Abstract
Neospora caninum is an Apicomplexa parasite that in the last two decades was acknowledged as the main pathogenic agent responsible for economic losses in the cattle industry. In the present study, the effectiveness of intranasal immunization with N. caninum membrane antigens plus CpG adjuvant was assessed in a murine model of intragastrically established neosporosis. Immunized mice presented a lower parasitic burden in the brain on infection with 5 × 107 tachyzoites, showing that significant protection was achieved by this immunization strategy. Intestinal IgA antibodies raised by immunization markedly agglutinated live N. caninum tachyzoites whereas previous opsonization with IgG antibodies purified from immunized mice sera reduced parasite survival within macrophage cells. Although an IgG1 : IgG2a ratio < 1 was detected in the immunized mice before and after infection, indicative of a predominant T helper type 1 immune response, no increased production of interferon-γ was detected in the spleen or mesenteric lymph nodes of the immunized mice. Altogether, these results show that mucosal immunization with N. caninum membrane proteins plus CpG adjuvant protect against intragastrically established neosporosis and indicate that parasite-specific mucosal and circulating antibodies have a protective role against this parasitic infection.
Keywords: antibody responses, CpG DNA, mucosal immunity, mucosal vaccines, parasitology
Introduction
Neospora caninum is an Apicomplexa parasite initially described as the causative agent of neuromuscular disease in dogs.1 Although canids have been identified as the definitive hosts of N. caninum, this parasite can infect a wide range of intermediate hosts including bovines.2 Infected cattle have increased incidence of abortion, which, together with the high efficiency of vertical transmission, makes neosporosis responsible for severe economic losses.3 Therefore, effective control methods that could prevent parasite spread are necessary. Lack of intervention carries too great a risk and a test and cull approach, despite its effectiveness, is too expensive. Coccidiostatic treatment also appears to be an expensive option that raises concerns regarding its use in animals for human consumption.4 Vaccination appears to be the best approach to effectively control neosporosis.5 However, no commercial vaccine is currently available for neosporosis, after the recent withdrawal of Bovilis® Neoguard, which nevertheless had limited efficacy.3 Therefore, development of a novel vaccine that could prevent this parasitic disease is a pressing necessity.
Attenuated N. caninum tachyzoites were successfully used to immunize mice6,7 or cattle9 against neosporosis. However, the use of attenuated strains is undesirable because of their short shelf life and the possible regression to a more virulent status.5 On the other hand, although it was reported that immunization with whole parasite lysates protected mice from N. caninum infection or vertical transmission,10–11 other studies showed that immunization using parasite lysates conferred little protection or even exacerbated the outcome of murine infection12,13 and failed to prevent vertical transmission in cattle.15 Recombinant N. caninum proteins have also been tested as potential vaccine candidates with promising although variable efficacy.16–22 Nonetheless, and despite the gastrointestinal mucosa being a natural infection route for N. caninum, mucosal (intranasal; i.n.) immunization against neosporosis has been attempted in a limited number of studies that yielded encouraging results.23,24 Despite the immunization route used therein, the immune response in the mucosae or associated lymphoid tissue was not specifically addressed.
Here, our previously described model of N. caninum infection established through the gastrointestinal tract26–27 was used to assess the protective effect of i.n. immunization against neosporosis by using N. caninum membrane proteins (NcMP) as target antigens. Our results show that immunization with NcMP plus CpG adjuvant conferred protection against the parasite infection. Moreover, by showing an in vitro effector function of mucosal and circulating antibodies, we provide evidence for a protective role of the humoral immune response against neosporosis.
Materials and methods
Animals
Seven-week-old female C57BL/6 mice were purchased from Charles River (Barcelona, Spain). Animals were kept at the Instituto de Ciências Biomédicas Abel Salazar animal facility throughout the experimental procedures. Interleukin-12 (IL-12)/IL-23 p40−/− C57BL/6 mice 7–11 weeks old, were purchased from Jackson Laboratories (Bar Harbor, ME) and bred at the same facility. Procedures involving mice were performed according to the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (ETS 123) and 86/609/EEC Directive and Portuguese rules (DL 129/92). Authorization for the experiments was issued by the animal welfare section of the competent national board, Direcção Geral de Veterinária (0420/000/000/2008).
Parasites
Neospora caninum tachyzoites (Nc1 isolate) were kept by serial passages in VERO cell cultures, maintained in minimal essential medium containing Earle’s salts (Sigma, St Louis, MO), supplemented with 10% fetal calf serum (PAA Laboratories, Pasching, Austria), l-glutamine (2 mm), penicillin (200 IU/ml) and streptomycin (200 g/ml) (all from Sigma), in a humidified atmosphere with 5% CO2 at 37°. Tachyzoites were maintained until 80% destruction of the host cell monolayer and were isolated as previously described.26 Briefly, free parasites and adherent cells were recovered using a cell scraper and centrifuged at 1500 g for 15 min. The pellet was passed through a 25-G needle and then washed three times in PBS by centrifugation at 1500 g for 15 min. The resulting pellet was resuspended and passed through a PD-10 desalting column, containing Sephadex™ G-25M (GE Healthcare, Freiburg, Germany). Tachyzoite concentration was determined in a haemocytometer. In these experiments the parasites used underwent fewer than 15 in vitro passages from the original ATCC vial. The viability of the used inocula was confirmed in IL-12/IL-23 p40−/− mice that were earlier shown to be highly susceptible to neosporosis.28
Preparation of whole tachyzoite lysates and cell-membrane extracts
The NcMP were extracted by using a modification of a previously described method.29–30 Briefly, free tachyzoites were resuspended in PBS containing 0·75% Triton X-114 (Sigma), incubated for 10 min on ice and centrifuged at 10 000 g for 30 min at 4°. The supernatant was recovered and placed in a water bath at 30° for 3 min. The procedure was repeated and the supernatant was centrifuged at 1000 g for 3 min at room temperature. The aqueous phase was discarded and the NcMP were precipitated with the addition of absolute ethanol, vortexed vigorously for 15 seconds and incubated for 1 hr on ice. The samples were centrifuged at 12 000 g for 20 min at 4° and the resulting pellet was dried, resuspended in PBS and stored at −20°. Whole N. caninum lysates were prepared by disruption of tachyzoites following sonication (26 cycles of 15 seconds at 100 W) with a Branson cell disrupter model W 185 D in an ice bath. The obtained N. caninum sonicates (NcS) were sequentially passaged through 0·45-µm and 0·2-μm pore-size filters and stored at −20°. Quantification of NcMP or NcS was performed using the Lowry protein assay. SDS–PAGE was performed following each protein extraction to determine and confirm the protein migration profile. Briefly a discontinuous SDS–PAGE (4–10% acrylamide) was loaded with 10 μg NcMP or NcS, previously heated at 95° for 5 min, and electrophoresis was carried out at a 25-mA constant current. Protein migration profiles were visualized using silver nitrate staining.
Electrophoretic analysis of NcMP
Neospora caninum tachyzoite membrane proteins were prepared for their use as target antigens in mucosal immunization. After extraction, prepared proteins were analysed by SDS–PAGE under reducing conditions and the protein migration profile was determined. As shown in the Supplementary material, Fig. S1, the membrane protein extraction displayed an enrichment of the proteins with molecular weights of approximately 55 000, 35 000 and 29 000, as compared with the proteins obtained in NcS preparations. Additionally, the extraction protocol yielded proteins with estimated molecular weights of 39 000 and 17 000 that were not visible in the NcS gel lane.
Immunizations and tissue sample collection
Eight-week-old female mice were used in three independent experiments with random distribution into four groups per experiment. The immunizations and procedures for collection of serum or vaginal or intestinal lavage fluids (VLF and ILF, respectively) are schematically described in the Supplementary material, Fig. S2. Mice were immunized i.n. at day zero under light isoflurane anaesthesia with 20 μl of PBS containing 30 μg NcMP (NcMP group) or 30 μg NcMP plus 10 μg CpG 1826 VacciGrade (Invivogen, San Diego, CA) (NcMP/CpG group). Sham-immunized control mice were treated with PBS alone (PBS group) or with PBS containing 10 μg CpG 1826 VacciGrade (CpG group). The immunization procedure was repeated 3 weeks after the first immunization. At 6 weeks, all mice were challenged intragastrically (i.g.) with 5 × 107 freshly isolated N. caninum tachyzoites as previously described.26 At 7 weeks, mice were killed by cervical dislocation and spleens and mesenteric lymph nodes (MLN) were aseptically removed for analysis of the immune response, while the brains were collected and stored at −20° for DNA extraction. One week after the boost immunization and 1 week after infection, serum was collected from all mice from the the submandibular vein for detection of N. caninum-specific IgG. At 4 and 7 weeks after the first immunization, vaginal and intestinal lavages were performed, respectively, for detection of N. caninum-specific IgA. The total number of mice used in the three experiments was 14 in the PBS and NcMP/CpG groups and 13 in the CpG and NcMP groups. Mice similarly immunized with NcMP/CpG or treated with CpG alone (n = 6 for both groups) were kept for 4 months after the boost immunization and then killed for analysis of intestinal IgA.
Antibody detection
Serum IgG1 and IgG2a antibodies specific for NcMP were quantified by ELISA. Briefly 96-well plates (Maxisorp; Nunc, Roskilde, Denmark) were coated overnight at 4° with NcMP diluted in PBS at a concentration of 5 μg/ml. All the wells were saturated with 2% BSA (Sigma) in TST buffer (150 mm NaCl, 10 mm EDTA and 0·05% Tween 20, pH 8) for 1 hr at room temperature. Serum samples were serially diluted in 1% BSA TST buffer and incubated for 1 hr at room temperature, followed by washing and addition of alkaline phosphatase-coupled goat anti-mouse IgG1 or IgG2a monoclonal antibodies (mAb) (Southern Biotechnology Associates, Birmingham, AL) and incubation for 1 hr at room temperature. After washing, the specifically bound antibodies were detected by adding the p-nitrophenyl phosphate (Sigma) substrate solution and on development the reaction was stopped by the addition of 0·1 m EDTA, pH 8 solution. The absorbance was measured at 405 nm, subtracting for each well the value of the absorbance at 570 nm. The antibody titres were expressed as the log10 value of the reciprocal highest dilution with an absorbance higher than the value of the control (no serum added). IgA antibodies specific for NcMP were quantified by ELISA as described above, using alkaline phosphatase-coupled goat anti-mouse anti-IgA mAb (Southern Biotech).
Purification of serum IgG antibodies and mucosal IgA
Mouse serum samples and ILF collected on the day of euthanasia were, respectively, used for IgG and IgA purification. Pooled sera collected from mice of the NcMP/CpG and CpG groups were used to purify IgG antibodies by using a HiTrap Protein G HP purification column (GE Healthcare), according to the manufacturer’s instructions. Recovered antibodies were buffer-exchanged against sterile PBS to a final concentration of 4·5 mg/ml as determined by Lowry protein assay and stored at −20°. The purified IgG fractions obtained from the sera of CpG or NcMP/CpG groups were, respectively, designated as IgG-CpG or IgG-NcMP/CpG. The NcMP-specific antibody titres of the IgG-CpG and IgG-NcMP/CpG preparations were below the detection limit and 1·559 × 109, respectively, as determined by ELISA.
To obtain IgA antibodies, pooled ILF were passed through a 20-μm pore-size filter before being introduced in a Protein L/Agarose (Invivogen) column. Antibody purification was carried out according to the manufacturer’s instructions. Recovered antibodies were buffer-exchanged against sterile PBS, and stored at −20°. The purified IgA fractions obtained from the ILF of the CpG or NcMP/CpG groups were, respectively, designated as IgA-CpG or IgA-NcMP/CpG. The total IgA titres for the IgA-CpG and IgA-NcMP/CpG preparations were 657 648 and 786 788, respectively, and were normalized to 650 × 103 for further use. The NcMP-specific IgA titres of the IgA-CpG and IgA-NcMP/CpG preparations were below the detection limit and 8995, respectively.
Antibody-binding and parasite-agglutination assays
To evaluate the ability of antibodies present in IgG-CpG, IgA-CpG, IgG-NcMP/CpG or IgA-NcMP/CpG to bind N. caninum, different dilutions of these preparations were incubated with 1 × 106 tachyzoites, for 25 min on ice. Detection of bound antibodies was made by using flow cytometry, for which parasites were further incubated with polyclonal anti-IgG antiserum, FITC-conjugated (Southern Biotech), or with anti-IgA FITC-conjugated (BD Biosciences Pharmingen, San Diego, CA) mAb (clone C10-3) for 25 min on ice and then washed with PBS containing 1% BSA and 10 mm sodium azide. Parasite samples were analysed in an EPICS XL flow cytometer using the EXPO32ADC software (Beckman Coulter, Miami, FL). The collected data files (100 000 events per sample) were converted for analysis with the CellQuest software, v3.2.1f1 by using FACS convert, v1.0 (both from Becton Dickinson, San Jose, CA). Agglutination assays were performed by incubating 1 × 106 tachyzoites with either IgA-CpG or IgA-NcMP/CpG or PBS alone for 1 hr at 4°. After incubation, smears of each sample were prepared on microscope slides that were fixed in cold methanol for 5 min. Samples were then stained with Hemacolor 2 and 3 (Merk, Darmstadt, Germany) according to the manufacturer’s instructions. Mounted slides were observed in a light microscope and 20 micrographs at 200 × and 400 × magnification were taken (Leica Qwin plus v3.5.1 Software, Leica Microsystems, Wetzlar, Germany) as a representative display of each slide. The number and size of parasite clusters were analysed using ImageJ software (Version 1.47, National Institutes of Health, Bethesda, MD).
Intracytoplasmic staining
For intracellular cytokine detection by flow cytometry, spleens and MLN were aseptically removed from the killed infected mice, homogenized in Hanks’ balanced salt solution (Sigma) and red blood cells were lysed. The remaining cells were counted and plated in round-bottom 96-well plates (Nunc), at a concentration of 1 × 106 cells/ml in RPMI-1640 (Sigma) supplemented with 10% fetal calf serum (PAA Laboratories), HEPES (10 mm), penicillin (200 IU/ml) and streptomycin (200 g/ml) (all from Sigma), β-mercaptoethanol (0·1 mm) (Merk) (RPMI-1640 complete medium). Cells were incubated in a humidified atmosphere with 5% CO2 at 37° for 5 hr under stimulation with 20 ng/ml PMA (Sigma), 200 ng/ml ionomycin (Merk) and 10 ng/ml brefeldin A (Epicentre Biotechologies, Madison, WI). Then, cells were recovered and non-specific antibody binding was prevented by the pre-incubation with anti-FcγR mAb followed by incubation with either anti-CD4 peridinin-chlorophyll protein-cychrome 5.5 (PerCP-Cy5.5) -conjugate (clone RM4-5) or anti-CD8 PerCP-Cy5.5-conjugate (clone 53-6.7) mAb (both from BD Biosciences). Following extracellular staining the cells were washed, fixed in 2% formaldehyde, washed again and permeabilized with 0·05% saponin (Sigma)/PBS solution. Intracytoplasmic staining was carried out with anti-interferon-γ (IFN-γ) FITC-conjugate (clone XMG1.2), anti-IL-4 phycoerythrin-conjugate (clone BVD4-1D11) and anti-IL-10 phycoerythrin-conjugate (clone JES5-16E3) (all from BD Biosciences) after pre-incubation of the cells with anti-FcγR mAb. Antibody-labelled cells were analysed in an EPICS XL flow cytometer using the EXPO32ADC software (Beckman Coulter). At least 150 000 events were acquired per sample. The collected data files were converted using FACS convert, v1.0 (Becton Dickinson) and analysed using Cell Quest software, v3.2.1f1 (Becton Dickinson).
In vitro cell cultures and cytokine detection
To assess in vitro cytokine production by NcMP-stimulated spleen and MLN cells, 5·0-ml aliquots of cell suspensions prepared as described above for intracytoplasmic staining, by homogenizing these organs in Hanks’ balanced salt solution (Sigma) followed by red blood cell lysis, were layered onto 2·5 ml of a polysucrose-sodium ditrizoate solution (Histopaque 1083®, Sigma) and centrifuged at 800 g for 20 min at room temperature. Mononuclear cells collected from the medium–Histopaque interface were washed, suspended in RPMI-1640 complete medium, plated (5·0 × 105/well) in round-bottom 96-well plates, and stimulated with NcMP (100 μg/ml) for 5 days at 37° and 5% CO2. Four animals from each group were used and triplicate wells were set for cells cultured from each animal. The concentrations of IFN-γ and IL-4 in cell culture supernatants were quantified with the Mouse IFN-γ DuoSet® ELISA development system (R&D Systems, Minneapolis, MN) and the IL-4 ELISA Ready-Set-Go!® (eBioscience, San Diego, CA) kits, respectively, both according to the manufacturer’s instructions.
Macrophage cell cultures and parasite opsonization survival
Murine bone marrow-derived macrophages were differentiated from bone marrow precursors. The bone marrow-derived macrophage cultures were generated in six-well plates (Nunc) by culturing 5 × 106 cells in 5 ml RPMI-1640 complete medium supplemented with 10% L-929 cell line conditioned medium and incubated at 37° in a 5% CO2 humidified chamber. On day 4, the cell culture medium was renewed and 5 ml of fresh medium supplemented with L-929 cell line conditioned medium was added. Differentiated macrophages were harvested on day 7 by gently scraping the wells. The cells were counted and plated in 24-well plates at a concentration of 1 × 106 cells/ml.
Macrophages were then infected at a multiplicity of infection of 1 : 1 with N. caninum tachyzoites incubated with IgG-NcMP/CpG or IgG-CpG, as described above, at different dilutions or with untreated parasites as control. Cells were incubated for 6 hr at 37° in a 5% CO2 humidified chamber.
DNA extraction
DNA from the brain of infected mice or from macrophages infected with the parasite was extracted as previously described.31 Briefly, brains were weighed and homogenized. Both sample types were incubated overnight at 55° in a solution containing 1% SDS and 1 mg/ml Proteinase K (Sigma). DNA was extracted by the phenol–chloroform (from Sigma and Merk, respectively) method followed by ammonium acetate/ethanol precipitation.
Real-time PCR analysis
The parasite burden in the brain of infected mice and macrophage cell cultures was assessed by a quantitative real-time PCR (qPCR) analysis of the parasite DNA performed in a Corbett rotor gene 6000 system (Corbett Life Science, Sydney, NSW, Australia). Brain analysis was performed using a Rotor-Gene probe PCR kit (Qiagen, Hilden, Germany), for the amplification of a 103-bp sequence of the Nc5 region of the N. caninum genome using the primers NcA 5′-GCTACCAACTCCCTCGGTT-3′ and NcS 5′-GTTGCTCTGCTGACGTGTCG-3′, both at a final concentration of 0·2 μm, and the fluorescent probe FAM-CCCGTTCACACACTATAGTCACAAACAAAA-BBQ at a final concentration of 0·1 μm (all designed and obtained from TIB-Molbiol, Berlin, Germany). The DNA samples were amplified using the following programme: 95° for 3 min, 95° for 5 seconds, 60° for 20 seconds with fluorescence acquisition. The second and third steps were repeated 50 times. Length of the amplified DNA was confirmed in a 3% agarose gel stained with ethidium bromide. Macrophage samples were analysed using Express Sybr green ER qPCR supermix universal (Invitrogen), for the amplification of a 337-bp sequence of the Nc5 region of the N. caninum genome using the primers Np21plus 5′-CCCAGTGCGTCCAATCCTGTAAC-3′ and Np6plus 5′-CTCGCCAGTCAACCTACGTCTTCT-3′ (both from TIB-Molbiol), both at a final concentration of 0·25 μm. The DNA samples were amplified using the following programme: 95° for 10 min, 95° for 30 seconds, 63° for 20 seconds, 72° for 45 seconds with fluorescence acquisition, the second, third and fourth steps were repeated 45 times. A melting curve was performed in each run to access the PCR-amplified fragments: from 65° to 95°, with increments of 1° for 5 seconds. In all runs, the parasite burden was determined by interpolation of a standard curve, ranging from 101 to 10−4 ng of DNA extracted from N. caninum tachyzoites included in each run and the data were analysed using the Rotor gene 6000 software v1.7 (Corbett Life Science).
Statistical analysis
Statistical analyses were performed using GraphPad software (Version 5.0, GraphPad Software, Inc. La Jolla, CA). In the scatter dot graphs the mean for each group was displayed as a horizontal line. Column graphs are represented showing the mean plus one standard error of the mean (SEM). Statistical analysis was performed using one-way analysis of variance with Newman–Keuls post-hoc analysis.
Results
Intestinal mucosa IgA produced on immunization binds to and agglutinates N. caninum tachyzoites
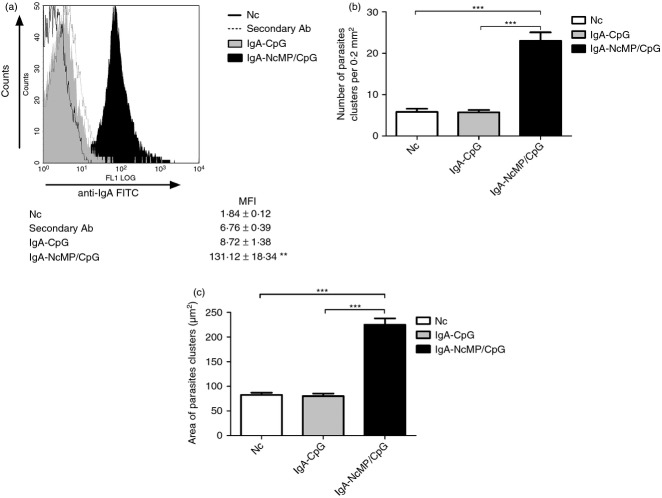
Production of IgA is the hallmark of specific immune responses at the mucosa.32 As IgA levels in the small intestine are difficult to ascertain without invasive procedures, NcMP-specific IgA titres were measured in VLF to monitor the effectiveness of the mucosal immunization. As shown in Fig. 1(a), higher titres of antigen-specific IgA were detected in the VLF of mice from the NcMP/CpG group than in the other groups at 7 days after the boosting i.n. immunization. Accordingly, elevated levels of NcMP-specific IgA were detected in ILF of the NcMP/CpG immunized mice, as compared with the other groups studied, 7 days after infection (Fig. 1b). Mice of the NcMP group also presented higher antibody levels in the VLF and ILF than control groups at both assessed time-points, although lower than those of the NcMP/CpG group, so highlighting the adjuvant effect of CpG. NcMP-specific IgA levels in the ILF of non-infected NcMP/CpG-immunized mice were sustained and were elevated 4 months after the boost immunization compared with those of CpG-treated controls (2·62 ± 0·31 mean log10 IgA titre ± 1 SD versus below detection limit values, respectively; n = 6/group). The ability of the raised IgA antibodies to bind N. caninum tachyzoites was confirmed using flow cytometry. As shown in Fig. 2(a), a significant increase in the mean fluorescence intensity value due to parasite-bound IgA was observed in tachyzoites incubated with IgA-NcMP/CpG compared with that of parasites incubated with IgA-CpG, so confirming the specificity of the antibodies raised by immunization. The agglutination of pathogens is one recognized effector function of IgA in the intestinal mucosa, so preventing their attachment to host cells.33 To assess the agglutinating capacity of the intestinal IgA produced on i.n. immunization, N. caninum tachyzoites were incubated with IgA-NcMP/CpG and IgA-CpG and the formation of parasitic agglutinates was assessed by optical microscopy. On incubation, a higher number of parasite clusters and a higher area per cluster (Fig. 2b,c, respectively) were found when the tachyzoites were incubated with IgA-NcMP/CpG than with IgA-CpG or PBS alone. Parasites incubated with IgA-CpG or PBS were mainly observed as single cells, whereas parasites treated with IgA-NcMP/CpG were mainly observed agglutinated in large bodies (see Supplementary material, Fig. S3). Together, these results show that mucosal parasite-agglutinating IgA antibodies are produced on i.n. immunization targeting NcMP.
Figure 1.
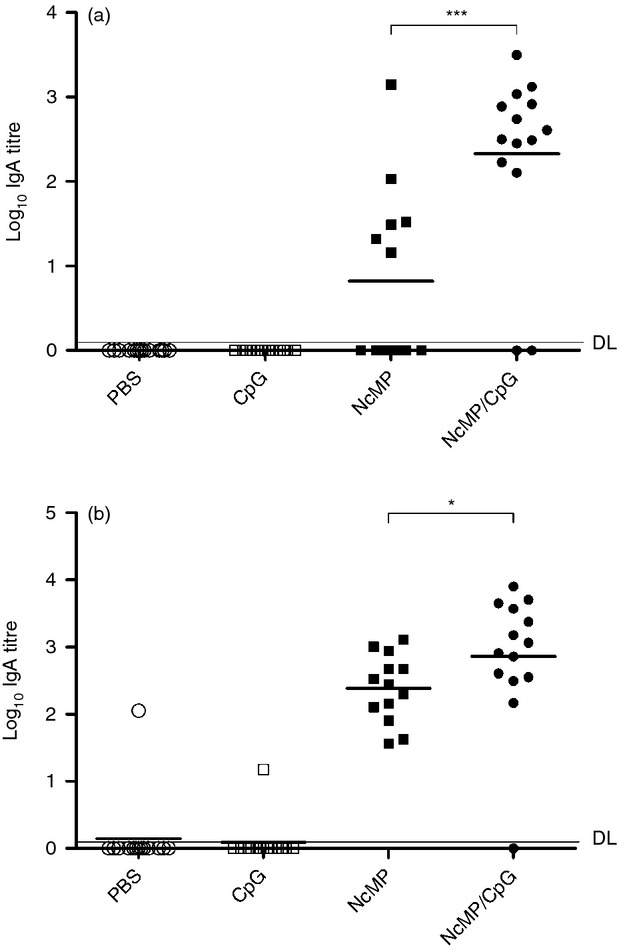
Neospora caninum membrane proteins (NcMP)-specific IgA titres detected by ELISA in vaginal and intestinal lavage fluids (VLF and ILF, respectively). IgA titres were determined by ELISA in (a) VLF collected 7 days after the second immunization from mice immunized twice intranasally with NcMP with or without CpG adjuvant (NcMP and NcMP/CpG, respectively) or sham-immunized with PBS (PBS) or CpG adjuvant alone (CpG); (b) ILF collected from the same groups 7 days after intragastric challenge with 5 × 107 N. caninum tachyzoites performed 3 weeks after the last immunizing administration. Results correspond to pooled data of three independent experiments (PBS n = 14; CpG n = 13; NcMP n = 13; NcMP/CpG n = 14). Each dot represents an individual mouse. Horizontal lines correspond to the mean value in each group [*P < 0·05; *** P < 0·001; detection limit (DL) is indicated by a horizontal line].
Figure 2.
(a) Flow cytometric analysis of anti-IgA-FITC monoclonal antibody (mAb) staining of Neospora caninum tachyzoites incubated with intestinaI IgA antibodies collected from mice 7 days after intragastric infection with 5 × 107 N. caninum tachyzoites 3 weeks after the second of two intranasal immunizations with N. caninum membrane proteins (NcMP) plus CpG adjuvant (IgA-NcMP/CpG) or similarly treated with CpG alone (IgA-CpG), or with anti-IgA-FITC alone (Secondary Ab), or untreated (Nc). Histograms are a representative example from three independent experiments with n = 3 for each condition. The mean fluorescence intensity (MFI) ± one SD is indicated. Statistical significance of the IgA-NcMP/CpG condition as compared with any of the other conditions is indicated (**P < 0·01). Parasite agglutination was assessed by Hemacolor staining of tachyzoites incubated with PBS (Nc) or IgA-CpG or IgA-NcMP/CpG fractions for 1 hr. Analysis of the number (b) and size (c) of parasite clusters was made with pooled results of 20 micrographs taken from each condition at a 200 × magnification. Parasite clustering was considered for four or more parasites appearing bound together. Bars correspond to tachyzoites incubated in: PBS alone (Nc), IgA-CpG alone (IgA-CpG) or with IgA-NcMP/CpG (IgA-NcMP/CpG), as indicated. Results are of one representative example out of three independent experiments. Each bar represents the mean value for each group. Error bar = SEM (***P < 0·001).
Serum IgG antibodies elicited on immunization with NcNP plus CpG bind to N. caninum and reduce parasite survival in infected macrophages
To determine whether parasite-specific IgG antibodies were also induced by the i.n. immunization, serum samples were analysed for the presence of NcMP-specific antibodies of that isotype. As shown in Fig. 3, the majority of the immunized mice presented high levels of antigen-specific IgG antibodies, detected before and after the i.g. parasitic challenge. All but one mouse treated i.n. with CpG or PBS alone presented no detectable serum IgG antibodies with this specificity by day 7 on the parasitic challenge. Analysis of the IgG isotype profile revealed a mixed IgG1 and IgG2a response in the NcMP and NcMP/CpG groups. However, disparate IgG1/IgG2a ratios were detected in these groups. Whereas in the mice immunized with NcMP alone, this ratio was > 1 before and after infection, a ratio < 1 was observed for the NcMP/CpG group (Fig. 3). As the IgG2a and IgG1 isotypes were, respectively, associated with a T helper type 1 (Th1) and a Th2-type immune response,34 these results indicate that a predominant Th1-type immune response was induced in the NcMP/CpG group whereas a Th2-type immune response was elicited by NcMP immunization in the absence of adjuvant. To determine the ability of serum IgG produced in the immunized mice to bind N. caninum parasites, tachyzoites were incubated with either IgG-NcMP/CpG or IgG-CpG and analysed by flow cytometry. As shown in Fig. 4(a), IgG-NcMP/CpG antibodies bound N. caninum tachyzoites more markedly than those in the IgG-CpG preparation.
Figure 3.
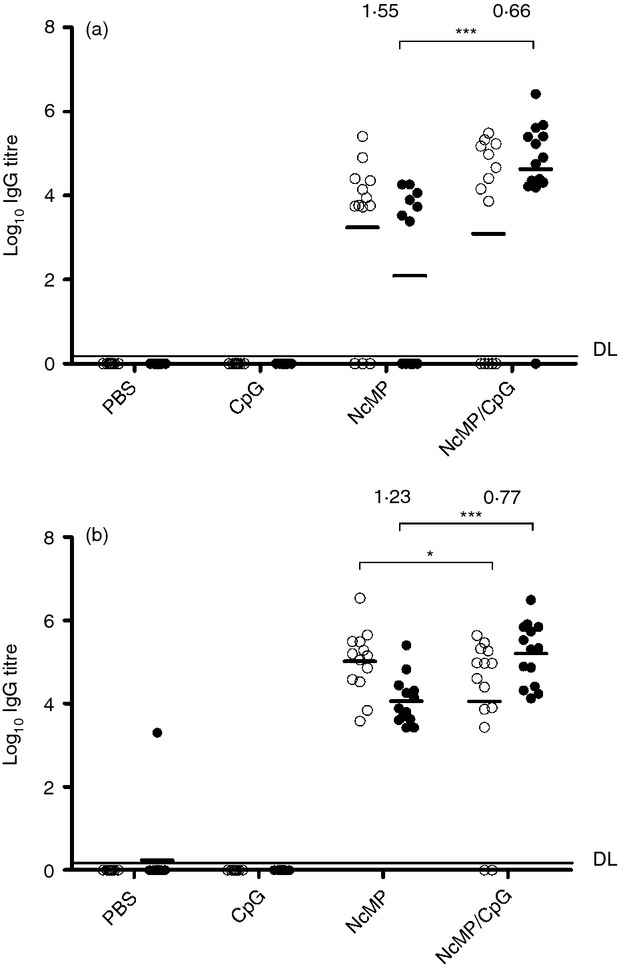
Titres of Neospora caninum membrane protein (NcMP) -specific serum IgG1 (open circles) and IgG2a (closed circles) antibodies determined by ELISA (a) 7 days after the second immunization in mice immunized twice intranasally with NcMP with or without CpG adjuvant (NcMP and NcMP/CpG, respectively) or sham-immunized with PBS (PBS) or CpG adjuvant alone (CpG) or (b) in the same groups, 7 days after an intragastric challenge with 5 × 107 N. caninum tachyzoites performed 3 weeks after the last immunizing administration. Numbers above each group represent the IgG1 : IgG2a ratio, calculated with the mean log10 titres for the correspondent IgG isotype. Results correspond to pooled data of three independent experiments (PBS n = 14; CpG n = 13; NcMP n = 13; NcMP/CpG n = 14). Each dot represents an individual mouse. Horizontal lines correspond to the mean value in each group (*P < 0·05; ***P < 0·001; detection limit (DL) is indicated by a horizontal line.
Figure 4.
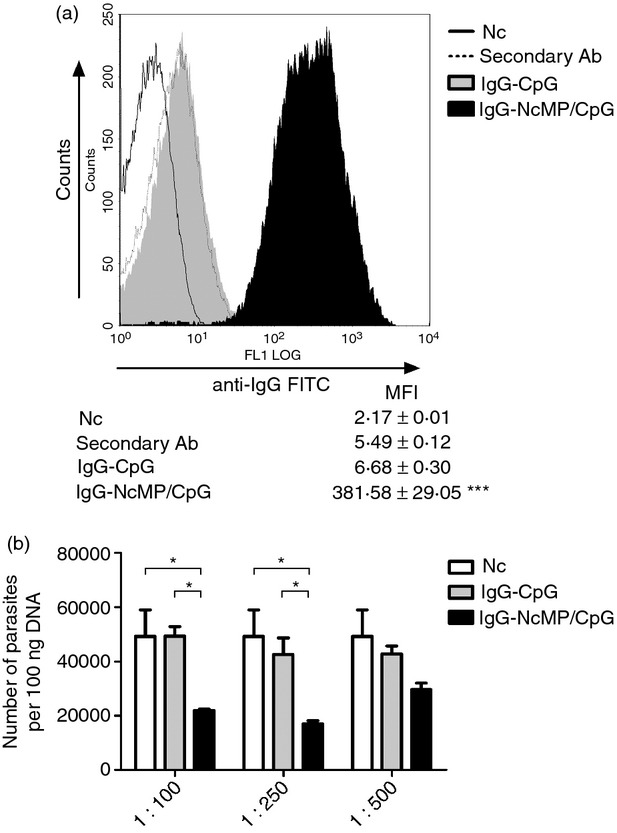
(a) Flow cytometric analysis of anti-IgG-FITC polyclonal antibody staining of Neospora caninum tachyzoites incubated with serum IgG antibodies collected from mice 7 days after intragastric infection with 5 × 107 N. caninum tachyzoites 3 weeks after being immunized twice intranasally with N. caninum membrane proteins (NcMP) plus CpG adjuvant (IgG-NcMP/CpG) or similarly treated with CpG alone (IgG-CpG), or with anti-IgG-FITC alone (Secondary Ab), or untreated (Nc). Histograms are a representative example from one of three independent experiments (n = 3 for each condition). The mean fluorescence intensity (MFI) ± one SD is indicated. Statistical significance of the IgG-NcMP/CpG condition as compared with any of the other conditions is indicated (***P < 0·001). (b) Number of parasites, assessed by quantitative PCR in bone marrow-derived macrophage cell cultures challenged at a multiplicity of infection (MOI) of 1 : 1 for 6 hr with 1 × 106 tachyzoites previously incubated with IgG-CpG or IgG-NcMP/CpG at the indicated dilutions, or untreated parasites (Nc). Results are of a representative example out of three independent experiments. Each bar represents the mean of three wells. Error bar = SEM (*P < 0·05).
Neospora caninum is an obligate intracellular parasite and consequently the capacity to infect new cells once inside the host is essential for its survival. Therefore, blocking the infection of new cells could be an important factor for parasite control. To test the effects of the IgG preparations in the capacity of N. caninum to survive in the macrophage cell cultures, tachyzoites were incubated with the IgG antibody preparations. The opsonized tachyzoites as well as non-opsonized counterparts were used to challenge macrophage cell cultures for 6 hr, after which the number of parasites therein was evaluated by qPCR. As shown in Fig. 4(b), parasite opsonization with IgG raised by immunization resulted in a dose-dependent reduction in the total number of parasites detected in the cultures.
Protective effect of mucosal immunization against i.g. established neosporosis
Neosporosis is thought to be horizontally transmitted by oocyst ingestion in naturally infected hosts.35 Therefore, it is conceivable that boosting the immune response in the gastrointestinal mucosa by parasite-specific immunization may increase host resistance against this parasitic disease. To assess whether the immunization procedures used here could be protective, we evaluated by using qPCR the parasitic burden in the brain of mice of the different groups, 7 days after i.g. administration of 5 × 107 N. caninum tachyzoites performed 3 weeks after the last immunization. As shown in Fig. 5, a significant reduction of the mean parasitic DNA level in mice of the NcMP/CpG group was detected, as compared with that in control mice, which received CpG or PBS alone. Moreover, the NcMP/CpG group showed the highest number of mice with absent or below detection-limit parasitic DNA (n = 4; n = 5 and n = 10 in the PBS, CpG and NcMP, and NcMP/CpG groups, respectively). Interestingly, the mouse within the NcMP/CpG group showing higher parasitic colonization was the only one presenting no detectable levels of IgA in either VLF or ILF, as shown in Fig. 1. No significant differences were found among any other groups, although the mean parasitic burden detected in the NcMP/CpG group was greatly reduced compared with that of the NcMP group. These results together show that i.n. immunization with NcMP plus CpG adjuvant confers protection against neosporosis established by the gastrointestinal tract.
Figure 5.
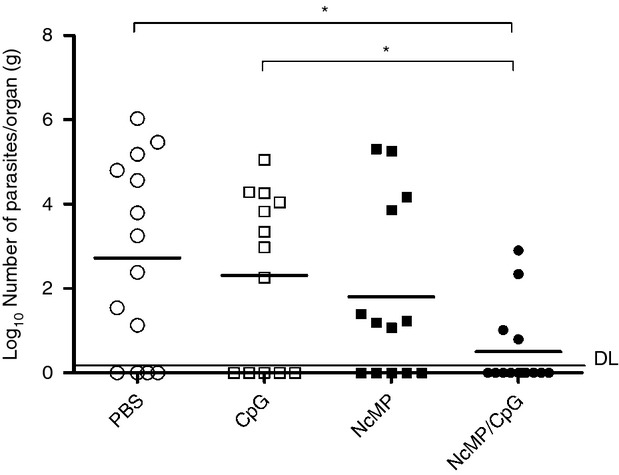
Parasitic load assessed by quantitative PCR 7 days after intragastric challenge with 5 × 107 Neospora caninum tachyzoites in the brain of mice previously immunized twice intranasally with N. caninum membrane proteins (NcMP) with or without CpG adjuvant (NcMP and NcMP/CpG, respectively) or sham-immunized with PBS (PBS) or CpG adjuvant alone (CpG). Results are from pooled data of three independent experiments. (PBS n = 14; CpG n = 13; NcMP n = 13; NcMP/CpG n = 14). Each dot represents an individual mouse. Horizontal lines correspond to the mean value in each group (*P < 0·05); detection limit (DL) is indicated by a horizontal line.
Cytokine production in the immunized mice
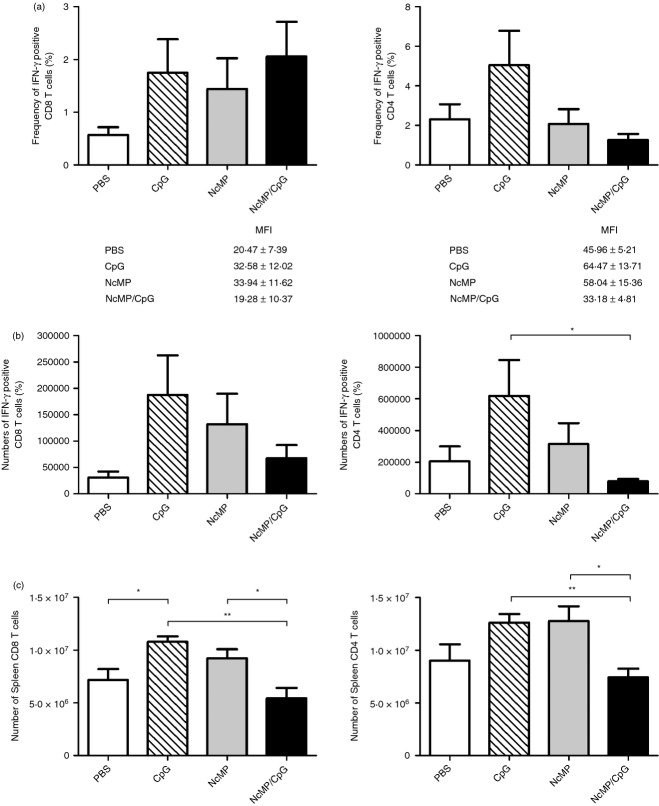
Host production of IFN-γ is associated with resistance to neosporosis whereas production of IL-4 and IL-10 are associated with susceptibility to this infection.36 Therefore, the frequency and numbers of cells producing these cytokines were assessed in the spleen and MLN of immunized mice and controls, 7 days after i.g. infection. Unexpectedly, no differences were observed among the different analysed groups in the frequencies of splenic CD4+ T cells producing any of these cytokines (Fig. 6a and see Supplementary material, Fig. S4). Similarly, no significant differences in the frequency of splenic IFN-γ-producing CD8+ T cells were observed among groups (Fig. 6a). To determine whether, despite being present in similar frequencies to those of controls, CD4+ IFN-γ+ and CD8+ IFN-γ+ T cells of immunized mice could be producing higher amounts of this cytokine, the mean fluorescence intensity due to the IFN-γ staining was assessed in these lymphocyte populations. Results shown in Fig. 6(a) indicate that this is not the case, as mean fluorescence intensity values detected among all assessed groups did not significantly vary. Nevertheless, and surprisingly, lower numbers of splenic CD4+ IFN-γ+ cells were observed in the NcMP/CpG group compared with the respective CpG control group. Similarly, lower numbers of CD8+ IFN-γ+ cells were observed in the NcMP/CpG group, although not reaching statistical significance (Fig. 6b). The NcMP/CpG group also presented significantly lower numbers of total CD4+ and CD8+ T cells, compared with the CpG and NcMP groups (Fig. 6c). Similar analyses to all those performed in the spleen were carried out in the MLN that did not show any significant differences among the analysed groups (data not shown). Moreover, no significant differences among groups were observed in the levels of IFN-γ or of IL-4 detected in cell culture supernatants of NcMP-stimulated splenocytes collected from the different analysed mice, 7 days after infection (see Supplementary material, Fig. S4). Cytokine levels in similarly stimulated MLN cell cultures were consistently found near or below detection limits (data not shown). Taken together, these results indicate that in the NcMP/CpG group, the cellular immune response elicited on parasitic challenge was comparable to that of non-immunized PBS-treated infected mice and less noticeable than in the mice that received CpG adjuvant or NcMP alone.
Figure 6.
Frequency (a) and total numbers (b) of splenic interferon-γ-positive (IFN-γ+) CD8+ and CD4+ T cells, or of (c) total splenic CD8+ and CD4+ T cells, detected 7 days after intragastric challenge with 5 × 107 Neospora caninum tachyzoites in mice previously immunized with N. caninum membrane proteins (NcMP) with or without CpG adjuvant (NcMP and NcMP/CpG, respectively) or sham-immunized with PBS (PBS) or CpG adjuvant alone (CpG). The mean fluorescence intensity (MFI) ± one SEM due to IFN-γ staining is indicated for each group in (a). Results correspond to pooled data of two independent experiments. PBS, CpG and NcMP n = 8; NcMP/CpG n = 9. Each bar represents the mean value for each group. Error bar = SEM (*P < 0·05; ** P < 0·01).
Discussion
We show here that mice i.n. immunized with membrane proteins extracted from N. caninum tachyzoites plus CpG adjuvant presented a lower parasitic burden in the brain when infected i.g. with N. caninum tachyzoites than controls receiving CpG or PBS alone. These results are in agreement with previous reports showing a host protective effect of i.n. immunization with N. caninum antigens in mice intraperitoneally challenged with this parasite.23,24 Yet, our data extend the protective effect of i.n. immunization to N. caninum infection established through the gastrointestinal tract, a route more closely resembling the one naturally used for parasite penetrance into the host in horizontally transmitted neosporosis.35 A higher number of mice without detectable parasite DNA was observed in the NcMP/CpG group than in the other studied groups. This might indicate that the immunization procedure used here could completely prevent parasite colonization in some of the infected mice. Nevertheless, further experiments will be necessary to more rigorously determine to what extent this may have happened as some of the mice that received CpG or PBS alone also presented parasite DNA below the detection limit.
CpG adjuvant typically promotes a Th1-type immune response.37 Therefore, as could be expected, the immunization procedure assessed here induced a predominant production of antigen-specific antibodies of the IgG2a isotype, which is associated with a Th1-type immune response.34 Nevertheless, the production of IgG1 antibodies was also raised by the i.n. immunization. As previously remarked, a balanced Th1/Th2 response might be more adequate in the course of neosporosis by conferring protection against the parasite, nonetheless avoiding fetal rejection.25 Immunization with NcMP alone also induced the production of antigen-specific IgG. However, and in contrast with NcMP plus CpG immunization, this response was mainly characterized by the production of IgG1, indicative of a predominant Th2-type response. These results may indicate that N. caninum structural antigens promote a Th2-type polarization of the immune response, associated with host susceptibility to neosporosis38 that could be overcome by the usage of CpG adjuvant. A similar effect of CpG was previously observed in mice subcutaneously immunized with N. caninum lysates or soluble antigen preparations.11 Our results also reinforce the adequateness of using CpG adjuvant to achieve immunoprotection against N. caninum infection, as described by others using alternative immunization strategies.11–39
Despite the fact that the serum IgG isotype profile might indicate that a Th1-type immune response was elicited in the NcMP/CpG group, no significant differences in the frequencies of splenic or MLN T cells producing IFN-γ, IL-10 and IL-4 could be detected among the studied groups. As the majority of mice from the NcMP/CpG group presented no detectable parasitic colonization in the absence of a significant increase in IFN-γ production the achieved protection could be, at least partially, independent of a strong Th1 response. Vaccine-induced protection in the absence of a Th1-type response was previously reported in mice infected by the protozoan Leishmania amazonensis.40 As the parasite-specific IgA obtained from the intestinal lumen of immunized mice was shown to agglutinate N. caninum parasites, it might be hypothesized that the lack of a noticeable Th1-type cytokine bias in the NcMP/CpG group could result from the impairment of host invasion across the intestinal mucosa and consequent antigenic stimulation. Supporting this hypothesis, the only animal in that group that did not present detectable levels of IgA in both analysed mucosa was the one showing higher parasite DNA levels. In this scenario, although a cell mediated immune response, polarized to a Th1-type response, might be generated by the immunization, its full activation upon infection in the immunized mice could have been prevented by lack of antigenic stimulation in the NcMP/CpG group. The lack of an exacerbated cellular immune response in the spleen and MLN of the NcMP/CpG group further supports this hypothesis. As a strong production of IFN-γ in dams has previously been shown to compromise fetus viability,41 it might be worth assessing the immunizing protocol attempted here in pregnant mice infected with N. caninum as we observed that protection could be attained without a marked Th1 response.
As N. caninum is an obligate intracellular pathogen a cell-mediated rather than a humoral immune response could be expected to be protective. Nevertheless, previous studies have reported a host protective role of intestinal IgA in cats42 and mice43 infected with the closely related protozoan Toxoplasma gondii, in agreement with the evidence presented here indicating that such a protective role may also be played by IgA in the course of N. caninum infection. Interestingly, non-infected mice immunized with NcMP plus CpG still presented antigen-specific intestinal IgA antibodies by 4 months after the last i.n. antigenic administration. Although preliminary, these results might indicate that this immunization procedure can induce a long-term IgA response in the gut, this would be worth assessing in more detail. Moreover, as we also show, IgG antibodies obtained from immunized mice can opsonize N. caninum tachyzoites and reduce parasite survival in murine macrophages challenged with this parasitic form, indicating that antibodies specific for N. caninum might contribute to host protection against this parasite. These results are in agreement with previous reports demonstrating that antibodies specific for different membrane proteins of N. caninum are capable of interfering with, and may even block, the parasite entry into host cells.44,45 However, such a protective role needs to be confirmed in further experiments, namely as the effect of IgG opsonization on leucocyte killing of tachyzoites of the closely related protozoan T. gondii remains controversial with previous studies showing either enhanced killing47 or no effect in this regard.48
Only a limited number of reports specifically studied the mucosal immune response to this parasite26–27 and to the best of our knowledge no study had previously addressed the immune response in the intestinal mucosa and associated lymphoid tissue of mice immunized with N. caninum antigens. The widespread usage of intraperitoneal inoculation in experimental studies on neosporosis probably contributed to the overlooking of the mucosal layer of immune defence against this parasitosis. By showing that intestinal lumen antibodies induced by mucosal immunization can agglutinate N. caninum tachyzoites we provide the first evidence indicating that stimulating antibody production in the gut by means of mucosal immunization may be worth attempting as a protective strategy against horizontally transmitted neosporosis.
Acknowledgments
Supported by FCT/MCTES (PIDDAC) and co-funded by FEDER through COMPETE, PTDC/CVT/115126/2009 and FCOMP-01-0124-FEDER-014679. Pedro Ferreirinha was supported by FCT grant SFRH/BD/76900/2011. Luzia Teixeira was supported by FSE and MCTES through POPH-QREN-Tipologia 4.2. Begoña Pérez Cabezas was supported by an EFIS-IL Short-term Fellowship. The authors acknowledge the excellent technical assistance of Encarnação Rebelo.
Glossary
- BMDM
bone marrow-derived macrophages
- CpG
oligodeoxynucleotides containing non methylated guanine-p-citosine motifs
- i.g.
intragastric
- IgA-CpG
IgA purified from the intestinal lavage fluids of mice from the CpG group
- IgA-NcMP/CpG
IgA purified from the intestinal lavage fluids of mice from the NcMP/CpG group
- IgG-CpG
IgG purified from the sera of mice from the CpG group
- IgG-NcMP/CpG
IgG purified from the sera of mice from the NcMP/CpG group
- i.n.
intranasal
- ILF
intestinal lavage fluids
- LCCM
L-929 cell condition medium
- mAb
monoclonal antibody
- MOI
multiplicity of infection
- MLN
mesenteric lymph nodes
- NcMP
Neospora caninum membrane proteins
- NcS
Neospora caninum sonicates
- PE
phycoerythrin
- PerCP-Cy5.5
peridinin-chlorophyll proteins-cychrome 5.5
- qPCR
quantitative real-time PCR
- RT
room temperature
- SD
standard deviation
- SEM
standard error of the mean
- VLF
vaginal lavage fluids
Disclosures
The authors have no financial or any other conflict of interests.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Electrophoretic migration profiles of Neospora caninum protein extracts.
Schematic representation of the time-line (in weeks) for the immunization protocol.
Microscopic analysis of Neospora caninum tachyzoites clustering assessed by Hemacolor staining.
Frequency (a) and total numbers (b) of splenic interleukin-10-positive (IL-10+) or IL-4+ CD4+ T cells detected 7 days after intragastric (i.g.) challenge with 5 × 107 Neospora caninum tachyzoites in mice previously immunized with N. caninum membrane proteins with or without CpG adjuvant (NcMP and NcMP/CpG, respectively) or sham-immunized with PBS (PBS) or CpG adjuvant alone (CpG).
References
- Dubey JP, Hattel AL, Lindsay DS, Topper MJ. Neonatal Neospora caninum infection in dogs: isolation of the causative agent and experimental transmission. J Am Vet Med Assoc. 1988;193:1259–63. [PubMed] [Google Scholar]
- Dubey JP, Schares G. Neosporosis in animals – the last five years. Vet Parasitol. 2011;180:90–108. doi: 10.1016/j.vetpar.2011.05.031. [DOI] [PubMed] [Google Scholar]
- Reichel MP, Alejandra Ayanegui-Alcerreca M, Gondim LF, Ellis JT. What is the global economic impact of Neospora caninum in cattle – the billion dollar question. Int J Parasitol. 2013;43:133–42. doi: 10.1016/j.ijpara.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Reichel MP, Ellis JT. If control of Neospora caninum infection is technically feasible does it make economic sense? Vet Parasitol. 2006;142:23–34. doi: 10.1016/j.vetpar.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Reichel MP, Ellis JT. Neospora caninum – how close are we to development of an efficacious vaccine that prevents abortion in cattle? Int J Parasitol. 2009;39:1173–87. doi: 10.1016/j.ijpara.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Miller C, Quinn H, Ryce C, Reichel MP, Ellis JT. Reduction in transplacental transmission of Neospora caninum in outbred mice by vaccination. Int J Parasitol. 2005;35:821–8. doi: 10.1016/j.ijpara.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Lindsay DS, Schurig GG, Boyle SM, Duncan RB, Vemulapalli R, Sriranganathn N. Vaccination with γ-irradiated Neospora caninum tachyzoites protects mice against acute challenge with N. caninum. J Eukaryot Microbiol. 2006;53:151–6. doi: 10.1111/j.1550-7408.2005.00083.x. [DOI] [PubMed] [Google Scholar]
- Bartley PM, Wright S, Chianini F, Buxton D, Innes EA. Inoculation of BALB/c mice with live attenuated tachyzoites protects against a lethal challenge of Neospora caninum. Parasitology. 2008;135:13–21. doi: 10.1017/S0031182007003526. [DOI] [PubMed] [Google Scholar]
- Williams DJ, Guy CS, Smith RF, Ellis J, Bjorkman C, Reichel MP, Trees AJ. Immunization of cattle with live tachyzoites of Neospora caninum confers protection against fetal death. Infect Immun. 2007;75:1343–8. doi: 10.1128/IAI.00777-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddell S, Jenkins MC, Collica CM, Dubey JP. Prevention of vertical transfer of Neospora caninum in BALB/c mice by vaccination. J Parasitol. 1999;85:1072–5. [PubMed] [Google Scholar]
- Ribeiro DP, Freitas MM, Cardoso MR, Pajuaba AC, Silva NM, Mineo TW, Silva JS, Mineo JR, Silva DA. CpG-ODN combined with Neospora caninum lysate, but not with excreted–secreted antigen, enhances protection against infection in mice. Vaccine. 2009;27:2570–9. doi: 10.1016/j.vaccine.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Baszler TV, McElwain TF, Mathison BA. Immunization of BALB/c mice with killed Neospora caninum tachyzoite antigen induces a type 2 immune response and exacerbates encephalitis and neurological disease. Clin Diagn Lab Immunol. 2000;7:893–8. doi: 10.1128/cdli.7.6.893-898.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunden A, Wright S, Allen JE, Buxton D. Immunisation of mice against neosporosis. Int J Parasitol. 2002;32:867–76. doi: 10.1016/s0020-7519(02)00024-3. [DOI] [PubMed] [Google Scholar]
- Teixeira L, Marques A, Meireles CS, et al. Characterization of the B-cell immune response elicited in BALB/c mice challenged with Neospora caninum tachyzoites. Immunology. 2005;116:38–52. doi: 10.1111/j.1365-2567.2005.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrianarivo AG, Rowe JD, Barr BC, et al. A POLYGEN-adjuvanted killed Neospora caninum tachyzoite preparation failed to prevent foetal infection in pregnant cattle following i.v./i.m. experimental tachyzoite challenge. Int J Parasitol. 2000;30:985–90. doi: 10.1016/s0020-7519(00)00088-6. [DOI] [PubMed] [Google Scholar]
- Cannas A, Naguleswaran A, Muller N, Eperon S, Gottstein B, Hemphill A. Vaccination of mice against experimental Neospora caninum infection using NcSAG1- and NcSRS2-based recombinant antigens and DNA vaccines. Parasitology. 2003;126:303–12. doi: 10.1017/s0031182002002895. [DOI] [PubMed] [Google Scholar]
- Cannas A, Naguleswaran A, Muller N, Gottstein B, Hemphill A. Reduced cerebral infection of Neospora caninum-infected mice after vaccination with recombinant microneme protein NcMIC3 and ribi adjuvant. J Parasitol. 2003;89:44–50. doi: 10.1645/0022-3395(2003)089[0044:RCIONC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Pinitkiatisakul S, Mattsson JG, Wikman M, Friedman M, Bengtsson KL, Stahl S, Lundén A. Immunisation of mice against neosporosis with recombinant NcSRS2 iscoms. Vet Parasitol. 2005;129:25–34. doi: 10.1016/j.vetpar.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Debache K, Guionaud C, Alaeddine F, Mevissen M, Hemphill A. Vaccination of mice with recombinant NcROP2 antigen reduces mortality and cerebral infection in mice infected with Neospora caninum tachyzoites. Int J Parasitol. 2008;38:1455–63. doi: 10.1016/j.ijpara.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Debache K, Alaeddine F, Guionaud C, Monney T, Muller J, Strohbusch M, Leib SL, Grandgirard D, Hemphill A. Vaccination with recombinant NcROP2 combined with recombinant NcMIC1 and NcMIC3 reduces cerebral infection and vertical transmission in mice experimentally infected with Neospora caninum tachyzoites. Int J Parasitol. 2009;39:1373–84. doi: 10.1016/j.ijpara.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Alaeddine F, Keller N, Leepin A, Hemphill A. Reduced infection and protection from clinical signs of cerebral neosporosis in C57BL/6 mice vaccinated with recombinant microneme antigen NcMIC1. J Parasitol. 2005;91:657–65. doi: 10.1645/GE-401R. [DOI] [PubMed] [Google Scholar]
- Tuo W, Zhao Y, Zhu D, Jenkins MC. Immunization of female BALB/c mice with Neospora cyclophilin and/or NcSRS2 elicits specific antibody response and prevents against challenge infection by Neospora caninum. Vaccine. 2011;29:2392–9. doi: 10.1016/j.vaccine.2011.01.041. [DOI] [PubMed] [Google Scholar]
- Debache K, Guionaud C, Alaeddine F, Hemphill A. Intraperitoneal and intra-nasal vaccination of mice with three distinct recombinant Neospora caninum antigens results in differential effects with regard to protection against experimental challenge with Neospora caninum tachyzoites. Parasitology. 2010;137:229–40. doi: 10.1017/S0031182009991259. [DOI] [PubMed] [Google Scholar]
- Debache K, Kropf C, Schutz CA, et al. Vaccination of mice with chitosan nanogel-associated recombinant NcPDI against challenge infection with Neospora caninum tachyzoites. Parasite Immunol. 2011;33:81–94. doi: 10.1111/j.1365-3024.2010.01255.x. [DOI] [PubMed] [Google Scholar]
- Debache K, Hemphill A. Differential effects of intranasal vaccination with recombinant NcPDI in different mouse models of Neospora caninum infection. Parasite Immunol. 2013;35:11–20. doi: 10.1111/pim.12013. [DOI] [PubMed] [Google Scholar]
- Teixeira L, Botelho AS, Batista AR, et al. Analysis of the immune response to Neospora caninum in a model of intragastric infection in mice. Parasite Immunol. 2007;29:23–36. doi: 10.1111/j.1365-3024.2006.00911.x. [DOI] [PubMed] [Google Scholar]
- Correia A, Ferreirinha P, Costa AA, et al. Mucosal and systemic T cell response in mice intragastrically infected with Neospora caninum tachyzoites. Vet Res. 2013;44:69. doi: 10.1186/1297-9716-44-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo TW, Benevides L, Silva NM, Silva JS. Myeloid differentiation factor 88 is required for resistance to Neospora caninum infection. Vet Res. 2009;40:32. doi: 10.1051/vetres/2009015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemphill A, Gottstein B. Identification of a major surface protein on Neospora caninum tachyzoites. Parasitol Res. 1996;82:497–504. doi: 10.1007/s004360050152. [DOI] [PubMed] [Google Scholar]
- Zintl A, Pennington SR, Mulcahy G. Comparison of different methods for the solubilisation of Neospora caninum (Phylum Apicomplexa) antigen. Vet Parasitol. 2006;135:205–13. doi: 10.1016/j.vetpar.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Inoue N, Xuan X, Nagasawa H, Igarashi I, Fujisaki K, Otsuka H, Mikami T. Protective efficacy of vaccination by recombinant vaccinia virus against Neospora caninum infection. Vaccine. 2001;19:1381–90. doi: 10.1016/s0264-410x(00)00389-3. [DOI] [PubMed] [Google Scholar]
- Meeusen EN. Exploiting mucosal surfaces for the development of mucosal vaccines. Vaccine. 2011;29:8506–11. doi: 10.1016/j.vaccine.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Horton RE, Vidarsson G. Antibodies and their receptors: different potential roles in mucosal defense. Front Immunol. 2013;4:200. doi: 10.3389/fimmu.2013.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens TL, Bossie A, Sanders VM, Fernandez-Botran R, Coffman RL, Mosmann TR, Vitetta ES. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255–8. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Schares G, Ortega-Mora LM. Epidemiology and control of neosporosis and Neospora caninum. Clin Microbiol Rev. 2007;20:323–67. doi: 10.1128/CMR.00031-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes EA. The host–parasite relationship in pregnant cattle infected with Neospora caninum. Parasitology. 2007;134:1903–10. doi: 10.1017/S0031182007000194. [DOI] [PubMed] [Google Scholar]
- Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2011;10:499–511. doi: 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa Y, Inoue N, Makala L, Nagasawa H. A role for balance of interferon-γ and interleukin-4 production in protective immunity against Neospora caninum infection. Vet Parasitol. 2003;116:175–84. doi: 10.1016/j.vetpar.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Jenkins M, Parker C, Tuo W, Vinyard B, Dubey JP. Inclusion of CpG adjuvant with plasmid DNA coding for NcGRA7 improves protection against congenital neosporosis. Infect Immun. 2004;72:1817–19. doi: 10.1128/IAI.72.3.1817-1819.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez MX, Barcante TA, Vilela L, Tafuri WL, Afonso LC, Vieira LQ. Vaccine-induced protection against Leishmania amazonensis is obtained in the absence of IL-12/23p40. Immunol Lett. 2006;105:38–47. doi: 10.1016/j.imlet.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Rosbottom A, Gibney EH, Guy CS, Kipar A, Smith RF, Kaiser P, Trees AJ, Williams DJ. Upregulation of cytokines is detected in the placentas of cattle infected with Neospora caninum and is more marked early in gestation when fetal death is observed. Infect Immun. 2008;76:2352–61. doi: 10.1128/IAI.01780-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata Y, Terada K, Taka A, Isamida T, Kanda M, Saito A. Positive evidence that anti-Toxoplasma gondii IgA antibody exists in the intestinal tract of infected cats and exerts protective activity against the infection. Vet Parasitol. 1997;73:1–11. doi: 10.1016/s0304-4017(97)00126-x. [DOI] [PubMed] [Google Scholar]
- Zorgi NE, Costa A, Galisteo AJ, Jr, do Nascimento N, Andrade HF., Jr Humoral responses and immune protection in mice immunized with irradiated T. gondii tachyzoites and challenged with three genetically distinct strains of T. gondii. Immunol Lett. 2011;138:187–96. doi: 10.1016/j.imlet.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Ike K, Kurotaki T, Ito A, Imai S. Monoclonal antibodies preventing invasion of Neospora caninum tachyzoites into host cells. J Vet Med Sci. 2004;66:1355–8. doi: 10.1292/jvms.66.1355. [DOI] [PubMed] [Google Scholar]
- Haldorson GJ, Stanton JB, Mathison BA, Suarez CE, Baszler TV. Neospora caninum: antibodies directed against tachyzoite surface protein NcSRS2 inhibit parasite attachment and invasion of placental trophoblasts in vitro. Exp Parasitol. 2006;112:172–8. doi: 10.1016/j.exppara.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Zhang H, Compaore MK, Lee EG, Liao M, Zhang G, Sugimoto C, Fujisaki K, Nishikawa Y, Xuan X. Apical membrane antigen 1 is a cross-reactive antigen between Neospora caninum and Toxoplasma gondii, and the anti-NcAMA1 antibody inhibits host cell invasion by both parasites. Mol Biochem Parasitol. 2007;151:205–12. doi: 10.1016/j.molbiopara.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Erbe DV, Pfefferkorn ER, Fanger MW. Functions of the various IgG Fc receptors in mediating killing of Toxoplasma gondii. J Immunol. 1991;146:3145–51. [PubMed] [Google Scholar]
- Fadul CE, Channon JY, Kasper LH. Survival of immunoglobulin G-opsonized Toxoplasma gondii in nonadherent human monocytes. Infect Immun. 1995;63:4290–4. doi: 10.1128/iai.63.11.4290-4294.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electrophoretic migration profiles of Neospora caninum protein extracts.
Schematic representation of the time-line (in weeks) for the immunization protocol.
Microscopic analysis of Neospora caninum tachyzoites clustering assessed by Hemacolor staining.
Frequency (a) and total numbers (b) of splenic interleukin-10-positive (IL-10+) or IL-4+ CD4+ T cells detected 7 days after intragastric (i.g.) challenge with 5 × 107 Neospora caninum tachyzoites in mice previously immunized with N. caninum membrane proteins with or without CpG adjuvant (NcMP and NcMP/CpG, respectively) or sham-immunized with PBS (PBS) or CpG adjuvant alone (CpG).