Abstract
Increasing evidence suggests that gut flora play an important role in the pathogenesis of non-alcoholic fatty liver disease (NAFLD). Our previous studies show that hepatic natural killer T (NKT) cells play a significant role in the pathogenesis of NAFLD. In this study, we explore the mechanism by which modification of gut flora leads to the alteration of hepatic NKT cells and improvement of steatosis. Mice were fed a high-fat (HF) diet to induce NAFLD. Some of them also received different doses of mixed-strain probiotics (VSL#3); single-strain probiotic (Bifidobacterium infantis) or antibiotics. Animal weight, glucose tolerance, liver steatosis and hepatic NKT cells were assessed. Lipid extracts from probiotics were tested for their ability to activate NKT cells. Toll-like receptor 4 (TLR4) knockout mice were also evaluated for their responses to HF diet. High-dose VSL#3 was more effective than low-dose VSL#3 and B. infantis for the improvement of hepatic NKT cell depletion and steatosis. The lipids extracted from VSL#3 stimulated NKT cells both in vivo and in vitro. In contrast, lipids from B. infantis decreased α-GalCer-mediated NKT cell activation in vitro, but were able to stimulate NKT cells. TLR4 knockout mice have a similar response to HF-diet-induced NKT cell depletion and obesity. These results suggest that alterations in the gut flora have profound effects on hepatic NKT cells and steatosis, which are both strain-specific and dose-dependent, but not through TLR4 signalling. Furthermore, these data suggest that probiotics may contain bacterial glycolipid antigens that directly modulate the effector functions of hepatic NKT cells.
Keywords: interleukin-2, natural killer T cells, non-alcoholic fatty liver disease, probiotics, steatosis
Introduction
Obesity and its related non-alcoholic fatty liver disease (NAFLD) have emerged as a major health problem around the world. More than 60 million adults are obese1 and 9·1 million individuals have NAFLD2 in the USA, the epicentre of this endemic. NAFLD encompasses a spectrum of entities including simple steatosis, non-alcoholic steatohepatitis, fibrosis and cirrhosis.3 Among the various environmental factors that might contribute to the rising incidence of obesity and NAFLD, dietary habits and gut flora merit particular consideration. Many studies have shown that changes in dietary fats are associated with an increased prevalence of NAFLD.4,5 Intestinal bacteria are also known to play a critical role in obesity and NAFLD.7 Germ-free mice colonized with gut bacteria from obese mice had higher weight gain than mice colonized with gut bacteria from lean mice on the same diet.8 Germ-free mice are also resistant to high-fat (HF) -diet-induced obesity related metabolic changes.9 Although many physicochemical determinants may affect the composition of gut bacteria, dietary factors have the most significant effect.10–11 Despite strong evidence supporting the role of intestinal bacteria in the pathogenesis of obesity and metabolic dysfunction,12 there is little knowledge of the mechanisms by which altered intestinal bacteria contribute to obesity and NAFLD.
Natural killer T (NKT) cells are a group of unique lymphocytes that are predominantly found in the liver, with significantly lower abundance in other lymphoid organs.13–14 Hepatic NKT cells are also implicated in the pathogenesis and disease progression of NAFLD, although the exact function of NKT cells in the pathogenesis of NAFLD remains controversial. A reduction in NKT cells has been shown in mice with fatty liver disease4–15 and in the peripheral blood of patients with NAFLD.16 Adoptive transfer of NKT cells improves steatosis and insulin resistance in an HF-diet-induced mouse model of NAFLD.17 Despite mounting evidence demonstrating the importance of NKT cells in regulating hepatic immune responses in NAFLD, there is little knowledge of how NKT cells themselves are regulated. Recent studies have shown that bacterial antigens can serve as ligands for NKT cells.18,19 In addition, probiotics, live bacteria that alter the host’s microflora and exert health benefits,21 can also influence the composition of hepatic NKT cells.17 This raises the possibility that endogenous gut microbiota and exogenous bacterial supplements (probiotics) may be able to regulate NKT cells, and in turn, modulate NAFLD.
In the current study, we evaluated the strain and dose effects of probiotics on hepatic NKT cells in a mouse model of NAFLD. More importantly, we explore the mechanism by which modification of gut flora by probiotics leads to the alteration of hepatic NKT cells and the improvement of steatosis. Results from this study may have profound therapeutic implications for the management of obesity-related fatty liver disease and insulin resistance.
Materials and methods
Animal experiments
Adult male wild-type C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). The mice were fed commercial high-fat diets (HF diet; 60% of the total calories from fat) or normal diet (ND diet; 11% of the total calories from fat, BioServ, Frenchtown, NJ) for 12 weeks. All mice were maintained in a temperature-controlled and light-controlled facility and allowed to consume water and food ad libitum. Some HF-diet-fed mice also received either high-dose (1·5 × 109 colonies/mouse/day) or low-dose (1·5 × 108 colonies/mouse/day) VSL#3 probiotics (a mixture of viable, lyophilized bifidobacteria, lactobacilli and Streptococcus thermophilus, Danisco – USA – Madison Plant, Madison, WI), or single strain probiotic Bifidobacterium infantis (Align, Procter & Gamble, Cincinnati, OH), or antibiotics (KCV, a mixture of kanamycin, colistin and vancomycin; Sigma, St Louis, MO) for the last 4 weeks. Some normal-diet-fed mice also received lipid extracts from probiotics VSL#3 or Align B. infantis (see below for detail) intraperitoneally. Toll-like receptor 4 (TLR4) knockout mice with C57BL/6 background were purchased from Jackson Laboratory (Bar Harbor, ME) and also evaluated for their response to an HF diet. All animal experiments fulfilled the National Institutes of Health and Johns Hopkins University criteria for the humane treatment of laboratory animals.
Liver histology and triglyceride content
Thin slices of liver tissue were stained with haematoxylin & eosin. Ten 200 × light microscope fields were assessed on each section and scored for the severity of steatosis.17 Total lipids were extracted from frozen liver tissue according to a published method.22 Hepatic triglyceride content was measured using a kit according to the manufacturer’s instructions (Sigma #TR0100).
Glucose tolerance test and HOMA-IR
Mice were given glucose (1·5 g/kg, intraperitoneally) following overnight fasting. Glucose was measured in blood collected from the tail vein using the OneTouch® ultra® monitoring system with a range of 20–600 mg/dl (LifeScan, Inc., Milpitas, CA) at various time-points (from 0 to 120 min after the glucose injection). The insulin concentration in mouse serum was determined by an ultra-sensitive mouse insulin ELISA kit according to the manufacturer’s instructions (Crystal Chem Inc., Downers Grove, IL). Insulin resistance was evaluated by the homeostasis model assessment method (HOMA-IR).23 HOMA-IR are calculated using the formula: HOMA-IR (mmol/l × μU/ml) = fasting glucose (mmol/l) × fasting insulin (μU/ml)/22·5.24
Isolation and cell surface labelling of hepatic mononuclear cells
Hepatic mononuclear cells were isolated as previously described4 and then labelled with CD3 and CD1d tetramer (NIH tetramer facility) loaded with a ligand (PBS-57, an analogue of α-GalCer). After surface labelling, the hepatic mononuclear cells were evaluated by flow cytometry (Becton Dickinson, Palo Alto, CA). The data were analysed using cell quest software (Becton Dickinson).
CFSE proliferation assay
Hepatic mononuclear cells were isolated and incubated for 10 min at 37° with 2·5 μm carboxyfluorescein succinimidyl ester (CFSE) using the CellTrace™ CFSE cell proliferation kit following the manufacturer’s instructions (Invitrogen, Eugene, OR). CFSE-labelled hepatic mononuclear cells were washed with PBS/0·1% fetal bovine serum and cultured with artificial antigen-presenting cells (aAPCs) loaded with VSL#3 extract or unloaded empty beads (used as a control). After 3 days of co-culture, cells were harvested, washed and stained with NKT tetramer. NKT cell proliferation was evaluated by flow cytometry. The data were analysed using flowjo software (Tree Star, INC, Ashland, OR).
Probiotic lipid extraction and antigen presentation
Either 9 × 1011 VSL#3 or 2·1 × 1010 Align B. infantis were dissolved in 18 ml water; 2 : 1 methanol : chloroform was used to extract bacterial lipids. Extracts were centrifuged at 500 × g for 15 min at room temperature. The supernatant containing the lipid was dried and resuspended in PBS. The total lipid contents were measured using a total lipid kit according to the manufacturer’s protocol (Biotron Diagnostics, Hemet, CA). CD1d-Ig-based aAPCs were generated according to the method described by Webb et al.25 Briefly, CD1d-Ig (DimerXI; BD Biosciences) was mixed with epoxy beads (Dynabeads, M-450, Epoxy, Invitrogen, Grand Island, NY). These CD1d-Ig beads were loaded with either 5 μg/ml of α-GalCer (Enzo Life Sciences, Plymouth Meeting, PA) or different concentrations of probiotic lipid extracts. After extensive washing, aAPCs loaded with α-GalCer or probiotic lipid extract (unloaded empty beads were used as a control) were co-cultured with the NKT cell hybridoma, DN32.D3, for 16–24 hr at 37° in 96-well U-bottom plates (2 × 105 aAPCs mixed with 5 × 104 NKT cells in each well). Interleukin-2 released by NKT hybridoma cells was measured by standard ELISA using an interleukin-2 ELISA kit according to the manufacturer’s instructions (Biolegend, San Diego, CA), and was used to indicate NKT cell activation.
Statistical analysis
All values are expressed as mean ± SD. Multiple comparisons were evaluated by analysis of variance (SPSS 11.5 for windows; SPSS Inc., Chicago, IL). The paired group means were compared by t-test using Microsoft Excel (Microsoft, Redmond, WA). The P values < 0·05 were considered statistically significant.
Results
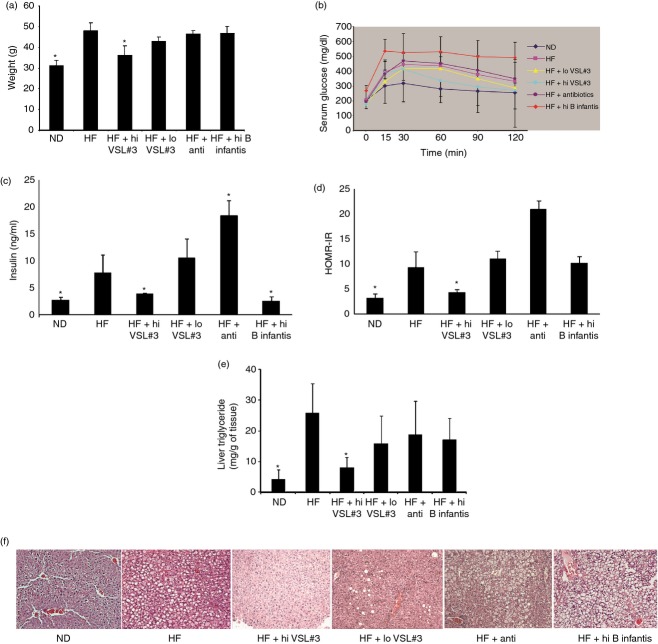
High-dose VSL#3 improves obesity and hepatic steatosis in HF-diet-fed mice
We have previously shown that probiotics improve NAFLD caused by an HF diet.17 To better understand the mechanisms by which the probiotics result in convalescence, we investigated the effects of different doses and evaluated whether the effects on NAFLD were strain specific. HF-diet-fed wild-type mice were treated with high or low doses of VSL#3 (mixed strains of probiotics) and a high-dose single strain of B. infantis. We also treated HF-diet-fed mice with a mixture of antibiotics. High doses of VSL#3 significantly reduced HF-diet-induced obesity (Fig. 1a). Although low-dose VSL#3 also improved weight gain in HF-diet-fed mice but the result did not reach statistical significance. A high dose of B. infantis and a mixture of antibiotics had little impact on animal weight (Fig. 1a). High doses of VSL#3 also significantly improved the glucose tolerance test (Fig. 1b) and reduced serum insulin level (Fig. 1c). Low-dose VSL#3 slightly improved the glucose tolerance test (Fig. 1b), but had no effect on serum insulin level (Fig. 1c). Treatment with B. infantis reduced the serum insulin level (Fig. 1c), but significantly worsened the glucose tolerance test (Fig. 1b) whereas the antibiotic mixture had little impact on the glucose tolerance test (Fig. 1b) but produced a significantly higher serum insulin level (Fig. 1c). HOMA-IR is the best indicator for insulin resistance,26–27 so it was calculated by using the above parameters. High doses of VSL#3 significantly improved HF-induced insulin resistance, as reflected by HOMA-IR, while a low dose of VSL#3 and B. infantis had little effect, and the antibiotic mixture even slightly worsened HOMA-IR (Fig. 1d). For HF-diet-induced hepatic steatosis, high doses of VSL#3 significantly reduced hepatic triglyceride content (Fig. 1e) and improved histology (Fig. 1f). A low dose of VSL#3 slightly improved steatosis on histology (Fig. 1f), but did not reach statistical significance for hepatic triglyceride content (Fig. 1e), whereas B. infantis and the antibiotic mixture had no effect on hepatic triglyceride content and histological steatosis.
Figure 1.
The dose- and strain-dependent effects of probiotics on high-fat (HF) diet-induced obesity, insulin resistance and hepatic steatosis. Wild-type (WT) C57BL/6 mice were fed either normal diet (ND) or HF diet and some of HF-diet-fed mice were also treated with high-dose VSL#3 (HF+hi VSL#3), or low-dose VSL#3 (HF+lo VSL#3), or antibiotics (HF+anti) or high dose of Bifidobacterium infantis (HF+hi B infantis), or continuous ND or HF diet. (a) Animal weight; (b) glucose tolerance tests; (c) serum insulin level; (d) insulin resistance measured by HOMA-IR; (e) hepatic triglyceride content; (f) representative haematoxylin & eosin of liver histology. *P < 0·05 versus HF-diet group.
Treatment with high-dose VSL#3 increases the percentage of hepatic NKT cells
Previously, probiotics were shown to improve HF-diet-induced hepatic NKT cell depletion.4–17 In the current study, we demonstrate that the effect of probiotics on hepatic NKT cells was also dose and strain dependent. Only high-dose VSL#3 improved hepatic NKT cell depletion in HF-diet-fed mice, whereas low-dose VSL#3, single strain B. infantis and antibiotics had little impact (Fig. 2).
Figure 2.
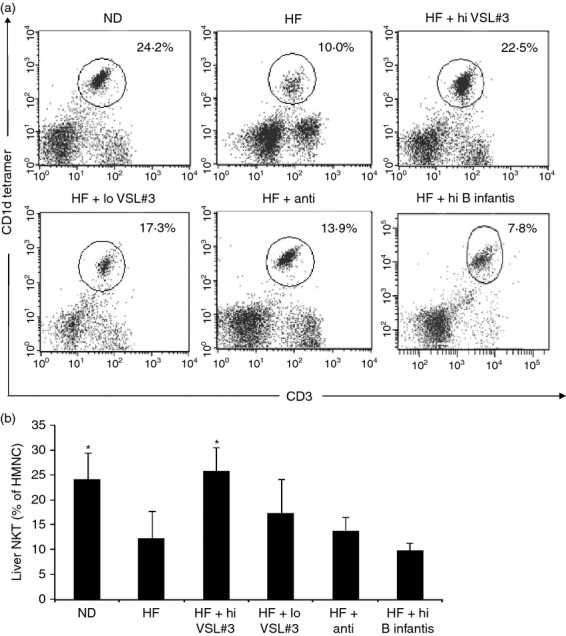
The dose- and strain-dependent effect of probiotics on hepatic natural killer T (NKT) cells. Animals were treated as described in Fig. 1. Hepatic mononuclear cells (HMNC) were isolated and labelled with various surface markers. (a) A representative dot plot of NKT cell staining (gated on total HMNC); (b) means ± SD of three independent experiments (n = 5 per group). *P < 0·05 versus high-fat (HF) -diet group.
Probiotic antigens stimulate NKT cells
To determine the potential mechanism of probiotic stimulation of NKT cells, lipid extractions were made from VSL#3 and B. infantis. The extracts were loaded onto aAPCs and then co-cultured with NKT hybridomas. Interleukin-2 released from the NKT hybridomas reflected the stimulation of NKT cells by probiotic antigens. The VSL#3 extracts stimulated NKT cells in a dose-dependent manner (Fig. 3). B. infantis extracts had little effect on NKT cells (Fig. 3a). Both extracts showed competitive binding against α-GalCer to stimulate NKT cells. To further determine whether probiotic antigens can stimulate NKT cells in vivo, low doses of lipid extractions from VSL#3 or B. infantis were administrated to mice intraperitoneally. Both VSL#3 and B. infantis lipid extract administration in vivo stimulated hepatic NKT cells (Fig. 3b), while α-GalCer in vivo caused hepatic NKT cell anergy as previously published.28 This result suggests that the lipids from probiotics and α-GalCer may have different mechanisms to induce NKT cell stimulation in vivo. To further investigate the mechanism of probiotic-induced NKT cell stimulation, an NKT cell proliferation assay was performed with CFSE labelling. Primary NKT cells showed increased proliferation after being stimulated with VSL#3 extract (Fig. 3c). The result confirms that the lipids from VSL#3 stimulate NKT cells and increase their proliferation.
Figure 3.
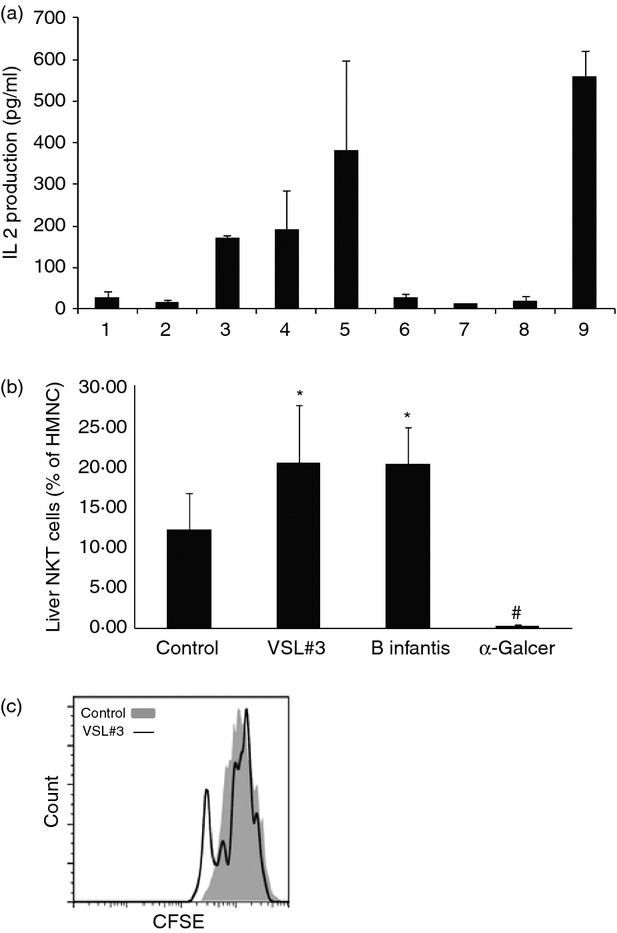
Probiotic antigens stimulate natural killer T (NKT) cells in vitro and in vivo. (a) Bacterial glycolipids extracted were co-cultured with NKT hybridoma. Interleukin-2 (IL-2) released by NKT hybridoma indicates their activation. 1 = medium only; 2 = artificial antigen-presenting cells (aAPCs) only; 3 = aAPCs loaded with low-dose VSL#3 extract; 4 = aAPCs loaded with high-dose VSL#3 extract; 5 = aAPCs loaded with high-dose VSL#3 extract plus α-GalCer; 6 = aAPCs loaded with low-dose Bifidobacterium infantis extract; 7 = aAPCs loaded with high-dose B. infantis extract; 8 = aAPCs loaded with high-dose B. infantis extract plus α-GalCer; and 9 = aAPCs loaded with α-GalCer. (b) Lipid extracts from VSL#3 or B. infantis, or α-GalCer (2 μg per mouse) were injected into C57BL/6 wild-type mice fed a normal diet. After 24 hr, the animals were killed and their hepatic NKT cells were evaluated as described in Fig 2. Means ± SD of the percentages of hepatic NKT cells (gated on CD3+ and CD1d Tetramer+) among hepatic mononuclear cells (HMNC) are shown (n = 5 per group). (c) A representative histogram of NKT cell proliferation assay. HMNC were labelled with CFSE and stimulated with aAPCs loaded with VSL#3 lipid extract or unloaded empty beads. *P < 0·025, #P < 0·002 versus control.
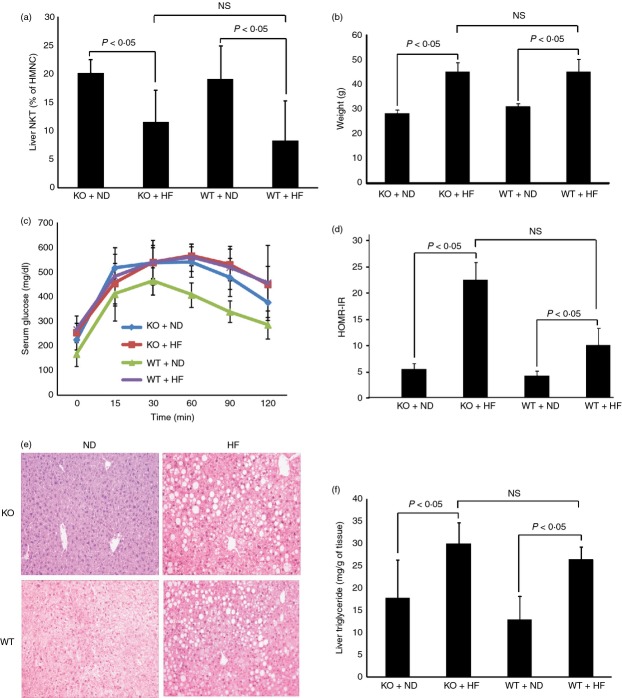
Disruption of TLR4 signalling does not protect HF-diet-induced NKT cell depletion and metabolic dysfunction
We further examined whether TLR4 played any role in HF-diet-induced hepatic NKT cell depletion. Wild-type and TLR4 knockout mice were fed normal or HF diet. Hepatic NKT cells and metabolic profiles were measured. Disrupting TLR4 had no impact on HF-diet-induced hepatic NKT cell depletion (Fig. 4a) nor on HF-diet-induced obesity (Fig. 4b), insulin resistance or hepatic steatosis (Fig. 4e,f). There is a slightly increased glucose intolerance in TLR4 knockout mice fed normal diet (Fig. 4c). However, it did not reach statistical significance. HOMA-IR also showed no difference between wild-type and TLR4 knockout groups on insulin resistance (Fig. 4d).
Figure 4.
Disrupting Toll-like receptor 4 (TLR4) signalling did not protect mice from high-fat (HF) -diet-induced non-alcoholic fatty liver disease (NAFLD). Wild-type and TLR4 knockout C57BL/6 mice were fed normal diet (ND) or HF diet for 12 weeks. (a) Hepatic natural killer T (NKT) cell content; (b) animal weight; (c) glucose tolerance tests; (d) insulin resistance as measured by HOMA-IR; (e) representative haematoxylin & eosin staining of liver histology; (f) hepatic triglyceride content. Results were means ± SD (n = 5 per group)
Discussion
Intestinal bacteria are known to play a critical role in obesity and NAFLD.7 Colonization of germ-free mice with conventional gut bacteria causes a significant increase in body fat, despite a decrease in food intake.29 In the current study, we show that modification of intestinal bacteria with probiotics has a significant impact on liver NKT cells in a dose- and strain-dependent manner. We also show that the effects of probiotics on hepatic NKT cells are probably, at least partially, due to probiotic antigens that can directly stimulate NKT cells, although the overall effect of probiotics may still be the balance of endogenous bacteria. In addition, VSL#3 has a much greater impact on hepatic NKT cells in vivo than single-strain B. infantis; even though B. infantis is part of the VSL#3 mixture, suggesting that the interaction between different strains among probiotics or between host and probiotic may also play an important role.
The exact role of NKT cells in the pathogenesis of NAFLD is still controversial. Although studies from our groups and others show a reduction of NKT cells in HF-diet-induced mouse fatty liver4–15 and in the peripheral blood of patients with NAFLD,16 another study reported increased hepatic NKT cells in methionine choline-deficient-diet-induced non-alcoholic steatohepatitis.30 There is also a report demonstrating that the number of hepatic NKT cells is increased in patients with steatosis.31 These controversies are most likely due to diverse NKT cell populations and to the lack of reliable and standard methods to detect NKT cells. Animal models of NAFLD generated by different diets are likely to have different intestinal bacteria, because dietary factors have the most significant influence on the composition of gut bacterial flora.10–11 A recent study showed that the phenotypes and functions of NKT cells are directly dependent on the gut bacterial antigen presented to them.32 High-fat and methionine choline-deficient diets fed to mice are likely to produce large differences in their gut bacterial contents. Our current study supports the notion that alteration of intestinal bacteria can modulate hepatic NKT cells. Further studies are currently underway in our laboratory to understand the effect of different diets on gut bacterial composition and regulation of hepatic NKT cells. We are also studying the mechanism of which B. infantis lipid extract had different effects on hepatic NKT cells in vitro compared with in vivo. This is probably a result of antigen modification and presentation when bacterial lipid extract was injected in vivo.
Similarly, dietary factors may also explain the controversial role of TLR4 in the pathogenesis of NAFLD. Previously, some studies have shown that TLR4 is involved in the development of methionine choline-deficient-diet-induced,33 as well as fructose-induced,34 NAFLD. However, in our current study, we found no difference in metabolic profiles, including steatosis, between wild-type and TLR4 knockout mice fed an HF diet. Again, different diets may alter intestinal bacteria and so have different effects on TLR4 signalling. Other studies also showed that the NKT cell responsiveness to gut bacteria did not require TLR signals.32
In summary, our study investigates the potential mechanisms by which dietary factors alter intestinal bacteria and lead to the formation NAFLD. Modification of intestinal bacteria by probiotics directly influences hepatic NKT cells that contribute to the pathogenesis of NAFLD. Our observation that probiotics can enhance NKT cell function has potentially broader implications for the treatment of various liver diseases including NAFLD and autoimmune diseases.
Acknowledgments
SL performed the experiments; ZL designed the research; TW provided technical training; SL analysed the data; SL and ZL wrote the paper. All authors read and approved the final manuscript. We thank Dr Ronald L Schnaar’s laboratory for providing technical support of lipid extraction, James Potter for editorial assistance, and the National Institutes of Health tetramer facility for providing mouse CD1d tetramer. This work was supported by NIH/NIDDK R01DK075990 (ZL).
Glossary
- aAPCs
artificial antigen-presenting cells
- CFSE
carboxyfluorescein succinimidyl ester
- HF
high-fat
- HOMA-IR
homeostasis model assessment method
- NAFLD
non-alcoholic fatty liver disease
- ND
normal diet
- NKT
natural killer T
- TLR4
Toll-like receptor 4
- VSL#3
mixed strain probiotics
Diclosures
The authors declare having no competing interests.
References
- Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–7. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- Parekh S, Anania FA. Abnormal lipid and glucose metabolism in obesity: implications for nonalcoholic fatty liver disease. Gastroenterology. 2007;132:2191–207. doi: 10.1053/j.gastro.2007.03.055. [DOI] [PubMed] [Google Scholar]
- Li Z, Soloski MJ, Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42:880–5. doi: 10.1002/hep.20826. [DOI] [PubMed] [Google Scholar]
- Hua J, Ma X, Webb T, Potter JJ, Oelke M, Li Z. Dietary fatty acids modulate antigen presentation to hepatic NKT cells in nonalcoholic fatty liver disease. J Lipid Res. 2010;51:1696–703. doi: 10.1194/jlr.M003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilar L, Oliveira CP, Faintuch J, Mello ES, Nogueira MA, Santos TE, Alves VA, Carrilho FJ. High-fat diet: a trigger of non-alcoholic steatohepatitis? Preliminary findings in obese subjects. Nutrition. 2008;24:1097–102. doi: 10.1016/j.nut.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Abu-Shanab A, Quigley EM. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010;7:691–701. doi: 10.1038/nrgastro.2010.172. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA. 2007;104:979–84. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–23. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107:14691–6. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121:2126–32. doi: 10.1172/JCI58109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4:231–7. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- Klugewitz K, Adams DH, Emoto M, Eulenburg K, Hamann A. The composition of intrahepatic lymphocytes: shaped by selective recruitment? Trends Immunol. 2004;25:590–4. doi: 10.1016/j.it.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Kremer M, Thomas E, Milton RJ, et al. Kupffer cell and interleukin-12-dependent loss of natural killer T cells in hepatosteatosis. Hepatology. 2010;51:130–41. doi: 10.1002/hep.23292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CF, Yu CH, Li YM, Xu L, Du J, Shen Z. Association of the frequency of peripheral natural killer T cells with nonalcoholic fatty liver disease. World J Gastroenterol. 2007;13:4504–8. doi: 10.3748/wjg.v13.i33.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Hua J, Li Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol. 2008;49:821–30. doi: 10.1016/j.jhep.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattner J, DeBord KL, Ismail N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–9. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- Kinjo Y, Tupin E, Wu D, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–86. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- Kinjo Y, Wu D, Kim G, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–5. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- Schrezenmeir J, de Vrese M. Probiotics, prebiotics, and synbiotics – approaching a definition. Am J Clin Nutr. 2001;73:361S–4S. doi: 10.1093/ajcn/73.2.361s. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Shoji T, Emoto M, et al. Insulin resistance as an independent predictor of cardiovascular mortality in patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:1894–900. doi: 10.1097/01.asn.0000019900.87535.43. [DOI] [PubMed] [Google Scholar]
- Webb TJ, Bieler JG, Schneck JP, Oelke M. Ex vivo induction and expansion of natural killer T cells by CD1d1-Ig coated artificial antigen presenting cells. J Immunol Methods. 2009;346:38–44. doi: 10.1016/j.jim.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai WW, Niessen S, Goebel N, Yates JR, 3rd, Guccione E, Montminy M. PRMT5 modulates the metabolic response to fasting signals. Proc Natl Acad Sci USA. 2013;110:8870–5. doi: 10.1073/pnas.1304602110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista JG, Soares-Jr JM, Maganhin CC, Simoes RS, Tomaz G, Baracat EC. Assessing the benefits of rosiglitazone in women with polycystic ovary syndrome through its effects on insulin-like growth factor 1, insulin-like growth factor-binding protein-3 and insulin resistance: a pilot study. Clinics (Sao Paulo) 2012;67:283–7. doi: 10.6061/clinics/2012(03)14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L, Wang CR, Joyce S, Van Kaer L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–83. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syn WK, Oo YH, Pereira TA, et al. Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology. 2010;51:1998–2007. doi: 10.1002/hep.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler M, Taylor S, Okebugwu K, Yee H, Fielding C, Fielding G, Poles M. Intrahepatic natural killer T cell populations are increased in human hepatic steatosis. World J Gastroenterol. 2011;17:1725–31. doi: 10.3748/wjg.v17.i13.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingender G, Stepniak D, Krebs P, et al. Intestinal microbes affect phenotypes and functions of invariant natural killer T cells in mice. Gastroenterology. 2012;143:418–28. doi: 10.1053/j.gastro.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csak T, Velayudham A, Hritz I, et al. Deficiency in myeloid differentiation factor-2 and toll-like receptor 4 expression attenuates nonalcoholic steatohepatitis and fibrosis in mice. Am J Physiol Gastrointest Liver Physiol. 2011;300:G433–41. doi: 10.1152/ajpgi.00163.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff SC, Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50:1094–104. doi: 10.1002/hep.23122. [DOI] [PubMed] [Google Scholar]