Abstract
Nuclear factor-κB-inducing kinase (NIK) is known to play a critical role in maintaining proper immune function. This is exemplified in the spontaneous mutant mouse lacking functional NIK, alymphoplasia (aly), which is simultaneously immune-compromised and autoimmune-prone. To investigate the role of NIK in αβ T-cell repertoire formation, we analysed T-cell development in aly/aly mice bearing a transgenic T-cell receptor (TCR). Although there were no apparent abnormalities in the mature αβ T cells of non-transgenic aly/aly mice, the maturation efficiency of idiotypehigh+ T cells in the TCR-transgenic mice was lower in aly/aly mice compared with those found in aly/+ mice, suggesting that the mature αβ T-cell repertoire could be altered by the absence of functional NIK. In one strain of TCR-transgenic aly/aly mice with a negatively selecting H-2 background, the proportion of CD8low+ idiotypehigh+ cells, which are thought to potentially represent the γδ lineage of T cells, was markedly decreased. When the γδ T cells in non-transgenic aly/aly mice were investigated, the proportion of γδ T cells in the peripheral organs of aly/aly mice was found to be one-half to one-fifth of those in aly/+ mice. Analyses of bone marrow chimera mice indicated that NIK in host cells, rather than in donor cells was important for generating a normal number of peripheral γδ T cells. Collectively, these results suggest that NIK could be involved in thymic positive selection of some αβ T cells and that NIK in non-haematopoietic cells is important for the optimal development and/or maintenance of γδ T cells.
Keywords: nuclear factor-κB-inducing kinase, repertoire formation, T-cell receptor-transgenic mouse
Introduction
The development of αβ T cells in the thymus is a multi-step process that depends crucially on signalling from T-cell receptors (TCRs). In the thymocytes in which successful recombination of the TCR-β gene segments has occurred, pre-TCR complexes composed of the TCR-β chain and the pTα chain are generated, and the ligand-independent triggering of the pre-TCR signal drives the thymocyte differentiation forward to CD4/CD8 double-positive (DP) stages.1 Further maturation requires signalling from TCR-αβ, but the signalling from TCR-αβ on DP thymocytes should be strictly controlled, because the strength and/or duration of the TCR signalling has to be converted into different signals in quality, leading the cells to different fates. Namely, while apoptosis would be induced in the DP cells bearing TCR-αβ that interact too strongly with self-peptide/MHC molecules, the DP thymocytes with moderate avidity with self-peptide/MHC would survive to mature into CD4 or CD8 single-positive (SP) cells, depending on the classes of MHC molecules with which they have interacted.
Signalling from TCR activates a number of transcription factors. Among them, nuclear factor-κB (NF-κB) has been demonstrated to be one of the important regulators for thymocyte differentiation.2 It has been demonstrated that NF-κB activation is observed at β-selection3 as well as positive and negative selection of αβ T cells.4,5 Although the PKCθ/Carma1/Bcl10/Malt1 pathways for IκB kinase activation seems pivotal for TCR-induced NF-κB activation in peripheral mature T cells, abrogation of this pathway does not affect TCR-induced NF-κB activation in thymocytes as much as in peripheral T cells.7,8 These observations indicate that the molecules that mediate TCR-induced NF-κB activation in immature thymocytes may be different from those in mature T cells.
The NF-κB-inducing kinase, NIK, is known to contribute to NF-κB activation,10 and to play diverse roles in various aspects of homeostasis. Its in vivo roles have been investigated mainly using an NIK-deficient mouse11 and a spontaneous mutant mouse, alymphoplasia (aly).12 The alymphoplasia mouse was isolated as a mutant mouse that lacked all lymph nodes and Peyer’s patches,12 and its causal mutation has been identified on the NIK gene.13 The mutation is a mis-sense mutation (G855R), which results in defective interaction with IκB kinase α, resulting in impaired phosphorylation of p100.14 Regarding the role of NIK in TCR signalling in thymocytes, it has been shown that NF-κB activation upon anti-CD3 stimulation was attenuated in aly/aly thymocytes,15 suggesting that NIK plays mandatory roles in TCR-mediated NF-κB activation in thymocytes. These results also suggested a possibility that the NIK in thymocytes may be involved in thymic selection, and so in peripheral T-cell repertoire formation.
In aly/aly mice, however, apparent abnormalities have not been found in T-cell development.12 The numbers of thymocytes or splenic T cells in aly/aly mice are normal, and the peripheral CD4+/CD8+ ratio is almost the same as that in wild-type (WT) mice. Nevertheless, it is still possible that the threshold of positive or negative selection may be shifted by the aly mutation, and that the mature T-cell repertoire in aly/aly mice may be different from that in WT mice. In such a case, the analyses should be performed with a fixed TCR, using TCR transgenic (Tg) mice, to follow the fate of the T cells expressing a particular TCR.
In contrast to the αβ T cells, information on the role of NIK or of NF-κB activation in the development of another subset of T cells, γδ T cells, is sparse. Although the genetic requirements in the development differ between αβ and γδ T cells,16 it is thought that, like αβ T cells, TCR signalling may be crucial for the maturation of (at least some populations of) γδ T cells in the thymus.17 Intriguingly, differentiation of thymic γδ T cells has been shown to be affected by the lymphotoxin β (LTβ) signalling upon interaction with DP αβ T cells.18 Given that NIK is critical in the signal transduction from LTβ receptor (LTβR),11 it appears quite possible that NIK may play some key roles in the development of γδ T cells, which still remain to be explored.
In the present study, development of αβ T cells and γδ T cells in aly/aly mice have been investigated using the TCR-αβ Tg mouse, to reveal the roles of NIK in the development of αβ and γδ T cells. The results suggested that the efficiency of the positive selection of at least some of αβ T cells could be affected by the lack of functional NIK. It was also suggested that peripheral maintenance and/or the development of γδ T cells may require functional NIK to be expressed in non-haematopoietic cells.
Materials and methods
Mice
C57BL/6J (H-2b), DBA/1 (H-2q), C3H/HeN (H-2k) mice were purchased from Charles River Japan, Inc. (Kanagawa, Japan). B10.S (H-2s) mice were purchased from Japan SLC, Inc. (Shizuoka, Japan). The alymphoplasia mice were obtained from Clea Japan, Inc. (Tokyo, Japan), and were bred onto C57BL/6J > 10 times before inter-breeding to produce the aly/aly mouse or breeding with other strains of mice. The QM11TCR-Tg mouse, possessing the transgenes for the α and β chains of TCR recognizing I-Ak as the allo-antigen, was described previously.19 In some experiments, analyses were performed using QM11TCR-Tg mice with RAG-2-deficient background.19 The green fluorescent protein (GFP) -Tg mouse of C57BL/6 background [C57BL/6 TgN (act-EGFP) OsbY01]20 was kindly provided from Dr Masaru Okabe (Osaka University) and was maintained in our animal facility. All mice used in this study were maintained in a specific pathogen-free facility of Kitasato University School of Medicine. The experimental procedure was approved by the Animal Experimentation and Ethics Committee of the Kitasato University School of Medicine, and all animal experiments were performed following the guidelines of the committee.
Antibodies and reagents
FITC-labelled anti-CD4 antibody (RM4-5), anti-Thy1.2 antibody (53-2.1), and anti-CD25 antibody (PC61) were purchased from BD Pharmingen (San Diego, CA). FITC-labelled antibodies to CD27 antibody (LG.3A10), CD122 antibody (5H4), phycoerythrin-labelled antibodies to γδ TCR (GL3) and CD8 (53-6.7), phycoerythrin-, and phycoerythrin-Cy5-labelled streptavidin were obtained from BioLegend (San Diego, CA). Biotinylated anti-idiotype antibody to QM11TCR was prepared in our laboratory.21 Antibodies to CD3 (2C11), and to FcγR II/III (2.4G2) were prepared from hybridomas in the laboratory.
Preparation of bone marrow chimeras
The recipient aly/aly or aly/+ mice were lethally irradiated (8·5 Gy) using an X-ray irradiator MBR-1505R (Hitachi Medico Co., Tokyo, Japan) with a filter (Cu: 0·5 mm, Al: 1 mm). The following day, the recipient mice were reconstituted with 1·0 × 107 of T-cell-depleted bone marrow (BM) cells from GFP-Tg, aly/aly or aly/+ mice. T-cell depletion was conducted by treating the cells with anti-CD4, anti-CD8 and anti-Thy1.2 antibody plus rabbit complement at 37° for 45 min. The chimeric mice were analysed 60–70 days after BM reconstitution.
Flow cytometry
Flow cytometric analyses were performed as described previously.19 Briefly, 2 × 105 to 10 × 105 cells were stained in and washed with ice-cold Hanks’ balanced salt solution containing 0·5% BSA and 0·02% sodium azide. Secondary staining was carried out in the same manner. Stained cells after washing were examined on FACSCalibur (BD Biosciences, Mountain View, CA) with cell quest software. Cell sorting was performed using FACSAria (BD Biosciences).
ELISA
The concentrations of interferon-γ (IFN-γ) in the culture supernatants were measured by sandwich ELISA, using ‘high-binding’ EIA/RIA plates (Costar, NY), purified or biotinylated antibodies (Caltag, CA), and horseradish peroxidase-conjugated streptavidin (Thermo Scientific, Rockford, IL). As the substrate for peroxidase, a TMB (3,3′,5,5′-tetramethylbenzidine) liquid substrate system (Sigma, St Louis, MO) was used, and the reaction was stopped by adding the same amount of 0.5 m H2SO4. After stopping the reaction, the absorbance at 450 nm was measured.
Results
The absence of functional NIK could affect the efficiency of thymic positive selection of T cells expressing a transgenic TCR
The fact that alymphoplasia mice have a normal number of T cells suggests that the absence of NIK may not have a significant impact on T-cell development. However, we examined the possibility that the aly mutation could affect the threshold of thymic selection by investigating the differentiation of T cells expressing a fixed, transgenic TCR. The TCR-Tg mouse used here was the QM11TCR-Tg mouse that we developed and have reported on previously.19 In this TCR-Tg system, several different selecting MHC molecules have been identified. Among these, class I MHC Dq/Lq and class II MHC I-Aq were identified to be the positively selecting elements to drive Tg-TCR+ T cells to differentiate CD8+ or CD4+ T cells, respectively.19 Hence, on an H-2q background, selecting MHC molecules of both class I and class II are available simultaneously. Indeed, in this situation, differentiation of idiotypehigh+ cells into both CD4SP and CD8SP cells could be observed.19
We crossed aly/aly mice with the QM11TCR-Tg mice and examined whether the differentiation of the idiotypehigh+ cells could be influenced by the aly mutation. As shown in Fig. 1a,b, in H-2q, QM11TCR-Tg, aly/aly mice, the efficiency of positive selection of idiotypehigh+ cells was decreased compared with that in aly/+ mice. The effect was more pronounced for differentiation into CD8SP cells than into CD4SP cells. It should be noted, however, that differentiation of idiotypehigh+ cells into CD4SP was also less effective in aly/aly mice, although it was not statistically significant. The same tendency was also observed in splenic mature T cells. Notably, the reduction of the proportion of idiotypehigh+ cells in aly/aly mice was statistically significant for both CD4+ and CD8+ T cells (Fig. 1c), whereas the total T-cell number was not decreased in aly/aly mice (data not shown).
Figure 1.
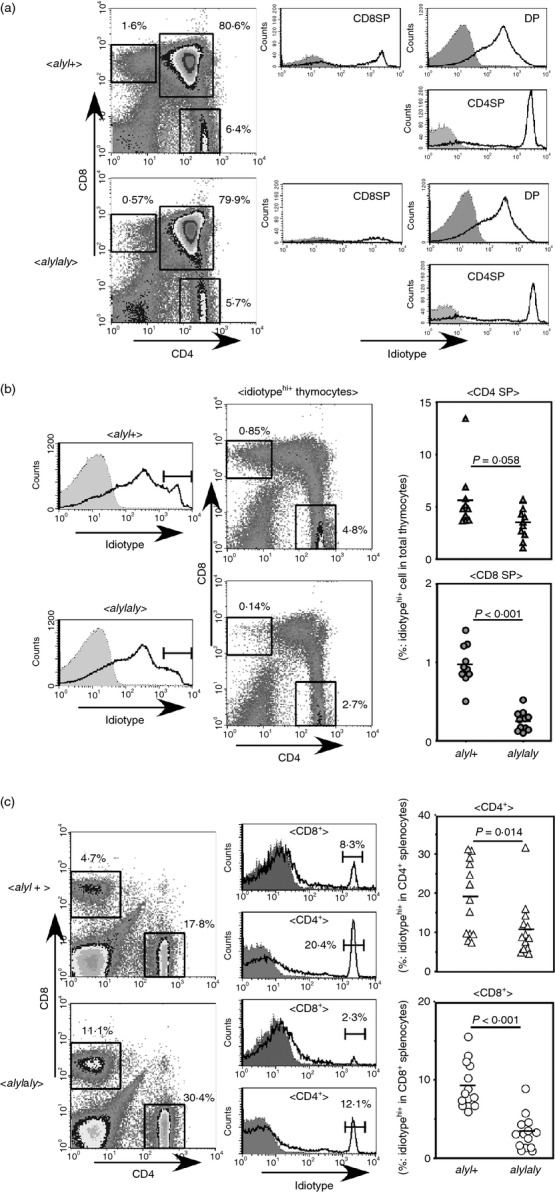
Inefficient positive selection of QM11T-cell receptor transgenic (QM11TCR-Tg) T cells in aly/aly mice. (a) The T cells expressing QM11TCR can be positively selected by class I MHC (Dq/Lq) or class II MHC (I-Aq) to mature into CD8 single-positive (SP) or CD4SP T cells, respectively. Expression of idiotype on CD4SP, CD8SP or CD4/CD8 double-positive (DP) thymocytes of H-2q (expressing both Dq/Lq and I-Aq) aly/+ (upper panels) and aly/aly (lower panels) mice are shown. Right panels indicate the expression of idiotype on CD4SP, CD8SP and DP thymocytes. Shaded histograms indicate negative control staining. A representative set of results from the analyses of > 10 mice [as in (b)] is shown. (b) Idiotypehigh+ cells in thymocytes from H-2q aly/+ (upper panels) and aly/aly (lower panels) mice were gated as indicated to show their expression of CD4 and CD8. The numbers indicate the percentage CD4SP idiotypehigh+ or CD8SP idiotypehigh+ cells in total thymocytes. In the right hand graphs, the percentages of CD4SP idiotypehigh+ cells (top) and CD8SP idiotypehigh+ cells (bottom) in total thymocytes from H-2q, QM11TCR-Tg, aly/+, or aly/aly mice are shown. In the left panels, shaded histograms indicate negative control staining. Mice aged from 8 to 18 weeks were analysed. The P values were determined by two-tailed Student’s t-test. (c) The splenocytes from H-2q aly/+ (upper panels) and aly/aly (lower panels) mice were analysed for idiotype expression on CD4+ and CD8+ T cells. A representative set of results for the expression of CD4 and CD8 (left panels) and the expression of idiotype on CD4+ or CD8+ T cells (middle panels) is shown. In the left panels, the numbers indicate the percentage of CD4+ or CD8+ T cells in total splenocytes. In aly/aly mice, B-cell survival is impaired12 owing to defective B cell activating factor belonging to the tumor necrosis factor family (BAFF) signalling, which may lead to relative increase of T-cell proportion in the spleen of aly/aly mice. In the middle panels, the shaded histograms show negative control staining, and the numbers indicate the percentages of idiotypehigh+ cells among CD4+ or CD8+ T cells. Fourteen mice (6–13 weeks old) for each strain were analysed and the results are shown in the right hand graphs.
These results were also reproduced when analysed on a RAG-2-deficient background (see Supplementary material, Fig. S1). The number of idiotypehigh+ cells either of CD4SP or CD8SP mature thymocytes were smaller in aly/aly mice compared with those in aly/+ mice. This was also true for the number of CD4+ or CD8+ T cells in the spleen.
These results indicate that NIK plays an important role in the optimal positive selection of at least some subsets of T cells, and so that the αβ T-cell repertoire in the aly/aly mouse may be different from that in the normal mouse.
The effects of aly mutation on the negative selection of T cells expressing the transgenic TCR
We next examined the impact of aly mutation on the negative selection in the QM11TCR-Tg system. In this setting, idiotypehigh+ cells were negatively selected by two different MHC molecules, one of which was I-Ak (original specificity of QM11TCR), and the other was the H-2s class I MHC molecule.19–21 Negative selection of idiotypehigh+ cells in QM11TCR-Tg mice was observed therefore in two strains of mouse; H-2bxk and H-2s. The total number of thymocytes in aly/aly mice of either H-2bxk or H-2s background was not different from that observed in aly/+ mice (Fig. 2a), where the proportion of CD4/CD8 DP subset was dramatically decreased in both aly/+ and aly/aly mice (Fig. 2b, data not shown), implying that negative selection of the cells specific to the antigens expressed in the thymus may be operated properly in aly/aly mice. Unexpectedly, however, it was noticed that in the H-2s background, the CD8low+ idiotypehigh+ cells were markedly reduced in aly/aly mice (Fig. 2c). The CD8low+ idiotypehigh+ cells were also reported in other TCR-Tg mice,22–23 and were demonstrated to exist in any H-2 backgrounds, although their existence is eminent particularly in negatively selecting background. Although the mechanism of their differentiation has not been fully clarified, it was suggested that these cells might be one of the populations that represents the γδ lineage of T cells in the TCR-Tg setting.24 In our system, this population was deleted in the H-2bxk background, probably because of co-receptor-independent recognition of I-Ak by the QM11TCR,25 and therefore the effect of the aly mutation on the generation of this population in the H-2bxk background could not be investigated. Nevertheless, the examination of mice on an H-2s background indicated that the generation of the CD8low+ idiotypehigh+ cells may require functional NIK.
Figure 2.
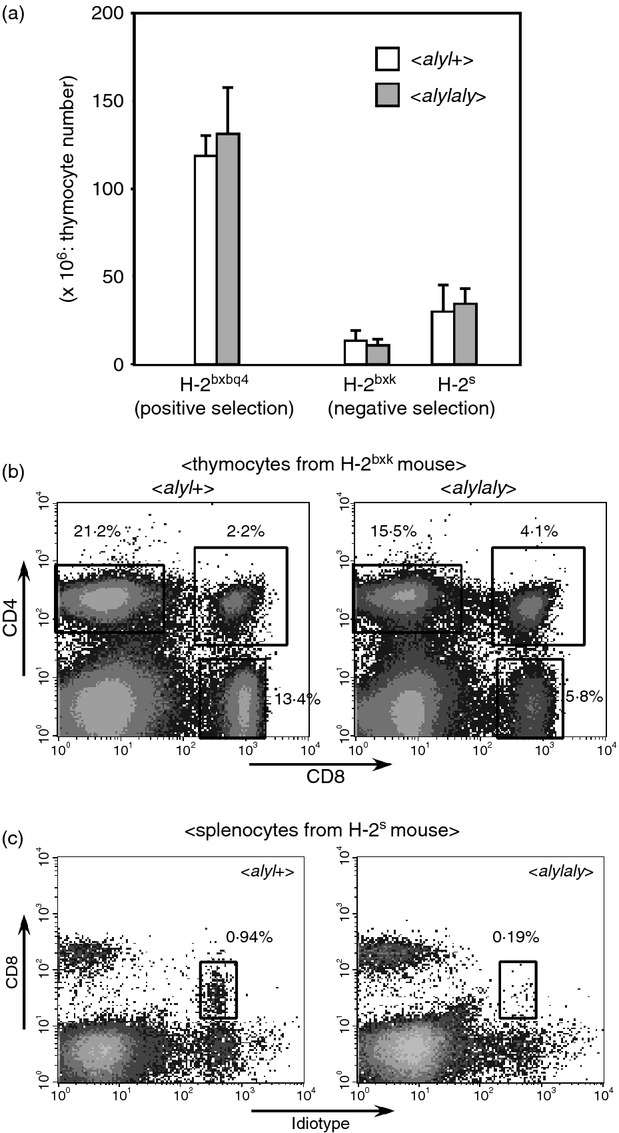
Negative selection of QM11T-cell receptor transgenic (QM11TCR-Tg) T cells occurred normally, but CD8low+ idiotypehigh+ cells failed to develop in aly/aly mice. (a) The thymocyte numbers of QM11TCR-Tg, aly/aly or aly/+ mice with indicated H-2 backgrounds are shown. Five to six mice aged 8 to 10 weeks for each strain were analysed. Error bars represent SD. (b) The expression of CD4 and CD8 on the thymocytes from QM11TCR-Tg, aly/aly (right) or aly/+ (left) mice with negatively selecting H-2bxk background is indicated. A representative set of results from the analyses of > 10 mice is shown. (c) The expression of CD8 and idiotype on the splenocytes from QM11TCR-Tg, aly/aly (right) or aly/+ (left) mice on an H-2s background are shown. A representative set of results from analyses of > 10 mice is shown. The percentage of CD8low+ idiotypehigh+ cells in aly/aly mice varied from 1/20 to 1/3 of that in aly/+ mice.
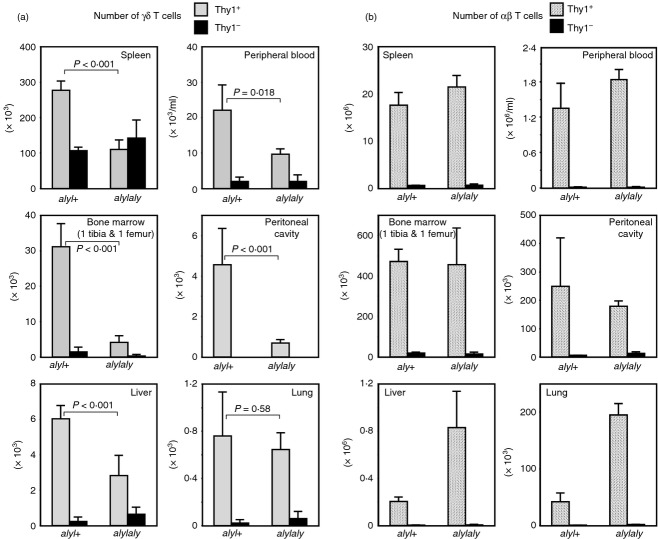
The proportion and the number of γδ T cells, but not αβ T cells, in the peripheral organs were decreased in aly/aly mice
The observation of impaired generation of CD8low+ idiotypehigh+ cells in the periphery of the H-2s, QM11TCR-Tg, aly/aly mice led us to investigate the development of γδ T cells in the non-transgenic aly/aly mouse. In the thymus, despite the total cell number being normal12 (data not shown), the proportion of γδ T cells was slightly reduced in aly/aly mice compared with that in aly/+ mice (Fig. 3a). However, the reduction in aly/aly mouse of the γδ T cells, especially of Thy1.2+ cells, was more evident in peripheral tissues, such as the spleen, peritoneal cavity, bone marrow, liver and lungs (Fig. 3b–g). This also held true in most, but not all, tissues when the absolute numbers of γδ T cells were compared (Fig. 4a). In contrast, as shown in Fig. 4(b), the number of αβ T cells did not decrease in the aly/aly mouse, or rather, it significantly increased in some tissues, such as liver and lung, which might be due to pathogenic infiltration of CD4+ αβ T cells.
Figure 3.
Reduced proportion of γδ T cells in aly/aly in comparison with that of aly/+ mice. (a) The average percentages (± SD) of γδ T cells in CD3high+ cells in thymi from aly/aly or aly/+ mice (10 times backcrossed to C57BL/6, 9–11 weeks old, n = 4) are demonstrated. The difference between aly/aly and aly/+ mice was statistically significant (P = 0·0029). (b–g) The mice analysed in (a) were examined for the expression of γδ T-cell receptor (TCR) and Thy1.2 on CD3+-gated cells in the indicated organs, and the average percentages ± SD in CD3+ cells are indicated. The difference between aly/aly and aly/+ mice was statistically significant in every organ indicated (P < 0·001).
Figure 4.
Absolute number of αβ or γδ T cells in aly/aly and aly/+ mice in the peripheral organs. The leucocytes were harvested from indicated organs from the mice analysed in Fig. 3, and the expression of CD3, T-cell receptor αβ (TCR-αβ), TCR-γδ and Thy1.2 on them was examined. Total numbers of γδ (a) or αβ (b) T cells from indicated organs from aly/aly or aly/+ mice are shown. Error bars represent SD.
The peripheral γδ T cells could be divided into specific subsets according to their expression of activation markers or cell surface molecules.26 We next assessed whether the decrease of peripheral γδ T cells in aly/aly mice may result from impaired generation of some particular γδ subsets, by comparing the expression of CD27, CD25, or CD122 on γδ T cells in the peripheral blood of aly/aly mice with those of aly/+ mice. As shown in Fig. 5, the proportion of cells positive for these markers was almost the same, suggesting that the reduction of the γδ T cells in aly/aly mice may be independent of expression for these markers.
Figure 5.
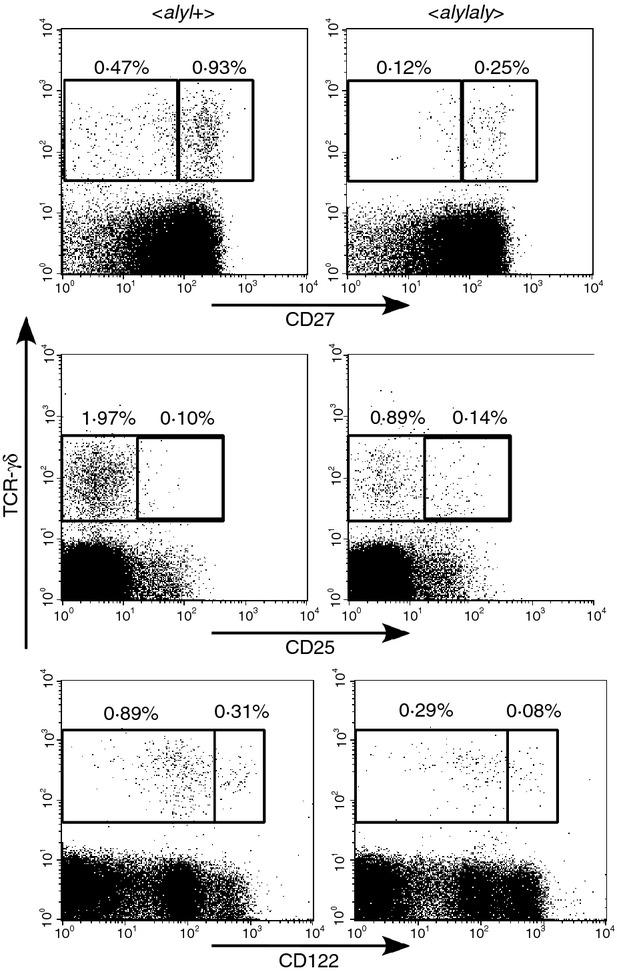
The majority of peripheral blood γδ T cells in aly/aly mice were CD27high+, CD25−, CD122low+, as were in aly/+ mice. Expression of CD25, CD27 or CD122 on γδ T cells in peripheral blood of aly/aly and aly/+ mice was investigated and the percentages of the cells positive or negative for each marker, in CD3+ cells are shown. Representative results obtained from three mice are indicated.
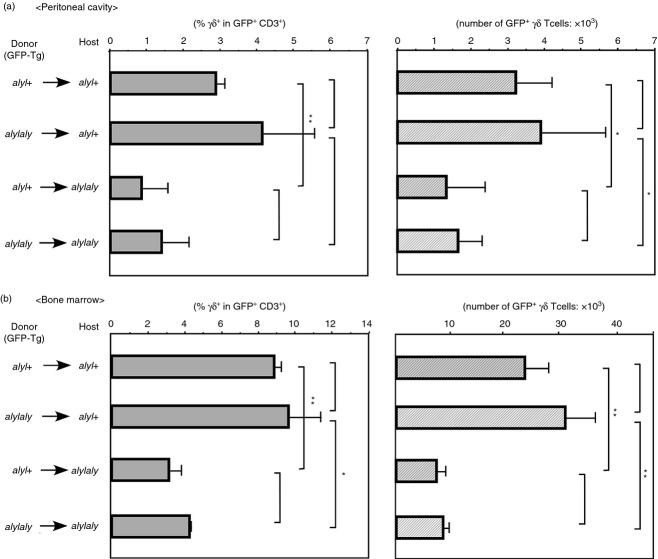
NIK in host cells may be necessary for maintaining a normal number of γδ T cells in the periphery
We next prepared the BM chimera mice to determine whether functional NIK in haematopoietic or host cells was important for the maintenance of a normal number of γδ T cells. For this purpose, the GFP-Tg mouse of B6 background was used to discriminate the donor cells from host cells. We developed the GFP-Tg, aly/aly or aly/+ mice and the BM cells from these mice were injected intravenously into non-Tg aly/aly or aly/+ mice that had been irradiated (8·5 Gy) the day before transfusion of donor cells. After 9 to 10 weeks, the number and proportion of GFP+ γδ T cells in several organs from those chimeric mice were analysed. For unknown reasons, in the BM chimeras, the number or proportion of peripheral γδ T cells was not statistically different in some sites other than peritoneal cavity or BM between the [GFP × aly/aly → aly/aly] mice and the [GFP × aly/+ → aly/+] mice, although there was still a tendency for the γδ T cells to be reduced in the [GFP × aly/aly → aly/aly] chimera compared with the [GFP × aly/+ → aly/+] chimera (Fig. S2, data not shown). In Fig. 6, the number and proportion of the GFP+ γδ T cells in the peritoneal cavity and the BM of each chimera are shown. Both the number and the proportion of γδ T cells were significantly reduced when aly/aly mice were used as hosts, as compared with those in aly/+ recipient mice, even when aly/+ donor cells were transplanted. By contrast, no significant differences in γδ T-cell number or proportion was found between the mice receiving aly/+ BM cells and the mice receiving aly/aly donor cells. These results indicate that NIK in non-haematopoietic cells, rather than γδ precursor cells, is important for supporting a normal number of γδ T cells in the periphery.
Figure 6.
Nuclear factor-κB-inducing kinase (NIK) in non-haematopoietic cells is important for maintaining a normal number of γδ T cells in peritoneal cavity and bone marrow. Bone marrow cells from green fluorescent protein transgenic (GFP-Tg) aly/aly or GFP-Tg aly/+ (B6 background) were injected into 8·5 Gy irradiated aly/aly or aly/+ mice (1 × 107/mouse). Nine to 10 weeks later, percentage of GFP+ γδ T cells in GFP+ CD3+ cells and absolute number of GFP+ γδ T cells in peritoneal cavity (left) and in bone marrow (right) were analysed. The average numbers ± SD from four recipients are shown. *P < 0.05, **P < 0.01.
Impaired IFN-γ production in CD27+ γδ T cells in aly/aly mice
To assess whether aly mutation would have any impact on the function of γδ T cells, IFN-γ production from splenic γδ T cells was examined. As it was shown that among splenic γδ T cells, CD27+ γδ T cells were the main sources of IFN-γ,27 we sorted CD27+ γδ T cells and stimulated the cells in vitro with plate-bound anti-CD3ε antibody. As shown in Fig. 7, the amount of IFN-γ secreted from aly/aly cells was smaller than that from normal aly/+ cells, suggesting that NIK may contribute not only to the development and/or peripheral distribution of γδ T cells but also to maximal production of IFN-γ from them.
Figure 7.
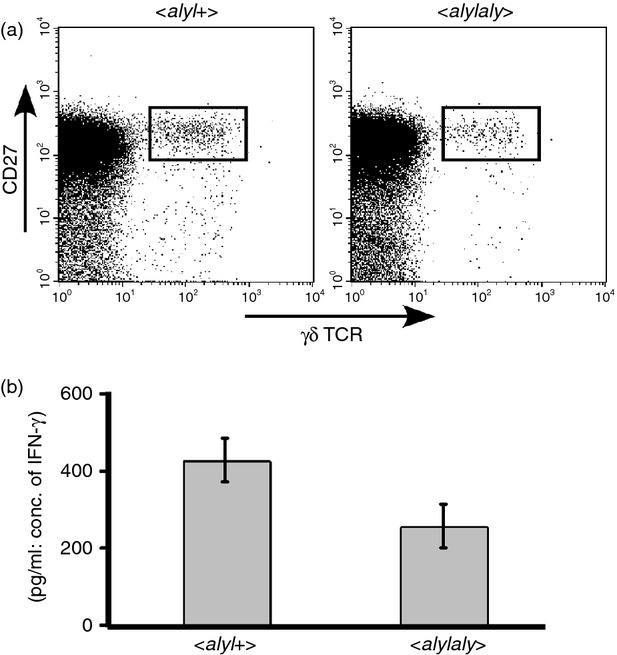
Impaired interferon-γ (IFN-γ) production from CD27high+ γδ T cells in aly/aly mouse. (a) The expression of CD27 and T-cell receptor (TCR) -γδ on splenocytes from aly/aly or aly/+ mice are shown. (b) CD27high+ γδ T cells in the spleen of aly/aly or aly/+ mice were sorted with cell sorter. Twenty thousand cells per well were stimulated with plate-bound anti-CD3 antibody (10 μg/ml) in a microtitre plate for 18 hr, and the concentration of IFN-γ in the supernatants was determined by ELISA. A representative result is shown. The average value of the ratio of IFN-γ secreted from aly/aly versus that secreted from aly/+ cells in three similar experiments was 0·55, and was statistically significant (P = 0·025).
Discussion
Nuclear factor-κB-inducing kinase, which was originally identified as a kinase mediating the signal from tumour mecrosis factor receptor, Fas or interleukin-1 receptor,10 is expressed in various types of cells, and plays a critical role in non-canonical pathways of NF-κB activation.28 The crucial role of NIK in the maintenance of proper immune function was highlighted by analyses of the alymphoplasia mouse, which was revealed to be defective in its ability to mount immune responses against allogeneic cells12 or viruses.29 On the other hand, the aly/aly mouse was also shown to develop autoimmune diseases in exocrine organs, such as lacrimal gland, lung, liver and salivary glands.30 Hence, in the aly/aly mouse, immune responses to foreign antigens seem to be impaired whereas those to autoantigens are more prone to be invoked compared with WT mouse. These observations indicate that the T-cell repertoire may not be appropriately formed in aly/aly mice. In fact, it was demonstrated that autoimmune diseases could be transferred to RAG2 knockout mouse by adoptively transferring a T-cell-enriched fraction of spleen from aly/aly mice.30 Nonetheless, no apparent abnormality was observed either in the thymocytes or splenic T cells in aly/aly mice.12
It has been shown that negative selection is defective in the aly/aly mouse, owing to impaired formation of medullary thymic epithelial cells,31 which were implicated in the deletion of auto-reactive T cells, especially the cells specific for peripheral tissue antigens. NIK is downstream of CD40 or RANK signalling and Akiyama et al.32 demonstrated that defective RANK/CD40-mediated signalling in medullary thymic epithelial cell precursor cells is responsible for impaired medullary thymic epithelial cell formation in aly/aly mouse.
The examination of negative selection in the QM11TCR-Tg, aly/aly mouse disclosed that elimination of T cells recognizing an auto-antigen expressed in the thymus could be properly carried out in aly/aly mice, which is consistent with a previous observation in the HY-TCR Tg mice.31 Hence, the defective self-tolerance in T cells of aly/aly mice appears to be limited against peripheral tissue antigens, particularly those in exocrine organs, although its underlying mechanism is at present unclear. In addition to imperfect negative selection in aly/aly mice, in the present study, we have demonstrated that positive selection in at least some subsets of T cells was indeed affected in aly/aly mice and suggested that NIK could be involved in shaping the αβ T-cell repertoire.
In the QM11TCR-Tg system, where both classes of selecting MHC molecules are available simultaneously, the differentiation of DP thymocytes into CD8SP was more severely affected than was CD4SP differentiation by the aly mutation. This is partly consistent with a previous study by Jimi et al.,4 demonstrating that the inhibition of NF-κB activity in thymocytes by transgenic expression of a ‘super-repressor’ form of IκBα, repressed the CD8SP cell differentiation, but had little impact on CD4SP cell differentiation in TCR-Tg mice. Substantial influence of CD4SP differentiation observed in the QM11TCR-Tg mouse could be due to the differences of TCR specificity, or it might be attributable to some functions of NIK other than NF-κB activation, as described below. We are currently preparing mice to develop BM chimeras so that we could determine whether impaired positive selection in aly/aly mice would be restored by transferring aly/+ BM cells.
In the TCR-Tg system, some unusual populations have been found, one of which is the CD8low+ idiotypehigh+ cells.22–23 Although these cells were initially thought to be an artefact of the TCR transgenic system, detailed analyses of these cells suggested that they might represent γδ T-cell lineage.24 This notion was further corroborated by the findings by Pennington et al.,33 who demonstrated that these cells expressed ‘γδ-biased genes’. Although the putative association between these two populations needs to be examined further in future studies, defective appearance of these populations in the periphery were also observed in our system of aly/aly mice.
Regarding the role of NIK in the development of γδ T cells, it was previously shown by Nanno et al.34 that the proportion of intestinal intraepithelial γδ T cells in aly/aly mice is smaller than that in aly/+ mice. This observation has been herein extended to show that the reduction of γδ T cells is more prominent in other peripheral organs. Thymic differentiation of γδ T cells has been shown to be regulated in trans by DP αβ thymocytes through stimulating the LTβR on γδ T cells;18 for this reason it was speculated that the development of γδ T cells may be influenced by the mutation of NIK which mediates signalling from LTβR.10 Unexpectedly, however, the BM chimera experiment suggested that NIK in host cells rather than donor cells is important for maintaining a normal number of γδ T cells in the peritoneal cavity and in the BM. This observation argues against the possibility that the impaired generation of γδ T cells in aly/aly mice may be the result of the impaired signalling from LTβR in γδ precursor. Rather, this result may indicate that the proper generation of γδ cells would require the intact thymic structure, as was observed in NKT cells.35 Alternatively, considering that the reduction of γδ T cells was more profound in the periphery than in the thymus, the cells in the peripheral tissue may require NIK expression for maintenance of a normal number of γδ T cells. It is also possible that more prominent reduction of γδ T cells in the periphery may result from a defect in emigration from the thymus.36 However, the impaired ability of IFN-γ production from splenic CD27+ γδ T cells from aly/aly mice may still possibly be due to defective trans-differentiation by LTβ stimulation.
The developmental pathways or the molecules involved in the differentiation of γδ T cells have not yet been clarified in comparison to those of αβ T cells. There are numbers of genes that were shown to be crucial in γδ T-cell development, but most of these are also mandatory for αβ T-cell differentiation.16 In terms of this, NIK may be a rare molecule whose absence affects the appearance of γδ but not αβ T cells. Furthermore, very few molecules have been described that need to be expressed in non-haematopoietic cells in contributing to proper γδ T-cell generation.
Although further investigation is essential to understand the molecular basis of NIK involvement in the maintenance of γδ cells, the function of NIK to support γδ T-cell differentiation could be independent of the non-canonical pathway of NF-κB activation, as it was shown that in mice deficient for either NF-κB2 or RelB, the proportion and number of γδ T cells remained unaltered.37 A similar situation has been reported that while the number of CD4+ FoxP3+ regulatory T cells is diminished in the aly/aly mouse, no such regulatory T-cell reduction was observed in NF-κB2-deficient mice.38–39 In this context, some studies have indicated that, in addition to NF-κB activation, NIK could participate in other signalling pathways, such as signal transducer and activator of transcription 340 or mitogen-activated protein kinases.41 Defining the pathways through which NIK takes part in γδ T-cell differentiation would lead to uncovering a novel function of NIK, which is being attempted in our ongoing study.
Acknowledgments
KE, MO, SK and HN designed and performed experiments, and analysed data. KE and KI wrote the manuscript. NS and KI supervised the research. This work was supported by a Grant-in-Aid for Scientific Research (KAKENHI) (22501024 to KE), and by the Private Universities Grant for Promotion of Fundamental Strategic Research from the Ministry of Education, Culture, Sports, Science and Technology.
Glossary
- Aly
alymphoplasia
- B6
C57BL/6
- BM
bone marrow
- DP
double positive
- LTβR
lymphotoxin β receptor
- MHC
major histocompatibility complex
- mTEC
medullary thymic epithelial cell
- NIK
nuclear factor-κB-inducing kinase
- RAG
recombination activating gene
- SP
single positive
- Tg
transgenic
- TCR
T-cell antigen receptor
- WT
wild-type
Disclosures
The authors declare no financial or commercial conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Defective positive selection of T cells expressingQM11T-cell receptor.
Impaired generation of γγ T cells in aly/aly host mice of bone marrow chimera.
References
- Yamasaki S, Ishikawa E, Sakuma M, et al. Mechanistic basis of pre-T cell receptor-mediated autonomous signaling critical for thymocyte development. Nat Immunol. 2006;7:67–75. doi: 10.1038/ni1290. [DOI] [PubMed] [Google Scholar]
- Siebenlist U, Brown K, Claudio E. Control of lymphocyte development by nuclear factor-κB. Nat Rev Immunol. 2005;5:435–45. doi: 10.1038/nri1629. [DOI] [PubMed] [Google Scholar]
- Voll RE, Jimi E, Phillips RJ, Barber DF, Rincon M, Hayday AC, Flavell RA, Ghosh S. NF-κB activation by the pre-T cell receptor serves as a selective survival signal in T lymphocyte development. Immunity. 2000;13:677–89. doi: 10.1016/s1074-7613(00)00067-4. [DOI] [PubMed] [Google Scholar]
- Jimi E, Strickland I, Voll RE, Long M, Ghosh S. Differential role of the transcription factor NF-κB in selection and survival of CD4+ and CD8+ thymocytes. Immunity. 2008;29:523–37. doi: 10.1016/j.immuni.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettmann T, Leiden JM. NF-κB is required for the positive selection of CD8+ thymocytes. J Immunol. 2000;165:5004–10. doi: 10.4049/jimmunol.165.9.5004. [DOI] [PubMed] [Google Scholar]
- Mora AL, Stanley S, Armistead W, Chan AC, Boothby M. Inefficient ZAP-70 phosphorylation and decreased thymic selection in vivo result from inhibition of NF-κB/Rel. J Immunol. 2001;167:5628–35. doi: 10.4049/jimmunol.167.10.5628. [DOI] [PubMed] [Google Scholar]
- Schulze-Leuhrmann J, Ghosh S. Antigen-receptor signaling to Nuclear Factor κB. Immunity. 2006;25:701–15. doi: 10.1016/j.immuni.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Morley SC, Weber KS, Kao H, Allen PM. Protein kinase C-θ is required for efficient positive selection. J Immunol. 2008;181:4696–708. doi: 10.4049/jimmunol.181.7.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost PJ, Weiss S, Ferch U, Gross O, Mak TW, Peschel C, Ruland J. Bcl10/Malt1 signaling is essential for TCR-induced NF-κB activation in thymocytes but dispensable for positive or negative selection. J Immunol. 2007;178:953–60. doi: 10.4049/jimmunol.178.2.953. [DOI] [PubMed] [Google Scholar]
- Malinin NL, Boldin MP, Kovalenko AV, Wallach D. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–4. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- Yin L, Wu L, Wesche H, Arthur CD, White JM, Goeddel DV, Schreiber RD. Defective lymphotoxin-β receptor-induced NF-κB transcriptional activity in NIK-deficient mice. Science. 2001;291:2162–5. doi: 10.1126/science.1058453. [DOI] [PubMed] [Google Scholar]
- Miyawaki S, Nakamura Y, Suzuka H, Koba M, Yasumizu R, Ikehara S, Shibata Y. A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur J Immunol. 1994;24:429–34. doi: 10.1002/eji.1830240224. [DOI] [PubMed] [Google Scholar]
- Shinkura R, Kitada K, Matsuda F, et al. Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-κB-inducing kinase. Nat Genet. 1999;22:74–7. doi: 10.1038/8780. [DOI] [PubMed] [Google Scholar]
- Sun S-C. The noncanonical NF-kB pathway. Immunol Rev. 2012;246:125–40. doi: 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Yamada T, Yoshinaga SK, Boone T, Horan T, Fujita S, Li Y, Mitani T. Essential role of NF-κB-inducing kinase in T cell activation through the TCR/CD3 pathway. J Immunol. 2002;169:1151–8. doi: 10.4049/jimmunol.169.3.1151. [DOI] [PubMed] [Google Scholar]
- Hayes SM, Love PE. A retrospective on the requirements for γδ T-cell development. Immunol Rev. 2007;215:8–14. doi: 10.1111/j.1600-065X.2006.00476.x. [DOI] [PubMed] [Google Scholar]
- Xiong N, Raulet DH. Development and selection of γδ T cells. Immunol Rev. 2007;215:15–31. doi: 10.1111/j.1600-065X.2006.00478.x. [DOI] [PubMed] [Google Scholar]
- Silva-Santos B, Pennington DJ, Hayday AC. Lymphotoxin-mediated regulation of γδ cell differentiation by αβ T cell progenitors. Science. 2005;307:925–8. doi: 10.1126/science.1103978. [DOI] [PubMed] [Google Scholar]
- Eshima K, Suzuki H, Shinohara N. Cross-positive selection of thymocytes expressing a single TCR by multiple major histocompatibility complex molecules of both classes: implications for CD4+ versus CD8+ lineage commitment. J Immunol. 2006;176:1628–36. doi: 10.4049/jimmunol.176.3.1628. [DOI] [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–9. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Eshima K, Takagaki Y, et al. Origin of a T cell clone with a mismatched combination of MHC restriction and coreceptor expression. J Immunol. 1994;153:4496–507. [PubMed] [Google Scholar]
- von Boehmer H, Kirberg J, Rocha B. An unusual lineage of α/β T cells that contains autoreactive cells. J Exp Med. 1991;174:1001–8. doi: 10.1084/jem.174.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JH, Meleedy-Rey P, McCulley DE, Sha WC, Nelson CA, Loh DY. Evidence for CD8-independent T cell maturation in transgenic mice. J Immunol. 1990;144:3318–25. [PubMed] [Google Scholar]
- Bruno L, Fehling HJ, von Boehmer H. The αβ T cell receptor can replace the γδ receptor in the development of γδ lineage cells. Immunity. 1996;5:343–52. doi: 10.1016/s1074-7613(00)80260-5. [DOI] [PubMed] [Google Scholar]
- Eshima K, Tachibana M, Suzuki H, Yamazaki S, Shinohara N. Co-receptor-independent signal transduction in a mismatched CD8+ major histocompatibility complex class II-specific allogeneic cytotoxic T lymphocyte. Eur J Immunol. 1997;27:55–61. doi: 10.1002/eji.1830270109. [DOI] [PubMed] [Google Scholar]
- Hayday AC. γδ T cells and the lymphoid stress–surveillance response. Immunity. 2009;31:184–96. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Ribot JC, deBarros A, Pang DJ, et al. CD27 is a thymic determinant of the balance between interferon-γ- and interleukin 17-producing γδ T cell subsets. Nat Immunol. 2009;10:427–36. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thu YM, Richmond A. NF-κB inducing kinase: a key regulator in the immune system and in cancer. Cytokine Growth Factor Rev. 2010;21:213–26. doi: 10.1016/j.cytogfr.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer U, Althage A, Odermatt B, Hengartner H, Zinkernagel RM. Immunodeficiency of alymphoplasia mice (alyalyin vivo: structural defect of secondary lymphoid organs and functional B cell defect. Eur J Immunol. 2000;30:2799–807. doi: 10.1002/1521-4141(200010)30:10<2799::AID-IMMU2799>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Tsubata R, Tsubata T, Hiai H, et al. Autoimmune disease of exocrine organs in immunodeficient alymphoplasia mice: a spontaneous model for Sjögren’s syndrome. Eur J Immunol. 1996;26:2742–8. doi: 10.1002/eji.1830261129. [DOI] [PubMed] [Google Scholar]
- Kajiura F, Sun S, Nomura T, et al. NF-κB-inducing kinase establishes self-tolerance in a thymic stroma-dependent manner. J Immunol. 2004;172:2067–75. doi: 10.4049/jimmunol.172.4.2067. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Shimo Y, Yanai H, et al. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity. 2008;29:423–37. doi: 10.1016/j.immuni.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Pennington DJ, Silva-Santos B, Shires J, et al. The inter-relatedness and interdependence of mouse T cell receptor γδ+ and αβ+ cells. Nat Immunol. 2003;4:991–8. doi: 10.1038/ni979. [DOI] [PubMed] [Google Scholar]
- Nanno M, Matsumoto S, Koike R, et al. Development of intestinal intraepithelial T lymphocytes is independent of Peyer’s patches and lymph nodes in aly mutant mice. J Immunol. 1994;153:2014–20. [PubMed] [Google Scholar]
- Nakagawa K, Iwabuchi K, Ogasawara K, et al. Generation of NK1.1+ T cell antigen receptor α/β+ thymocytes associated with intact thymic structure. Proc Natl Acad Sci U S A. 1997;94:2472–7. doi: 10.1073/pnas.94.6.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franki AS, Van Beneden K, Dewint P, et al. A unique lymphotoxin αβ-dependent pathway regulates thymic emigration of Vα14 invariant natural killer T cells. Proc Natl Acad Sci U S A. 2006;103:9160–5. doi: 10.1073/pnas.0508892103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powolny-Budnicka I, Riemann M, Tänzer S, Schmid RM, Hehlgans T, Weih F. RelA and RelB transcription factors in distinct thymocyte populations control lymphotoxin-dependent interleukin-17 production in γδ T cells. Immunity. 2011;34:364–74. doi: 10.1016/j.immuni.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Zhu M, Chin RK, Christiansen PA, Lo JC, Liu X, Ware C, Siebenlist U, Fu YX. NF-κB2 is required for the establishment of central tolerance through an Aire-dependent pathway. J Clin Invest. 2006;116:2964–71. doi: 10.1172/JCI28326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Wang Z, Ding J, Peterson P, Gunning WT, Ding HF. NF-κB2 is required for the control of autoimmunity by regulating the development of medullary thymic epithelial cells. J Biol Chem. 2006;281:38617–24. doi: 10.1074/jbc.M606705200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadiminty N, Chun JY, Hu Y, Dutt S, Lin X, Gao AC. LIGHT, a member of the TNF superfamily, activates Stat3 mediated by NIK pathway. Biochem Biophys Res Commun. 2007;359:379–84. doi: 10.1016/j.bbrc.2007.05.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foehr ED, Bohusla J, Chen LF, DeNoronha C, Geleziunas R, Lin X, O’Mahony A, Greene WC. The NF-κB-inducing kinase induces PC12 cell differentiation and prevents apoptosis. J Biol Chem. 2000;275:34021–4. doi: 10.1074/jbc.C000507200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Defective positive selection of T cells expressingQM11T-cell receptor.
Impaired generation of γγ T cells in aly/aly host mice of bone marrow chimera.