Abstract
Insomnia persistently affects the quality and quantity of sleep. Currently approved treatments for insomnia primarily target γ-aminobutyric acid-A (GABA-A) receptor signalling and include benzodiazepines and GABA-A receptor modulators. These drugs are used to address this sleep disorder, but have the potential for side effects such as tolerance and dependence, making them less attractive as maintenance therapy. Forward and reverse genetic approaches in animals have implicated orexin signalling (also referred to as hypocretin signalling) in the control of vigilance and sleep/wake states. Screening for orexin receptor antagonists using in vitro and in vivo methods in animals has identified compounds that block one or other of the orexin receptors (single or dual orexin receptor antagonists [SORAs and DORAs], respectively) in animals and humans. SORAs have primarily been used as probes to further elucidate the roles of the individual orexin receptors, while a number of DORAs have progressed to clinical development as pharmaceutical candidates for insomnia. The DORA almorexant demonstrated significant improvements in a number of clinically relevant sleep parameters in animal models and in patients with insomnia but its development was halted. SB-649868 and suvorexant have demonstrated efficacy and tolerability in Phase II and III trials respectively. Furthermore, suvorexant is currently under review by the Food and Drug Administration for the treatment of insomnia. Based on the publication of recent non-clinical and clinical data, orexin receptor antagonists potentially represent a targeted, effective and well-tolerated new class of medications for insomnia.
Linked ArticlesThis article is part of a themed section on Orexin Receptors. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2014.171.issue-2
Keywords: orexin receptor antagonist, insomnia, GABA, sleep
Introduction
According to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision, (American Psychiatric Association, 2000), insomnia is defined as difficulty initiating or maintaining sleep or non-restorative sleep for at least 1 month, which causes clinically significant distress or impairment in social, occupational or other important areas of functioning. This sleep disturbance should not occur exclusively during the course of narcolepsy, a breathing-related sleep disorder, a circadian rhythm sleep disorder, parasomnias or a mental disorder (e.g. major depressive disorder), or as a result of substance abuse or another medical condition. More commonly, this sleep disorder is classified as secondary (attributable to a medical or psychiatric cause) or primary insomnia (idiopathic or psychophysiological in nature) (Morgenthaler et al., 2006). A main aim of treatment for insomnia is to improve sleep onset and, in particular, sleep maintenance without next-day ‘hangover’ effects. Currently available treatments primarily rely on the modulation of GABA-A receptor-mediated mechanisms, a therapeutic strategy that is helpful to many patients but also associated with central nervous system-related adverse events, for example morning sedation and cognitive hangover effects (Hindmarch et al., 2006; Roth, 2007; Otmani et al., 2008; Hoque and Chesson Jr, 2009; Roehrs and Roth, 2012). An idealized pharmacotherapy for the treatment of insomnia has been suggested by the British Association of Psychopharmacology (Figure 1) (Wilson et al., 2010), and includes rapid sleep onset, with maintenance throughout the night, lacking next day impairment and minimal adverse effects.
Figure 1.
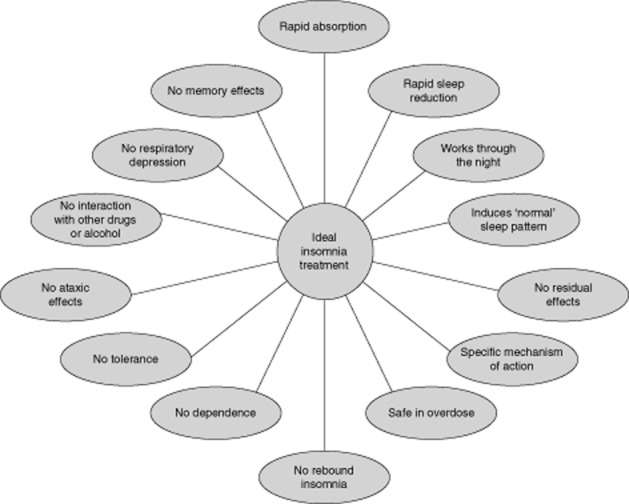
The characteristics of a hypothetical ideal insomnia treatment. Wilson et al., J. Psychopharmacol (vol. no. 24, issue no. 11) pp. 1577–1601. © 2010 by Wilson et al. Reprinted by permission of SAGE (Wilson et al., 2010).
As described previously in this special issue, the orexin signalling system (also called the hypocretin system) was discovered and characterized in rodent and dog models of narcolepsy, using both forward and reverse genetic approaches (de Lecea et al., 1998; Sakurai et al., 1998; Chemelli et al., 1999; Lin et al., 1999; Nishino et al., 2000). The revelation of the genetic basis for regulation of sleep and wake by the orexin-signalling system has made modulation of orexin receptors an attractive target for pharmaceutical development of treatments for insomnia. Further, due to the remarkable level of genetic and functional conservation of the orexin system in mammals, there is a high level of translatability of efficacy from rodent to human (Brisbare-Roch et al., 2007; Gotter et al., 2012b) that has facilitated drug development.
Many new drug mechanisms fail during pharmaceutical development due primarily to a lack of efficacy. Targets with genetic validation have a higher probability of success but are rare (Renger and Kern, 2011). Understanding of the genetic basis of arousal governed by orexins provides a model system for the generation of proof of concept data and for the testing of novel small molecules for the treatment of insomnia. Furthermore, characterization of the orexin 1 and orexin 2 receptors (OX1R and OX2R) as G-coupled protein receptors (Sakurai et al., 1998) facilitated the development of medications that could attenuate their activation and responses and consequently impact on sleep/wake control in the brain selectively.
Identification of novel medications for insomnia has relied upon in vitro assays and in vivo preclinical screens of libraries of molecules in order to identify compounds that selectively act on the target receptors. This review will discuss the development of orexin receptor antagonists to date and describe how some of these molecules are used to further delineate the function of the orexin receptors, with some proceeding through clinical development to become potential novel medications for the treatment of insomnia.
Orexin receptor antagonists identified by high-throughput screening
The orexin system plays a key role in promoting wakefulness across species. Orexin neuron activity oscillates throughout the day, with the greatest activity occurring during the normal wake period and falling silent during the normal sleep period (Taheri et al., 2000; Zeitzer et al., 2003; Grady et al., 2006). In genetic studies in rodents and dogs, complete loss of orexin signalling over time results in fragmented sleep and arousal states but leaves the overall amounts of sleep and wake constant over a 24 h period (Hara et al., 2001; Beuckmann et al., 2004).
In patients with narcolepsy, post-mortem studies have shown very few surviving orexin-producing neurons and chronically reduced levels of orexin-A in their cerebrospinal fluid; these findings indicated that near complete loss of normal orexin signalling has a significant effect on consolidation of sleep and arousal in humans (Peyron et al., 2000; Mignot et al., 2002; Thannickal et al., 2003; Crocker et al., 2005). Clinically, narcolepsy is characterized by excessive daytime sleepiness and the occurrence of characteristic waking symptoms associated with disrupted rapid eye movement (REM) sleep, such as cataplexy (sudden loss of muscular tone), sleep paralysis and hypnagogic (associated with the rapid transition to sleep) and hypnopompic hallucinations (associated with the transition from sleep) (Morgenthaler et al., 2007).
The role of the individual orexin neuropeptides and of the orexin receptors in producing these effects is not fully understood. However, characterization of acute pharmacological blockade versus complete pathological loss of signalling is now being pursued via use of novel orexin receptor antagonists. Interestingly, to date, no group has reported a pharmacological recapitulation of the narcoleptic phenotype via pharmacological blockade of the orexin system (Brisbare-Roch et al., 2007; Winrow et al., 2011; Herring et al., 2012b).
Single orexin receptor antagonists
The first small molecules identified during screening reportedly inhibited only single orexin receptors (single orexin-receptor antagonists; SORAs). Some of these, for example, SB-334867 (a heterocyclic urea developed by GSK that bound to OX1R) and JNJ-10397049 (an OX2R antagonist developed by Johnson & Johnson), have been used as receptor-specific probes. By inhibiting the activity of one receptor subtype in animal models, it was possible to observe how the absence of its downstream signal affected arousal and sleep architecture. In this way, studies using SORAs and/or rat knockouts indicated that arousal was primarily governed by OX2R signalling while switching between vigilance states (and stages in sleep architecture) was primarily impacted by both receptors (Dugovic et al., 2009; Gozzi et al., 2011; Gotter et al., 2012b).
There are potential complications when using these agents as orexin receptor probes. For example, since the selectivity of SB-334867 for OX1R is only approximately 50-fold higher than that for OX2R (Haynes et al., 2000; Porter et al., 2001), at higher doses, SB-334867 is likely to block both orexin receptors, complicating interpretation of results in high dose studies. In addition, SB-334867 has demonstrated binding activity with a number of other receptors and transporters (Winrow et al., 2012a). Moreover, SB-334867 reportedly degrades when stored as a solution for use in preclinical in vivo and in vitro tests and can decompose to an inactive form when kept as a hydrochloride salt (McElhinny Jr et al., 2012). These findings introduce a number of confounding effects to studies employing SB-334867 as a single receptor probe and caution should be used in interpreting data regarding the functional roles of individual receptors based on these studies alone.
Dual orexin receptor antagonists
Evidence from murine knockout models indicated that loss of prepro-orexin peptide (a precursor of both orexin neuropeptides) (Chemelli et al., 1999) or orexin neurons (Hara et al., 2001) results in a more robust sleep phenotype than loss of function of either one of the receptor subtypes alone. Therefore, development of orexin receptor antagonists for the treatment of insomnia has focused on inhibiting both receptor subtypes by the use of dual orexin receptor antagonists (DORAs). A number of DORAs have emerged from molecular screens across a variety of structural classes and several of these have progressed to clinical development as treatments for insomnia; to date, SORAs have not been reported to have reached clinical development.
Currently, the most widely discussed DORA molecules in the literature are SB-649868 (a piperidine amide) developed by GSK, almorexant (a tetrahydroisoquinolone) developed by Actelion, and suvorexant (MK-4305; a diazepane) and MK-6096 (a piperidine carboxamide) that have both been developed by Merck. Other classes of compounds with orexin receptor antagonist activity include pyrrolidine carboxamides, proline amides, diazaspirodecanes, indoles, heteroaryl piperidines, amidoethylthioether derivatives, sulfonamides, spirobenzodioxanes and acyclic diamines (Coleman and Renger, 2010).
Preclinical, pharmacological and pharmacokinetic data for selected orexin receptor antagonists
While numerous orexin receptor antagonists have been identified using screening techniques similar to those outlined above, most have not continued on to clinical development. These include both SORAs – EMPA (selectivity >900 greater for OX2R over OX1R), JNJ-1037049 (selectivity 630 times greater for OX2R over OX1R), GSK-1059865 (selectivity 79 times greater for OX1R over OX2R), SB-334867 (selectivity 50 times greater for OX1R over OX2R), SB-408124 (selectivity 64 times greater for OX1R over OX2R) and SB-674042 (selectivity 130 times greater for OX1R over OX2R) – and the DORAs, DORA-1 (selectivity 0.1-0.2 times greater for OX2R over OX1R), DORA-12 (selectivity 1.0–10.5 times greater for OX2R over OX1R) and DORA-22 (selectivity 3.2–15 times greater for OX2R over OX1R) (Smart et al., 2001; Langmead et al., 2004; McAtee et al., 2004; Bergman et al., 2008; Malherbe et al., 2009; Cox et al., 2010; Faedo et al., 2012).
Pharmacological and pharmacokinetic data for the most widely described orexin-receptor antagonists – almorexant, DORA-22, MK-6096 (DORA-28), SB-649868 and suvorexant (MK-4305) – are summarized in Tables 2000 and 2008. With the exception of almorexant and DORA-22, all of these are in various stages of clinical development with suvorexant being the most advanced.
Table 1.
Selectivity, binding affinities and dissociation constants for selected dual orexin receptor antagonists at human orexin receptors
| Drug name | Stage of development | Selectivity | pKi (binding affinity) (nM) | pKb (dissociation constant) (nM) | References |
|---|---|---|---|---|---|
| Almorexant | No longer in development despite completion of Phase III trial RESTORA I | OX2R 1.6X OX1R | OX1R: 2.7 | OX1R: 128.4 | Brisbare-Roch et al., 2007; Winrow et al., 2012b |
| OX2R: 0.2 | OX2R: 118.9 | ||||
| DORA-22 | Preclinical development | OX2R 3.2-15X OX1R | OX1R: 9.7 | OX1R: 32 | Winrow et al., 2012b |
| OX2R: 0.6 | OX2R: 10 | ||||
| MK-6096 | Phase II clinical trials | OX2R 1.0-8.1X OX1R | OX1R: 2.5 | OX1R: 11 | Winrow et al., 2012b |
| OX2R: 0.3 | OX2R: 11 | ||||
| SB-649868 | Phase II trials | OX2R 0.6-0.8X OX1R | OX1R: 9.5 | NA | Faedo et al., 2012 |
| OX2R: 9.4 | NA | ||||
| Suvorexant (MK-4305) | Phase III trials (currently undergoing FDA review) | OX2R 0.9-1.6X OX1R | OX1R: 1.2 | OX1R: 50 | Cox et al., 2010 |
| OX2R: 0.60 | OX2R: 56 |
FDA, Food and Drug Administration; NA, not available.
Table 2.
Pharmacokinetics of selected dual orexin receptor antagonists. aPreviously unpublished observations; bApparent terminal t½
| Drug name | Bioavailability (%) | Tmax (h) | Cmax (ng mL−1; nM) | t1/2 (h) | AUC0-∞ (ng·h mL−1; μM*hr) | Refs |
|---|---|---|---|---|---|---|
| Almorexant | ||||||
| Dog | 18–49 | 0.5–2.0 | NA | 8.0–9.0 | NA | Brisbare-Roch et al., 2007; Hoch et al., 2012c |
| Rat (100–300 mg·kg−1) | 8–34 | NA | NA | NA | NA | |
| Human (200 mg) | 11.2 | 0.9 | 154.0 | 38.4 | 523.0 | |
| DORA-22 | ||||||
| Dog (3 mg·kg−1) | NA | 0.8 | 1140 | 2.5 | 4.6 | Winrow et al., 2012b |
| Dog (30 mg·kg−1) | NA | 1.0 | 7300 | 2.5 | 64.3 | |
| Rat (10 mg·kg−1) | 32a | 0.5a | 670 | 0.5 | 2.5 | |
| MK-6096 | ||||||
| Dog (0.25 mg·kg−1) | 49 | 0.8 | 194 | 1.7 | 0.4 | Coleman et al., 2012; Winrow et al., 2012b |
| Dog (0.5 mg·kg−1) | 49 | 0.4 | 468 | 1.7 | 1.3 | |
| Rat (15 mg·kg−1) | 25 | 0.4 | 1900 | 0.5 | 2.3 | |
| SB-649868 | ||||||
| Dog | NA | NA | NA | <1.0 | NA | Renzulli et al., 2011; Bettica et al., 2012a |
| Rat | NA | NA | NA | <1.0 | NA | |
| Human (30 mg) | NA | 4.0 | 1200 | 4.8 | 8300 | |
| Human (5 mg) | NA | 2.5 | 158 | 3.5 | NA | |
| Human (15 mg) | NA | 2.5 | 624 | 4.8 | NA | |
| Human (30 mg) | NA | 3.0 | 964 | 5.1 | NA | |
| Suvorexant | ||||||
| Dog (3 mg·kg−1) | 56 | 0.4a | 817 | 3.3 | 4.0 | Cox et al., 2010; Winrow et al., 2011; Sun et al., 2013 |
| Rat (10 mg·kg−1) | 19 | 3.3a | 1600 | 0.6 | 12.4 | |
| Human (10 mg) | NA | 3.0 | 440 | 9.0b | 6.7 | |
| Human (50 mg) | NA | 3.0 | 870 | 10.8b | 10.9 | |
| Human (100 mg) | NA | 3.0 | 2120 | 13.1b | 29.8 |
NA, not available.
Characterizing orexin signalling
Early studies noted increased feeding behaviour secondary to arousal with exogenous administration of orexins in rodents. Research into the potential role of orexin signalling blockade in treating metabolic disorders has failed to progress and it is now thought that the effects of orexin on feeding may be subsequent to their role in arousal (Gotter et al., 2012b). To date, pharmacological studies of novel orexin receptor antagonists in animals have not indicated a clear relationship with changes in feeding.
JNJ-10397049 (an OX2R-specific SORA) decreased latency to sleep and locomotor activity and increased REM sleep, non-REM sleep and total sleep time in rats; SB-408124 (an OX1R SORA) did not exhibit any of these effects (Dugovic et al., 2009). Of note, when SB-408124 and JNJ-10397049 were co-administered, sleep induction and prolongation of non-REM sleep by the OX2R-specific antagonist were partially attenuated while latency to REM sleep was shortened and REM sleep duration was slightly prolonged. Dugovic et al. interpreted their results to indicate that the two orexin receptor subtypes do not contribute equally to the modulation of arousal and shifts in sleep states and to also reflect the complex interactions that lead to sleep induction and arousal (Dugovic et al., 2009).
In preclinical in vivo studies, administration of the DORA SB-649868 attenuated grooming activity evoked by injection of orexin A in rats. Moreover, in this study SB-649868 (3–30 mg·kg−1) significantly reduced latency to and increased the duration of non-REM and REM sleep compared with placebo (P < 0.001 for all comparisons) (Di Fabio et al., 2011). SB-649868 did not impair motor co-ordination in rats, whereas both zolpidem and ethanol were detrimental to motor performance and potentiated each other's effects. These results may indicate that zolpidem has broad downstream effects as a result of impacting global GABA signalling, while the effects of the orexin receptor antagonists are specific to arousal (Di Fabio et al., 2011).
Almorexant, the first DORA reported to enter clinical development, demonstrated dose-dependent increases in REM and non-REM sleep, and decreased orexin A-induced locomotion in mice and rats (Brisbare-Roch et al., 2007; 2008,; Dugovic et al., 2009; Li and Nattie, 2010; Mang et al., 2012). By contrast, treatment of rats with the GABA-A receptor modulator zolpidem resulted in longer non-REM sleep but no prolongation of REM sleep (Brisbare-Roch et al., 2007; 2008,). Furthermore, almorexant did not induce sleep in knockout mice lacking orexin receptors, providing proof of concept for the mechanism of action of this compound (Mang et al., 2012). Almorexant did not reduce next-day motor performance in a rat model of sedation and muscular relaxation, whereas rats given zolpidem or ethanol exhibited hangover effects, which were exacerbated when both of the latter agents were co-administered (Steiner et al., 2011).
Pharmacokinetic studies with almorexant have revealed drug-drug interactions via CYP3A4 inhibition. Almorexant increased the maximum concentration, half-life and overall exposure of the benzodiazepine midazolam and increased the maximum concentration and overall exposure of the hypolipidaemic drug simvastatin (Hoch et al., 2012b). A slight food effect (delayed time to maximum plasma concentration, higher overall exposure and prolonged half-life) has been reported when almorexant is taken with a high-fat meal; however, the authors of this study indicated that precaution need not be exercised with regard to almorexant and meal times (Hoch et al., 2011b). In addition, no dose adjustment is required with almorexant when taken by Japanese patients compared with Caucasian individuals despite small differences in the pharmacokinetics of this DORA in these populations (Hoch et al., 2011a).
Suvorexant reduced locomotor activity and promoted sleep in rats, dogs and rhesus monkeys in a dose-dependent manner (Winrow et al., 2011). Retention of the sleep-inducing effects of suvorexant across multiple species in preclinical studies provided a strong scientific basis for pursuing the development of suvorexant as a therapy for insomnia.
MK-6096 significantly decreased latency to slow wave sleep (P < 0.05) and increased duration of stage II slow wave sleep in dogs (P < 0.01) in a dose-dependent manner (Winrow et al., 2012b). In rats, MK-6096 also decreased latency to slow wave non-REM sleep and REM sleep (P < 0.01) and increased the duration of REM sleep (P < 0.001) at all doses (Winrow et al., 2012b). Results were similar in this study with the MK-6096 analogue, DORA-22. Sleep-promoting effects were not observed in murine orexin receptor knockouts with DORA-22, and MK-6096 had no significant off-target activities against a large battery of other receptors, indicating the high level of selectivity and specificity of these DORAs (Winrow et al., 2012b).
Overview of clinical data
Current treatments
Benzodiazepines and GABA-A receptor modulators are currently the mainstay treatments for insomnia although other newer treatments such as melatonin agonists are available. Antipsychotics and antidepressants have also become a treatment approach for insomnia – despite a paucity of clinical efficacy data and not having labelling for an insomnia indication – as well as over the counter antihistamines (Schutte-Rodin et al., 2008; Wilson et al., 2010).
There is strong evidence from at least one meta-analysis of randomized, controlled trials to support the efficacy of both benzodiazepines and GABA-A receptor modulators for the short-term treatment of insomnia (Wilson et al., 2010). With short-term treatment, these drugs can improve sleep-onset latency, total sleep time, sleep efficiency, sleep quality and depending on the molecule, may prevent early waking according to both subjective and objective measures (Wilson et al., 2010). The relationships of sedative hypnotics' pharmacokinetic properties, such as half-life and concentration achieved, on therapeutic activity have been reported (Lieberman and Neubauer, 2007). Hypnotics with longer half-lives have correspondingly longer durations of activity provided that they persist at a minimally effective concentration (Figure 2). This may be problematic if the drug remains at therapeutic levels beyond the required rest period. Moreover, dose is also a consideration as increased dose can cause the compound to persist for longer depending on its half-life (Figure 2) (Lieberman and Neubauer, 2007). Insomnia is often a chronic condition and use of benzodiazepines and GABA-A receptor modulators for the long-term treatment of insomnia is not generally recommended based on the evaluated evidence (Wilson et al., 2010). However, it should be noted that while some countries have short-term use restrictions on the labels of some GABA-A receptor modulators, other GABA-A receptor modulators can be used in the long term, for example, eszopiclone, which has 6-month data in its indication label (Sunovion Pharmaceuticals Inc, 2012).
Figure 2.
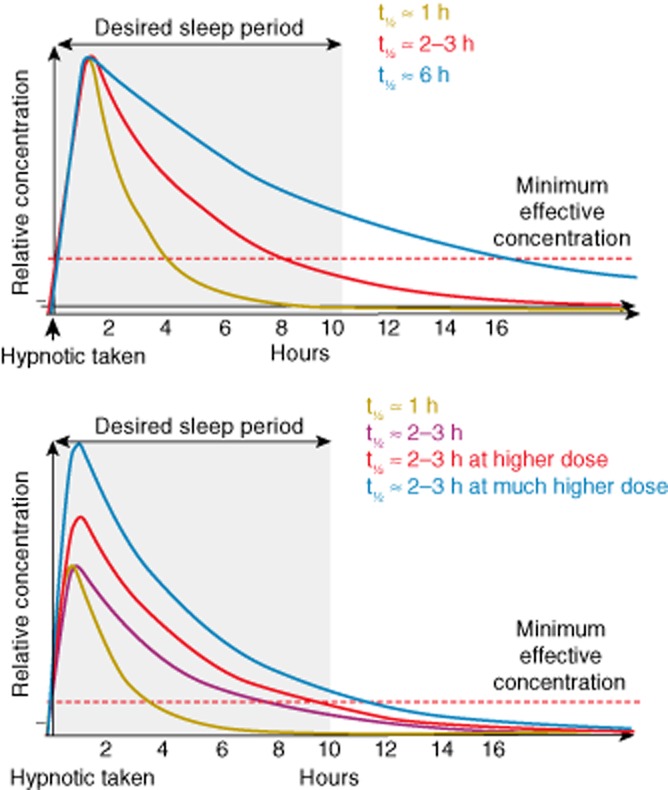
Relationships between the pharmacokinetic profiles of benzodiazepines and GABA-A receptor modulators and their therapeutic activity by half-life (top panel) and concentration (lower panel) (taken from Lieberman and Neubauer, 2007).
Choice of benzodiazepine or GABA-A receptor modulator depends on patient-specific treatment goals. For example, some benzodiazepines and GABA-A receptor modulators are effective in inducing sleep onset, while others result in a longer duration of sleep or later waking (Schutte-Rodin et al., 2008). Other major considerations in the choosing of treatments for insomnia are safety and tolerability, which will be discussed in a later section.
Evidence for the use of antipsychotics and antidepressants in the treatment of insomnia is relatively weak compared with that for the use of benzodiazepines and GABA-A receptor modulators (Schutte-Rodin et al., 2008; Wilson et al., 2010). Current guidelines suggest that use of antipsychotics and antidepressants is most appropriate in patients with a psychiatric disorder that is co-morbid with or causative for insomnia (Wilson et al., 2010). There is limited evidence for the efficacy of antihistamines in the treatment of insomnia and their potential for anticholinergic side effects reduces their utility as a long-term treatment (Schutte-Rodin et al., 2008; Wilson et al., 2010). Melatonin agonists were a relatively novel approach at the time of the development of the most recent insomnia treatment guidelines. The mechanism of action of the melatonin agonists relates to the role of endogenous melatonin in inducing drowsiness during circadian regulation of sleep and wake as opposed to the more general hypnotic mechanism historically used to treat insomnia. Early melatonin agonists had relatively short half-lives which may have limited their efficacy in terms of prolonging sleep duration; however, extended-release formulations of these drugs have assisted in overcoming this issue and the evidence base for melatonin agonists in the treatment of insomnia is growing (Wilson et al., 2010). The first melatonin agonist to be approved in the US (in 2005) was ramelteon (Takeda Pharmaceuticals America Inc, 2010). However, ramelteon became approximately 33% less effective at improving latency to persistent sleep in adults in long-term studies as compared with placebo (over 6 months), suggesting that the efficacy of this mechanism may wane over time (Mayer et al., 2009).
Orexin receptor antagonists
There are presently a limited number of published studies that provide clinical data for the DORAs in development.
For SB-649868, Phase I polysomnography data indicated that time to persistent sleep was significantly shorter (P < 0.001) and that total sleep time was significantly improved (P < 0.001) with SB-649868 30 mg and 60 mg versus placebo in healthy volunteers (Bettica et al., 2012a). In addition, REM sleep duration was significantly increased and REM latency (time from sleep onset to first epoch of REM sleep) decreased with SB-649868 versus placebo. However, duration of wake after sleep onset was not significantly improved with either dose of SB-649868 in this study (Bettica et al., 2012a). In a traffic noise model of situational insomnia in healthy volunteers, treatment with SB-649868 (30 mg) compared with zolpidem (10 mg) resulted in significantly greater increases in total sleep time (P < 0.001) as well as significant reductions in time to achieving persistent sleep (P < 0.001) (Bettica et al., 2012b). Moreover, compared with placebo, SB-649868 (30 mg) significantly increased REM sleep duration (P = 0.002); conversely, zolpidem (10 mg) resulted in a significantly reduced duration of REM sleep (P = 0.049) (Bettica et al., 2012b). These results, alongside favourable pharmacokinetic and safety data, led to the Phase II evaluation of SB-649868. In a Phase II randomized, double-blind, placebo-controlled study of 52 patients with primary insomnia, SB-649868 significantly reduced latency to persistent sleep (P < 0.001 for all doses), improved duration of wake after sleep onset (P ≤ 0.001 for 30 mg and 60 mg) and increased total sleep time (P < 0.001 for all doses) in a dose-dependent manner (Bettica et al., 2012c). Furthermore, the duration of stage II and REM sleep increased significantly with SB-649868 30 mg and 60 mg (P < 0.005). Subjective measures of sleep quantity and quality were also significantly improved in this study (Bettica et al., 2012c).
Almorexant at doses of 100–1000 mg, given in the morning or evening, has been evaluated in healthy volunteers. Following evening administration of the higher almorexant doses, polysomnography indicated that patients had shorter sleep latency, including latency to REM sleep, and had REM sleep of longer duration (Hoever et al., 2012a). When almorexant was administered in the morning, subjects experienced drowsiness and cognitive deficits indicative of sleep induction (Hoever et al., 2012a). In a second study conducted in healthy volunteers, almorexant at doses of ≥200 mg significantly reduced latency to stage 2 sleep (P ≤ 0.03) and increased sleep efficiency (P < 0.05) and total sleep time (P < 0.05) compared with baseline measurements (Brisbare-Roch et al., 2007). Zolpidem did not significantly reduce sleep latency in this study. In a double-blind, Phase II randomized study in 161 patients with primary insomnia, almorexant 400 mg significantly improved sleep efficiency versus placebo after the first dose (mean treatment effect 14.4%, P < 0.001). In addition, sleep latency was reduced by a mean of 18 min versus placebo (P = 0.02) and duration of wake after sleep onset was reduced by a mean of 54 min (P < 0.001) (Hoever et al., 2012b). Almorexant also significantly (P < 0.05) improved patient-reported measures of sleep (Hoever et al., 2012b).
In a Phase II randomized, double-blind, 4-week study of 254 patients with primary insomnia, suvorexant 10–80 mg significantly improved sleep efficiency from the first night compared with placebo (P ≤ 0.01) in a dose-dependent manner, and maintained this treatment difference to the end of the study (P ≤ 0.01). In addition, all suvorexant doses significantly improved wake after sleep onset at both time points (P ≤ 0.001). Sleep latency also improved after first treatment with suvorexant 40 and 80 mg (Herring et al., 2012b). General dose-dependent improvements in the total Insomnia Severity Index score were observed compared with placebo for suvorexant 20 mg (−2.0; P ≤ 0.01), 40 mg (−1.8; P ≤ 0.01) and 80 mg (−1.6; P ≤ 0.05). Furthermore, other than subjective refreshed feeling on waking, higher suvorexant doses (40–80 mg) improved patient-reported sleep outcomes on the first night of treatment and at study end (Herring et al., 2012b).
Safety: a rationale for targeting orexin pathway in the treatment of insomnia
Current treatments
Most current medications for insomnia interact with the GABA system, which has multiple functions throughout the brain, resulting in the potential for a broad spectrum of side effects and adverse events. In addition, the pharmacokinetic profiles of classic GABA-mediated medications for insomnia are particularly important as treatments with longer half-lives may result in residual sleepiness and next-day hangover effects such as cognitive impairment, while compounds with short half-lives may not persist within the body for a sufficient period to maintain sleep or reduce instances of wake after sleep onset (Schutte-Rodin et al., 2008; Wilson et al., 2010). Examples of adverse events resulting from the global effects of modulating GABA signalling as well as next-day hangover effects include daytime sedation, confusion, anterograde amnesia and increased falls (Rush et al., 1998; Hindmarch et al., 2006; Roth, 2007; Otmani et al., 2008; Hoque and Chesson Jr, 2009; Roehrs and Roth, 2012). In addition, dependence and tolerance are potential problems with long-term use of benzodiazepines and GABA-A receptor modulators as brain GABA receptor function can change in response to treatment (Wilson et al., 2010).
Orexin receptor antagonists
The main functions of signalling through the orexin system appear to be the promotion of arousal and consolidation of sleep and wakefulness, although orexins have also been implicated preclinically in several areas including reward pathway modulation and changes in animal models of depression. Overall, orexin signalling is not associated with the broad range of roles of the GABA system. It has been posited that the narrower functional remit of the orexin system may indicate that DORAs are a targeted treatment strategy for insomnia with reduced potential for adverse events compared with other commonly targeted treatment pathways (Gotter et al., 2012a,b2012b; Hoever et al., 2012b).
A number of hypothetical safety issues have been investigated during the development of the orexin receptor antagonists based on the mechanism of action. As mentioned earlier, cataplexy – a sudden loss of muscle tone in parts or the whole of the body – occurs in a small proportion of patients with narcolepsy. Even though narcolepsy appears to occur in individuals with near complete and persistent loss of orexin signalling, it has been suggested that attenuating or blocking orexin signalling using pharmacological orexin receptor antagonists may result, not just in the promotion of sleep, but also in the induction of cataplexy. Furthermore, as orexin peptides have a role in maintaining normal sleep architecture, it has been hypothesized that orexin receptor antagonists may dysregulate REM and non-REM sleep stages, resulting in side effects such as sleep fragmentation, hallucinations and sleep paralysis. Additional clinical data are needed to understand these theoretical effects. To date, there have been no reports of cataplexy with almorexant or suvorexant in clinical or preclinical studies (Brisbare-Roch et al., 2007; 2008,; Winrow et al., 2011; Hoever et al., 2012a,b2012b; Herring et al., 2012b).
Although SB-649868 is currently listed as undergoing assessment in Phase II trials in the GlaxoSmithKline product pipeline (GlaxoSmithKline, 2012) and data from Phase I and II studies have recently been published (Bettica et al., 2012a,c2012c), an unspecified preclinical toxicity resulted in the development programme for this orexin receptor antagonist being put on hold in 2007 (Scammell and Winrow, 2011). In healthy volunteers, published safety data for SB-649868 showed cognitive impairment versus placebo using the Digit Symbol Substitution Test at peak drug levels. However, when the test was repeated next morning (at drug nadir) this impairment did not persist (Bettica et al., 2012a). In patients with insomnia, the most commonly reported adverse events associated with SB-649868 treatment were headache (in the placebo and SB-649868 10 mg groups), nasopharyngitis (SB-649868 30 mg group) and dry mouth (SB-649868 60 mg group) (Bettica et al., 2012c). In this study, results of cognitive tests performed the morning after treatment were generally comparable between SB-649868 and placebo, although the number of correctly remembered words on the Verbal Learning and Memory Test was significantly lower with active treatment (P ≤ 0.022) (Bettica et al., 2012c).
Overall, findings have been positive regarding almorexant in published reports. In one recent trial, adverse events associated with almorexant (dizziness, nausea, fatigue, headache and dry mouth) were dose-dependent, generally transient and mild to moderate in severity (Hoever et al., 2012b). Almorexant appears to affect sleep architecture in a dose-dependent manner increasing both non-REM and REM sleep, with higher doses decreasing the time to the onset of REM sleep (shortening the duration of non-REM sleep) and also increasing the duration of REM sleep (Hoever et al., 2012b). In this study of patients with primary insomnia, residual treatment effects of almorexant using subjective measures were not reported except for a small increase in mean reaction time (34.7 ms) for almorexant at the highest dose tested (400 mg). No other notable deficits were reported in the cognitive tests performed on waking (Hoever et al., 2012b).
These initial positive findings in humans regarding lack of next-day, residual effects had been portended in animal studies. Treatment with almorexant did not reduce motor performance or grip strength on waking in rats, whereas zolpidem and ethanol not only reduced motor performance but, when given concomitantly, exacerbated each other's effects (Steiner et al., 2011). Unlike zolpidem, almorexant treatment of rats did not potentiate the next-day sedating effects of alcohol – a finding that has since been reproduced in human volunteers (Hoch et al., 2012a). These results indicate that while alcohol and GABA-A receptor modulators produce hangover effects, almorexant treatment permits full alertness on waking. Furthermore, almorexant co-administration did not engender residual sleepiness the next day. Almorexant administration in rats did not lead to the development of tolerance after five nights of treatment; by contrast, zolpidem tolerance was reported with repeated dosing in this study (Brisbare-Roch et al., 2007; 2008,). The half-life of almorexant in humans is almost 40 h (Table 2008) and is much longer than other DORAs analysed clinically. Although results from animal models and subjective studies in humans indicate that next-day effects with almorexant were not significant, an exceedingly prolonged half-life may nevertheless lead to hangover effects. Yet, despite promising clinical efficacy and safety results, almorexant development was halted in 2011 due to undisclosed adverse effects in clinical trials.
Suvorexant has been reported to be in late clinical development (Herring et al., 2012a,c2012c). In the earlier Phase II trial by Herring and colleagues discussed above, the most common adverse event associated with suvorexant was somnolence, which showed a dose-related increase in events across treatment groups of 1 (1.6%), 3 (4.9%), 6 (10.2%) and 7 (11.5%) for suvorexant 10, 20, 40 and 80 mg, respectively, compared with 1 (0.4%) for placebo. Other adverse events reported in ≥2% of patients were headache 4.9%, dizziness 4.9%, abnormal dreams 4.9%, upper respiratory and urinary tract infection 3.3% for both. One patient discontinued treatment in the suvorexant arm (compared with three patients in the placebo arm) due to experiencing a mild hypnagogic hallucination. Two patients reported transient sleep paralysis (of 2–10 min duration), two patients reported visual hallucinations and one patient reported excessive daytime sleepiness that lasted for 4 h (Herring et al., 2012b). Anterograde amnesia, a side effect that has been associated with GABA-A receptor modulator use, was not reported, nor were there adverse events indicative of potential for an abuse liability. No consistent pattern suggestive of rebound insomnia or withdrawal effects was observed after 4 weeks of treatment with suvorexant. Notably, no consistent evidence of next-day residual effects on psychomotor performance (assessed by both the Digit Symbol Substitution Test and the Digit Symbol Copying Test) was observed (Herring et al., 2012b).
Current nonclinical evidence suggests that receptor occupancy of approximately 70–80% is required to block the effects of endogenous orexin and promote sleep (data on file). The necessity for a high-level of receptor occupancy means that a DORA with sleep-promoting effects must maintain a relatively high plasma concentration throughout the designated rest period; however, a requirement for >70% receptor occupancy may reduce the potential for next day effects.
Conclusion
The identification of orexin neuropeptides and their involvement in the regulation of sleep/wake states spurred the pharmaceutical development of new targeted treatments for insomnia. Observations in animal models that functional loss of orexinergic activity was associated with increased sleepiness and fragmented wake led to the notion that pharmacological blockade of orexin receptors might be able to address an underlying cause of insomnia. Early preclinical work provided proof of concept for the orexin receptor blockade hypothesis and validated the orexin receptor antagonist mechanism of action in the induction of sleep. Normally, orexinergic diurnal variation occurs such that orexin activity is highest during waking hours and lowest during the normal sleep period. Administration of DORAs during this latter, inactive phase did not lead to robust sleep effects in healthy animals as endogenous orexin levels were at their nadir. By contrast, effects on sleep promotion were seen when DORAs were administered during times of high orexin activity, namely during the wake phase.
Available clinical data regarding the orexin receptor antagonists indicate that these molecules have many of the desired characteristics of an ideal treatment for patients with chronic insomnia, including both onset and maintenance effects without significant tolerability issues or withdrawal effects (Herring et al., 2012b) (Figure 1).
Benzodiazepines and GABA-A receptor modulators improve certain insomnia symptoms but concerns regarding residual/hangover effects, tolerability and withdrawal limit the widespread and long-term use of some of these medicines for treatment of chronic insomnia.
The orexin receptor antagonists described herein, particularly the DORAs, have subtle differences in terms of their effects on arousal and sleep architecture. Some variability in tolerability profiles, presumably due to differences in pharmacokinetics and binding selectivity for the two receptor subtypes, has been reported. However, DORAs have demonstrated efficacy in clinical trials, resulting in improved sleep latency, increased duration of sleep and decreased wake after sleep onset. Of the DORAs in development, suvorexant is the most clinically advanced, having completed Phase III trials. Final Phase III reports are awaited although top line data have been presented in the past year (Herring et al.,2012a,c,).
The ability to create animal models and cell lines for screening of novel molecules that block the orexin receptors presents, not only a means of testing promising therapeutics for insomnia, but also the opportunity to use orexinergic compounds in an exploratory manner and to investigate the role of orexin signalling in other putative indications, including, for example, addiction (see elsewhere in this review), depression, pain and migraine prophylaxis.
Acknowledgments
We thank Jane Bryant, PhD from Complete Medical Communications, who provided medical writing support funded by Merck & Co., Inc. and Anthony Gotter, PhD from Merck & Co., Inc. for his helpful comments on the manuscript.
Glossary
- DORA
dual orexin receptor antagonist
- REM
rapid eye movement
- SORA
single orexin receptor antagonist
Conflicts of interest
Christopher Winrow and John Renger are full-time employees of Merck & Co., Inc.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth edn. Washington, DC: American Psychiatric Association.; 2000. Text Revision. [Google Scholar]
- 2.Bergman JM, Roecker AJ, Mercer SP, Bednar RA, Reiss DR, Ransom RW, et al. Proline bis-amides as potent dual orexin receptor antagonists. Bioorg Med Chem Lett. 2008;18:1425–1430. doi: 10.1016/j.bmcl.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Bettica P, Nucci G, Pyke C, Squassante L, Zamuner S, Ratti E, et al. Phase I studies on the safety, tolerability, pharmacokinetics and pharmacodynamics of SB-649868, a novel dual orexin receptor antagonist. J Psychopharmacol. 2012a;26:1058–1070. doi: 10.1177/0269881111408954. [DOI] [PubMed] [Google Scholar]
- 4.Bettica P, Squassante L, Groeger JA, Gennery B, Winsky-Sommerer R, Dijk DJ. Differential effects of a dual orexin receptor antagonist (SB-649868) and zolpidem on sleep initiation and consolidation, SWS, REM sleep, and EEG power spectra in a model of situational insomnia. Neuropsychopharmacology. 2012b;37:1224–1233. doi: 10.1038/npp.2011.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bettica P, Squassante L, Zamuner S, Nucci G, Danker-Hopfe H, Ratti E. The orexin antagonist SB-649868 promotes and maintains sleep in men with primary insomnia. Sleep. 2012c;35:1097–1104. doi: 10.5665/sleep.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beuckmann CT, Sinton CM, Williams SC, Richardson JA, Hammer RE, Sakurai T, et al. Expression of a poly-glutamine-ataxin-3 transgene in orexin neurons induces narcolepsy-cataplexy in the rat. J Neurosci. 2004;24:4469–4477. doi: 10.1523/JNEUROSCI.5560-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brisbare-Roch C, Clozel M, Jenck F. Effects of repeated oral administration of the orexin receptor antagonist almorexant in male rats and dogs. Sleep. 2008;31:A38–A38. [Google Scholar]
- 8.Brisbare-Roch C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S, et al. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13:150–155. doi: 10.1038/nm1544. [DOI] [PubMed] [Google Scholar]
- 9.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 10.Coleman PJ, Renger JJ. Orexin receptor antagonists: a review of promising compounds patented since 2006. Expert Opin Ther Pat. 2010;20:307–324. doi: 10.1517/13543770903567085. [DOI] [PubMed] [Google Scholar]
- 11.Coleman PJ, Schreier JD, Cox CD, Breslin MJ, Whitman DB, Bogusky MJ, et al. Discovery of [(2R,5R)-5-{[(5-fluoropyridin-2-yl)oxy]methyl}-2-methylpiperidin-1-yl][5-methyl-2 -(pyrimidin-2-yl)phenyl]methanone (MK-6096): a dual orexin receptor antagonist with potent sleep-promoting properties. ChemMedChem. 2012;7:415–424. doi: 10.1002/cmdc.201200025. 337. [DOI] [PubMed] [Google Scholar]
- 12.Cox CD, Breslin MJ, Whitman DB, Schreier JD, McGaughey GB, Bogusky MJ, et al. Discovery of the dual orexin receptor antagonist [(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methy l-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the treatment of insomnia. J Med Chem. 2010;53:5320–5332. doi: 10.1021/jm100541c. [DOI] [PubMed] [Google Scholar]
- 13.Crocker A, Espana RA, Papadopoulou M, Saper CB, Faraco J, Sakurai T, et al. Concomitant loss of dynorphin, NARP, and orexin in narcolepsy. Neurology. 2005;65:1184–1188. doi: 10.1212/01.wnl.0000168173.71940.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Lecea L, Kilduff TS, Peyron C, Gao XB, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Fabio R, Pellacani A, Faedo S, Roth A, Piccoli L, Gerrard P, et al. Discovery process and pharmacological characterization of a novel dual orexin 1 and orexin 2 receptor antagonist useful for treatment of sleep disorders. Bioorg Med Chem Lett. 2011;21:5562–5567. doi: 10.1016/j.bmcl.2011.06.086. [DOI] [PubMed] [Google Scholar]
- 16.Dugovic C, Shelton JE, Aluisio LE, Fraser IC, Jiang XH, Sutton SW, et al. Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J Pharmacol Exp Ther. 2009;330:142–151. doi: 10.1124/jpet.109.152009. [DOI] [PubMed] [Google Scholar]
- 17.Faedo S, Perdona E, Antolini M, Di FR, Merlo PE, Corsi M. Functional and binding kinetic studies make a distinction between OX1 and OX2 orexin receptor antagonists. Eur J Pharmacol. 2012;692:1–9. doi: 10.1016/j.ejphar.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 18.GlaxoSmithKline. 2012. GlaxoSmithKline product pipeline. Available at: http://www.gsk.com/investors/product-pipeline.html (accessed 11/12/2012)
- 19.Gotter AL, Roecker AJ, Hargreaves R, Coleman PJ, Winrow CJ, Renger JJ. Orexin receptors as therapeutic drug targets. Prog Brain Res. 2012a;198:163–188. doi: 10.1016/B978-0-444-59489-1.00010-0. [DOI] [PubMed] [Google Scholar]
- 20.Gotter AL, Webber AL, Coleman PJ, Renger JJ, Winrow CJ. International Union of Basic and Clinical Pharmacology. LXXXVI. Orexin receptor function, nomenclature and pharmacology. Pharmacol Rev. 2012b;64:389–420. doi: 10.1124/pr.111.005546. [DOI] [PubMed] [Google Scholar]
- 21.Gozzi A, Turrini G, Piccoli L, Massagrande M, Amantini D, Antolini M, et al. Functional magnetic resonance imaging reveals different neural substrates for the effects of orexin-1 and orexin-2 receptor antagonists. PLoS ONE. 2011;6:e16406. doi: 10.1371/journal.pone.0016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grady SP, Nishino S, Czeisler CA, Hepner D, Scammell TE. Diurnal variation in CSF orexin-A in healthy male subjects. Sleep. 2006;29:295–297. doi: 10.1093/sleep/29.3.295. [DOI] [PubMed] [Google Scholar]
- 23.Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 24.Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, et al. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept. 2000;96:45–51. doi: 10.1016/s0167-0115(00)00199-3. [DOI] [PubMed] [Google Scholar]
- 25.Herring WJ, Connor K, Ivgy-May N, Snavely D, Snyder E, Liu K, et al. 2012a. Efficacy and safety of suvorexant, a dual orexin receptor antagonist, in patients with primary insomnia: results from two pivotal trials.
- 26.Herring WJ, Snyder E, Budd K, Hutzelmann J, Snavely D, Liu K, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012b;79:2265–2274. doi: 10.1212/WNL.0b013e31827688ee. [DOI] [PubMed] [Google Scholar]
- 27.Herring WJ, Snyder E, Paradis E, Liu M, Snavely D, Roth T, et al. Long term safety and efficacy of suvorexant in patients with primary insomnia. J Sleep Disord Res. 2012c;35:A217. abstract 0641. [Google Scholar]
- 28.Hindmarch I, Legangneux E, Stanley N, Emegbo S, Dawson J. A double-blind, placebo-controlled investigation of the residual psychomotor and cognitive effects of zolpidem-MR in healthy elderly volunteers. Br J Clin Pharmacol. 2006;62:538–545. doi: 10.1111/j.1365-2125.2006.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoch M, Hay JL, Hoever P, de Kam ML, Te Beek ET, van Gerven JM, et al. Dual orexin receptor antagonism by almorexant does not potentiate impairing effects of alcohol in humans. Eur Neuropsychopharmacol. 2012a;23:107–117. doi: 10.1016/j.euroneuro.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Hoch M, Hoever P, Alessi F, Marjason J, Dingemanse J. Pharmacokinetics and tolerability of almorexant in Japanese and Caucasian healthy male subjects. Pharmacology. 2011a;88:121–126. doi: 10.1159/000330098. [DOI] [PubMed] [Google Scholar]
- 31.Hoch M, Hoever P, Alessi F, Theodor R, Dingemanse J. Pharmacokinetic interactions of almorexant with midazolam and simvastatin, two CYP3A4 model substrates, in healthy male subjects. Eur J Clin Pharmacol. 2012b;69:523–532. doi: 10.1007/s00228-012-1403-6. [DOI] [PubMed] [Google Scholar]
- 32.Hoch M, Hoever P, Haschke M, Krahenbuhl S, Dingemanse J. Food effect and biocomparison of two formulations of the dual orexin receptor antagonist almorexant in healthy male subjects. J Clin Pharmacol. 2011b;51:1116–1121. doi: 10.1177/0091270010377634. [DOI] [PubMed] [Google Scholar]
- 33.Hoch M, Hoever P, Zisowsky J, Priestley A, Fleet D, Dingemanse J. Absolute oral bioavailability of almorexant, a dual orexin receptor antagonist, in healthy human subjects. Pharmacology. 2012c;89:53–57. doi: 10.1159/000335367. [DOI] [PubMed] [Google Scholar]
- 34.Hoever P, de Haas SL, Dorffner G, Chiossi E, van Gerven JM, Dingemanse J. Orexin receptor antagonism: an ascending multiple-dose study with almorexant. J Psychopharmacol. 2012a;26:1071–1080. doi: 10.1177/0269881112448946. [DOI] [PubMed] [Google Scholar]
- 35.Hoever P, Dorffner G, Benes H, Penzel T, Danker-Hopfe H, Barbanoj MJ, et al. Orexin receptor antagonism, a new sleep-enabling paradigm: a proof-of-concept clinical trial. Clin Pharmacol Ther. 2012b;91:975–985. doi: 10.1038/clpt.2011.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoque R, Chesson AL., Jr Zolpidem-induced sleepwalking, sleep related eating disorder, and sleep-driving: fluorine-18-flourodeoxyglucose positron emission tomography analysis, and a literature review of other unexpected clinical effects of zolpidem. J Clin Sleep Med. 2009;5:471–476. [PMC free article] [PubMed] [Google Scholar]
- 37.Langmead CJ, Jerman JC, Brough SJ, Scott C, Porter RA, Herdon HJ. Characterisation of the binding of [3H]-SB-674042, a novel nonpeptide antagonist, to the human orexin-1 receptor. Br J Pharmacol. 2004;141:340–346. doi: 10.1038/sj.bjp.0705610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li AH, Nattie E. Antagonism of rat orexin receptors by almorexant attenuates central chemoreception in wakefulness in the active period of the diurnal cycle. J Physiol. 2010;588:2935–2944. doi: 10.1113/jphysiol.2010.191288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lieberman JAI, Neubauer DN. Understanding insomnia: diagnosis and management of a common sleep disorder. J Fam Pract. 2007;56:35A–49A. [PubMed] [Google Scholar]
- 40.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin XY, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 41.Malherbe P, Borroni E, Gobbi L, Knust H, Nettekoven M, Pinard E, et al. Biochemical and behavioural characterization of EMPA, a novel high-affinity, selective antagonist for the OX(2) receptor. Br J Pharmacol. 2009;156:1326–1341. doi: 10.1111/j.1476-5381.2009.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mang GM, Durst T, Burki H, Imobersteg S, Abramowski D, Schuepbach E, et al. The dual orexin receptor antagonist almorexant induces sleep and decreases orexin-induced locomotion by blocking orexin 2 receptors. Sleep. 2012;35:1625–1635. doi: 10.5665/sleep.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mayer G, Wang-Weigand S, Roth-Schechter B, Lehmann R, Staner C, Partinen M. Efficacy and safety of 6-month nightly ramelteon administration in adults with chronic primary insomnia. Sleep. 2009;32:351–360. doi: 10.1093/sleep/32.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McAtee LC, Sutton SW, Rudolph DA, Li X, Aluisio LE, Phuong VK, et al. Novel substituted 4-phenyl-[1,3]dioxanes: potent and selective orexin receptor 2 (OX(2)R) antagonists. Bioorg Med Chem Lett. 2004;14:4225–4229. doi: 10.1016/j.bmcl.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 45.McElhinny CJ, Jr, Lewin AH, Mascarella SW, Runyon S, Brieaddy L, Carroll FI. Hydrolytic instability of the important orexin 1 receptor antagonist SB-334867: possible confounding effects on in vivo and in vitro studies. Bioorg Med Chem Lett. 2012;22:6661–6664. doi: 10.1016/j.bmcl.2012.08.109. [DOI] [PubMed] [Google Scholar]
- 46.Mignot E, Lammers GJ, Ripley B, Okun M, Nevsimalova S, Overeem S, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–1562. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 47.Morgenthaler T, Kramer M, Alessi C, Friedman L, Boehlecke B, Brown T, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An american academy of sleep medicine report. Sleep. 2006;29:1415–1419. [PubMed] [Google Scholar]
- 48.Morgenthaler TI, Kapur VK, Brown T, Swick TJ, Alessi C, Aurora RN, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30:1705–1711. doi: 10.1093/sleep/30.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 50.Otmani S, Demazieres A, Staner C, Jacob N, Nir T, Zisapel N, et al. Effects of prolonged-release melatonin, zolpidem, and their combination on psychomotor functions, memory recall, and driving skills in healthy middle aged and elderly volunteers. Hum Psychopharmacol. 2008;23:693–705. doi: 10.1002/hup.980. [DOI] [PubMed] [Google Scholar]
- 51.Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 52.Porter RA, Chan WN, Coulton S, Johns A, Hadley MS, Widdowson K, et al. 1,3-Biarylureas as selective non-peptide antagonists of the orexin-1 receptor. Bioorg Med Chem Lett. 2001;11:1907–1910. doi: 10.1016/s0960-894x(01)00343-2. [DOI] [PubMed] [Google Scholar]
- 53.Renger JJ, Kern JT. Preface to the special issue ‘Novel pharmaconeurogenetic approaches arising from progress in translational genetics. J Neurogenet. 2011;25:117–119. doi: 10.3109/01677063.2011.629355. [DOI] [PubMed] [Google Scholar]
- 54.Renzulli C, Nash M, Wright M, Thomas S, Zamuner S, Pellegatti M, et al. Disposition and metabolism of [14C]SB-649868, an orexin 1 and 2 receptor antagonist, in humans. Drug Metab Dispos. 2011;39:215–227. doi: 10.1124/dmd.110.035386. [DOI] [PubMed] [Google Scholar]
- 55.Roehrs T, Roth T. Insomnia pharmacotherapy. Neurother. 2012;9:728–738. doi: 10.1007/s13311-012-0148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roth T. A physiologic basis for the evolution of pharmacotherapy for insomnia. J Clin Psychiatry. 2007;68(Suppl. 5):13–18. [PubMed] [Google Scholar]
- 57.Rush CR, Armstrong DL, Ali JA, Pazzaglia PJ. Benzodiazepine-receptor ligands in humans: acute performance-impairing, subject-rated and observer-rated effects. J Clin Psychopharmacol. 1998;18:154–165. doi: 10.1097/00004714-199804000-00008. [DOI] [PubMed] [Google Scholar]
- 58.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 59.Scammell TE, Winrow CJ. Orexin receptors: pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2011;51:243–266. doi: 10.1146/annurev-pharmtox-010510-100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- 61.Smart D, Sabido-David C, Brough SJ, Jewitt F, Johns A, Porter RA, et al. SB-334867-A: the first selective orexin-1 receptor antagonist. Br J Pharmacol. 2001;132:1179–1182. doi: 10.1038/sj.bjp.0703953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steiner MA, Lecourt H, Strasser DS, Brisbare-Roch C, Jenck F. Differential effects of the dual orexin receptor antagonist almorexant and the GABA(A)-alpha1 receptor modulator zolpidem, alone or combined with ethanol, on motor performance in the rat. Neuropsychopharmacology. 2011;36:848–856. doi: 10.1038/npp.2010.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun H, Kennedy WP, Wilbraham D, Lewis N, Calder N, Li X, et al. Effects of suvorexant, an orexin receptor antagonist, on sleep parameters as measured by polysomnography in healthy men. Sleep. 2013;36:259–267. doi: 10.5665/sleep.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sunovion Pharmaceuticals Inc. 2012. Lunesta product label. Available at: http://www.lunesta.com/PostedApprovedLabelingText.pdf (accessed 28/2/2013)
- 65.Taheri S, Sunter D, Dakin C, Moyes S, Seal L, Gardiner J, et al. Diurnal variation in orexin A immunoreactivity and prepro-orexin mRNA in the rat central nervous system 3. Neurosci Lett. 2000;279:109–112. doi: 10.1016/s0304-3940(99)00955-6. [DOI] [PubMed] [Google Scholar]
- 66.Takeda Pharmaceuticals America Inc. 2010. Rozerem product label. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021782s011lbl.pdf (accessed 28/2/2013)
- 67.Thannickal TC, Siegel JM, Nienhuis R, Moore RY. Pattern of hypocretin (orexin) soma and axon loss, and gliosis, in human narcolepsy. Brain Pathol. 2003;13:340–351. doi: 10.1111/j.1750-3639.2003.tb00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilson SJ, Nutt DJ, Alford C, Argyropoulos SV, Baldwin DS, Bateson AN, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. J Psychopharmacol. 2010;24:1577–1601. doi: 10.1177/0269881110379307. [DOI] [PubMed] [Google Scholar]
- 69.Winrow CJ, Gotter AL, Coleman PJ, Hargreaves R, Renger J. Recent chronology of orexin pharmacology and its potential as a treatment for primary insomnia. In: Rankovic Z, Bingham M, Nestler EJ, Hargreaves R, editors. Drug Discovery for Psychiatric Disorders. London: The Royal Society of Chemistry; 2012a. pp. 416–442. [Google Scholar]
- 70.Winrow CJ, Gotter AL, Cox CD, Doran SM, Tannenbaum PL, Breslin MJ, et al. Promotion of sleep by suvorexant – a novel dual orexin receptor antagonist. J Neurogenet. 2011;25:52–61. doi: 10.3109/01677063.2011.566953. [DOI] [PubMed] [Google Scholar]
- 71.Winrow CJ, Gotter AL, Cox CD, Tannenbaum PL, Garson SL, Doran SM, et al. Pharmacological characterization of MK-6096 – a dual orexin receptor antagonist for insomnia. Neuropharmacology. 2012b;62:978–987. doi: 10.1016/j.neuropharm.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 72.Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci. 2003;23:3555–3560. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


