brózik M, Hudecz F. Filaggrin peptides with citrullin for diagnosis and monitoring autoantibodies in rheumatoid arthritis. Collect Symp Ser. 2011;13:1–6. [Google Scholar]
- Babos F, Szarka E, Nagy G, Majer Z, Sarmay G, Magyar A, Hudecz F. Role of N- or C-terminal biotinylation in autoantibody recognition of citrullin containing filaggrin epitope peptides in rheumatoid arthritis. Bioconjug Chem. 2013;24:817–27. doi: 10.1021/bc400073z. [DOI] [PubMed] [Google Scholar]
- Masson-Bessiere C, Sebbag M, Girbal-Neuhauser E, Nogueira L, Vincent C, Senshu T, Serre G. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the α- and β-chains of fibrin. J Immunol. 2001;166:4177–84. doi: 10.4049/jimmunol.166.6.4177. [DOI] [PubMed] [Google Scholar]
- Suwannalai P, van de Stadt LA, Radner H, et al. Avidity maturation of anti-citrullinated protein antibodies in rheumatoid arthritis. Arthritis Rheum. 2012;64:1323–8. doi: 10.1002/art.33489. [DOI] [PubMed] [Google Scholar]
- van der Woude D, Rantapaa-Dahlqvist S, Ioan-Facsinay A, et al. Epitope spreading of the anti-citrullinated protein antibody response occurs before disease onset and is associated with the disease course of early arthritis. Ann Rheum Dis. 2010;69:1554–61. doi: 10.1136/ard.2009.124537. [DOI] [PubMed] [Google Scholar]
- Willemze A, Bohringer S, Knevel R, et al. The ACPA recognition profile and subgrouping of ACPA-positive RA patients. Ann Rheum Dis. 2012;71:268–74. doi: 10.1136/annrheumdis-2011-200421. [DOI] [PubMed] [Google Scholar]
- Trouw LA, Mahler M. Closing the serological gap: promising novel biomarkers for the early diagnosis of rheumatoid arthritis. Autoimmun Rev. 2012;12:318–22. doi: 10.1016/j.autrev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Ioan-Facsinay A, el-Bannoudi H, Scherer HU, et al. Anti-cyclic citrullinated peptide antibodies are a collection of anti-citrullinated protein antibodies and contain overlapping and non-overlapping reactivities. Ann Rheum Dis. 2011;70:188–93. doi: 10.1136/ard.2010.131102. [DOI] [PubMed] [Google Scholar]
- Lundberg K, Bengtsson C, Kharlamova N, et al. Genetic and environmental determinants for disease risk in subsets of rheumatoid arthritis defined by the anticitrullinated protein/peptide antibody fine specificity profile. Ann Rheum Dis. 2013;72:652–8. doi: 10.1136/annrheumdis-2012-201484. [DOI] [PubMed] [Google Scholar]
- Ioan-Facsinay A, Willemze A, Robinson DB, et al. Marked differences in fine specificity and isotype usage of the anti-citrullinated protein antibody in health and disease. Arthritis Rheum. 2008;58:3000–8. doi: 10.1002/art.23763. [DOI] [PubMed] [Google Scholar]
- Fisher BA, Plant D, Lundberg K, Charles P, Barton A, Venables PJ. Heterogeneity of anticitrullinated peptide antibodies and response to anti-tumor necrosis factor agents in rheumatoid arthritis. J Rheumatol. 2012;39:929–32. doi: 10.3899/jrheum.111315. [DOI] [PubMed] [Google Scholar]
- Anzilotti C, Riente L, Pratesi F, Chimenti D, Delle Sedie A, Bombardieri S, Migliorini P. IgG, IgA, IgM antibodies to a viral citrullinated peptide in patients affected by rheumatoid arthritis, chronic arthritides and connective tissue disorders. Rheumatology (Oxford) 2007;46:1579–82. doi: 10.1093/rheumatology/kem193. [DOI] [PubMed] [Google Scholar]
- Conrad K, Roggenbuck D, Reinhold D, Dorner T. Profiling of rheumatoid arthritis associated autoantibodies. Autoimmun Rev. 2010;9:431–5. doi: 10.1016/j.autrev.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Reparon-Schuijt CC, van Esch WJ, van Kooten C, Schellekens GA, de Jong BA, van Venrooij WJ, Breedveld FC, Verweij CL. Secretion of anti-citrulline-containing peptide antibody by B lymphocytes in rheumatoid arthritis. Arthritis Rheum. 2001;44:41–7. doi: 10.1002/1529-0131(200101)44:1<41::AID-ANR6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Bellatin MF, Han M, Fallena M, et al. Production of autoantibodies against citrullinated antigens/peptides by human B cells. J Immunol. 2012;188:3542–50. doi: 10.4049/jimmunol.1100577. [DOI] [PubMed] [Google Scholar]