Abstract
Multiple homeostatic systems are regulated by orexin (hypocretin) peptides and their two known GPCRs. Activation of orexin receptors promotes waking and is essential for expression of normal sleep and waking behaviour, with the sleep disorder narcolepsy resulting from the absence of orexin signalling. Orexin receptors also influence systems regulating appetite/metabolism, stress and reward, and are found in several peripheral tissues. Nevertheless, much remains unknown about the signalling pathways and targets engaged by native receptors. In this review, we integrate knowledge about the orexin receptor signalling capabilities obtained from studies in expression systems and various native cell types (as presented in Kukkonen and Leonard, this issue of British Journal of Pharmacology) with knowledge of orexin signalling in different tissues. The tissues reviewed include the CNS, the gastrointestinal tract, the pituitary gland, pancreas, adrenal gland, adipose tissue and the male reproductive system. We also summarize the findings in different native and recombinant cell lines, especially focusing on the different cascades in CHO cells, which is the most investigated cell line. This reveals that while a substantial gap exists between what is known about orexin receptor signalling and effectors in recombinant systems and native systems, mounting evidence suggests that orexin receptor signalling is more diverse than originally thought. Moreover, rather than being restricted to orexin receptor ‘overexpressing’ cells, this signalling diversity may be utilized by native receptors in a site-specific manner.
Linked ArticlesThis article is part of a themed section on Orexin Receptors. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2014.171.issue-2
Keywords: orexin, hypocretin, neuron, adrenal gland, adipose tissue, depolarization, plasticity, cell death, hormone release, second messenger
Introduction
The landmark discoveries of the orexin (hypocretin) peptides (orexin-A and orexin-B) and their two known GPCRs (OX1 and OX2 receptors) in 1998 (de Lecea et al., 1998; Sakurai et al., 1998) set the stage for a new understanding of the brain's arousal system and the sleep disorder narcolepsy – a disorder characterized by excessive sleepiness, sleep attacks and the sudden loss of postural muscle tone (cataplexy) (for review, Siegel and Boehmer, 2006). Beyond arousal, orexins are implicated in the regulation of appetite, metabolism, stress, reward and autonomic function (for review, see Carter et al., 2009; Sharf et al., 2010; Girault et al., 2012; Nattie and Li, 2012), suggesting that these peptides work globally to organize and adjust homeostatic responses (see Li and de Lecea, 2013). In spite of these advances, many questions remain concerning how the orexin system operates. One critical question relates to the nature of the signalling cascades and effectors utilized by native orexin receptors. The answer to this question is important both from a functional perspective and from the perspective of developing new pharmacotherapeutics focused on these receptors (see Winrow and Renger, 2013) or their signalling pathways. As indicated in our sister review (Kukkonen and Leonard, 2013), the signalling of orexin receptors is multifaceted and complex. However, the widest spectrum of responses is seen in recombinant cell lines, like CHO. Do these diverse responses result from receptor overexpression or can native orexin receptors also couple to so many signal cascades? And if so, are all the cascades utilized in all tissues or is there specificity perhaps based on specific tissue needs? This review summarizes what is known about native orexin receptor signalling in the brain and peripheral tissues and aims at answering these questions. We also try to correlate orexin responses with particular orexin receptor subtypes. As orexin receptor identification by antibodies or by use of a reputedly selective agonist is unreliable (reviewed in Kukkonen, 2012; 2013), we have focused on studies utilizing mRNA detection, selective receptor antagonists and receptor knockout data.
CNS
The CNS is the most important orexin target tissue as orexin peptides are synthesized in the hypothalamus by a small group of neurons (de Lecea et al., 1998; Sakurai et al., 1998) with axons that are widely distributed in the CNS (Peyron et al., 1998; Nambu et al., 1999; van den Pol, 1999). Consequently, CNS neurons are the most extensively investigated native cells for orexin responses. However, CNS neurons are not a single cell type but represent an unknown number of different subtypes, with multiple subtypes often interdigitated within the same structures. This, of course, makes biochemical and pharmacological analysis difficult or impossible, and therefore, we have focused primarily on single cell electrophysiology and Ca2+ imaging approaches in vitro, mainly in brain slices, which have cellular resolution.
Based on in situ hybridization, both known orexin receptors are distributed throughout the CNS, each having a different, yet overlapping, pattern (Trivedi et al., 1998; Marcus et al., 2001). Moreover, several single-cell RT-PCR studies indicate that, at least in some places, mRNA for both receptors can be co-expressed (Eriksson et al., 2001; Brown et al., 2002; Korotkova et al., 2003; van den Top et al., 2003). By comparing the published distribution of orexin receptor mRNA with the results from in vitro recordings, it is clear that there is excellent correspondence between receptor mRNA expression and functional receptors, as determined by the presence of postsynaptic orexin responses (see Supporting Information Table S1). It is also clear that while the response to orexin application has been tested for many cell groups, there are regions expressing modest to high levels of mRNA that have not yet been studied (e.g. taenia tecta, intergeniculate leaflet and numerous hypothalamic nuclei; compare Supporting Information Table S1 to Marcus et al., 2001, table 1). There are also regions where mRNA was not noted but where neurons have functional receptors (e.g. thalamic parafascicular nucleus, area postrema and sublaterodorsal nucleus) where more detailed in situ analysis is needed (see Mieda et al., 2011). Finally, there are a couple of regions where neurons show direct postsynaptic responses to applied orexin but have mRNA levels appearing no higher than background (e.g. substantia nigra pars reticulata, medial dorsal nucleus of the thalamus), suggesting that functional receptors may be present in neurons having low mRNA levels or in regions with low densities of expressing neurons. Thus, it is possible that functionally important groups of orexin-responding neurons have not yet been detected.
Orexin receptor actions in the CNS
So far, for neurons that respond to exogenous orexin-A or orexin-B with a change in membrane potential, orexins produce a slow and long-lasting depolarization that can be large enough to initiate firing, or − if the neuron is already firing − to increase its firing rate. Thus, orexins are universally considered excitatory neuropeptides because their receptors couple to effectors that produce depolarization.
Although there are a few cases where the effectors underlying these depolarizations have been identified at a molecular level (Kukkonen and Leonard, 2013), in most cases, depolarization has been attributed to three categories of action: (i) closure of K+ channels active at rest (Figure 1A; Horvath et al., 1999; Ivanov and Aston-Jones, 2000; Brown et al., 2001; Hwang et al., 2001; Bayer et al., 2002; Grabauskas and Moises, 2003; Hoang et al., 2003; 2004; van den Top et al., 2003; Yang and Ferguson, 2003; Yang et al., 2003; Bayer et al., 2004; Wu et al., 2004; Murai and Akaike, 2005; Bisetti et al., 2006; Govindaiah and Cox, 2006; Huang et al., 2006; Kolaj et al., 2008; Doroshenko and Renaud, 2009; Zhang et al., 2010; 2011); (ii) activation of an electrogenic sodium-calcium exchanger (NCX) (Figure 1B; Eriksson et al., 2001; Wu et al., 2002; 2004; Burdakov et al., 2003; Acuna-Goycolea and van den Pol, 2009; Zhang et al., 2011); and (iii) the activation of non-selective cation channels (NSCCs) (Figure 1C; Eriksson et al., 2001; Hwang et al., 2001; Brown et al., 2002; Burlet et al., 2002; Liu et al., 2002; Wu et al., 2002; 2004; Yang and Ferguson, 2002; Yang and Ferguson, 2003; Huang et al., 2006).
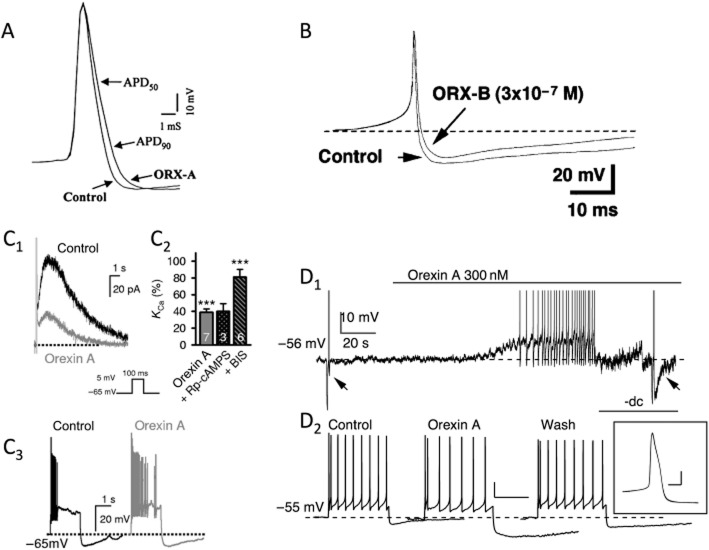
Figure 1.
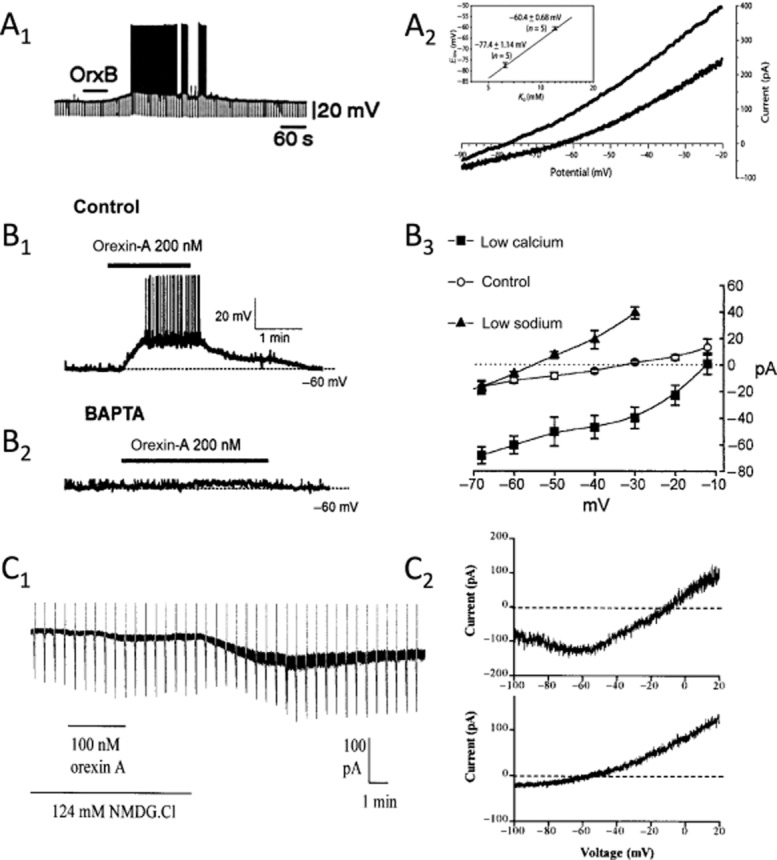
Orexin-mediated depolarizations in the CNS. (A) Orexins close leak-like K+ channels. The orexin-B-mediated (100 nM) depolarization in layer 6b neocortical pyramidal neurons, which causes firing, is associated with an increase in membrane resistance (note the larger negative-going voltage deflections produced by constant current pulses during the depolarization (A1). The I-V curve for the orexin-activated current reverses near the predicted K+ equilibrium potential in an extracellular [K+] of 6.25 mM (upper I-V curve) and 12 mM (lower I-V curve) (A2). The inset shows the mean reversal potential measured at each extracellular [K+]. (B) Orexins stimulate electrogenic NCX. Orexin-A produces a suprathreshold depolarization in type C arcuate nucleus neurons of the hypothalamus (B1). The depolarizing current is insensitive to lowering extracellular [Ca2+] (not shown) but is completely blocked by strong buffering of intracellular Ca2+ (BAPTA, 10 mM), consistent with it being triggered by the release of Ca2+ from intracellular stores (B2). The reversal potential of the orexin-activated exchanger current shifts with changes of Na+ and Ca2+ gradients (B3). (C) Orexins activate a ‘noisy’ non-selective cation current that is not reduced by low extracellular [Ca2+] or strong buffering of intracellular [Ca2+]. The orexin-A-activated current in dorsal raphé neurons was initiated, but was almost absent in low extracellular [Na+] (26 mM; NMDG). The current was reinstated by switching back to normal extracellular [Na+] (150 mM; C1). The reversal potential of this cation current is near 0 mV in normal extracellular [Na+] (C2, upper subfigure) but shifts to near −60 mV in low extracellular [Na+] (C2, lower subfigure). Subfigures (A), (B) and (C) are adapted from Bayer et al. (2004); Burdakov et al. (2003); and Brown et al. (2002), respectively and are reproduced with permission.
As noted in Kukkonen and Leonard (2013), closure of K+ channels active at rest has been relatively straightforward to demonstrate following application of orexin. For example, the membrane depolarization produced by orexin in layer 6b neurons (Bayer et al., 2004) results from a reduction in membrane current that has a reversal potential that shifts in a Nernstian manner with the K+ equilibrium potential (Figure 1A). In contrast, the distinction between currents arising from the NCX and NSCC are more problematic, especially in the absence of detailed biophysical analyses because these currents can have similar reversal potentials, are difficult to isolate from other membrane currents, are lacking highly selective antagonists (Clapham, 2003; Törok, 2007; Numata et al., 2011) and can be interdependent (Rosker et al., 2004; Louhivuori et al., 2010). Further complicating the matter, simultaneous closure of K+ channels and activation of one or both of the other effectors appears to underlie orexin actions in some neurons. Thus, we feel that identification of either NCX or NSCCs as targets for orexin receptor activation in most neurons should be considered provisional. Nevertheless, in some neurons, distinction between a NCX and a NSCC underlying the orexin-mediated depolarization appears warranted (see Supporting Information Table S1; Figure 1B,C). For example, in type C GABA cells of the arcuate nucleus (Burdakov et al., 2003), the membrane depolarization produced by orexin is mediated by a current that reverses near −30 mV under control conditions and is strongly reduced both by the NCX antagonist KB-R7943 and by buffering intracellular Ca2+ to very low levels with 10 mM BAPTA [1,2-bis(O-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid], suggesting that elevation of intracellular Ca2+ triggers an inward NCX current in these neurons. It was also shown that lowering extracellular Na+ shifts the reversal potential of this current to more negative values, while lowering extracellular Ca2+ shifts the reversal potential to more positive values – changes that at least qualitatively fit expectations for an NCX current (Luo and Rudy, 1994). In contrast, the orexin-mediated depolarization in dorsal raphé neurons appears mainly mediated by NSCCs as it is not blocked by strong buffering of intracellular Ca2+ with BAPTA (Liu et al., 2002) or low extracellular Ca2+ (Brown et al., 2002; Liu et al., 2002; Kohlmeier et al., 2008), is not effectively blocked by KB-R7943 (Liu et al., 2002; Kohlmeier et al., 2008) or by intracellular (Liu et al., 2002) and extracellular Cs+ (Kohlmeier et al., 2008), and has a reversal potential − in low extracellular Ca2+ − that shifts from near 0 mV in normal extracellular Na+ to near −50 mV in 26 mM extracellular Na+ (Brown et al., 2002).
A hallmark orexin action in expression systems is the elevation of intracellular Ca2+. Both OX1 and OX2 receptors release Ca2+ from intracellular stores and, at lower orexin concentrations, a primary Ca2+ influx is evoked (Kukkonen and Leonard, 2013). The effect of orexin on intracellular Ca2+ levels has been examined in only a few types of central neurons (van den Pol et al., 1998; 2001; van den Pol, 1999; Uramura et al., 2001; Lambe and Aghajanian, 2003; Kohlmeier et al., 2004; 2008; Muroya et al., 2004; Ishibashi et al., 2005; Tsujino et al., 2005). Interestingly, there are only a few examples where orexin actions may depend on release from intracellular stores (Korotkova et al., 2002; Burdakov et al., 2003; Muroya et al., 2004). In one study, chelation of intracellular Ca2+ with BAPTA, but not reduction of extracellular Ca2+, prevented orexin depolarizations (Burdakov et al., 2003), while in another, thapsigargin reduced orexin-mediated firing (Korotkova et al., 2002). In the third study, direct Ca2+ measurements showed that part of the orexin-induced Ca2+ elevation was prevented by pretreatment with thapsigargin (Muroya et al., 2004), but the noisy responses make firm conclusions difficult. In other neurons, the orexin-mediated Ca2+ elevation is mediated by depolarization and the activation of voltage-gated Ca2+ channels (VGCCs) (van den Pol et al., 1998; Uramura et al., 2001; Kohlmeier et al., 2004; 2008,; Ishibashi et al., 2005) as it was blocked by lowering extracellular Ca2+ and, in some cases, was shown to require depolarization, was insensitive to store depletion and/or was sensitive to blockers of VGCCs. Orexin also produces an enhancement of Ca2+ influx mediated by VGCCs in some neurons (Kohlmeier et al., 2008; see also Kukkonen and Leonard, 2013). Nevertheless, evidence indicates that orexin receptors can activate PKC (see below) in CNS neurons, which likely requires PLC activity and thus may coincide with inositol-1,4,5-trisphosphate (IP3)-triggered Ca2+ release. It is therefore noteworthy that Ca2+ imaging studies in central neurons have been limited to somata and proximal dendrites and space-averaged Ca2+ signals, as orexin receptor-mediated release of Ca2+ from intracellular stores may occur in a very localized manner and/or in neuronal compartments not yet examined such as the dendrites or synaptic terminals. Methods having high spatial resolution, like 2-photon imaging, should be utilized to address these possibilities.
In addition to mediating depolarization, there is emerging evidence that native orexin receptors alter the integrative properties of their target neurons by modulating postsynaptic ion channels and thereby strongly influence how synaptic inputs from other sources get converted into firing. Orexin-A (400 nM), acting through OX1 receptors and PKC, reduces the H-current (mediated by hyperpolarization-activated, cyclic nucleotide-gated HCN channels) and produces a negative shift in its voltage dependence in layer 5 prefrontal pyramidal neurons (Li et al., 2010). The prevalence of this action remains to be established but it could profoundly alter excitability and synaptic integration, as these channels are strongly expressed in dendrites (Magee, 1999). Orexins have also been reported to slow spike repolarization (Figure 2A) in solitary tract neurons (Yang and Ferguson, 2003; Yang et al., 2003) and reduce the post-spike afterhyperpolarization (AHP; Figure 2B) in noradrenergic locus coeruleus neurons (Horvath et al., 1999; Murai and Akaike, 2005) consistent with inhibition of a repolarizing K+ current mediated by voltage-dependent K+ channels or large-conductance Ca2+-activated K+ channels, which have recently been shown to be inhibited by orexin in smooth muscle (Squecco et al., 2011). Orexins also inhibit the long-lasting slow AHP that builds up following multiple spikes in paraventricular thalamic neurons (Figure 2C) (Zhang et al., 2010). These cells show robust low-threshold Ca2+ spiking, which drives rhythmic bursting, in part, via a Ca2+-dependent K+ current, termed the slow AHP current (IsAHP). This current, along with a Na+-dependent K+ current, produces strong spike-frequency adaptation in these cells when firing is elicited by prolonged depolarizing current pulses. Orexins inhibit both these currents via PKC, thereby enhancing the excitability of these neurons to sustained depolarizing input (Zhang et al., 2009; 2010). IsAHP is found in many neurons and, although multiple channels appear to mediate this current, it is well known to be suppressed by neurotransmitters via GPCRs that couple to Gs and Gq and it may be regulated by phosphatidylinositol-4,5-bisphosphate (PIP2) (for review, see Andrade et al., 2012).
Figure 2.
In addition to producing depolarization, orexins also modulate the integrative properties of neurons in the CNS. (A) Orexin-A (ORX-A, 10 nM) slowed action potential repolarization and broadened the spike without altering the magnitude of AHP in nucleus of the solitary tract neurons. The duration of the action potential at both 50% (APD50) and 90% (APD90) recovery was longer following orexin. This was associated with a reduction in non-inactivating voltage-dependent outward currents (not shown). (B) In dissociated locus coeruleus neurons, orexin-A and orexin-B (ORX-B) reduced the magnitude and duration of the AHP. This too was associated with a reduction in a non-inactivating, voltage-dependent outward current (not shown), which may be different from that reduced in (A). Reprinted from Murai and Akaike (2005), with permission from Elsevier. (C) In paraventricular thalamic neurons, orexins reduce an IsAHP and decrease spike-frequency adaptation. These neurons have a classical IsAHP, which is mediated mainly by a Ca2+-dependent K+ current and a Na+-dependent K+ current, which is activated with stronger stimuli. IsAHP was strongly reduced by orexin-A (200 nM; C1) and by stimulating either the PKA and PKC pathways (not shown). The orexin-A-mediated reduction was blocked by an inhibitor of PKC but not an inhibitor of PKA(C2). The reduction of this current by orexins (and PKA and PKC activators) decreases spike-frequency adaptation and increases the number of spikes fired for the same input current in these cells (C3). (D) Orexin-A (300 nM) depolarizes serotonergic dorsal raphé neurons and also enhances a late AHP. Orexin-A (300 nM) produced a suprathreshold depolarization (D1). The late AHP was evoked (5 spikes at 20 Hz) before orexin application (left arrow) and during orexin (right arrow) after returning to the same baseline membrane potential by current injection (-dc). Orexin made the late AHP larger (198 ± 19 % of control, n = 20) and longer (429 ± 52 % of control half recovery time, n = 20). This orexin-enhanced AHP slowed steady-state firing in response to 100 pA (2 s) injected current pulses (D2) without changing the shape of the action potential (spikes superimposed from before and after orexin; D2 inset). Subfigures (A), (B) and (C) are adapted from Yang and Ferguson (2003), Murai and Akaike (2005), Zhang et al. (2010) and are reproduced with permission. Subfigure (D) was adapted from Ishibashi M, Gumenchuk I, Leonard CS (unpubl. data).
Recently, we found a functionally opposite action of orexin-A on serotonergic dorsal raphé neurons. These neurons have a large, medium-duration post-spike AHP that contributes to their characteristic pacemaker firing and strong spike-frequency adaptation (Aghajanian and Vandermaelen, 1982), which is almost entirely mediated by small-conductance Ca2+-activated K+ channels (see Rouchet et al., 2008). Orexin-A enhances this K+ current and induces a longer-lasting, unidentified Ca2+-dependent late AHP current that appears distinct from IsAHP. Together, these orexin-enhanced outward currents increase spike-frequency adaptation by slowing down steady-state firing and producing a delayed decrease in excitability (Ishibashi et al., 2012). Collectively, and in contrast to a simple depolarizing action, these modulatory actions imply that orexin receptor activation can finely tune how target neurons will respond to other inputs. Future studies aimed at understanding the mechanisms, prevalence and impact of this emerging class of actions could provide important insights into how orexins normally regulate information processing in the brain and how this is perturbed in the absence of orexins in narcolepsy.
In addition to postsynaptic actions, orexins have been recognized since the earliest studies in hypothalamic cultures to release neurotransmitters independently of tetrodotoxin-sensitive impulses (van den Pol et al., 1998), suggesting that orexin receptors are located at, or near, presynaptic terminals of some glutamate- and GABA-releasing neurons. This ability to release glutamate and/or GABA by increasing miniature postsynaptic current frequency has been observed in the CNS (van den Pol et al., 1998; Li et al., 2002; Smith et al., 2002; Davis et al., 2003; Acuna-Goycolea and van den Pol, 2009), although the underlying signalling mechanisms have not been explored. In some structures, orexin preferentially releases GABA (Davis et al., 2003) or glutamate (Smith et al., 2002; Acuna-Goycolea and van den Pol, 2009), typically in conjunction with postsynaptic actions. Presumably, this allows the orexins to amplify or attenuate information transmission arising from particular afferents.
Another mechanism by which orexins can influence the release of neurotransmitter from presynaptic terminals is by retrograde paracrine signalling via the endocannabinoid 2-AG (2-arachidonoylglycerol) (see Orexins and endocannabinoids in Kukkonen and Leonard, 2013). This has so far been investigated in only a few nuclei: postsynaptic orexin action is supposed to lead to production of 2-AG, which upon exit to the synaptic space stimulates presynaptic CB1 receptors, leading to a decrease in transmitter release, glutamate in the dorsal raphé nucleus (Haj-Dahmane and Shen, 2005) and GABA in the ventrolateral periaqueductal grey matter (Ho et al., 2011). The mechanism of stimulation of production of 2-AG and its substrate DAG is not clear, but if it is related to the ability of orexin receptors to activate PLC and/or induce Ca2+ influx, as expected (see Orexins and endocannabinoids in Kukkonen and Leonard, 2013), then the same mechanism is likely operational at other central orexin targets too.
In addition to dynamically adjusting the pre- and postsynaptic properties of their targets, orexins also regulate synaptic plasticity in the hippocampus (Selbach et al., 2004; 2010,) and ventral tegmental area (for review, see Baimel and Borgland, 2012). Remarkably, in the ventral tegmental area, this plasticity is critical to behavioural sensitization resulting from cocaine administration (Borgland et al., 2006) and involves an initial but transient increase in the number of postsynaptic NMDA-type glutamate receptors. While it is yet unclear whether regulation of NMDA (or AMPA receptors; Shin et al., 2009) is a prevalent orexin function, it has recently been shown that orexins potentiate the NMDA receptor-driven release of noradrenaline from the somata and dendrites of locus coeruleus neurons (Chen et al., 2008b). These effects on NMDA receptors were sensitive to OX1 and PKC inhibitors (Borgland et al., 2006; Chen et al., 2008b), which hints at involvement of the PLC–PKC pathway. In the VTA, a similar potentiation of NMDA receptor-mediated synaptic currents was produced by orexin-B in the presence of the OX1 antagonist SB-334867 (Borgland et al., 2008). This effect was sensitive to the OX2 antagonist JNJ-10397049 and PKC inhibitors, suggesting that both OX1 and OX2 induce this type of regulation by a common signalling pathway. It will be important to determine if glutamate receptors are regulated by orexins in other neurons.
Do orexin receptor subtypes show effector preferences in the CNS?
The tools available to identify which receptor underlies orexin action on central neurons are quite limited. Expression analysis, which could exclude the presence of one subtype, is hampered by receptor antibodies that are unreliable (Kukkonen, 2012; 2013). Hence, assessment of receptor mRNA and pharmacological analysis utilizing concentration–response relations and receptor subtype-selective antagonists have mainly been used. For orexin agonists, it is generally believed − based on the originally reported affinities and potencies (Sakurai et al., 1998) − that an equal potency of orexin-A and orexin-B indicates OX2, while a 10-fold greater potency of orexin-A indicates OX1. The synthetic ligand [Ala11, d-Leu15]-orexin-B, has been reported to be OX2-selective. However, agonist potency profiles alone are unreliable due to possible biased agonism/agonist trafficking of receptor responses or other processes (discussed in Kenakin, 1997,2011; Kukkonen, 2012,2013). Such issues have been identified for orexin-A, orexin-B and [Ala11, d-Leu15]-orexin-B (Putula et al., 2011), and therefore, agonist-based determinations of orexin receptor subtypes without additional supporting evidence should be considered provisional. In a few cases, receptor knockout mice have also been used to assess whether one or the other receptor is necessary to mediate an orexin action (see below), but this approach is also not without potential problems (Routtenberg, 1996). The use of orexin receptor subtype-selective antagonists requires a pharmacological analysis to show that inhibition occurs with the expected potency, since the available antagonists are not absolutely selective. In the best case, selective antagonists for both subtypes would be used.
Based on the results from expression systems and receptor sequence analysis, there is no reason that both receptors would not be able to couple to any of the effector families described (Kukkonen and Leonard, 2013), although this issue has not been thoroughly studied. The evidence indicates that both native orexin receptors in the CNS inhibit K+ currents and activate NSCCs. For example, OX2 receptor expression is enriched in layer 6 of the neocortex in rat (Trivedi et al., 1998; Marcus et al., 2001). Layer 6b neocortical neurons are depolarized by reduction of a resting K+ current and show equivalent responses to 30 nM orexin-A and orexin-B, consistent with OX2 receptors mediating this action (Bayer et al., 2004). In contrast, rat layer 2/3 prefrontal cortex primarily expresses OX1 mRNA (Trivedi et al., 1998; Marcus et al., 2001). These pyramidal neurons are depolarized by both reduction of a resting K+ current and activation of an NSCC, and the entire response to 400 nM orexin-A is blocked by 1 μM of the OX1 antagonist SB-334867 while no response is triggered by the ‘selective’ OX2 agonist [Ala11, d-Leu15]-orexin-B (Yan et al., 2012), consistent with OX1 receptors reducing a resting K+ current.
Similarly in rat dorsal raphé nucleus, mRNA for both orexin receptors are co-distributed (Trivedi et al., 1998; Marcus et al., 2001) and can be recovered from the same cells (Brown et al., 2002). Orexin-A and orexin-B activate a NSCC with a similar potency in rat (Brown et al., 2002; Liu et al., 2002; Soffin et al., 2004) but recordings from slices made from OX1 and OX2 knockout mice indicate that either receptor is sufficient to mediate the NSCC current (Leonard et al., 2011).
In contrast, reports of NCX activation by orexin are associated with responses thought to be mediated by OX2 receptors (Eriksson et al., 2001; Wu et al., 2002; 2004,; Burdakov et al., 2003). For example, in the tuberomammilary nucleus, NCX is reported to drive the orexin-mediated depolarization and this appears entirely mediated by OX2 as only OX2 mRNA is detected by in situ hybridization in rat (Marcus et al., 2001), orexin-evoked Ca2+ transients are absent in the OX2 knockout mice (Willie et al., 2003), and orexin-stimulated firing can be rescued by re-expressing OX2 in tuberomammillary nucleus neurons of conditional OX2 knockout mice (Mochizuki et al., 2011). In the lateral vestibular nucleus, the orexin-mediated depolarization is suggested to be mediated by both the closure of K+ channels and the activation of NCX (Zhang et al., 2011). The depolarization appeared partly mediated by either orexin receptor subtype, although it was not resolved whether OX2 activation was necessary for the NCX component of the depolarization. Future studies aimed at identifying both the receptors and evoked currents at the molecular level (see Orexin receptor actions in the CNS) will be needed to determine if the correlation of OX2 and NCX is of functional importance, and if so, how it might be mediated.
Finally, while it is likely that orexins mediate the same major physiological responses in the CNS of different species, the expression of individual receptor subtypes may vary between species, and thus the use of techniques with resolving power for the function of receptor subtypes (e.g. subtype-selective antagonists) is required. Moreover, mouse shows alternative splicing of the OX2 gene with two functional receptor proteins as a result (Chen and Randeva, 2004; Chen et al., 2006), which may complicate the analysis.
Molecular signalling mechanisms in the CNS
Much less is known about the signalling cascades engaged by orexin receptors in the brain than in expression systems. Direct studies suggest orexin receptors couple to (at least) Gi/o proteins in rat brain stem and to Gi/o, Gs and Gq in rat hypothalamus (see Kukkonen and Leonard, 2013 for details). Other evidence is indirect. A number of studies have implicated the PLC–PKC pathway based on the effectiveness of PKC inhibitors in blocking the orexin-mediated depolarization (Yang et al., 2003; Muroya et al., 2004; Xia et al., 2005), elevation of intracellular Ca2+ (Uramura et al., 2001; Kohlmeier et al., 2004; 2008,; Muroya et al., 2004), inhibition of the sAHP current (Zhang et al., 2010), enhancement of NMDA receptor currents (Borgland et al., 2006; Chen et al., 2008b) and suppression of H-current (Li et al., 2010); in addition, sensitivity to the PLC inhibitor U73122 is found in some studies (Muroya et al., 2004; Borgland et al., 2006). However, it has also been reported that the orexin-mediated depolarization of dorsal raphé neurons, which is mainly mediated by a NSCC, was not blocked by inhibitors of PKC, PKA or the ERK cascade (Brown et al., 2002). In these same neurons, a PKC inhibitor blocked the orexin-enhanced Ca2+ transients, but the orexin-mediated activation of the NSCC current was still detectable in some of the same neurons, even though fewer neurons showed this response (Kohlmeier et al., 2008), suggesting the presence of PKC-dependent and PKC-independent processes. Yet, in other neurons, the orexin-mediated depolarizations are blocked by inhibitors of PKA, but not PKC (Korotkova et al., 2002; van den Top et al., 2003; Nakamura et al., 2010). Analyses of the signal coupling are hampered by multiple signalling mechanisms of these receptors and possibly even multiple similar channel types in the selected cell population or even in single cells, together with the shortage of good tools (Kukkonen and Leonard, 2013). The situation is further complicated by potential signalling pathway cross-coupling and convergence onto common effectors. For example, somatostatin and met-enkephalin activate inward rectifier K+ 3.1/3.2 (Kir3.1/3.2, a.k.a. GIRK) channels in rat locus coeruleus neurons via pertussis toxin-sensitive G-proteins, while substance P suppresses the induced activity of these channels via a pertussis toxin-insensitive mechanism (Velimirovic et al., 1995). Orexin is able to both induce the rapid activity and inhibit the sustained activity of the channels via pertussis toxin-sensitive and -insensitive mechanisms respectively (Hoang et al., 2003).
Thus, in the CNS, there is evidence that orexin receptors couple to multiple effectors including those leading to Ca2+ and Na+ influx − for which, in most cases, we do not know the channel type or activation mechanism. Moreover, it is evident that that in some central neurons, PLC−PKC and AC −PKA pathways are engaged as has been elaborated in expression systems and other tissues. Given the relative paucity of orexin receptor signalling studies in the CNS, it is plausible that the diverse signalling pathways described in other systems may also be found in the CNS as these studies develop.
How these signalling pathways function in the CNS may depend on the nature of the orexin signals to be detected. Orexins may operate as paracrine signals that act over substantial diffusion distances at very low concentrations or they may operate as more localized synaptic signals at high concentrations. Evidence for the former mode is suggested by the accumulation of orexin in the cerebrospinal fluid over the entire active periods (Zeitzer et al., 2004) and the ability of either intracerebroventricular orexin delivery or ectopic re-expression of the pre-propeptide to rescue orexin-deficient genetic models (Mieda et al., 2004; Liu et al., 2011). Evidence for the latter is suggested by the ability to record orexin-mediated synaptic potentials following electrical stimulation (Yamuy et al., 2004) and rapid arousal responses following optogenetic in vivo stimulation (Adamantidis et al., 2007) of orexin neurons.
Orexin receptor modulation of central circuits
Orexin receptor signalling influences multiple homeostatic systems, including those regulating arousal, appetite, metabolism, reward, stress and autonomic function (for review, see Carter et al., 2009; Sharf et al., 2010; Girault et al., 2012; Nattie and Li, 2012). Perhaps most strikingly, orexin signalling is essential for the normal regulation of sleep and waking as its disruption results in narcolepsy with cataplexy – a sleep disorder characterized by excessive sleepiness, sleep attacks, sudden loss of postural muscle tone in response to strong emotions (cataplexy), and state instability and fragmentation produced by shorter and more frequent bouts of waking and sleep (for review, see Siegel and Boehmer, 2006). Overall, the circuits controlling these systems are not known precisely and undoubtedly involve overlapping populations of neurons in many cases (for review, see Brown et al., 2012). Thus, our understanding of the role played by orexin receptor signalling at particular nodes in these circuits is currently quite incomplete.
In the control of arousal and behavioural state, orexin actions have been identified at many cell groups thought to be important for maintaining arousal including the orexin neurons themselves (Yamanaka et al., 2010) and all tested ascending cholinergic systems (Eggermann et al., 2001; Burlet et al., 2002; Wu et al., 2002; 2004,; Hoang et al., 2004; Kohlmeier et al., 2004; 2008,; Arrigoni et al., 2010; Leonard et al., 2011) and monoaminergic systems (Hagan et al., 1999; Horvath et al., 1999; Ivanov and Aston-Jones, 2000; Nakamura et al., 2000; Bayer et al., 2001; Brown et al., 2001; 2002,; Eriksson et al., 2001; Uramura et al., 2001; Liu et al., 2002; van den Pol et al., 2002; Yamanaka et al., 2002; Hoang et al., 2003; Willie et al., 2003; Kohlmeier et al., 2004; 2008,; Korotkova et al., 2003; Soffin et al., 2004; Haj-Dahmane and Shen, 2005; Murai and Akaike, 2005; Borgland et al., 2006; Leonard et al., 2011; Mochizuki et al., 2011; Ishibashi et al., 2012) which form part of the ascending reticular activating system and project to the thalamus and/or cortex (for review, see Jones, 2005). Moreover, orexins excite neurons in the sublaterodorsal region (Brown et al., 2006; 2008,), which is an area containing neurons with multiple transmitter phenotypes, and recently recognized as critical in regulating rapid eye movement sleep expression and muscle tone during sleep (Boissard et al., 2002; Lu et al., 2006). At the level of thalamocortical systems, orexin actions appear highly suited to promote arousal and suppress sleep because orexins excite midline, intralaminar and reticular thalamic nuclei, which are part of the ‘non-specific’ projection system, without exciting ‘specific’ thalamocortical neurons (Bayer et al., 2002; Govindaiah and Cox, 2006). Except for prefrontal cortex, orexin actions in the neocortex appear restricted to deep layer (6b) neurons, which make intracortical connections to layer 1 where they can interact with midline and intralaminar thalamic inputs (Bayer et al., 2004). Thus, orexins can promote neocortical arousal through both non-specific thalamocortical pathways and the numerous pre-cortical afferent systems that are directly activated by orexins.
Consistent with the idea that thalamocortical actions of orexin are critical for waking, orexin action at these sites appears mediated mainly by OX2 receptors (Bayer et al., 2002; 2004,). OX2 receptors are particularly important for normal waking as the defect in canine narcolepsy is a null OX2 receptor (Lin et al., 1999) and mice lacking OX2 receptors have a narcolepsy phenotype with shorter wake bouts and sleep attacks (Willie et al., 2003), unlike OX1 null mice that do not have a narcolepsy phenotype (Mieda, 2012). Moreover, in the absence of OX2, the ability of intracerebroventricularly infused orexin to promote arousal and suppress sleep through OX1 receptors is impaired (Mieda, 2012). However, recent findings utilizing a conditional OX2 receptor knockout mouse indicate that re-expression of OX2 in the tuberal region of the hypothalamus, including the histaminergic tuberomammillary nucleus, is sufficient to reinstate normal wake bout durations although fragmentation of sleep was not rescued (Mochizuki et al., 2011). This indicates, however, that thalamocortical orexin actions are not necessary for maintaining normal wake bouts. Given the apparent redundancies in arousal systems, orexin actions in thalamocortical systems may still be sufficient to stabilize normal wake bouts and/or it may be required for other aspects of waking (e.g. vigilance) yet to be determined.
Beyond the control of arousal and behavioural state, orexins influence numerous other systems as noted earlier. Several lines of evidence indicate orexins act on the neural substrates regulating motivated behaviour and reward. For example, OX1 receptors depolarize ventral tegmental area GABA and dopamine neurons (Nakamura et al., 2000; Uramura et al., 2001; Korotkova et al., 2003), and actions of orexin here appear necessary for behavioural sensitization to cocaine administration by up-regulating synaptic NMDA receptors (see above). Orexins depolarize neurons in many of the structures that are efferent targets of the ventral tegmental area system including neurons in the shell region of the nucleus accumbens via OX2 (Mukai et al., 2009), the bed nucleus of the stria terminals (Lungwitz et al., 2012) and neurons that provide input to the nucleus accumbens, including the central nucleus of the amygdala via OX2 receptors (Bisetti et al., 2006), paraventricular thalamic nucleus (Ishibashi et al., 2005; Huang et al., 2006; Kolaj et al., 2007; Doroshenko and Renaud, 2009; Zhang et al., 2009; 2010,) and prefrontal cortex (Li et al., 2010; Yan et al., 2012). Orexins also depolarize neurons in structures that provide afferent input to the ventral tegmental area including laterodorsal tegmentum (Burlet et al., 2002; Kohlmeier et al., 2004; 2008,; Leonard et al., 2011; Ishibashi et al., 2012; Takahashi et al., 2002) and pedunculopontine tegmentum (Kim et al., 2009a; 2009b) via OX1 receptors.
Orexins stimulate feeding (Sakurai et al., 1998) and orexin neurons respond to signals of metabolic status (Yamanaka et al., 2003; Burdakov et al., 2006; Karnani et al., 2011). They provide strong output to the arcuate nucleus where they excite orexigenic neuropeptide Y/agouti-related protein (NPY/AgRP) neurons and produce rhythmic bursting (van den Top et al., 2004) via OX2 receptors and activation of NCX, likely stimulated by Ca2+ release from intracellular stores (Burdakov et al., 2003; Muroya et al., 2004). Orexins also can excite the anorexigenic preproopiomelanocortin (POMC) arcuate neurons, many of which project into the median eminence, by apparent activation of NCX (Acuna-Goycolea and van den Pol, 2009). This result contradicts a previous study that concluded orexins indirectly decrease firing in POMC arcuate neurons by suppressing spontaneous excitatory and increasing spontaneous inhibitory synaptic currents (Ma et al., 2007). As noted by the authors, the orexin-mediated depolarization reversed near −40 mV and in the prior study, orexin actions were studied at depolarized membrane potentials suggesting that the depolarizing action might have been missed. While future studies should resolve the basis for these differences, these studies serve to remind us that the pathways and effectors engaged by orexin receptors may well be sensitive to the internal state of the cell, yielding different actions under different conditions. NCX might serve such a purpose as its activity is tied to metabolic factors as well as signalling pathways (DiPolo and Beauge, 2006; Törok, 2007).
Orexins strongly activate the autonomic nervous system and regulate visceral sensory-motor control (Hwang et al., 2001; Follwell and Ferguson, 2002; Smith et al., 2002; Yang and Ferguson, 2002; 2003; Davis et al., 2003; Grabauskas and Moises, 2003; van den Top et al., 2003; Yang et al., 2003; Dergacheva et al., 2005; 2012; Huang et al., 2010). Finally, orexins also can also influence the somatic sensory-motor systems, including superficial dorsal horn neurons of the spinal cord (van den Pol, 1999; Grudt et al., 2002) and ventrolateral periaqueductal grey neurons (Ho et al., 2011), both of which regulate pain transmission, and descending vestibulospinal neurons, which can powerfully influence posture and motor performance (Zhang et al., 2011).
In conclusion, native orexin receptors throughout the CNS drive membrane depolarization, increase intracellular Ca2+ and increase transmitter release from presynaptic terminals. Emerging evidence indicates that these receptors also strongly modulate cell excitability through multiple means and can regulate synaptic plasticity. Multiple effectors and signalling mechanisms are involved but our limited knowledge mostly precludes molecular identification of the signalling pathways and effectors mediating these actions. Future approaches should make use of the recently developed pharmacological tools to inhibit signal cascades [e.g. (phosphor)lipases and kinases] and orexin receptor subtypes. In addition, better resolution of the membrane currents regulated by orexins is necessary for their identification. This will require better electrophysiological isolation to enable their study over wider range of voltages, more extensive ion substitution experiments, development and use of better pharmacological blockers and ultimately utilization of knockout or knockdown approaches.
Orexin responses outside the CNS
Gastrointestinal tract
OX1 and OX2 mRNA was detected in rat and guinea pig myenteric plexi (Kirchgessner and Liu, 1999). Orexin-A (30 nM) excited guinea pig ileal submucosal neurons, and an increase in input resistance was seen. Afterhyperpolarization was also reduced. Both responses suggest inhibition of K+ channels (Kirchgessner and Liu, 1999). However, orexin-A also reduced the amplitude of the evoked excitatory postsynaptic potentials. No synaptic/action potential block was applied in these studies. In another study, orexin-A depolarized guinea pig ileal myenteric neurons; reduced input resistance, current reversal around the equilibrium potential of K+ and resistance to synaptic block suggest postsynaptic inhibition of K+ channels (Katayama et al., 2003). Orexin also enhanced evoked cholinergic potentials but did not affect direct acetylcholine responses, suggesting presynaptic facilitation. Acetylcholine overflow and contraction was seen in an orexin-A-stimulated guinea pig ileal preparation; results with tetrodotoxin, atropine and SB-334867 suggest that orexin-A acts on neuronal OX1 receptors increasing acetylcholine overflow, which depolarizes the muscle via muscarinic receptors (Matsuo et al., 2002).
Mouse duodenum was also contracted by orexin-A (300 nM) (Squecco et al., 2011). The contraction was partially inhibited by the L-type VGCC blocker nifedipine (1 μM), nearly fully by 10 μM 2-aminoethoxydiphenyl borate [inhibitor of IP3 receptors, orai1 and some transient receptor potential (TRP) channels] and fully by 50 μM Ni2+ and 1 mM tetraethylammonium chloride. Multiple channels were affected by orexin-A: activity of voltage-gated Na+ channels as well as VGCCs of T- and L-type was enhanced while BK-type K+ channels were inhibited. In addition, activation of receptor- and store-operated Ca2+ channels was suggested (Squecco et al., 2011). The results demonstrate the problems orexin researchers are facing from the multiple signal pathways utilized by orexin receptors and the limitations of the pharmacological inhibitors.
STC-1 mouse gastric endocrine carcinoid tumour cells, apparently isolated from genetically oncogene-harbouring mice, express mRNA for both OX1 and OX2 receptors (Larsson et al., 2003). Orexin-A potently stimulated cholecystokinin secretion from these cells, putatively by inducing depolarization and activation of L-type VGCCs. No obvious Ca2+ or cAMP response to orexin-A was seen, but the former may be hidden under the strong spontaneous Ca2+ oscillations.
OX1 receptors have been suggested to be expressed in primary human colorectal tumours and also in many cell lines established from primary tumours but not in the normal colon epithelium (Voisin et al., 2011; see Cell lines natively expressing orexin receptors below and Cell death in Kukkonen and Leonard, 2013).
Pituitary gland
It is somewhat unclear which parts of the pituitary express which orexin receptors in which species (see Kukkonen, 2013). In rat corticotropes, orexin-A inhibits adrenocorticotropin release in response to corticotropin-releasing hormone (Samson and Taylor, 2001). An inhibitor of PKC blocks the orexin response, while pertussis toxin does not; the target of PKC is unknown. In isolated sheep somatotropes, orexin-A, instead, enhances L-type VGCC current in a PKC-dependent manner and augments growth hormone release (Chen and Xu, 2003). Whether the latter is mediated by the PKC pathway was not assessed.
Adrenal gland
Orexin receptor activation of G-proteins has been investigated by Randeva and co-workers in adrenal cortex utilizing the GTP-azidoanilide labelling methods (see Orexin receptor coupling partners: heterotrimeric G-proteins in Kukkonen and Leonard, 2013). In human and rat cells, coupling to Gs, Gi and Gq is seen (Karteris et al., 2001,2005; Randeva et al., 2001). Whether mainly OX1 or OX2 receptors are expressed in man is unsettled (Karteris et al., 2001; Randeva et al., 2001; Mazzocchi et al., 2001b), while rat has been reported to express mRNA for both OX1 and OX2 (Karteris et al., 2005). Both human and rat cells respond to orexin by increasing glucocorticoid (cortisol and corticosterone, respectively) synthesis/release via the AC−PKA pathway (Malendowicz et al., 1999; Mazzocchi et al., 2001b). This could be mediated by Gs, but this has not been verified, and PLC is also activated by orexins in these cells (Randeva et al., 2001; Karteris et al., 2005).
H295R human adrenocortical carcinoma cells express both OX1 and OX2 receptor mRNA, although OX2 is more highly expressed (Ramanjaneya et al., 2008; Wenzel et al., 2009). Orexin receptor activation stimulates cortisol release and expression of proteins involved in steroidogenesis like steroidogenic acute regulatory protein (StAR; mRNA and protein), different cytochrome P450 (CYP) species (mRNA) and 3β-hydroxysteroid dehydrogenase (HSD3B2; mRNA) (Ramanjaneya et al., 2008; Wenzel et al., 2009). Ca2+ is elevated at high orexin concentrations, but the MAPKs, ERK and p38 are phosphorylated in the low nanomolar range (Ramanjaneya et al., 2009; Wenzel et al., 2009). PKC phosphorylation is responsible for the ERK activation, and inhibition of either one inhibits most of the HSD3B2 mRNA induction (Ramanjaneya et al., 2009; Wenzel et al., 2009). The results are complicated and partly contradictory, and it remains unclear whether ERK activation is responsible for all the steroidogenic actions (only HSD3B2 mRNA induction was assessed). The result concerning the PKC−ERK cascade would appear contradictory to the results with the primary adrenal cortical cells. This could be explained by a distorted cancer phenotype of the cell line; however, it is also conceivable that the PKC−ERK response seen in H295R cells relates to cell differentiation rather than regular stimulation of steroid biosynthesis.
Many studies find no response to orexins with respect to aldosterone or catecholamine release, but recently, amperometric recordings showed catecholamine release via OX1 receptors in cultured rat chromaffin cells (Chen et al., 2008a). Primary human pheochromocytomas (OX2 mRNA) also release catecholamines upon orexin stimulation (Mazzocchi et al., 2001a). PLC, but not AC, is stimulated in these cells, and catecholamine release is suggested to be mediated by the PLC−PKC pathway. Aldosterone release from cultured porcine chromaffin cells is stimulated only at high orexin concentrations (Nanmoku et al., 2002). The signal pathways are unclear.
Endocrine and exocrine pancreas
Whether (exogenous) orexinergic stimulation is capable of regulating release of pancreatic glucagon and insulin or other processes in endocrine pancreas is under debate. Some reports show expression of orexin receptor mRNA in endocrine pancreas and even responses in isolated tissue fragment, but the signal cascades have not been analysed. InR1-G9 cells are glucagon-secreting cells subcloned from Syrian golden hamster insulinoma cells. It is unclear, which orexin receptors subtype these cells express (as this was only determined by antibodies), but the cells functionally responded to orexin-A in nanomolar range by phosphorylation of phosphoinositide-dependent kinase 1 (PDK1) and PKB, which suggests activation of the PI3K pathway (Göncz et al., 2008). Orexin-A stimulated phosphorylation of the PKB-target protein, transcription factor Forkhead box protein O1 (FOXO1), and inhibition of PI3K or knockdown of FOXO1 reversed orexin-A-mediated decrease of proglucagon mRNA (Göncz et al., 2008). GPCRs are known to utilize several pathways for activation of class Ia and class Ib PI3Ks; it is thus far unclear how orexin receptors do it in these cells. There are a few other indications of PI3K activation by orexin receptors (Ammoun et al., 2006a; White and brown adipose tissue). In the paper (Göncz et al., 2008), the authors use rat insulinoma Ins-1 cells as a positive control for OX1 receptors; however, there are no published reports indicating the presence of OX1 receptors in Ins-1 cells.
There are no reports of orexin signalling in the exocrine pancreas except for the rat pancreatic acinar tumour AR42J cells, which express OX2 receptors. Orexins rather weakly stimulated Ca2+ mobilization in these cells but with high potency (subnanomolar) (Harris et al., 2002). Amylase secretion was stimulated 100-fold less potently (Harris et al., 2002) and cell death, in the long run, by a further 10-fold lower potency (Voisin et al., 2006). Causal relationships between these responses have, unfortunately, not been determined.
Male reproductive system
OX1 and OX2 receptor mRNA has been detected in different sites in the male reproductive tract of man and rat (Jöhren et al., 2001; Barreiro et al., 2004; Karteris et al., 2004). Orexin-A (possibly via OX1 receptors) stimulates testosterone production and decreases mRNA for anti-Müllerian hormone in rat testis via unknown pathways (Barreiro et al., 2004,2005). In human male testicular membranes, orexin-A stimulates PLC (Karteris et al., 2004).
White and brown adipose tissue
Human primary white adipocytes of subcutaneous and omental adipose tissue express mRNA for both OX1 and OX2 receptors (Digby et al., 2006). Orexin responses (orexin-A and orexin-B, 100 nM) were investigated. In subcutaneous fat, PPARγ2 mRNA was increased, while in omental fat, hormone-sensitive lipase mRNA was decreased (Digby et al., 2006). In differentiated mouse white adipocyte-like 3T3-L1 cells (OX1 and OX2 mRNA), orexin-A stimulated PI3K and, in PI3K-dependent manner, glucose uptake and triglyceride synthesis (Skrzypski et al., 2011). Orexin-A stimulated ERK phosphorylation, increased PPARγ2 mRNA and stimulated adiponectin release, while siRNA against PPARγ (γ2?) blocked orexin-A-induced increase in triglyceride content and adiponectin release. In contrast, baseline lipolysis was inhibited. Orexin-A has been reported to stimulate proliferation (but not differentiation) of undifferentiated 3T3-L1 cells (and also of another fibroblast cell line, NIH3T3); however, the concentration–response relationship and the response magnitude is very different in the two studies (Zwirska-Korczala et al., 2007; Skrzypski et al., 2012). Orexin-A also activated ERK but not PKB (Skrzypski et al., 2012). This appears contradictory to the finding that PI3K would be stimulated in differentiated cells; however, the authors have not assessed the PKB in undifferentiated cells or phosphatidylinositol-3,4,5-trisphosphate in differentiated cells. ERK activity was suggested to be protective against cell death by serum starvation as the protective effect of orexin-A was blocked by MAPK/ERK kinase 1 (MEK1) inhibition. Glucose uptake and PPARγ2 expression was increased in rat primary pre-adipocytes by orexin-A, but differentiation to adipocytes was not stimulated (Skrzypski et al., 2011; 2012).
Plancentally produced orexins were recently identified as pivotally contributing to the prenatal development of brown adipose tissue in mice (Sellayah et al., 2011). Orexin-A, via OX1 receptors, triggered the differentiation programme in mouse primary preadipocytes and mouse embryonic fibroblasts (MEFs), C3H10T1/2 mouse mesenchymal stem cells and HIB1b mouse preadipocytes; differentiation was evaluated by morphological and mRNA markers. The signal pathways inducing differentiation are unclear because signal transduction was mainly assessed in already differentiated C3H10T1/2 cells. In these cells and in mouse primary brown pre-adipocytes, orexin stimulation activated p38 and the transcription factor SMAD1/5. Upon more detailed investigations in C3H10T1/2 cells, orexin-A was seen to induce expression of mRNA for both bone morphogenic protein 7 (BMP7) and BMPR1A receptor during differentiation. The signal cascade, based on pharmacological inhibitors, was suggested to proceed from OX1 receptors partly via BMP7/BMPR1A and partly via p38 MAPK. Even BMP7 signalling was suggested to partially require p38 (Sellayah et al., 2011).
Haematopoietic cells
Despite some reports, firm evidence is lacking for the expression of orexin receptors in hematopoietic cells or their precursors. See discussion in Kukkonen (2013).
Cell lines natively expressing orexin receptors
A number of cancer cell lines were identified to express orexin receptor mRNA by RT-PCR. The cell lines expressing OX1 mRNA include human neuroblastoma SK-N-MC and human colon carcinoma cells HT29-D4, Caco-2, SW480 and LoVo (Rouet-Benzineb et al., 2004) (please note that not all the cell lines have been examined for expression of OX2). OX2 receptor mRNA is expressed in rat pancreatic acinar tumour line AR42J (Harris et al., 2002). These cell lines show decreased cell division and enhanced cell death upon orexin exposure (Rouet-Benzineb et al., 2004; Voisin et al., 2006); however, it has not been examined whether the former results from a decrease in proliferation or from the proceeding cell death. There also may be heterogeneity in these cell lines (as in most cell lines); we, for instance, have not been able to measure orexin responses in our SK-N-MC cells (Kukkonen et al., unpubl. data).
H295R human adrenocortical carcinoma cells and pancreatic tumour cells are addressed under the sections Adrenal gland and Endocrine and exocrine pancreas. Also, some other cell lines have been reported to respond to orexin, but they have found no wider use, or are problematic, for instance, by responding to orexins only at high concentrations.
In summary, many peripheral tissues express orexin receptors and respond (sometimes strongly) to exogenous orexins. However, a major question remaining is whether orexin (or another ligand) can reach these receptors under physiological (or pathological) conditions (see the Conclusions section). While this seems to be so for brown adipose tissue, the question remains open for other tissues. For possible orexin system-targeting therapy (see, e.g. Cell death in Kukkonen and Leonard, 2013, and the Conclusions section), such physiological access would, however, be less significant.
Recombinant cells
Recombinant cell lines have found much use in signalling research for orexin receptors as native orexin receptor-expressing cells have been difficult to isolate in suitable quantities, and native cell lines are scarce. Recombinant cells allow for genetic and physiological methods of analysis that are difficult to apply to native cells. However, recombinant cell lines are unrelated to, or only distantly related to, native orexin receptor-expressing cells. Therefore, results may be difficult to extrapolate to native situations. Nevertheless, remarkably similar responses have been seen: orexins, for instance, regulate NSCCs in both neurons and recombinant neuronal and non-neuronal cells (see Orexin receptor actions in the CNS, and Ca2+ and Depolarization and synaptic functions in Kukkonen and Leonard, 2013). Heterologous receptor expression platforms are often also criticized for causing artificial signal coupling not present in native cells. While being a justified concern, there is no clear evidence for this with orexin receptors. Moreover, the orexin receptor levels are unknown in native cells, and receptor levels could also be high at postsynaptic regions. Moreover, human orexin receptor signalling can be studied in recombinant expression systems in contrast to studies on native cells, which have focused mainly on rodent cells. Thus, it is possible that species-specific differences in signalling and regulation are yet to be discovered.
CHO cells (based on the CHO-K1 clone) are the cells most used for orexin receptor studies. These were also the first stable recombinant cell lines to be produced, probably simultaneously, in several laboratories (Sakurai et al., 1998; Smart et al., 1999; Lund et al., 2000; Ammoun et al., 2003). The human OX1 receptor has been most extensively investigated; the fact that the same cell lines have been used in a number of studies allows comparison of the potency of activation of different signal cascades, which are summarized in Figure 3. As discussed in Kukkonen and Leonard (2013), multiple signalling cascades are activated in these cells. G-proteins of the families Gq, Gi and Gs are suggested to be activated (Lund et al., 2000; Holmqvist et al., 2005), but there is mainly indirect evidence for this (see Orexin receptor coupling partners: heterotrimeric G-proteins in Kukkonen and Leonard, 2013). Downstream, a potent activation of Ca2+ influx/inward current and the phospholipases cytosolic phospholipase A2 (cPLA2), PLC (hydrolysing phosphoinositides PI and/or PIP) and phospholipase D1 (PLD1) is seen (Lund et al., 2000; Kukkonen and Åkerman, 2001; Larsson et al., 2005; Ammoun et al., 2006a; Johansson et al., 2007; 2008; Turunen et al., 2010; 2012; reviewed in Kukkonen and Leonard, 2013) (Figure 3). Fairly potently activated signal cascades go to ERK, cell death (p38 MAPK) and diacylglycerol lipase (DGL) (via the PLC pathway) (Ammoun et al., 2006a,b; Turunen et al., 2012; reviewed in Kukkonen and Leonard, 2013), while hydrolysis of PIP2 (and generation of IP3) and activation of Gs require relatively high orexin concentrations (Holmqvist et al., 2005; Johansson et al., 2008; reviewed in Kukkonen and Leonard, 2013) (Figure 3). We have assessed some of the signal cascades via their downstream effectors, like PLC and/or PLD signalling via DGL activity, translocation of protein kinase Cγ-C1 domain to the plasma membrane and the novel PKC (nPKC)-dependent component of AC activation (Figure 3). The signal cascades are dependent on each other, as determined utilizing a pharmacological analysis. The cPLA2 cascade contributes to the Ca2+ influx, nPKC is required for PLD1 activation, both PLD1 and PLC cascades contribute to the PKC translocation/activation, PLC cascade produces the DAG required for DGL, and IP3 induces Ca2+ release (Ekholm et al., 2007; Johansson et al., 2008; Jäntti et al., 2012; Turunen et al., 2012) (Figure 3). PLC, PLD and cPLA2 cascades should be linked via PIP2 as well: PLD should stimulate PIP2 generation, and PIP2 is required for PLD activity and it may also enhance cPLA2α activity (reviewed in Ghosh et al., 2006; Kukkonen, 2011). The ERK cascade can be partially inhibited by inhibiting many signal cascades (Ammoun et al., 2006a). The most perplexing findings relate to Ca2+ influx and nPKC. Ca2+ influx seems to amplify or gate many of the pathways via an unknown mechanism, but it is also itself regulated by one of these pathways, cPLA2 (Turunen et al., 2010; 2012; see Ca2+ in Kukkonen and Leonard, 2013). Some nPKC (possibly PKCδ) gates PLD1 activation by orexin, but this seems to be independent of PLC activity (Jäntti et al., 2012). OX2 signalling cascades have been more seldom assessed. OX2 receptor stimulation equally well elevates Ca2+ and activates ERK in CHO cells (Sakurai et al., 1998; Smart et al., 1999; Ammoun et al., 2003; 2006a; Putula et al., 2011), and also activates PLC (Tang et al., 2008; Kukkonen et al., unpubl. data). Some of the putative relationships of the signal cascades are presented in Figure 4.
Figure 3.
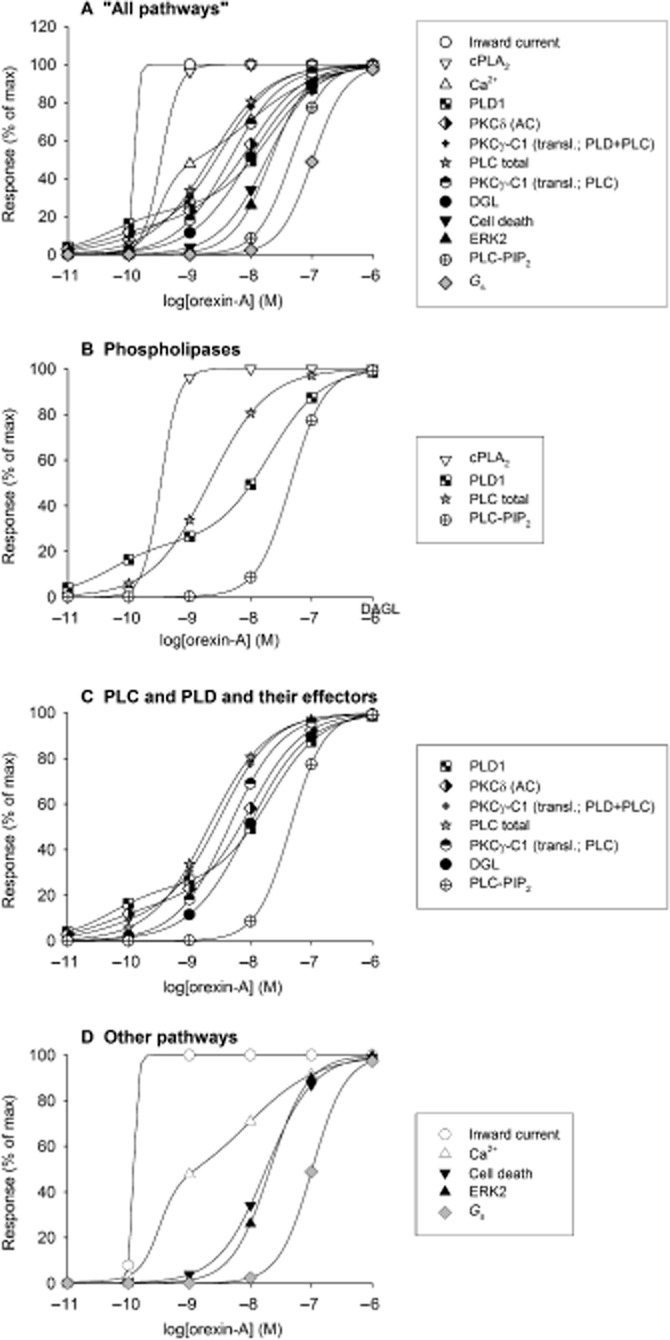
Summary of concentration–response relations for different responses produced by orexin-A at the human OX1 receptor. This illustrates the different potencies with which orexin-A stimulates different signalling cascades under conditions of equal receptor expression levels in the same clone of CHO cells. Thus, cell background and receptor expression levels are equal across cases so potencies are directly comparable. The results are expressed as % of maximal response for each output. The responses measured are: ‘inward current’ (whole-cell patch-clamp recordings), ‘cPLA2’ (pyrrophenone-sensitive and tetrahydrolipstatin-insensitive AA release chromatographically measured in 14C-AA-labelled cells), ‘Ca2+’ (cytosolic Ca2+ elevation measured with fura-2 parallel to the patch-clamp recordings), ‘PLD1’ (PLD transphosphatidylation assay in 14C-oleic acid-labelled cells with chromatographic separation; PLD1 defined by pharmacological PLD1 and PLD2 inhibitors), ‘PKCδ (AC)’ [nPKC (likely PKCδ)-dependent AC stimulation measured chromatographically in 3H-adenine-labelled cells], ‘PKCγ-C1 (transl.; PLD + PLC)’ (DAG generation by both PLC and PLD measured by translocation of the fusion protein of C1-domain of PKCγ and green fluorescent protein to the plasma membrane), ‘PLC total’ (total inositol phosphate release from 3H-inositol-labelled cells determined by chromatography), ‘PKCγ-C1 (transl.; PLC)’ (DAG generation by PLC alone measured by translocation of the fusion protein of C1-domain of PKCγ and green fluorescent protein to the plasma membrane in cells expressing dominant-negative PLD), ‘DGL’ (tetrahydrolipstatin-sensitive and pyrrophenone-insensitive 2-AG release chromatographically measured in 14C-AA-labelled cells), ‘cell death’ (assessed by staining and counting of viable cells), ‘ERK2’ [ERK2 phosphorylation assessed by Western blotting against the phosphorylated (active) ERK1/2 species], ‘PLC-PIP2’ (IP3 release assessed by a IP3-binding protein kit and by translocation of the PH-domain of PLCδ1 from the plasma membrane upon PLC activity towards PIP2), and ‘Gs’ (Gαs-dependent AC stimulation measured chromatographically in 3H-adenine-labelled cells). For the sake of clarity, only curve-fitting results and not the original data points are presented. The curves can be identified with the symbols added on the traces. The measurements originate from the following studies: Holmqvist et al. (2005); Larsson et al. (2005); Ammoun et al. (2006a,b2006b); Johansson et al. (2008); Jäntti et al. (2012); Turunen et al. (2012).
Figure 4.
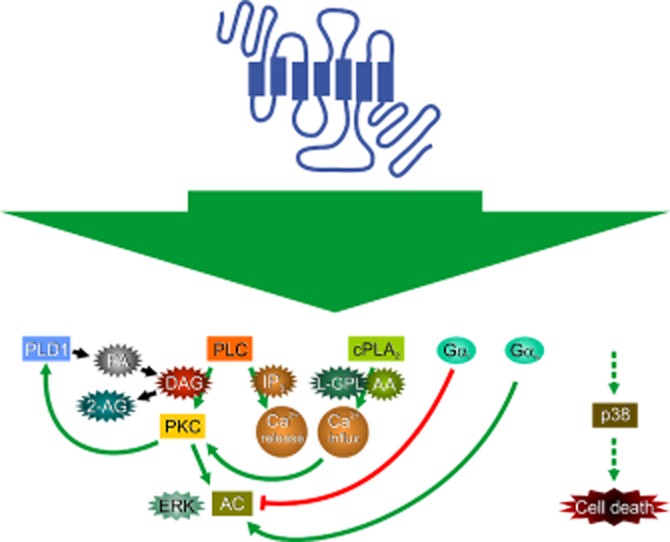
Schematic summary of some of the relationships of human OX1 receptor signalling cascades in CHO cells as described in Figure 3. References are as for Figure 3. While Gq may underlie PLC activation (and possibly other responses), this has not been explicitly assessed and is thus not indicated in the figure. L-GPL, lyso-glycerophospholipid; PA, phosphatidic acid.
CHO-S cells, which represent a suspension-adapted clone of CHO-K1, have been used in the studies of Laburthe and co-workers on cell death (see Cell death in Kukkonen and Leonard, 2013). In these cells, OX1 receptors elevate Ca2+, activate PLC and induce cell death (Voisin et al., 2008; El Firar et al., 2009). The authors have also shown that both rat orexin receptor subtypes induce apoptosis of these cells (Voisin et al., 2006).
HEK-293 cells are the next most used cell line. Stable clones, including tetracyclin-inducible ones, have been established in several laboratories (Ellis et al., 2006; Magga et al., 2006; Tang et al., 2008; Ward et al., 2011), and the cells have also been utilized for transient orexin receptor expression. Human OX1 receptors are known to connect to Ca2+ elevation (Magga et al., 2006). Transient expression has shown the same for human OX2 receptors, and both receptors couple to PLC as well (Tang et al., 2008; Putula et al., 2011; Putula and Kukkonen, 2012). Ca2+ signalling of OX1 receptors utilizes both influx and release; the mechanisms of the Ca2+ influx-dependent oscillations are described under Ca2+ in Kukkonen and Leonard (2013). We have seen release of arachidonic acid (AA) and 2-AG from stably and transiently OX1-expressing cells upon OX1 receptor stimulation (Peltonen et al., 2009; Turunen et al., 2012); the origins of these have not been determined, but it is reasonable to assume that at least 2-AG results from PLC activity. Transiently expressed OX1 receptors have been shown to engage β-arrestin in their signalling to ERK in HEK-293 (see Orexin receptor coupling partners: interaction with other proteins in Kukkonen and Leonard, 2013). OX2 receptors also activate AC and ERK and p38 MAPK cascades; both PKA and PKC are suggested to contribute to the ERK but not to the p38 cascade (Tang et al., 2008).
As an attempt to create a more neuronal model for orexin signalling studies, we established stable human orexin receptor-expressing clones of neuro-2a mouse neuroblastoma and PC12 rat pheochromocytoma cells (Holmqvist et al., 2002). Unfortunately, the cells have remained rather unexplored. The original study showed that both receptor subtypes couple to Ca2+ signalling (likely both influx and release as in CHO and HEK-293 cells) and PLC in these cell lines (Holmqvist et al., 2002). Ca2+ release was shown to require IP3 while the receptor-operated Ca2+ influx was independent of IP3 in neuro-2a-OX1 cells, similar to CHO cells (Ekholm et al., 2007). Also, ERK is activated upon OX1 receptor stimulation in these cells (Ekholm et al., 2007). In neuro-2a-OX1 cells, but not in PC12-OX1 cells, 2-AG and AA release is seen upon orexin stimulation; the source, like in HEK-293 cells, is undetermined (Turunen et al., 2012).
Stable MEF-hOX1 cells have been used in studies of cell death to assess the role of Gq/11 in the response. In these cells, orexin-A (1 μM) induces cell death and stimulates PLC, but the responses are absent when MEF cells from the Gq/11-knockout are used (Voisin et al., 2008).
Also, some other cell lines have been constructed, but they have found no wider use, or show some problems. Transient expression of orexin receptors utilizing liposome-based transfection or transduction with mammalinized baculovirus has also been reported in IMR-32, CHO and MEF cells (Näsman et al., 2006; Louhivuori et al., 2010; Jäntti et al., 2013) (and also for HEK-293 cells as described earlier). Differentiated IMR-32 cells show Ca2+ transients related to the action of NSCCs and NCX (see Ca2+ in Kukkonen and Leonard, 2013). MEF and HEK-293 cells have been utilized for translocation and β-arrestin studies (see Orexin receptor coupling partners: interaction with other proteins in Kukkonen and Leonard, 2013). Transiently transfected HEK-293 cells have also been utilized for the investigation of orexin receptor coupling to Kir3.1/3.2 channels; orexin receptors inhibit these channels, which may mimic their action in the CNS (Hoang et al., 2003) (see Central nervous system and Depolarization and synaptic functions in Kukkonen and Leonard, 2013).
Conclusions
In these two reviews (the present study and Kukkonen and Leonard, 2013), we have gathered and analysed current knowledge about orexin receptor signalling. It is obvious that orexin receptors possess the ability to signal in multiple ways. The mechanisms that determine which pathway is used in which cell type and situation are unknown. The analysis of signal cascades and target proteins is not easily performed in native cells; this is especially difficult in neurons, and as a consequence, relatively little molecular data are available. Thus, most of the signalling analysis has been performed in recombinant cell lines, mainly CHO cells, and with respect to some specific responses, in certain native cell lines. We believe that the responses seen in recombinant cell lines indeed represent physiological signalling mechanisms of orexin receptors, but where these different pathways are utilized remain mostly obscure as signalling pathways in native cells have been underexplored. The information obtained in cell lines could be utilized to formulate specific tests for native cells especially by utilizing novel pharmacological inhibitors that would be expected to reveal important new information. Thus far, the information flow in this direction seems limited, and traditional, less conclusive approaches with inhibitors having less selective or even doubtful pharmacological profiles (e.g. D609, U73122, KB-R7943) are still often used without adequate controls. However, it must be noted that the pharmacological tools for this type of research are still very limited, which thus calls for even more judicious use. More information is constantly generated and novel tools developed, which will hopefully allow, for instance, distinction of TRP channel isoforms.
One major question relating to orexin receptor signalling is its physiological importance. In CNS neurons, orexin receptors act at all levels of the neuroaxis to effect depolarization. Emerging evidence suggests that orexin receptor action is more subtle than this simple excitation, and that they dynamically adjust presynaptic activity and postsynaptic responsiveness. Orexin receptors also can regulate the expression of synaptic plasticity and potentially the expression of target neuron phenotypes. Nevertheless, linking the action mediated by orexin receptors at particular sites to overall system or circuit functions remains quite challenging, although the use of receptor/peptide deletion models and orexin receptor antagonists has been fruitful is some cases. The major effect of orexin peptide/receptor dysfunction is dysregulation of sleep-arousal behaviours, but other phenotypes, while perhaps less obvious, have (Kayaba et al., 2003) and will be discovered, considering the widespread influence of the peptide-receptor system. Being harder to uncover does not mean they are less important, but that they may involve processes protected by redundancy in regulatory systems or developmental compensation. For the peripheral systems, no clear phenotypes have yet been described, except for the developmental failure of brown adipocytes. While we find orexin receptor mRNA expression and functional responses in many peripheral tissues, preproorexin expression is found at very few sites − preproorexin mRNA expression has been reported in the gastrointestinal tract, testis, adrenal gland, pancreas and kidney (Sakurai et al., 1998; Kirchgessner and Liu, 1999; Jöhren et al., 2001; Nakabayashi et al., 2003; Karteris et al., 2004) − while there are probably no circulating orexins. Would then the peripheral orexin receptors and the responses they show to exogenous orexin stimulation have any physiological functions? One possibility, assuming there is a reason that these receptors are expressed, is that there are natural ligands for these receptors other than preproorexin-derived peptides. Based mainly on a single study (Nakabayashi et al., 2003), it seems that preproorexin expression might be more widespread in peripheral organs of man than rodents; could there be species-specific functions? If the peripheral orexin receptors have physiological significance in man, these may be manifested in side effects of orexin receptor antagonist therapy (Winrow and Renger, 2013).
The orexin system is an obvious therapeutic target for disorders of sleep and wakefulness. Both the native ligands (peptide processing, degradation) and the receptors (agonists, antagonists, allosteric modulation) could be targeted for drug development. Many orexin receptor antagonists (mainly either OX2-selective or non-selective) are under development or testing (Roecker and Coleman, 2008) and the first one of these dual receptor antagonists, suvorexant (MK-4305), is likely to be released in the US market soon (Winrow and Renger, 2013). Synthetic orexin receptor agonists, in contrast, have not been reported on except for one patent (Yanagisawa, 2010), although they would likely be useful in increasing/stabilizing wakefulness and as a treatment for excessive sleepiness (e.g. in narcolepsy) and for killing cancer cells (Cell death in Kukkonen and Leonard, 2013). For the receptor agonists, we might also expect other responses, like increased metabolic rate and peripheral responses; whether these were therapeutically useful for other indications or detrimental as side effects remains to be shown.
An interesting feature of the multiple orexin receptor responses is their relation to the agonist concentration. This is most clearly seen in the recombinant OX1 receptor-expressing CHO cells, in which different responses are triggered with distinct potencies spanning three orders of magnitude (Figure 3). Similar findings have also been reported in native OX2-expressing AR42J cells (see Endocrine and exocrine pancreas). This could, most simply, be explained by response amplification differences obtained via mutual affinities and efficacies of the specific signal transduction cascade components and/or thresholds, but it may also require other processes like receptor dimerization or trafficking. Would such orexin peptide concentration dependence of the response also be seen in physiological context? This would open up interesting possibilities of completely different responses mediated by synaptic and extrasynaptic orexin receptors. Another aspect of orexin responses are the roles played by the functional orexin peptides orexin-A and orexin-B. The original studies in recombinant CHO cells presented an often-cited selectivity of OX1 receptor between these peptides. While seen also in many other recombinant cell types with respect to Ca2+ elevation or PLC activation, this does not hold true for other responses (Kukkonen, 2013). We would thus like to suggest that orexin peptides display biased agonism (Kenakin, 2011), that is, that different agonists, like the endogenous orexin peptides, may preferentially activate different receptor responses. This could be further complicated by each receptor subtype having a different bias, and homo-oligomerization among orexin receptor ‘monomers’ or hetero-oligomerization with other GPCRs could affect these response profiles as well. Finally, if orexin-A and orexin-B were released together, their time courses of action could differ according to their relative stabilities (Yoshida et al., 2003). In native cells, there are some dramatic examples of orexin-B being more potent or efficacious than orexin-A. As this has not observed in any recombinant receptor expression system (with exception of Tang et al., 2008), it raises the question of whether it is brought about by biased agonism (via receptor monomers or oligomers), or if there are one or more additional receptors for orexin peptides or if some other pharmacodynamic and pharmacokinetic properties of these peptides are involved?
In conclusion, studies with both recombinant and native cells support the view that orexin receptors are able to regulate many physiologically important molecular signalling cascades, but that not all cells utilize all possible cascades and that these cascades in native cells are not necessarily activated with the same efficacy as in recombinant systems. While it is clear that orexin signalling is diverse in the brain, future studies are needed to narrow the gap between what is known there and in recombinant systems. Advances in this area should benefit from the application of available pharmacological and molecular biological tools and knowledge of signalling patterns in recombinant systems. Finally, what regulates how orexin receptors (and other GPCRs) select a particular signalling pathway from all possible pathways at different sites is not known and is expected to be an important topic for future investigations.
Acknowledgments
This study was supported by the Academy of Finland (J. P. K.), the Magnus Ehrnrooth Foundation (J. P. K.), the Liv & Hälsa Foundation (J. P. K.) and grants NS027881 and HL064150 from the NIH of the USPHS (C. S. L.).
Glossary
- 2-AG
2-arachidonoylglycerol
- AA
arachidonic acid
- (s)AHP
(slow) afterhyperpolarization
- AR42J
a rat pancreatic acinar tumour cell line
- BAPTA
1,2-bis(O-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- Caco-2
HT29-D4, LoVo and SW480, human colon carcinoma cell lines, cPLA2, cytosolic (Ca2+-sensitive) phospholipase A2
- DGL
DAG lipase
- H-current
a non-selective cation current mediated by hyperpolarization-activated, cyclic nucleotide-gated (HCN) channels
- HSD3B2
3β-hydroxysteroid dehydrogenase
- IMR-32 and SK-N-MC
human neuroblastoma cell lines
- IP3
inositol-1,4,5-trisphosphate
- IsAHP
sAHP current
- Kir channels
inward rectifier K+ channels
- MEF
mouse embryonic fibroblast
- MEK1
MAPK/ERK kinase 1
- NCX
Na+/Ca2+-exchanger
- neuro-2a
a mouse neuroblastoma cell line
- nPKC
novel PKC
- NSCC
non-selective cation channel
- OX1 and OX2
OX1 and OX2 orexin receptors, respectively
- PC12
a rat pheochromocytoma cell line
- PIP
phosphatidylinositol-4-phosphate
- PIP2
phosphatidylinositol-4,5-bisphosphate
- TRP (channel)
transient receptor potential (channel)
- VGCC
voltage-gated Ca2+ channel
Conflicts of interest
None.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site: http://dx.doi.org/10.1111/bph.12296
Table S1 Postsynaptic orexin actions in the CNS.
References
- 1.Acuna-Goycolea C, van den Pol AN. Neuroendocrine proopiomelanocortin neurons are excited by hypocretin/orexin. J Neurosci. 2009;29:1503–1513. doi: 10.1523/JNEUROSCI.5147-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aghajanian GK, Vandermaelen CP. Intracellular recordings from serotonergic dorsal raphe neurons: pacemaker potentials and the effect of LSD. Brain Res. 1982;238:463–469. doi: 10.1016/0006-8993(82)90124-x. [DOI] [PubMed] [Google Scholar]
- 4.Ammoun S, Holmqvist T, Shariatmadari R, Oonk HB, Detheux M, Parmentier M, et al. Distinct recognition of OX1 and OX2 receptors by orexin peptides. J Pharmacol Exp Ther. 2003;305:507–514. doi: 10.1124/jpet.102.048025. [DOI] [PubMed] [Google Scholar]
- 5.Ammoun S, Johansson L, Ekholm ME, Holmqvist T, Danis AS, Korhonen L, et al. OX1 orexin receptors activate extracellular signal-regulated kinase (ERK) in CHO cells via multiple mechanisms: the role of Ca2+ influx in OX1 receptor signaling. Mol Endocrinol. 2006a;20:80–99. doi: 10.1210/me.2004-0389. [DOI] [PubMed] [Google Scholar]
- 6.Ammoun S, Lindholm D, Wootz H, Åkerman KE, Kukkonen JP. G-protein-coupled OX1 orexin/hcrtr-1 hypocretin receptors induce caspase-dependent and -independent cell death through p38 mitogen-/stress-activated protein kinase. J Biol Chem. 2006b;281:834–842. doi: 10.1074/jbc.M508603200. [DOI] [PubMed] [Google Scholar]
- 7.Andrade R, Foehring RC, Tzingounis AV. The calcium-activated slow AHP: cutting through the Gordian knot. Front Cell Neurosci. 2012;6:47. doi: 10.3389/fncel.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arrigoni E, Mochizuki T, Scammell TE. Activation of the basal forebrain by the orexin/hypocretin neurones. Acta Physiol (Oxf) 2010;198:223–235. doi: 10.1111/j.1748-1716.2009.02036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baimel C, Borgland SL. Hypocretin modulation of drug-induced synaptic plasticity. Prog Brain Res. 2012;198:123–131. doi: 10.1016/B978-0-444-59489-1.00008-2. [DOI] [PubMed] [Google Scholar]
- 10.Barreiro ML, Pineda R, Gaytan F, Archanco M, Burrell MA, Castellano JM, et al. Pattern of orexin expression and direct biological actions of orexin-A in rat testis. Endocrinology. 2005;146:5164–5175. doi: 10.1210/en.2005-0455. [DOI] [PubMed] [Google Scholar]
- 11.Barreiro ML, Pineda R, Navarro VM, Lopez M, Suominen JS, Pinilla L, et al. Orexin 1 receptor messenger ribonucleic acid expression and stimulation of testosterone secretion by orexin-A in rat testis. Endocrinology. 2004;145:2297–2306. doi: 10.1210/en.2003-1405. [DOI] [PubMed] [Google Scholar]
- 12.Bayer L, Eggermann E, Saint-Mleux B, Machard D, Jones BE, Muhlethaler M, et al. Selective action of orexin (hypocretin) on nonspecific thalamocortical projection neurons. J Neurosci. 2002;22:7835–7839. doi: 10.1523/JNEUROSCI.22-18-07835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayer L, Eggermann E, Serafin M, Saint-Mleux B, Machard D, Jones B, et al. Orexins (hypocretins) directly excite tuberomammillary neurons. Eur J Neurosci. 2001;14:1571–1575. doi: 10.1046/j.0953-816x.2001.01777.x. [DOI] [PubMed] [Google Scholar]
- 14.Bayer L, Serafin M, Eggermann E, Saint-Mleux B, Machard D, Jones BE, et al. Exclusive postsynaptic action of hypocretin-orexin on sublayer 6b cortical neurons. J Neurosci. 2004;24:6760–6764. doi: 10.1523/JNEUROSCI.1783-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bisetti A, Cvetkovic V, Serafin M, Bayer L, Machard D, Jones BE, et al. Excitatory action of hypocretin/orexin on neurons of the central medial amygdala. Neuroscience. 2006;142:999–1004. doi: 10.1016/j.neuroscience.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P, Luppi PH. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur J Neurosci. 2002;16:1959–1973. doi: 10.1046/j.1460-9568.2002.02257.x. [DOI] [PubMed] [Google Scholar]
- 17.Borgland SL, Storm E, Bonci A. Orexin B/hypocretin 2 increases glutamatergic transmission to ventral tegmental area neurons. Eur J Neurosci. 2008;28:1545–1556. doi: 10.1111/j.1460-9568.2008.06397.x. [DOI] [PubMed] [Google Scholar]
- 18.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown RE, McKenna JT, Winston S, Basheer R, Yanagawa Y, Thakkar MM, et al. Characterization of GABAergic neurons in rapid-eye-movement sleep controlling regions of the brainstem reticular formation in GAD67-green fluorescent protein knock-in mice. Eur J Neurosci. 2008;27:352–363. doi: 10.1111/j.1460-9568.2008.06024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown RE, Sergeeva O, Eriksson KS, Haas HL. Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology. 2001;40:457–459. doi: 10.1016/s0028-3908(00)00178-7. [DOI] [PubMed] [Google Scholar]
- 22.Brown RE, Sergeeva OA, Eriksson KS, Haas HL. Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline) J Neurosci. 2002;22:8850–8859. doi: 10.1523/JNEUROSCI.22-20-08850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown RE, Winston S, Basheer R, Thakkar MM, McCarley RW. Electrophysiological characterization of neurons in the dorsolateral pontine rapid-eye-movement sleep induction zone of the rat: intrinsic membrane properties and responses to carbachol and orexins. Neuroscience. 2006;143:739–755. doi: 10.1016/j.neuroscience.2006.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O'Kelly I, et al. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50:711–722. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 25.Burdakov D, Liss B, Ashcroft FM. Orexin excites GABAergic neurons of the arcuate nucleus by activating the sodium–calcium exchanger. J Neurosci. 2003;23:4951–4957. doi: 10.1523/JNEUROSCI.23-12-04951.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burlet S, Tyler CJ, Leonard CS. Direct and indirect excitation of laterodorsal tegmental neurons by hypocretin/orexin peptides: implications for wakefulness and narcolepsy. J Neurosci. 2002;22:2862–2872. doi: 10.1523/JNEUROSCI.22-07-02862.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter ME, Borg JS, de Lecea L. The brain hypocretins and their receptors: mediators of allostatic arousal. Curr Opin Pharmacol. 2009;9:39–45. doi: 10.1016/j.coph.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen C, Xu R. The in vitro regulation of growth hormone secretion by orexins. Endocrine. 2003;22:57–66. doi: 10.1385/ENDO:22:1:57. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Karteris E, Collins D, Randeva HS. Differential expression of mouse orexin receptor type-2 (OX2R) variants in the mouse brain. Brain Res. 2006;1103:20–24. doi: 10.1016/j.brainres.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Randeva HS. Genomic organization of mouse orexin receptors: characterization of two novel tissue-specific splice variants. Mol Endocrinol. 2004;18:2790–2804. doi: 10.1210/me.2004-0167. [DOI] [PubMed] [Google Scholar]
- 31.Chen XW, Huang W, Yan JA, Fan HX, Guo N, Lu J, et al. Reinvestigation of the effect of orexin A on catecholamine release from adrenal chromaffin cells. Neurosci Lett. 2008a;436:181–184. doi: 10.1016/j.neulet.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 32.Chen XW, Mu Y, Huang HP, Guo N, Zhang B, Fan SY, et al. Hypocretin-1 potentiates NMDA receptor-mediated somatodendritic secretion from locus ceruleus neurons. J Neurosci. 2008b;28:3202–3208. doi: 10.1523/JNEUROSCI.4426-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 34.Davis SF, Williams KW, Xu W, Glatzer NR, Smith BN. Selective enhancement of synaptic inhibition by hypocretin (orexin) in rat vagal motor neurons: implications for autonomic regulation. J Neurosci. 2003;23:3844–3854. doi: 10.1523/JNEUROSCI.23-09-03844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dergacheva O, Wang X, Huang ZG, Bouairi E, Stephens C, Gorini C, et al. Hypocretin-1 (orexin-A) facilitates inhibitory and diminishes excitatory synaptic pathways to cardiac vagal neurons in the nucleus ambiguus. J Pharmacol Exp Ther. 2005;314:1322–1327. doi: 10.1124/jpet.105.086421. [DOI] [PubMed] [Google Scholar]
- 36.Dergacheva O, Bateman R, Byrne P, Mendelowitz D. Orexinergic modulation of GABAergic neurotransmission to cardiac vagal neurons in the brain stem nucleus ambiguus changes during development. Neuroscience. 2012;209:12–20. doi: 10.1016/j.neuroscience.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Digby JE, Chen J, Tang JY, Lehnert H, Matthews RN, Randeva HS. Orexin receptor expression in human adipose tissue: effects of orexin-A and orexin-B. J Endocrinol. 2006;191:129–136. doi: 10.1677/joe.1.06886. [DOI] [PubMed] [Google Scholar]
- 39.DiPolo R, Beauge L. Sodium/calcium exchanger: influence of metabolic regulation on ion carrier interactions. Physiol Rev. 2006;86:155–203. doi: 10.1152/physrev.00018.2005. [DOI] [PubMed] [Google Scholar]
- 40.Doroshenko P, Renaud LP. Acid-sensitive TASK-like K+ conductances contribute to resting membrane potential and to orexin-induced membrane depolarization in rat thalamic paraventricular nucleus neurons. Neuroscience. 2009;158:1560–1570. doi: 10.1016/j.neuroscience.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Eggermann E, Serafin M, Bayer L, Machard D, Saint-Mleux B, Jones BE, et al. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108:177–181. doi: 10.1016/s0306-4522(01)00512-7. [DOI] [PubMed] [Google Scholar]
- 42.Ekholm ME, Johansson L, Kukkonen JP. IP(3)-independent signalling of OX(1) orexin/hypocretin receptors to Ca(2+) influx and ERK. Biochem Biophys Res Commun. 2007;353:475–480. doi: 10.1016/j.bbrc.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 43.El Firar A, Voisin T, Rouyer-Fessard C, Ostuni MA, Couvineau A, Laburthe M. Discovery of a functional immunoreceptor tyrosine-based switch motif in a 7-transmembrane-spanning receptor: role in the orexin receptor OX1R-driven apoptosis. FASEB J. 2009;23:4069–4080. doi: 10.1096/fj.09-131367. [DOI] [PubMed] [Google Scholar]
- 44.Ellis J, Pediani JD, Canals M, Milasta S, Milligan G. Orexin-1 receptor-cannabinoid CB1 receptor heterodimerization results in both ligand-dependent and -independent coordinated alterations of receptor localization and function. J Biol Chem. 2006;281:38812–38824. doi: 10.1074/jbc.M602494200. [DOI] [PubMed] [Google Scholar]
- 45.Eriksson KS, Sergeeva O, Brown RE, Haas HL. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci. 2001;21:9273–9279. doi: 10.1523/JNEUROSCI.21-23-09273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Follwell MJ, Ferguson AV. Cellular mechanisms of orexin actions on paraventricular nucleus neurones in rat hypothalamus. J Physiol. 2002;545:855–867. doi: 10.1113/jphysiol.2002.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghosh M, Tucker DE, Burchett SA, Leslie CC. Properties of the group IV phospholipase A2 family. Prog Lipid Res. 2006;45:487–510. doi: 10.1016/j.plipres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Girault EM, Yi CX, Fliers E, Kalsbeek A. Orexins, feeding, and energy balance. Prog Brain Res. 2012;198:47–64. doi: 10.1016/B978-0-444-59489-1.00005-7. [DOI] [PubMed] [Google Scholar]
- 49.Govindaiah G, Cox CL. Modulation of thalamic neuron excitability by orexins. Neuropharmacology. 2006;51:414–425. doi: 10.1016/j.neuropharm.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 50.Grabauskas G, Moises HC. Gastrointestinal-projecting neurones in the dorsal motor nucleus of the vagus exhibit direct and viscerotopically organized sensitivity to orexin. J Physiol. 2003;549:37–56. doi: 10.1113/jphysiol.2002.029546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grudt TJ, van den Pol AN, Perl ER. Hypocretin-2 (orexin-B) modulation of superficial dorsal horn activity in rat. J Physiol. 2002;538:517–525. doi: 10.1113/jphysiol.2001.013120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Göncz E, Strowski MZ, Grotzinger C, Nowak KW, Kaczmarek P, Sassek M, et al. Orexin-A inhibits glucagon secretion and gene expression through a Foxo1-dependent pathway. Endocrinology. 2008;149:1618–1626. doi: 10.1210/en.2007-1257. [DOI] [PubMed] [Google Scholar]
- 53.Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haj-Dahmane S, Shen RY. The wake-promoting peptide orexin-B inhibits glutamatergic transmission to dorsal raphe nucleus serotonin neurons through retrograde endocannabinoid signaling. J Neurosci. 2005;25:896–905. doi: 10.1523/JNEUROSCI.3258-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harris DM, Go VL, Reeve JR, Jr, Wu SV. Stimulation of amylase release by orexin is mediated by orexin 2 receptor in AR42J cells. Pancreas. 2002;25:405–410. doi: 10.1097/00006676-200211000-00014. [DOI] [PubMed] [Google Scholar]
- 56.Ho YC, Lee HJ, Tung LW, Liao YY, Fu SY, Teng SF, et al. Activation of orexin 1 receptors in the periaqueductal gray of male rats leads to antinociception via retrograde endocannabinoid (2-arachidonoylglycerol)-induced disinhibition. J Neurosci. 2011;31:14600–14610. doi: 10.1523/JNEUROSCI.2671-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoang QV, Bajic D, Yanagisawa M, Nakajima S, Nakajima Y. Effects of orexin (hypocretin) on GIRK channels. J Neurophysiol. 2003;90:693–702. doi: 10.1152/jn.00001.2003. [DOI] [PubMed] [Google Scholar]
- 58.Hoang QV, Zhao P, Nakajima S, Nakajima Y. Orexin (hypocretin) effects on constitutively active inward rectifier K+ channels in cultured nucleus basalis neurons. J Neurophysiol. 2004;92:3183–3191. doi: 10.1152/jn.01222.2003. [DOI] [PubMed] [Google Scholar]
- 59.Holmqvist T, Johansson L, Östman M, Ammoun S, Åkerman KE, Kukkonen JP. OX1 orexin receptors couple to adenylyl cyclase regulation via multiple mechanisms. J Biol Chem. 2005;280:6570–6579. doi: 10.1074/jbc.M407397200. [DOI] [PubMed] [Google Scholar]
- 60.Holmqvist T, Åkerman KEO, Kukkonen JP. Orexin signaling in recombinant neuron-like cells. FEBS Lett. 2002;526:11–14. doi: 10.1016/s0014-5793(02)03101-0. [DOI] [PubMed] [Google Scholar]
- 61.Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, et al. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–159. [PubMed] [Google Scholar]
- 62.Huang H, Ghosh P, van den Pol AN. Prefrontal cortex-projecting glutamatergic thalamic paraventricular nucleus-excited by hypocretin: a feedforward circuit that may enhance cognitive arousal. J Neurophysiol. 2006;95:1656–1668. doi: 10.1152/jn.00927.2005. [DOI] [PubMed] [Google Scholar]
- 63.Huang SC, Dai YW, Lee YH, Chiou LC, Hwang LL. Orexins depolarize rostral ventrolateral medulla neurons and increase arterial pressure and heart rate in rats mainly via orexin 2 receptors. J Pharmacol Exp Ther. 2010;334:522–529. doi: 10.1124/jpet.110.167791. [DOI] [PubMed] [Google Scholar]
- 64.Hwang LL, Chen CT, Dun NJ. Mechanisms of orexin-induced depolarizations in rat dorsal motor nucleus of vagus neurones in vitro. J Physiol. 2001;537:511–520. doi: 10.1111/j.1469-7793.2001.00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ishibashi M, Gumenchuk I, Leonard CS. Orexin-A enhances a slow afterhyperpolarization (sAHP) and slows steady firing in serotonergic dorsal raphe (DR) neurons. 2012. Neuroscience 2012; New Orleans, LA. Society for Neuroscience: Washington, DC. Program No. 486.28.
- 66.Ishibashi M, Takano S, Yanagida H, Takatsuna M, Nakajima K, Oomura Y, et al. Effects of orexins/hypocretins on neuronal activity in the paraventricular nucleus of the thalamus in rats in vitro. Peptides. 2005;26:471–481. doi: 10.1016/j.peptides.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 67.Ivanov A, Aston-Jones G. Hypocretin/orexin depolarizes and decreases potassium conductance in locus coeruleus neurons. Neuroreport. 2000;11:1755–1758. doi: 10.1097/00001756-200006050-00031. [DOI] [PubMed] [Google Scholar]
- 68.Johansson L, Ekholm ME, Kukkonen JP. Multiple phospholipase activation by OX(1) orexin/hypocretin receptors. Cell Mol Life Sci. 2008;65:1948–1956. doi: 10.1007/s00018-008-8206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johansson L, Ekholm ME, Kukkonen JP. Regulation of OX(1) orexin/hypocretin receptor-coupling to phospholipase C by Ca(2+) influx. Br J Pharmacol. 2007;150:97–104. doi: 10.1038/sj.bjp.0706959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26:578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 71.Jäntti MH, Putula J, Somerharju P, Frohman MA, Kukkonen JP. OX(1) orexin/hypocretin receptor activation of phospholipase D. Br J Pharmacol. 2012;165:1109–1123. doi: 10.1111/j.1476-5381.2011.01565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jäntti MH, Putula J, Turunen PM, Näsman J, Reijonen S, Kukkonen JP. Autocrine endocannabinoid signaling potentiates orexin receptor signaling upon CB1 cannabinoid-OX1 orexin receptor coexpression. Mol Pharmacol. 2013;83:621–632. doi: 10.1124/mol.112.080523. [DOI] [PubMed] [Google Scholar]
- 73.Jöhren O, Neidert SJ, Kummer M, Dendorfer A, Dominiak P. Prepro-orexin and orexin receptor mRNAs are differentially expressed in peripheral tissues of male and female rats. Endocrinology. 2001;142:3324–3331. doi: 10.1210/endo.142.8.8299. [DOI] [PubMed] [Google Scholar]
- 74.Karnani MM, Apergis-Schoute J, Adamantidis A, Jensen LT, de Lecea L, Fugger L, et al. Activation of central orexin/hypocretin neurons by dietary amino acids. Neuron. 2011;72:616–629. doi: 10.1016/j.neuron.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 75.Karteris E, Chen J, Randeva HS. Expression of human prepro-orexin and signaling characteristics of orexin receptors in the male reproductive system. J Clin Endocrinol Metab. 2004;89:1957–1962. doi: 10.1210/jc.2003-031778. [DOI] [PubMed] [Google Scholar]
- 76.Karteris E, Machado RJ, Chen J, Zervou S, Hillhouse EW, Randeva HS. Food deprivation differentially modulates orexin receptor expression and signalling in the rat hypothalamus and adrenal cortex. Am J Physiol Endocrinol Metab. 2005;288:E1089–E1100. doi: 10.1152/ajpendo.00351.2004. [DOI] [PubMed] [Google Scholar]
- 77.Karteris E, Randeva HS, Grammatopoulos DK, Jaffe RB, Hillhouse EW. Expression and coupling characteristics of the CRH and orexin type 2 receptors in human fetal adrenals. J Clin Endocrinol Metab. 2001;86:4512–4519. doi: 10.1210/jcem.86.9.7849. [DOI] [PubMed] [Google Scholar]
- 78.Katayama Y, Homma T, Honda K, Hirai K. Actions of orexin-A in the myenteric plexus of the guinea-pig small intestine. Neuroreport. 2003;14:1515–1518. doi: 10.1097/00001756-200308060-00023. [DOI] [PubMed] [Google Scholar]
- 79.Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, et al. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R581–R593. doi: 10.1152/ajpregu.00671.2002. [DOI] [PubMed] [Google Scholar]
- 80.Kenakin T. Functional selectivity and biased receptor signaling. J Pharmacol Exp Ther. 2011;336:296–302. doi: 10.1124/jpet.110.173948. [DOI] [PubMed] [Google Scholar]
- 81.Kenakin T. Pharmacologic Analysis of Drug-Receptor Interaction. 1st edn. New York: Raven Press; 1997. [Google Scholar]
- 82.Kim J, Nakajima K, Oomura Y, Wayner MJ, Sasaki K. Electrophysiological effects of orexins/hypocretins on pedunculopontine tegmental neurons in rats: an in vitro study. Peptides. 2009a;30:191–209. doi: 10.1016/j.peptides.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 83.Kim J, Nakajima K, Oomura Y, Wayner MJ, Sasaki K. Orexin-A and ghrelin depolarize the same pedunculopontine tegmental neurons in rats: an in vitro study. Peptides. 2009b;30:1328–1335. doi: 10.1016/j.peptides.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 84.Kirchgessner AL, Liu M. Orexin synthesis and response in the gut. Neuron. 1999;24:941–951. doi: 10.1016/s0896-6273(00)81041-7. [DOI] [PubMed] [Google Scholar]
- 85.Kohlmeier KA, Inoue T, Leonard CS. Hypocretin/Orexin peptide signalling in the ascending arousal system: elevation of intracellular calcium in the mouse dorsal raphe and laterodorsal tegmentum. J Neurophysiol. 2004;92:221–235. doi: 10.1152/jn.00076.2004. [DOI] [PubMed] [Google Scholar]
- 86.Kohlmeier KA, Watanabe S, Tyler CJ, Burlet S, Leonard CS. Dual orexin actions on dorsal raphe and laterodorsal tegmentum neurons: noisy cation current activation and selective enhancement of Ca2+ transients mediated by L-type calcium channels. J Neurophysiol. 2008;100:2265–2281. doi: 10.1152/jn.01388.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kolaj M, Coderre E, Renaud LP. Orexin peptides enhance median preoptic nucleus neuronal excitability via postsynaptic membrane depolarization and enhancement of glutamatergic afferents. Neuroscience. 2008;155:1212–1220. doi: 10.1016/j.neuroscience.2008.06.059. [DOI] [PubMed] [Google Scholar]
- 88.Kolaj M, Doroshenko P, Yan Cao X, Coderre E, Renaud LP. Orexin-induced modulation of state-dependent intrinsic properties in thalamic paraventricular nucleus neurons attenuates action potential patterning and frequency. Neuroscience. 2007;147:1066–1075. doi: 10.1016/j.neuroscience.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 89.Korotkova TM, Eriksson KS, Haas HL, Brown RE. Selective excitation of GABAergic neurons in the substantia nigra of the rat by orexin/hypocretin in vitro. Regul Pept. 2002;104:83–89. doi: 10.1016/s0167-0115(01)00323-8. [DOI] [PubMed] [Google Scholar]
- 90.Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kukkonen JP. A ménage à trois made in heaven: G-protein-coupled receptors, lipids and TRP channels. Cell Calcium. 2011;50:9–26. doi: 10.1016/j.ceca.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 92.Kukkonen JP. Physiology of the orexinergic/hypocretinergic system: a revisit in 2012. Am J Physiol Cell Physiol. 2013;301:C2–C32. doi: 10.1152/ajpcell.00227.2012. [DOI] [PubMed] [Google Scholar]
- 93.Kukkonen JP. Recent progress in orexin/hypocretin physiology and pharmacology. Biomol Concepts. 2012;3:447–463. doi: 10.1515/bmc-2012-0013. [DOI] [PubMed] [Google Scholar]
- 94.Kukkonen JP, Leonard CS. Orexin/hypocretin receptor signalling cascades. Br J Pharmacol. 2013 doi: 10.1111/bph.12324. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kukkonen JP, Åkerman KEO. Orexin receptors couple to Ca2+ channels different from store-operated Ca2+ channels. Neuroreport. 2001;12:2017–2020. doi: 10.1097/00001756-200107030-00046. [DOI] [PubMed] [Google Scholar]
- 96.Lambe EK, Aghajanian GK. Hypocretin (orexin) induces calcium transients in single spines postsynaptic to identified thalamocortical boutons in prefrontal slice. Neuron. 2003;40:139–150. doi: 10.1016/s0896-6273(03)00598-1. [DOI] [PubMed] [Google Scholar]
- 97.Larsson KP, Peltonen HM, Bart G, Louhivuori LM, Penttonen A, Antikainen M, et al. Orexin-A-induced Ca2+ entry: evidence for involvement of TRPC channels and protein kinase C regulation. J Biol Chem. 2005;280:1771–1781. doi: 10.1074/jbc.M406073200. [DOI] [PubMed] [Google Scholar]
- 98.Larsson KP, Åkerman KEO, Magga J, Uotila S, Kukkonen JP, Näsman J, et al. Orexin-A stimulates cholecystokinin release from intestinal neuroendocrine STC-1 cells by a Ca2+-dependent mechanism. Biochem Biophys Res Commun. 2003;309:209–216. doi: 10.1016/s0006-291x(03)01563-8. [DOI] [PubMed] [Google Scholar]
- 99.Leonard CS, Kalogiannis M, Kohlmeier KA. Hypocretin/orexin receptor functions in mesopontine systems regulating sleep, arousal and cataplexy. In: Baumann CR, Bassetti CL, Scammell TE, editors. Narcolepsy: Pathophysiology, Diagnosis, and Treatment. Berlin: Springer; 2011. pp. 139–152. [Google Scholar]
- 100.Li H, de Lecea L. The orexins as integrators of multiple physiological functions. Br J Pharmacol. 2013 doi: 10.1111/bph.12415. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li B, Chen F, Ye J, Chen X, Yan J, Li Y, et al. The modulation of orexin A on HCN currents of pyramidal neurons in mouse prelimbic cortex. Cereb Cortex. 2010;20:1756–1767. doi: 10.1093/cercor/bhp241. [DOI] [PubMed] [Google Scholar]
- 102.Li Y, Gao XB, Sakurai T, van den Pol AN. Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36:1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- 103.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 104.Liu M, Blanco-Centurion C, Konadhode R, Begum S, Pelluru D, Gerashchenko D, et al. Orexin gene transfer into zona incerta neurons suppresses muscle paralysis in narcoleptic mice. J Neurosci. 2011;31:6028–6040. doi: 10.1523/JNEUROSCI.6069-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu RJ, van den Pol AN, Aghajanian GK. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J Neurosci. 2002;22:9453–9464. doi: 10.1523/JNEUROSCI.22-21-09453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Louhivuori LM, Jansson L, Nordstrom T, Bart G, Nasman J, Akerman KE. Selective interference with TRPC3/6 channels disrupts OX1 receptor signalling via NCX and reveals a distinct calcium influx pathway. Cell Calcium. 2010;48:114–123. doi: 10.1016/j.ceca.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 107.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 108.Lund PE, Shariatmadari R, Uustare A, Detheux M, Parmentier M, Kukkonen JP, et al. The orexin OX1 receptor activates a novel Ca2+ influx pathway necessary for coupling to phospholipase C. J Biol Chem. 2000;275:30806–30812. doi: 10.1074/jbc.M002603200. [DOI] [PubMed] [Google Scholar]
- 109.Lungwitz EA, Molosh A, Johnson PL, Harvey BP, Dirks RC, Dietrich A, et al. Orexin-A induces anxiety-like behavior through interactions with glutamatergic receptors in the bed nucleus of the stria terminalis of rats. Physiol Behav. 2012;107:726–732. doi: 10.1016/j.physbeh.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Luo CH, Rudy Y. A dynamic model of the cardiac ventricular action potential. I. Simulations of ionic currents and concentration changes. Circ Res. 1994;74:1071–1096. doi: 10.1161/01.res.74.6.1071. [DOI] [PubMed] [Google Scholar]
- 111.Ma X, Zubcevic L, Bruning JC, Ashcroft FM, Burdakov D. Electrical inhibition of identified anorexigenic POMC neurons by orexin/hypocretin. J Neurosci. 2007;27:1529–1533. doi: 10.1523/JNEUROSCI.3583-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Magee JC. Dendritic lh normalizes temporal summation in hippocampal CA1 neurons. Nat Neurosci. 1999;2:508–514. doi: 10.1038/9158. [DOI] [PubMed] [Google Scholar]
- 113.Magga J, Bart G, Oker-Blom C, Kukkonen JP, Åkerman KE, Näsman J. Agonist potency differentiates G protein activation and Ca2+ signalling by the orexin receptor type 1. Biochem Pharmacol. 2006;71:827–836. doi: 10.1016/j.bcp.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 114.Malendowicz LK, Tortorella C, Nussdorfer GG. Orexins stimulate corticosterone secretion of rat adrenocortical cells, through the activation of the adenylate cyclase-dependent signaling cascade. J Steroid Biochem Mol Biol. 1999;70:185–188. doi: 10.1016/s0960-0760(99)00110-7. [DOI] [PubMed] [Google Scholar]
- 115.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 116.Matsuo K, Kaibara M, Uezono Y, Hayashi H, Taniyama K, Nakane Y. Involvement of cholinergic neurons in orexin-induced contraction of guinea pig ileum. Eur J Pharmacol. 2002;452:105–109. doi: 10.1016/s0014-2999(02)02300-2. [DOI] [PubMed] [Google Scholar]
- 117.Mazzocchi G, Malendowicz LK, Aragona F, Rebuffat P, Gottardo L, Nussdorfer GG. Human pheochromocytomas express orexin receptor type 2 gene and display an in vitro secretory response to orexins A and B. J Clin Endocrinol Metab. 2001a;86:4818–4821. doi: 10.1210/jcem.86.10.7929. [DOI] [PubMed] [Google Scholar]
- 118.Mazzocchi G, Malendowicz LK, Gottardo L, Aragona F, Nussdorfer GG. Orexin A stimulates cortisol secretion from human adrenocortical cells through activation of the adenylate cyclase-dependent signaling cascade. J Clin Endocrinol Metab. 2001b;86:778–782. doi: 10.1210/jcem.86.2.7233. [DOI] [PubMed] [Google Scholar]
- 119.Mieda M. Differential regulation of non-REM and REM sleep by orexinergic neurons. 2012. Neuroscience 2012; New Orleans, LA. Society for Neuroscience: Washington, DC. Program No. 822.05.
- 120.Mieda M, Hasegawa E, Kisanuki YY, Sinton CM, Yanagisawa M, Sakurai T. Differential roles of orexin receptor-1 and -2 in the regulation of non-REM and REM sleep. J Neurosci. 2011;31:6518–6526. doi: 10.1523/JNEUROSCI.6506-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mieda M, Willie JT, Hara J, Sinton CM, Sakurai T, Yanagisawa M. Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. Proc Natl Acad Sci U S A. 2004;101:4649–4654. doi: 10.1073/pnas.0400590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mochizuki T, Arrigoni E, Marcus JN, Clark EL, Yamamoto M, Honer M, et al. Orexin receptor 2 expression in the posterior hypothalamus rescues sleepiness in narcoleptic mice. Proc Natl Acad Sci U S A. 2011;108:4471–4476. doi: 10.1073/pnas.1012456108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mukai K, Kim J, Nakajima K, Oomura Y, Wayner MJ, Sasaki K. Electrophysiological effects of orexin/hypocretin on nucleus accumbens shell neurons in rats: an in vitro study. Peptides. 2009;30:1487–1496. doi: 10.1016/j.peptides.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 124.Murai Y, Akaike T. Orexins cause depolarization via nonselective cationic and K+ channels in isolated locus coeruleus neurons. Neurosci Res. 2005;51:55–65. doi: 10.1016/j.neures.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 125.Muroya S, Funahashi H, Yamanaka A, Kohno D, Uramura K, Nambu T, et al. Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca2+ signaling in a reciprocal manner to leptin: orexigenic neuronal pathways in the mediobasal hypothalamus. Eur J Neurosci. 2004;19:1524–1534. doi: 10.1111/j.1460-9568.2004.03255.x. [DOI] [PubMed] [Google Scholar]
- 126.Nakabayashi M, Suzuki T, Takahashi K, Totsune K, Muramatsu Y, Kaneko C, et al. Orexin-A expression in human peripheral tissues. Mol Cell Endocrinol. 2003;205:43–50. doi: 10.1016/s0303-7207(03)00206-5. [DOI] [PubMed] [Google Scholar]
- 127.Nakamura T, Uramura K, Nambu T, Yada T, Goto K, Yanagisawa M, et al. Orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Brain Res. 2000;873:181–187. doi: 10.1016/s0006-8993(00)02555-5. [DOI] [PubMed] [Google Scholar]
- 128.Nakamura Y, Miura S, Yoshida T, Kim J, Sasaki K. Cytosolic calcium elevation induced by orexin/hypocretin in granule cell domain cells of the rat cochlear nucleus in vitro. Peptides. 2010;31:1579–1588. doi: 10.1016/j.peptides.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 129.Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- 130.Nanmoku T, Isobe K, Sakurai T, Yamanaka A, Takekoshi K, Kawakami Y, et al. Effects of orexin on cultured porcine adrenal medullary and cortex cells. Regul Pept. 2002;104:125–130. doi: 10.1016/s0167-0115(01)00356-1. [DOI] [PubMed] [Google Scholar]
- 131.Nattie E, Li A. Respiration and autonomic regulation and orexin. Prog Brain Res. 2012;198:25–46. doi: 10.1016/B978-0-444-59489-1.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Numata T, Kiyonaka S, Kato K, Takahashi N, Mori Y. Activation of TRP channels in mammalian systems. In: Zhu MX, editor. TRP Channel. Boca Raton, FL: CRC Press; 2011. pp. 43–90. [PubMed] [Google Scholar]
- 133.Näsman J, Bart G, Larsson K, Louhivuori L, Peltonen H, Åkerman KE. The orexin OX1 receptor regulates Ca2+ entry via diacylglycerol-activated channels in differentiated neuroblastoma cells. J Neurosci. 2006;26:10658–10666. doi: 10.1523/JNEUROSCI.2609-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peltonen HM, Magga JM, Bart G, Turunen PM, Antikainen MS, Kukkonen JP, et al. Involvement of TRPC3 channels in calcium oscillations mediated by OX(1) orexin receptors. Biochem Biophys Res Commun. 2009;385:408–412. doi: 10.1016/j.bbrc.2009.05.077. [DOI] [PubMed] [Google Scholar]
- 135.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Putula J, Kukkonen JP. Mapping of the binding sites for the OX(1) orexin receptor antagonist, SB-334867, using orexin/hypocretin receptor chimaeras. Neurosci Lett. 2012;506:111–115. doi: 10.1016/j.neulet.2011.10.061. [DOI] [PubMed] [Google Scholar]
- 137.Putula J, Turunen PM, Jäntti MH, Ekholm ME, Kukkonen JP. Agonist ligand discrimination by the two orexin receptors depends on the expression system. Neurosci Lett. 2011;494:57–60. doi: 10.1016/j.neulet.2011.02.055. [DOI] [PubMed] [Google Scholar]
- 138.Ramanjaneya M, Conner AC, Chen J, Kumar P, Brown JE, Johren O, et al. Orexin-stimulated MAP kinase cascades are activated through multiple G-protein signalling pathways in human H295R adrenocortical cells: diverse roles for orexins A and B. J Endocrinol. 2009;202:249–261. doi: 10.1677/JOE-08-0536. [DOI] [PubMed] [Google Scholar]
- 139.Ramanjaneya M, Conner AC, Chen J, Stanfield PR, Randeva HS. Orexins stimulate steroidogenic acute regulatory protein expression through multiple signaling pathways in human adrenal H295R cells. Endocrinology. 2008;149:4106–4115. doi: 10.1210/en.2007-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Randeva HS, Karteris E, Grammatopoulos D, Hillhouse EW. Expression of orexin-A and functional orexin type 2 receptors in the human adult adrenals: implications for adrenal function and energy homeostasis. J Clin Endocrinol Metab. 2001;86:4808–4813. doi: 10.1210/jcem.86.10.7921. [DOI] [PubMed] [Google Scholar]
- 141.Roecker AJ, Coleman PJ. Orexin receptor antagonists: medicinal chemistry and therapeutic potential. Curr Top Med Chem. 2008;8:977–987. doi: 10.2174/156802608784936746. [DOI] [PubMed] [Google Scholar]
- 142.Rosker C, Graziani A, Lukas M, Eder P, Zhu MX, Romanin C, et al. Ca(2+) signaling by TRPC3 involves Na(+) entry and local coupling to the Na(+)/Ca(2+) exchanger. J Biol Chem. 2004;279:13696–13704. doi: 10.1074/jbc.M308108200. [DOI] [PubMed] [Google Scholar]
- 143.Rouchet N, Waroux O, Lamy C, Massotte L, Scuvee-Moreau J, Liegeois JF, et al. SK channel blockade promotes burst firing in dorsal raphe serotonergic neurons. Eur J Neurosci. 2008;28:1108–1115. doi: 10.1111/j.1460-9568.2008.06430.x. [DOI] [PubMed] [Google Scholar]
- 144.Rouet-Benzineb P, Rouyer-Fessard C, Jarry A, Avondo V, Pouzet C, Yanagisawa M, et al. Orexins acting at native OX(1) receptor in colon cancer and neuroblastoma cells or at recombinant OX(1) receptor suppress cell growth by inducing apoptosis. J Biol Chem. 2004;279:45875–45886. doi: 10.1074/jbc.M404136200. [DOI] [PubMed] [Google Scholar]
- 145.Routtenberg A. Reverse piedpiperase: is the knockout mouse leading neuroscientists to a watery end? Trends Neurosci. 1996;19:471–472. doi: 10.1016/S0166-2236(96)20051-7. [DOI] [PubMed] [Google Scholar]
- 146.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 147.Samson WK, Taylor MM. Hypocretin/orexin suppresses corticotroph responsiveness in vitro. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1140–R1145. doi: 10.1152/ajpregu.2001.281.4.R1140. [DOI] [PubMed] [Google Scholar]
- 148.Selbach O, Bohla C, Barbara A, Doreulee N, Eriksson KS, Sergeeva OA, et al. Orexins/hypocretins control bistability of hippocampal long-term synaptic plasticity through co-activation of multiple kinases. Acta Physiol (Oxf) 2010;198:277–285. doi: 10.1111/j.1748-1716.2009.02021.x. [DOI] [PubMed] [Google Scholar]
- 149.Selbach O, Doreulee N, Bohla C, Eriksson KS, Sergeeva OA, Poelchen W, et al. Orexins/hypocretins cause sharp wave- and theta-related synaptic plasticity in the hippocampus via glutamatergic, gabaergic, noradrenergic, and cholinergic signaling. Neuroscience. 2004;127:519–528. doi: 10.1016/j.neuroscience.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 150.Sellayah D, Bharaj P, Sikder D. Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab. 2011;14:478–490. doi: 10.1016/j.cmet.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 151.Sharf R, Sarhan M, Dileone RJ. Role of orexin/hypocretin in dependence and addiction. Brain Res. 2010;1314:130–138. doi: 10.1016/j.brainres.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shin HS, Cho HS, Sung KW, Yoon BJ. Orexin-A increases cell surface expression of AMPA receptors in the striatum. Biochem Biophys Res Commun. 2009;378:409–413. doi: 10.1016/j.bbrc.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 153.Siegel JM, Boehmer LN. Narcolepsy and the hypocretin system – where motion meets emotion. Nat Clin Pract Neurol. 2006;2:548–556. doi: 10.1038/ncpneuro0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Skrzypski M, Kaczmarek P, Le TT, Wojciechowicz T, Pruszynska-Oszmalek E, Szczepankiewicz D, et al. Effects of orexin A on proliferation, survival, apoptosis and differentiation of 3T3-L1 preadipocytes into mature adipocytes. FEBS Lett. 2012;586:4157–4164. doi: 10.1016/j.febslet.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 155.Skrzypski M, Le TT, Kaczmarek P, Pruszynska-Oszmalek E, Pietrzak P, Szczepankiewicz D, et al. Orexin A stimulates glucose uptake, lipid accumulation and adiponectin secretion from 3T3-L1 adipocytes and isolated primary rat adipocytes. Diabetologia. 2011;54:1841–1852. doi: 10.1007/s00125-011-2152-2. [DOI] [PubMed] [Google Scholar]
- 156.Smart D, Jerman JC, Brough SJ, Rushton SL, Murdock PR, Jewitt F, et al. Characterization of recombinant human orexin receptor pharmacology in a Chinese hamster ovary cell-line using FLIPR. Br J Pharmacol. 1999;128:1–3. doi: 10.1038/sj.bjp.0702780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Smith BN, Davis SF, Van Den Pol AN, Xu W. Selective enhancement of excitatory synaptic activity in the rat nucleus tractus solitarius by hypocretin 2. Neuroscience. 2002;115:707–714. doi: 10.1016/s0306-4522(02)00488-8. [DOI] [PubMed] [Google Scholar]
- 158.Soffin EM, Gill CH, Brough SJ, Jerman JC, Davies CH. Pharmacological characterisation of the orexin receptor subtype mediating postsynaptic excitation in the rat dorsal raphe nucleus. Neuropharmacology. 2004;46:1168–1176. doi: 10.1016/j.neuropharm.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 159.Squecco R, Garella R, Luciani G, Francini F, Baccari MC. Muscular effects of orexin A on the mouse duodenum: mechanical and electrophysiological studies. J Physiol. 2011;589:5231–5246. doi: 10.1113/jphysiol.2011.214940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Takahashi K, Koyama Y, Kayama Y, Yamamoto M. Effects of orexin on the laterodorsal tegmental neurones. Psychiatry Clin Neurosci. 2002;56:335–336. doi: 10.1046/j.1440-1819.2002.00967.x. [DOI] [PubMed] [Google Scholar]
- 161.Tang J, Chen J, Ramanjaneya M, Punn A, Conner AC, Randeva HS. The signalling profile of recombinant human orexin-2 receptor. Cell Signal. 2008;20:1651–1661. doi: 10.1016/j.cellsig.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 162.Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- 163.Tsujino N, Yamanaka A, Ichiki K, Muraki Y, Kilduff TS, Yagami K, et al. Cholecystokinin activates orexin/hypocretin neurons through the cholecystokinin A receptor. J Neurosci. 2005;25:7459–7469. doi: 10.1523/JNEUROSCI.1193-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Turunen PM, Ekholm ME, Somerharju P, Kukkonen JP. Arachidonic acid release mediated by OX(1) orexin receptors. Br J Pharmacol. 2010;159:212–221. doi: 10.1111/j.1476-5381.2009.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Turunen PM, Jäntti MH, Kukkonen JP. OX1 orexin/hypocretin receptor signaling via arachidonic acid and endocannabinoid release. Mol Pharmacol. 2012;82:156–167. doi: 10.1124/mol.112.078063. [DOI] [PubMed] [Google Scholar]
- 166.Törok TL. Electrogenic Na+/Ca2+-exchange of nerve and muscle cells. Prog Neurobiol. 2007;82:287–347. doi: 10.1016/j.pneurobio.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 167.Uramura K, Funahashi H, Muroya S, Shioda S, Takigawa M, Yada T. Orexin-a activates phospholipase C- and protein kinase C-mediated Ca2+ signaling in dopamine neurons of the ventral tegmental area. Neuroreport. 2001;12:1885–1889. doi: 10.1097/00001756-200107030-00024. [DOI] [PubMed] [Google Scholar]
- 168.van den Pol AN. Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J Neurosci. 1999;19:3171–3182. doi: 10.1523/JNEUROSCI.19-08-03171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.van den Pol AN, Ghosh PK, Liu RJ, Li Y, Aghajanian GK, Gao XB. Hypocretin (orexin) enhances neuron activity and cell synchrony in developing mouse GFP-expressing locus coeruleus. J Physiol. 2002;541:169–185. doi: 10.1113/jphysiol.2002.017426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.van den Pol AN, Patrylo PR, Ghosh PK, Gao XB. Lateral hypothalamus: early developmental expression and response to hypocretin (orexin) J Comp Neurol. 2001;433:349–363. doi: 10.1002/cne.1144. [DOI] [PubMed] [Google Scholar]
- 172.van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci. 2004;7:493–494. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- 173.van den Top M, Nolan MF, Lee K, Richardson PJ, Buijs RM, Davies CH, et al. Orexins induce increased excitability and synchronisation of rat sympathetic preganglionic neurones. J Physiol. 2003;549:809–821. doi: 10.1113/jphysiol.2002.033290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ward RJ, Pediani JD, Milligan G. Ligand-induced internalization of the orexin OX(1) and cannabinoid CB(1) receptors assessed via N-terminal SNAP and CLIP-tagging. Br J Pharmacol. 2011;162:1439–1452. doi: 10.1111/j.1476-5381.2010.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Velimirovic BM, Koyano K, Nakajima S, Nakajima Y. Opposing mechanisms of regulation of a G-protein-coupled inward rectifier K+ channel in rat brain neurons. Proc Natl Acad Sci U S A. 1995;92:1590–1594. doi: 10.1073/pnas.92.5.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wenzel J, Grabinski N, Knopp CA, Dendorfer A, Ramanjaneya M, Randeva HS, et al. Hypocretin/orexin increases the expression of steroidogenic enzymes in human adrenocortical NCI H295R cells. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1601–R1609. doi: 10.1152/ajpregu.91034.2008. [DOI] [PubMed] [Google Scholar]
- 177.Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, et al. Distinct narcolepsy syndromes in orexin receptor-2 and Orexin null mice: molecular genetic dissection of non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 178.Winrow CJ, Renger JJ. Discovery and development of orexin receptor antagonists as therapeutics for insomnia. Br J Pharmacol. 2013 doi: 10.1111/bph.12261. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Voisin T, El Firar A, Fasseu M, Rouyer-Fessard C, Descatoire V, Walker F, et al. Aberrant expression of OX1 receptors for orexins in colon cancers and liver metastases: an openable gate to apoptosis. Cancer Res. 2011;71:3341–3351. doi: 10.1158/0008-5472.CAN-10-3473. [DOI] [PubMed] [Google Scholar]
- 180.Voisin T, El Firar A, Rouyer-Fessard C, Gratio V, Laburthe M. A hallmark of immunoreceptor, the tyrosine-based inhibitory motif ITIM, is present in the G protein-coupled receptor OX1R for orexins and drives apoptosis: a novel mechanism. FASEB J. 2008;22:1993–2002. doi: 10.1096/fj.07-098723. [DOI] [PubMed] [Google Scholar]
- 181.Voisin T, Firar AE, Avondo V, Laburthe M. Orexin-induced apoptosis: the key role of the seven-transmembrane domain orexin type 2 receptor. Endocrinology. 2006;147:4977–4984. doi: 10.1210/en.2006-0201. [DOI] [PubMed] [Google Scholar]
- 182.Wu M, Zaborszky L, Hajszan T, van den Pol AN, Alreja M. Hypocretin/orexin innervation and excitation of identified septohippocampal cholinergic neurons. J Neurosci. 2004;24:3527–3536. doi: 10.1523/JNEUROSCI.5364-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wu M, Zhang Z, Leranth C, Xu C, van den Pol AN, Alreja M. Hypocretin increases impulse flow in the septohippocampal GABAergic pathway: implications for arousal via a mechanism of hippocampal disinhibition. J Neurosci. 2002;22:7754–7765. doi: 10.1523/JNEUROSCI.22-17-07754.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Xia J, Chen X, Song C, Ye J, Yu Z, Hu Z. Postsynaptic excitation of prefrontal cortical pyramidal neurons by hypocretin-1/orexin A through the inhibition of potassium currents. J Neurosci Res. 2005;82:729–736. doi: 10.1002/jnr.20667. [DOI] [PubMed] [Google Scholar]
- 185.Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 186.Yamanaka A, Tabuchi S, Tsunematsu T, Fukazawa Y, Tominaga M. Orexin directly excites orexin neurons through orexin 2 receptor. J Neurosci. 2010;30:12642–12652. doi: 10.1523/JNEUROSCI.2120-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yamanaka A, Tsujino N, Funahashi H, Honda K, Guan JL, Wang QP, et al. Orexins activate histaminergic neurons via the orexin 2 receptor. Biochem Biophys Res Commun. 2002;290:1237–1245. doi: 10.1006/bbrc.2001.6318. [DOI] [PubMed] [Google Scholar]
- 188.Yamuy J, Fung SJ, Xi M, Chase MH. Hypocretinergic control of spinal cord motoneurons. J Neurosci. 2004;24:5336–5345. doi: 10.1523/JNEUROSCI.4812-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yan J, He C, Xia JX, Zhang D, Hu ZA. Orexin-A excites pyramidal neurons in layer 2/3 of the rat prefrontal cortex. Neurosci Lett. 2012;520:92–97. doi: 10.1016/j.neulet.2012.05.038. [DOI] [PubMed] [Google Scholar]
- 190.Yanagisawa M. 2010. Small-molecule agonist for type-2 orexin receptor. USA.
- 191.Yang B, Ferguson AV. Orexin-A depolarizes dissociated rat area postrema neurons through activation of a nonselective cationic conductance. J Neurosci. 2002;22:6303–6308. doi: 10.1523/JNEUROSCI.22-15-06303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yang B, Ferguson AV. Orexin-A depolarizes nucleus tractus solitarius neurons through effects on nonselective cationic and K+ conductances. J Neurophysiol. 2003;89:2167–2175. doi: 10.1152/jn.01088.2002. [DOI] [PubMed] [Google Scholar]
- 193.Yang B, Samson WK, Ferguson AV. Excitatory effects of orexin-A on nucleus tractus solitarius neurons are mediated by phospholipase C and protein kinase C. J Neurosci. 2003;23:6215–6222. doi: 10.1523/JNEUROSCI.23-15-06215.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yoshida Y, Fujiki N, Maki RA, Schwarz D, Nishino S. Differential kinetics of hypocretins in the cerebrospinal fluid after intracerebroventricular administration in rats. Neurosci Lett. 2003;346:182–186. doi: 10.1016/s0304-3940(03)00571-8. [DOI] [PubMed] [Google Scholar]
- 195.Zeitzer JM, Buckmaster CL, Lyons DM, Mignot E. Locomotor-dependent and -independent components to hypocretin-1 (orexin A) regulation in sleep-wake consolidating monkeys. J Physiol. 2004;557:1045–1053. doi: 10.1113/jphysiol.2004.061606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang J, Li B, Yu L, He YC, Li HZ, Zhu JN, et al. A role for orexin in central vestibular motor control. Neuron. 2011;69:793–804. doi: 10.1016/j.neuron.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 197.Zhang L, Kolaj M, Renaud LP. Ca2+-dependent and Na+-dependent K+ conductances contribute to a slow AHP in thalamic paraventricular nucleus neurons: a novel target for orexin receptors. J Neurophysiol. 2010;104:2052–2062. doi: 10.1152/jn.00320.2010. [DOI] [PubMed] [Google Scholar]
- 198.Zhang L, Renaud LP, Kolaj M. Properties of a T-type Ca2+ channel-activated slow afterhyperpolarization in thalamic paraventricular nucleus and other thalamic midline neurons. J Neurophysiol. 2009;101:2741–2750. doi: 10.1152/jn.91183.2008. [DOI] [PubMed] [Google Scholar]
- 199.Zwirska-Korczala K, Adamczyk-Sowa M, Sowa P, Pilc K, Suchanek R, Pierzchala K, et al. Role of leptin, ghrelin, angiotensin II and orexins in 3T3 L1 preadipocyte cells proliferation and oxidative metabolism. J Physiol Pharmacol. 2007;58(Suppl. 1):53–64. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Postsynaptic orexin actions in the CNS.