Abstract
Orexin (hypocretin) peptides and their two known G-protein-coupled receptors play essential roles in sleep–wake control and powerfully influence other systems regulating appetite/metabolism, stress and reward. Consequently, drugs that influence signalling by these receptors may provide novel therapeutic opportunities for treating sleep disorders, obesity and addiction. It is therefore critical to understand how these receptors operate, the nature of the signalling cascades they engage and their physiological targets. In this review, we evaluate what is currently known about orexin receptor signalling cascades, while a sister review (Leonard & Kukkonen, this issue) focuses on tissue-specific responses. The evidence suggests that orexin receptor signalling is multifaceted and is substantially more diverse than originally thought. Indeed, orexin receptors are able to couple to members of at least three G-protein families and possibly other proteins, through which they regulate non-selective cation channels, phospholipases, adenylyl cyclase, and protein and lipid kinases. In the central nervous system, orexin receptors produce neuroexcitation by postsynaptic depolarization via activation of non-selective cation channels, inhibition of K+ channels and activation of Na+/Ca2+ exchange, but they also can stimulate the release of neurotransmitters by presynaptic actions and modulate synaptic plasticity. Ca2+ signalling is also prominently influenced by these receptors, both via the classical phospholipase C−Ca2+ release pathway and via Ca2+ influx, mediated by several pathways. Upon longer-lasting stimulation, plastic effects are observed in some cell types, while others, especially cancer cells, are stimulated to die. Thus, orexin receptor signals appear highly tunable, depending on the milieu in which they are operating.
Linked ArticlesThis article is part of a themed section on Orexin Receptors. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2014.171.issue-2
Keywords: G-protein-coupled receptor, intracellular Ca2+, phospholipase, endocannabinoid, adenylyl cyclase, plasticity, cell death, non-selective cation channel, K+ channel, Na+/K+ exchanger
Introduction
Since the discovery of the orexin (hypocretin) peptides, orexin-A and orexin-B, and their two known G-protein-coupled receptors (GPCRs), OX1 and OX2 receptors (the nomenclature according to BJP's CGtP; Alexander et al., 2013), in 1998 (de Lecea et al., 1998; Sakurai et al., 1998), much has been learnt about the organization and function of this system at the molecular, cellular and systems levels. The discovery that orexins are essential for the normal consolidation of sleep and waking and importantly influence homeostatic systems regulating appetite, stress and reward (see Borgland, 2013; Li and de Lecea, 2013) has kindled interest in this system as a target for developing novel pharmacotherapeutics (see Winrow and Renger, 2013). Nevertheless, fundamental questions remain about how this system operates at all levels. Of particular importance for understanding the cellular functions of orexin receptors is knowledge of the signalling cascades utilized and the effectors that are engaged. However, these cascades and effectors are not well understood in native orexin receptor expressing cells. In order to help advance such understanding, we review orexin receptor signalling from two viewpoints. First, we examine what is known about orexin receptor mechanisms and cascades, primarily from work in expression systems. Second, we examine what is known about orexin receptor signalling in different regions of the brain and other tissues. The first viewpoint is presented in this review, while the second is presented in our sister review (Leonard and Kukkonen, 2013).
Early studies in recombinant CHO cells showed that (human) orexin receptors strongly couple to Ca2+ elevation (Sakurai et al., 1998; Smart et al., 1999) and to phospholipase C (PLC; Lund et al., 2000; Holmqvist et al., 2002). Thus, orexin receptors were considered to be Gq-protein-coupled GPCRs, which signal via Gαq → PLC → IP3 (inositol-1,4,5-trisphosphate) → IP3 receptor → Ca2+ release from the endoplasmic reticulum (ER), and were expected to induce cell responses mainly via Ca2+ and diacylglycerol (DAG)-mediated pathways, such as those resulting from protein kinase C (PKC) activity. However, it was soon found that orexin receptor signalling, both in native tissues and in recombinant cell lines, was considerably more complicated and versatile (see, e.g. Malendowicz et al., 1999; Lund et al., 2000; Karteris et al., 2001; Randeva et al., 2001). In the following sections, we first present the known coupling partners of orexin receptors and then analyze the evidence for the ensuing signal transduction pathways. In each case, we consider the physiological actions resulting from these pathways, if known.
Orexin receptor coupling partners: heterotrimeric G-proteins
Orexin receptors are GPCRs, and as such, are expected to utilize heterotrimeric G-proteins as the main mediators of signal transduction. Both orexin receptors are very promiscuous in their signalling, and so far have been shown − by direct or indirect means − to couple to members of three of the four heterotrimeric G-protein families, namely Gq, Gi/o and Gs. Based on the results from expression systems (see below), nothing would preclude either receptor from coupling to any of these effector families, although this issue has not been sufficiently studied. Despite the long history of studying GPCR−G-protein coupling, few methods exist for direct measurements of G-protein activation and subtype-selective G-protein inhibition (Kukkonen, 2004); hence, conclusive results on orexin receptors are scarce.
GTP-azidoanilide methods can be used to selectively label G-proteins for identification. Cells are incubated with GTP-azidoanilide with 32P- or 33P-label, which, upon receptor stimulation, binds to the G-proteins. Exposure to UV light creates a covalent bond between Gα and the label, allowing the 32/33P-labelled proteins to be identified using antibodies. This technique has revealed the ability of OX2 orexin receptors to differentially couple to Gq, Gi/o and Gs proteins in different tissues (Karteris et al., 2001; Randeva et al., 2001; Karteris et al., 2005; see also Hypothalamus and Adrenal gland in Leonard and Kukkonen, 2013). It should be noted that this technique requires plasma membrane permeabilization prior to GTP-azidoanilide incubation, which may disrupt the signalling milieu and distort the results. Similar to GTPγS binding (below), GTP-azidoanilide may be more prone to labelling Gi/o proteins. The specificity of antibodies to detect the Gα subunits may also be variable. The only studies in the central nervous system (CNS) have involved the hypothalamus, where coupling to Gi, Go, Gs and Gq has been observed (Karteris et al., 2005).
35S-GTPγS binding is another method for measurement of G-protein activation in permeabilized cells. Due to the GTP/GDP affinity, GDP release rates and expression levels of different G-proteins, agonist-stimulated gross GTPγS binding is an effective measure of Gi/o binding only (when used without antibody separation). GTPγS can also be utilized for autoradiography (Laitinen, 2004). Orexin-A stimulates GTPγS binding to rat brain stem sites, putatively indicating receptor (unidentified subtype) coupling to the Gi/o proteins (Bernard et al., 2002; 2003).
Chimeric G-proteins, that is, Gα-subunits with the receptor-coupling parts of one G-protein fused with the effector-coupling part of another G-protein, have been used in one study with recombinant human OX1 receptors expressed in HEK-293 cells (Magga et al., 2006). In this study, different Gα-subunits, Gαo1, Gα11 and Gα16, were directed to Gαs signalling [adenylyl cyclase (AC) stimulation]. Only Gα11 and Gα16 fusion proteins, but not the Gαo1 fusion protein nor the wild-type Gs, were detected to couple to OX1 receptors. This appeared to contradict co-immunoprecipitation experiments performed in the same study, where Gq but also Gi and Gs, but not Go were immunoprecipitated together with the OX1 receptors (Magga et al., 2006). However, chimeric G-proteins may not interact with the orexin receptor or AC in the same way as do native G-proteins.
Indirect methods for determination of G-protein coupling of GPCRs include utilization of toxins, drugs, dominant-negative proteins, blocking peptides, antibodies or knockdown/knockout to block distinct G-proteins. Pertussis toxin (PTx) has until recently been the only commercially available G-protein inhibitor. It ADP-ribosylates Gi/o family G-proteins, which results in inactivation. In most cases, PTx may be a fairly reliable tool, but one should still be careful when using it as it may have other effects that are dependent on or independent of its enzymatic activity (Schneider et al., 2007; Mangmool and Kurose, 2011). The use of PTx also requires long incubation (usually at least overnight) to inactivate the entire target G-proteins, and this may induce plastic changes in the cells. We very often observe changes in cell morphology with PTx, which may indicate involvement of proteins other than Gi/o family G-proteins (J. P. Kukkonen, unpublished). Some cells may be less sensitive to PTx, either due to weak entry of PTx (low number of ‘PTx receptors’) or expression of the PTx-insensitive Gi/o family member Gz. Thus, the lack of ability of PTx to inhibit a particular response should not directly be interpreted as lack of Gi/o involvement; often, the effectiveness of the PTx treatment is not verified by cAMP measurements. Dominant-negative G-proteins have been used in two studies. However, dominant-negative G-proteins often do not show very good specificity (Kukkonen, 2004), which also seems to be reflected in these results (Tang et al., 2008; Ramanjaneya et al., 2009). Drugs, blocking peptides, antibodies or knockdown/knockout have not been utilized to analyze orexin receptor G-protein coupling, except for one study with an anti-Gs antibody (see below).
Yet a more indirect way of determining G-protein coupling is to measure the expected downstream responses mediated by particular G-proteins. Both orexin receptor subtypes efficiently couple to PLC-Ca2+ release, suggesting signalling via Gq proteins. This is observed both in multiple recombinant cells of neuronal and non-neuronal type (CHO, HEK-293, neuro-2a, PC12) and in some native cells (see Phospholipase C). We consider that Gq, coupling the receptors to PLCβ, is a rather fair conclusion in these cases, but one still has to consider the possibility of engagement of other PLC isoforms via receptor signals other than Gq (reviewed in Kukkonen, 2011; see also Phospholipase C). One also has to consider the propensity of orexin receptors to couple to receptor-operated Ca2+ influx (see Ca2+). AC is another classical GPCR target both for positive and for negative regulation. However, there are many regulating factors, and alterations in AC activity cannot be ascribed to a particular G-protein without further analysis (see Adenylyl cyclase). This is especially pertinent to orexin receptors, which prominently elevate Ca2+ and activate PKC, both of which are important regulators of ACs. Human OX1, expressed in CHO cells, appear to couple to Gi family proteins, as revealed by the sensitivity of AC inhibition to PTx, and to Gs family proteins, as revealed by AC stimulation and its inhibition by anti-Gs antibodies or by ‘competing’ Gs stimulation (Holmqvist et al., 2005). However, in these cells, Gs is activated only at high orexin concentrations, while the Gi and the putative Gq cascades are activated 100-fold more potently (see Recombinant cells and figure 3 in Leonard and Kukkonen 2013). This may not be the case in other cell types (see Hypothalamus and Adrenal gland in Leonard and Kukkonen, 2013).
Figure 3.
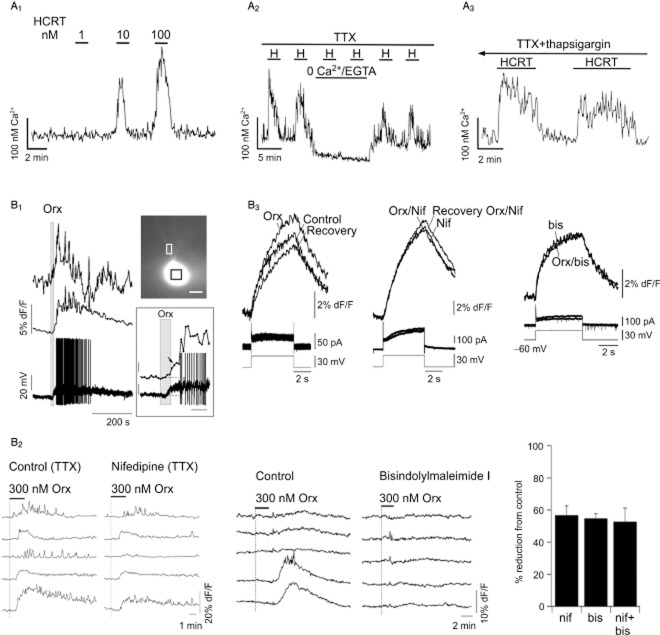
Ca2+ influx in native orexin receptor-expressing neurons. (A) Cultured rat hypothalamic neurons loaded with fura-2 AM. (A1) Response to orexin-B (‘HCRT’) is concentration-dependent. Ca2+ influx rather than release from intracellular stores is likely required for the response since it is blocked by removal of extracellular Ca2+ (‘0 Ca2+/EGTA’; (A2) but not by 2 μM thapsigargin (A3). TTX, tetrodotoxin (1 μM). The response was also blocked by 100 μM Cd2+ and 1 μM bisindolylmaleimide I, a PKC inhibitor (not shown). (B) Mouse dorsal raphé neurons recorded in acute brain slices. (B1) Upper right, a single neuron loaded with the Ca2+ indicator bis-fura-2 via the patch pipette. Upper left, fluorescence (dF/F) traces (indicating changes in intracellular [Ca2+]) from the same cell. Bottom trace is membrane potential. Middle and uppermost traces are simultaneous Ca2+-dependent fluorescence (average dF/F) from the soma (black box on the cell image) and from a proximal dendrite (white box on the cell image) respectively. The somatic Ca2+ trace and the current clamp recording are magnified in the right-hand bottom corner, demonstrating that the depolarization and somatic Ca2+ elevation occur before the action potential firing is triggered. The calibration bars indicate 10% dF/F and 10 mV for fluorescence and voltage trace, respectively, and 20 s. Orx, 300 nM orexin-A. (B2) Ca2+-dependent fluorescence (dF/F) from neurons loaded with fura-2 AM. Orexin-A (300 nM; ‘Orx’) produces a Ca2+ influx that is attenuated by the L-type VGCC blocker, nifedipine (left), and the PKC inhibitor bisindolylmaleimide I (1 μM; right). (B3) Ca2+ -dependent bis-fura-2 fluorescence (dF/F) recorded as in B1 under voltage-clamp conditions. Orexin-A (300 nM; ‘Orx’) reversibly enhances the somatic Ca2+ transient (left column, top trace) produced by a voltage jump from −60 to −30 mV (bottom trace) without changing the total membrane current (left column, middle trace). Nifedipine (‘Nif’, 1 μM; middle column) and bisindolylmaleimide (‘bis’, 1 μM; right column) fully attenuate the orexin-enhancement of the Ca2+ transient. These data indicate that orexin-A stimulates Ca2+ influx both by depolarising these neurons followed by opening of VGCCs and by a PKC-dependent enhancement of Ca2+ influx via L-type VGCCs. A1−3 adapted from van den Pol et al. (1998); B1 and B3 adapted from Kohlmeier et al. (2008); B2 adapted from Kohlmeier et al. (2004). The figures are reproduced with permission.
In conclusion, both orexin receptors are capable of coupling to several G-protein species. Hence, the common conception that OX1 couples exclusively to Gq, and that OX2 couples to Gq and Gi/o is dubious and other coupling possibilities need to be considered. The direct or less direct methods for assessing G-protein coupling of orexin receptors have been applied in only a few cases, and the conclusions about the importance of particular G-proteins for orexin receptor responses, even in these cases, must be tempered by the limitations in these methods. There are also many responses, as described below and in the sister review (Leonard and Kukkonen, 2013), that cannot be easily ascribed to a particular G-protein. Nevertheless, it is not unreasonable to assume that the Gq−PLC pathway plays an important role in many cases.
Orexin receptor coupling partners: interaction with other proteins
Many, if not all, GPCRs also interact with proteins other than heterotrimeric G-proteins (reviewed in Ritter and Hall, 2009). Novel interactions are often explored utilizing yeast-2-hybrid (Y2H) screening (or a similar method) and further verified by co-immunoprecipitation, glutathione S-transferase (GST)-pull-down, Förster/fluorescence energy transfer (FRET), etc., and even indirect means such as RNA interference (RNAi). These other proteins can transduce GPCR signals, but they can also affect GPCR trafficking or anchoring; however, in many cases, their functional roles are still unclear (reviewed in Ritter and Hall, 2009).
β-Arrestin appears to be an equally common interaction partner for GPCRs (reviewed in Rajagopal et al., 2010), as illustrated in the seminal work of Robert J. Lefkowitz, Nobel Laureate in Chemistry 2012. Classically, β-arrestins are involved in homologous desensitization. GPCR activity would lead to activation of GPCR kinases (GRKs) and receptor phosphorylation, which would recruit β-arrestin, which may, depending on the receptor type, lead to internalization of the receptor. Receptor-containing endosomes would then either recirculate to the plasma membrane or enter lysosomal degradation (reviewed in Rajagopal et al., 2010; Shenoy and Lefkowitz, 2011). However, β-arrestin is not only involved in trafficking but also acts as a signalling scaffold, for example, for the mitogen-activated protein kinase (MAPK) pathways of extracellular signal-regulated kinase (ERK) (reviewed in Rozengurt, 2007; Rajagopal et al., 2010). However, this is not the only way MAPK cascades are regulated by GPCRs (reviewed in Rozengurt, 2007). In transiently OX1-transfected CHO-K1 cells, human OX1 receptor activation attracts β-arrestin 1- and 2-GFP to the membrane and, in the continued presence of orexin-A, the fluorescence moves into intracellular puncta (Evans et al., 2001). When investigated using TAMRA (carboxytetramethylrhodamine)-labelled orexin-A, the GFP fluorescence of β-arrestin 1 and TAMRA fluorescence of orexin-A were co-localized (Evans et al., 2001). GRK2 was co-expressed in the cells and the cells were not assessed in the absence of heterologous GRK2. OX1 receptor−β-arrestin 2 interaction was later investigated in transiently transfected HEK-293 in the absence of heterologous GRK2 (Milasta et al., 2005). OX1 receptors were shown to co-localize with β-arrestin-GFP (or β-arrestin-red fluorescent protein) and to enter acidified endosomes. The interaction was verified by co-immunoprecipitation. The interaction epitope in the OX1 receptor was assessed by truncation and point mutations, which suggest that at least part of the interaction takes place via the receptor's C-terminus; weakened interaction led to a more transient ERK activation (Figure 1). In transiently transfected mouse embryonic fibroblast (MEF) cells from β-arrestin-1/2 double-knockout animals, no OX1 internalization was observed, unless β-arrestin-2 was reintroduced (Milasta et al., 2005).
Figure 1.

Comparison of the C-terminals of human and mouse OX1 receptors with respect to the suggested protein–protein interactions. The sequences are aligned to ease the comparison. The wild-type sequences are presented first for each species, and the mutant sequences (truncated and point-mutated) underneath. The human receptor data are obtained from investigations of the interaction of the receptor with β-arrestin-2 by assessment of the co-localization in HEK-293 cells upon endocytosis (Milasta et al., 2005). The mouse receptor data depict the assessment of the interaction of the receptor C-terminus with Dynlt1 with the Y2H method (Duguay et al., 2011). The strength of the interaction is indicated by the symbols after each sequence.
OX1 receptors have been suggested to interact with the protein phosphatase SHP-2 (Voisin et al., 2008; El Firar et al., 2009). This is discussed in detail under Cell death.
The Y2H method was utilized to reveal novel interaction partners for the C-terminus of the mouse OX1 receptor (Duguay et al., 2011). The screen suggested interaction with the dynein light chain Tctex-type 3. A focused testing also identified interaction between the dynein light chain Tctex-type 1 (Dynlt1) and either human orexin receptor. Co-immunoprecipitation in mammalian cells verified the interaction between OX1 and Dynlt1. The amino acids in either protein important for the interaction were further investigated using the Y2H method and the functional consequences assessed in recombinant HEK-293 cells. OX1-mediated ERK phosphorylation became more transient upon overexpression of Dynlt1 and more sustained when Dynlt1 levels were reduced using RNAi (Duguay et al., 2011). As dynein is involved in trafficking along microtubules, the effect observed could be related to a difference in receptor trafficking. However, no significant differences were observed in the resting plasma membrane localization or internalization upon agonist exposure (Duguay et al., 2011). It is noteworthy that both Duguay et al. (2011) and Milasta et al. (2005; see above) identified a partly overlapping region of the OX1 C-terminus to be involved in protein−protein interaction of OX1 receptors (Figure 1). In both cases, ERK signalling, but not gross receptor internalization, was affected. However, it seems that β-arrestin promotes ERK signalling, while Dynlt1 inhibits it, and Duguay et al. (2011) suggest that this could be due to competition between these proteins for binding to the C-terminus of OX1. However, there could also be other protein interactions in this domain, contributing to the receptor response. Unfortunately, the β-arrestin and Dynlt1 responses were only tested at high orexin-A concentration (500 nM).
GPCRs have also been reported to engage in dimerization/oligomerization processes. Such complexes may be homomeric, but heteromeric complexes have also been reported. While the role of homomeric complexes is difficult to assess, heteromeric complexes have sometimes been shown to affect receptor trafficking, signalling or pharmacology (reviewed in Bulenger et al., 2005; Milligan, 2009). Human OX1 receptors in recombinant HEK-293 cells have been shown, by native gel electrophoresis and FRET studies, to form homomeric complexes, and stimulation with orexin-A has been suggested to increase complex formation (Xu et al., 2011). It is, however, difficult to determine the number of receptors in a complex on the non-denaturing gel used. So far, only CB1 cannabinoid receptors have been identified as a heterodimerization partner for OX1 receptors − but it seems that other receptors have not yet been investigated either. Complexes between human OX1 and CB1 receptors have been verified by co-immunoprecipitation and fluorescence and bioluminescence energy transfer (BRET and FRET, respectively) in recombinant HEK-293 cells (Ward et al., 2011a). The physiological importance of this, however, has recently been questioned (see Orexins and endocannabinoids).
In conclusion, it is difficult to interpret the physiological significance of the putative orexin receptor interaction with proteins other than heterotrimeric G-proteins. One reason for this is that these have not yet been assessed in native orexin receptor-expressing cells, for example, by utilizing RNAi.
Adenylyl cyclase
AC regulation is a classical GPCR response. There are nine plasma membrane-bound AC isoforms, which all show different regulation, and one cytosolic variant (reviewed in Sunahara and Taussig, 2002). A typical feature of membrane-bound ACs is their synergistic regulation by different intracellular signals. The only common regulator is Gαs (AC activator), while the impact of other positive or negative regulators like Gαi, Gβγ, Ca2+ or PKC depends on the AC isoform (reviewed in Sunahara and Taussig, 2002). Orexin receptors are certainly capable of regulating AC (Malendowicz et al., 1999; Randeva et al., 2001; Mazzocchi et al., 2001b; Holmqvist et al., 2005; Karteris et al., 2005; Tang et al., 2008), although this seems less prominent than coupling to the PLC and Ca2+ cascades; however, not all tissues and cell types investigated have been examined for cAMP responses. Some of the AC stimulation may relate to the ability of orexin receptors to couple to Gs proteins (Orexin receptor coupling partners: heterotrimeric G-proteins, and Central nervous system and Adrenal gland in Leonard and Kukkonen, 2013), but also the other significant cascades such as Ca2+ and PLC−PKC may play a part. Unfortunately, we usually do not know how AC stimulation by orexin receptors is mediated even at sites where this is known to happen. Nevertheless, AC regulation by orexin receptors has a significant role in some tissue responses like corticosteroid release from adrenal cortex (Adrenal gland in Leonard and Kukkonen, 2013).
Phospholipase C
Mammalian phospholipase C, which we here refer to as PLC, is a family of cytosolic phosphoinositide-specific enzymes (reviewed in Kukkonen, 2011). Their most studied substrate is phosphatidylinositol-4,5-bisphosphate (PIP2), but they also hydrolyze other phosphoinositides, including phosphatidylinositol (PI) and phosphatidylinositol-4-phosphate/phosphatidylinositol-5-phosphate (commonly PIP). Hydrolysis takes place on the phosphoester bond between the phosphate and the glycerol, yielding DAG and inositol phosphates. DAG, as well as IP3, are the messengers produced upon PIP2 hydrolysis. It is also noteworthy that PIP2 itself has emerged as a major regulator of effectors (e.g. voltage-gated M-type K+ channels [KV7.2/3], N-type Ca2+ channels [CaV2.2] and transient receptor potential [TRP] channels, plasma membrane Ca2+ ATPase and Na+/Ca2+-exchanger [NCX]) (reviewed in Gamper and Shapiro, 2007; Suh and Hille, 2008).
The PLC family is divided into subfamilies of β, γ, δ, ε, ζ and η, with a total of 12 members. The substrate specificity of the members may vary, but this has not been thoroughly investigated. Regulation of the subfamilies and their members is very versatile (reviewed in Kukkonen, 2011). GPCRs can target multiple PLC isoforms. PLCβ is the classical target via Gαq family members, and Gβγ, but also PLCη, can be regulated by Gβγ. GPCRs can regulate, more indirectly, PLCδ and -ζ via Ca2+, PLCγ via Src (a protein tyrosine kinase) or phosphatidylinositol-3,4,5-trisphosphate (PIP3), and PLCε via Ras and Rho family monomeric G-proteins.
Direct measurements of PLC activity (total inositol phosphate or IP3 generation, PIP2 hydrolysis) show that both OX1 and OX2 receptors strongly activate PLC in many recombinant expression systems, including CHO, HEK-293, neuro-2a and PC12 cells (Lund et al., 2000; Holmqvist et al., 2002; Putula and Kukkonen, 2012; J. P. Kukkonen et al., unpublished). A reasonable assumption is that this takes place via the Gq family G-proteins, but in the absence of selective pharmacological inhibitors, this has not been verified. Indeed, in recombinant CHO cells, human OX1 receptors activate two apparently different PLC activities with different specificities for phosphoinositides (Johansson et al., 2008). Among native orexin receptor-expressing cells, PLC activation has been shown in explants of human primary pheochromocytomas (OX2 mRNA) and in membrane preparations of rat adrenal cortex and hypothalamus and human reproductive tract (mixed receptor populations) (Randeva et al., 2001; Mazzocchi et al., 2001a; Karteris et al., 2004; 2005,). Indirect indications of PLC activity are obtained from the use of PLC inhibitors or observation of Ca2+ release from the intracellular stores. It should be noted that PLC inhibitors, like U73122, may show non-specific toxicity and inhibit responses independent of their action on PLC (Taylor and Broad, 1998). Ca2+ elevation is not always determined to originate from Ca2+ release, and not even all Ca2+ release is IP3-dependent (reviewed in Konieczny et al., 2012). For orexin receptor signalling, pharmacological analysis has been used mainly with recombinant cells, where PLC activation is often known from direct measurements. Even more indirect indication for PLC activation has come from evidence of PKC involvement, which is usually rather easily assessed utilizing a suitable panel of pharmacological inhibitors. The members of the conventional (c) and novel (n) subfamilies are activated by DAG (reviewed in Newton, 2010). An nPKC, possibly PKCδ, is involved in human OX1 receptor stimulation of AC and phospholipase D (PLD) in recombinant CHO-hOX1 cells (Holmqvist et al., 2005; Jäntti et al., 2012), while cPKC may be involved in the activation of ERK in these cells (Ammoun et al., 2006a). PKC activation is also indicated by PKC translocation upon orexin receptor activation in these cells (Holmqvist et al., 2005; Ammoun et al., 2006a; Johansson et al., 2008; Ekholm et al., 2010). PKC involvement has been assessed, using inhibitors, in some neuronal preparations (Yang et al., 2003; Borgland et al., 2006; Zhang et al., 2010; see Central nervous system in Leonard and Kukkonen, 2013). However, PKC inhibitors have been applied surprisingly seldom. It should be noted that DAG is not only generated by PLC activity but can also originate from the PLD or reverse action of sphingomyelin synthase, and it is also generated (although not in the plasma membrane) during phospholipid synthesis (reviewed in Kukkonen, 2011).
DAG is principally metabolized either to phosphatidic acid (PA) by DAG kinase or sn2-monoacylglycerol by DAG lipase (DGL) (reviewed in Kukkonen, 2011). Both PA and sn2-monoacylglycerol may act as messengers (see Phospholipase D and Orexins and endocannabinoids).
Prokaryotes and protozoans possess phosphatidylcholine-specific PLC (PC-PLC). Such an enzyme has not been isolated in mammalian cells, but nevertheless, some studies report similar enzymatic activity. The reputed PC-PLC inhibitor, D609, has been used to garner evidence of PLC involvement. However, this inhibitor is not specific for ‘PC-PLC’ and it definitely is not an inhibitor of PI-PLC, although it has not been tested for all PLC isoforms (reviewed in Kukkonen, 2011; Kukkonen, 2013b). Therefore, even though some orexin responses have been blocked with D609 (reviewed in Kukkonen, 2013b), we think the conclusion of involvement of the putative mammalian PC-PLC in orexin receptor signalling is premature and requires further scrutiny.
In conclusion, PLC activation may be a very central orexin receptor cascade, although in most cases, the coupling has not been adequately assessed by either direct or indirect means. Whether the role of PLC in these cascades is to produce Ca2+ elevation (via release or Ca2+ channel regulation) or to elevate DAG for possible PKC activation is an important question remaining to be resolved.
Phospholipase D
PLD family enzymes hydrolyze the glycerophospholipid phosphoester bond between the head group and the phosphorus. The common isoforms are PLD1 and -2, which hydrolyze PC generating PA and choline (reviewed in Kukkonen, 2011). Generation of PA is the major signal mediated by PLD1 and -2. PA binds to signalling proteins, but it also has an impact on membrane curvature. PA is metabolized via hydrolysis to either DAG or lyso-PA (LPA), both of which may have messenger roles. It should be noted that DAG and PA from the PLC and PLD pathways, respectively, have different fatty acid compositions due to their different phospholipid sources and may have different messenger roles. We have recently observed that human OX1 receptors in CHO cells potently activate PLD (Jäntti et al., 2012). Pharmacological analysis indicates that the isoform activated is PLD1 and that the activation cascade goes via an nPKC but not Rho-family monomeric G-proteins. So far, this is the only report of PLD activation by orexin receptors. However, it should be straightforward to address the potential role of PLD in native orexin receptor function utilizing new commercially available PLD inhibitors (Monovich et al., 2007; Scott et al., 2009; Su et al., 2009).
Phospholipase A2 cascade
The PLA2 family comprises a vast number of enzymes with the capability to hydrolyze the sn2-ester bond of glycerophospholipids (reviewed in Dennis et al., 2011). Some of these enzymes, most notably the class IV and VI members, are involved in cytosolic signalling. Class IV is also known as cytosolic (Ca2+-sensitive) PLA2 (cPLA2) and these enzymes are activated by Ca2+ and by phosphorylation. Class VI is also known as iPLA2. These enzymes are not activated by Ca2+ but are possibly inhibited by it, while they may be activated by ATP, oligomerization, phosphorylation or proteolytic cleavage (reviewed in Balsinde and Balboa, 2005; Dennis et al., 2011). PLA2 enzymes are well known for the release of arachidonic acid (AA), but many of these enzymes show low fatty acid specificity. The other hydrolysis ‘fragment’, lysoglycerophospholipid, itself may also be a messenger or lead to messenger generation. AA is classically a substrate for eicosanoid synthesis, but it may also act as a messenger on its own (Kukkonen, 2011). Please note that AA can also result from the breakdown of endocannabinoids (see Orexins and endocannabinoids).
We recently identified significant release of 3H-label following orexin-A stimulation of OX1-expressing CHO and HEK-293 labelled with [3H]-AA (Peltonen et al., 2009; Turunen et al., 2010). We further investigated this in CHO cells and found label residing in both free AA as well as in 2-arachidonoylglycerol (2-AG) (Turunen et al., 2012). 2-AG was produced via the PLC−DGL pathway, while the free AA originated from both the PLA2 activity and the 2-AG breakdown. In recombinant human OX1-expressing HEK-293 and neuro-2a cells, orexin receptor stimulation liberated both 2-AG and AA, although the source of AA was not investigated. The PLA2 isoform in CHO cells was identified as cPLA2 (likely isoform α) (Turunen et al., 2012). The activation mechanism of cPLA2 in these cells remains elusive, but it would be tempting to suggest Ca2+ elevation, perhaps by influx, given the Ca2+ sensitivity of this isoform. However, orexin receptor-operated Ca2+ influx itself appears to require cPLA2 activity, at least in these cells (Turunen et al., 2012), leaving the situation unresolved (see Ca2+).
To our knowledge, these are the only reports of PLA2 activation in orexin signalling, so its role in native orexin receptor operation remains obscure. Similar to PLD, at least the role of cPLA2(α) in native systems should be easily assessed utilizing the novel pharmacological tool pyrrophenone (N-[[(2S,4R)-1-[2-(2,4-difluorobenzoyl)benzoyl]-4-[(triphenylmethyl)thio]-2-pyrrolidinyl]methyl]-4-[(Z)-(2,4-dioxo-5-thiazolidinylidene)methyl]-benzamide; Ono et al., 2002).
Ca2+
The earliest reports of orexin receptor activation indicated that these receptors trigger Ca2+ elevation in both recombinant and native cells (Sakurai et al., 1998; van den Pol et al., 1998). However, it soon became apparent that this response strongly relied on extracellular Ca2+ (Smart et al., 1999; Lund et al., 2000) (Figures 2 and 3).
Figure 2.
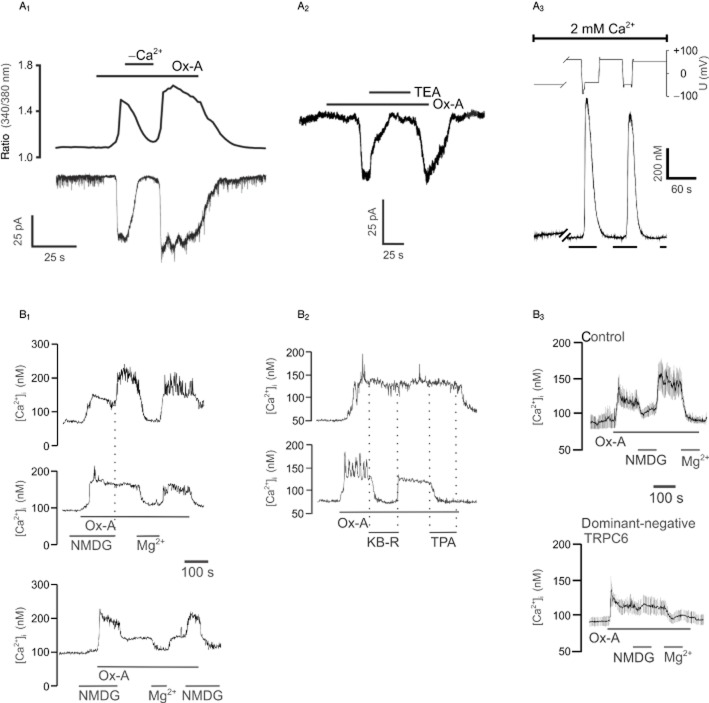
Ca2+ influx in recombinant human OX1 receptor-expressing cells. (A) Ca2+ influx is required for orexin responses in CHO-hOX1 cells. (A1) 0.3 nM orexin-A produces Ca2+ elevation (top trace) and a simultaneous inward current (lower trace). Removal of extracellular Ca2+ (‘−Ca2+’) attenuates both responses. The current is also effectively attenuated by substituting tetraethylammonium chloride for NaCl (TEA, 70 mM [70 mM NaCl replaced]; A2). This research (A1−2) was originally published in the Journal of Biological Chemistry. Larsson KP, Peltonen HM, Bart G, Louhivuori LM, Penttonen A, Antikainen M, Kukkonen JP, Åkerman, KE (2005). Orexin-A-induced Ca2+ entry: evidence for involvement of TRPC channels and protein kinase C regulation. J Biol Chem. 2005; 280: 1771–1781. © the American Society for Biochemistry and Molecular Biology. (A3) Removal of the driving force for Ca2+ entry by strong depolarization (see the voltage trace; top) abolishes the Ca2+ response (bottom) to 10 nM orexin-A (presence indicated by vertical bars under the Ca2+ trace). This research was originally published in the Journal of Biological Chemistry. Lund PE, Shariatmadari R, Uustare A, Detheux M, Parmentier M, Kukkonen JP, Åkerman, KEO. The orexin OX1 receptor activates a novel Ca2+ influx pathway necessary for coupling to phospholipase C. J Biol Chem. 2005; 275: 30806–30812. © the American Society for Biochemistry and Molecular Biology. (B) IMR-32 neuroblastoma cells transduced with hOX1 baculovirus show Ca2+ responses regulated by extracellular Na+ likely via NCX. (B1) Removal of extracellular Na+ (all NaCl replaced with N-methyl-d-glucamine [NMDG]) inhibits orexin-A (1 nM) responses in some cells (upper trace), while in other cells the response is stimulated (lower trace) or not affected (middle trace). 10 mM MgCl2 blocks the response in all three types of cells. (B2) The NCX blockers KB-R7943 (10 μM; lower trace) and SN-6 (1 μM; not shown) block most of the Ca2+ elevation in the cells showing Na+-dependent elevation whereas the cells with Na+-independent Ca2+ elevation are not affected (upper trace). The Na+-dependence of the responses is not shown in the figure. Both KB-R7943 and SN-6 are more potent inhibitors of the reverse mode of NCX. The effect of 100 nM 12-O-tetradecanoylphorbol-13-acetate (TPA) is also shown in B2. (B3) Expression of the dominant-negative TRPC6 subunit abolishes the Na+-dependent component of the response. Average traces are shown in B3. Reprinted from Cell Calcium, 48(2−3), Louhivuori LM, Jansson L, Nordström T, Bart G, Näsman J, Åkerman KEO, Selective interference with TRPC3/6 channels disrupts OX1 receptor signalling via NCX and reveals a distinct calcium influx pathway, pp. 114–123, © 2010, with permission from Elsevier. Figures adapted from Lund et al. (2000), Larsson et al. (2005), and Louhivuori et al. (2010) as indicated. The figures are reproduced with permission.
Classically, PLC activation generates IP3, which binds ER IP3 receptors and releases Ca2+ into the cytosol via these receptor channels (reviewed in Konieczny et al., 2012). Second, ER Ca2+ depletion signals the plasma membrane to allow Ca2+ influx to replenish the stores. This ‘store-operated’ or ‘capacitative’ Ca2+ influx, common in non-excitable cells, was recently shown to involve the ER Ca2+ sensor and response transducer stromal interaction molecule 1 (STIM1) and the plasma membrane Ca2+ channel orai1, although other proteins (e.g. of the TRP family) may also contribute (reviewed in Konieczny et al., 2012; Yuan et al., 2012). Capacitative Ca2+ entry not only recharges the store, but this entire release-influx ‘detour’ may function to create a high-Ca2+ microdomain near the plasma membrane for regulation of Ca2+ responsive ion channels, enzymes and other proteins (reviewed in Konieczny et al., 2012). Ca2+ influx can also be regulated via other channels and transporters in GPCR signalling. Voltage-gated Ca2+ channels (VGCCs) are directly activated by depolarization and can be modulated by second messenger systems. Known receptor-operated Ca2+ channels include non-selective cation channels (NSCCs) of the TRP family with a high diversity of activation signals (reviewed in Kukkonen, 2011), the ARC AA-regulated channels, which have recently been suggested to be composed of orai1 and -3 subunits (Shuttleworth, 2009) and the cyclic nucleotide-gated channels. Ca2+ influx can also be generated via reversible Na+/Ca2+(-K+) exchangers (NCX, NCKX), which can pump in Ca2+ by extruding Na+ (reviewed in Lytton, 2007).
Several lines of evidence indicate that NSCCs contribute to orexin responses. Indeed, orexins depolarize many types of neurons by activation of non-selective cation current (see Depolarization and synaptic functions); however, neither the identity of the channels nor their selectivity for Na+ and Ca2+ has been adequately examined. In recombinant human OX1-expressing CHO cells, these responses have been analyzed in some detail. OX1 stimulation activates a Ca2+ influx, which can be selectively visualized utilizing Mn2+ as Ca2+ substituent (Lund et al., 2000) and whole-cell patch clamp (Larsson et al., 2005) (Figure 2A1−2). This Ca2+ influx is activated at significantly lower orexin concentrations than Ca2+ release and it can be blocked by reversal of the driving force for Ca2+ entry (Lund et al., 2000) (Figure 2A3) and some TRP channel blockers (Kukkonen and Åkerman, 2001; Johansson et al., 2007) (Figure 2A2). This Ca2+ influx does not require IP3 (Ekholm et al., 2007), but instead cPLA2 activity, as inhibition of cPLA2 with methyl arachidonoyl fluorophosphonate or pyrrophenone blocks it (Turunen et al., 2010; 2012). Further studies utilizing dominant-negative TRPC channels suggest that TRPC1 and -3 channels contribute to this response in these cells (Larsson et al., 2005).
Näsman et al. (2006) also analyzed the responses in differentiated IMR-32 neuroblastoma cells, a model of human sympathetic neurons. Human OX1 receptors transiently expressed in these cells couple both to Ca2+ release and Ca2+ influx with Ca2+ influx as the primary response at low orexin concentration (1 nM). The pharmacological profile of response inhibition was similar for orexin-A-induced Ca2+ influx and dioctanoylglycerol-induced Ca2+ influx. Expression of dominant-negative TRPC6 was able to partly inhibit both responses, suggesting that both are mediated by DAG-sensitive TRPC (canonical subfamily of TRP) channels, that is, TRPC3, -6 and/or -7 (Näsman et al., 2006). In a further study, the authors found distinct subpopulations of these cells having Ca2+ responses that were either dependent on or independent of extracellular Na+ (Louhivuori et al., 2010) (Figure 2B1). The Na+-dependent responses required reverse-mode operation of NCX, which elevates intracellular Ca2+ upon elevation of intracellular Na+ and its extrusion, while the Na+-independent responses appeared to involve channel-mediated Ca2+ influx (Figure 2B2). Interestingly, TRPC3 channels may be the source of elevated Na+, as both RNAi with small hairpin-RNA and expression of dominant-negative TRPC6, which forms inactive complexes with TRPC3, inhibited most of the Na+-dependent response without inhibiting the Na+-independent responses (Louhivuori et al., 2010) (Figure 2B3). These findings suggest a mechanism by which orexin-stimulated Na+ entry via TRPC channels drives reverse mode operation of the NCX to elevate intracellular Ca2+. While channels formed by TRPC3 and -6 subunits show some preference for Ca2+ over Na+ (Gees et al., 2010), they likely still allow more Na+ influx under physiological conditions given the much greater extracellular [Na+].
In HEK-293 cells, both human orexin receptors also connect to PLC and both Ca2+ influx and release (Magga et al., 2006; Tang et al., 2008; Putula et al., 2011; Putula and Kukkonen, 2012). At low orexin-A concentrations (1 nM), regular Ca2+ influx-dependent oscillations are observed (Peltonen et al., 2009). OX1 receptors activate protein kinase D1 and -3 (PKD1 and -3, respectively), as indicated by their translocation to the plasma membrane and phosphorylation at Ser-916 (Peltonen et al., 2010). Influx-dependent Ca2+ oscillations are inhibited by dominant-negative constructs of either TRPC3 or PKD3 by 60%, suggesting involvement of these proteins in the process. However, while PKC inhibition with GF109203X inhibits PKD3 phosphorylation at Ser-916 by 70%, it does not affect Ca2+ oscillations (Peltonen et al., 2010).
Ca2+ measurements utilizing fluorescent probes do not easily distinguish Ca2+ influx and release, while electrophysiological techniques may miss/abolish some influx pathways. Some tricks can be utilized to pinpoint the influx in fluorescence measurements, such as removal of extracellular Ca2+ or replacement with other divalent cations (e.g. Mn2+ mentioned earlier), reduction of the driving force for Ca2+ entry (see above) or blocking of the influx with drugs or molecular biological inhibitors. All of these have their own advantages and problems, which cannot be reviewed here. In the absence of potent and specific channel inhibitors, less specific methods, including the drastic means of removal of extracellular Ca2+, must be used. Removal of Ca2+ − and less pronouncedly channel block − also alters other orexin receptor responses. In OX1-expressing CHO cells, the PLC (and Ca2+) response is shifted towards higher orexin-A concentrations and the ERK, cAMP, PLA2 and PLD responses are almost totally abolished (Ammoun et al., 2006a; Johansson et al., 2007; Turunen et al., 2010; Jäntti et al., 2012). Why is removal of extracellular Ca2+ more effective as an inhibitor of these responses than channel block or reduction of the driving force for Ca2+ influx? Could extracellular Ca2+ be required for orexin binding to its receptors? This has not been assessed by direct experiment, but some indirect evidence is inconsistent with this hypothesis (Lund et al., 2000). Also, if this was the case, it would be unlikely that the sensitivity for extracellular Ca2+ is different in different assays and different cell lines. Another hypothesis is that some types of orexin receptor signalling require a priming Ca2+ influx, which would allow coupling to other responses. Some circumstantial evidence supports this concept (Ammoun et al., 2006a). However, there is no direct molecular evidence for the hypothesis, and it is often difficult to find the operant and operand; for instance, Ca2+ influx seems to be required for PLA2 activation by orexin receptors, but PLA2 activity is also required for Ca2+ influx (Turunen et al., 2010; 2012,). A third explanation is that removal of Ca2+ affects the signal pathways downstream of the receptors, but there is no clear evidence to support this. Thus, the Ca2+ sensitivity of (some) orexin responses is currently without an unequivocal molecular explanation. Evidently, there also are orexin responses that are robust even when extra- and intracellular Ca2+ levels are significantly reduced (Kohlmeier et al., 2008).
Orexin-mediated Ca2+ responses have also been observed in neurons but in only a few cases has the source of the Ca2+ elevation been defined (van den Pol et al., 1998; 2001,; van den Pol, 1999; Uramura et al., 2001; Lambe and Aghajanian, 2003; Kohlmeier et al., 2004; 2008,; Ishibashi et al., 2005; Tsujino et al., 2005). Moreover, Ca2+ measurements in neurons have usually been made without simultaneous measurement of membrane potential. As orexin receptors induce depolarization (see Depolarization and synaptic functions), the simplest source for Ca2+ elevation would be orexin receptor-induced depolarization and subsequent activation of VGCCs. Accordingly, Ca2+ influx has been observed (van den Pol et al., 1998) (Figure 3A) and it often depends on the activation of VGCCs of N- and L-types (Uramura et al., 2001; Kohlmeier et al., 2004; 2008) (Figure 3B1−2). In addition, orexins have also been shown to potentiate Ca2+ transients resulting from L-type VGCCs under voltage-clamp conditions (Kohlmeier et al., 2008) (Figure 3B3). Thus, depolarization and activation of both potentiated and un-potentiated influx pathways contribute to the orexin-mediated elevation of intracellular Ca2+ in neurons. PKC has been identified in the response in some cases (van den Pol et al., 1998; Uramura et al., 2001; Kohlmeier et al., 2004; 2008) (Figure 3B); however, it remains unclear what is the target of the phosphorylation and how the transients are potentiated (e.g. altered voltage dependence, inactivation, conductance state). Without the ability to control membrane potential, the situation is even less clear since an impact on the membrane potential (e.g. via K+ channel closure) or directly on Ca2+ channels cannot be distinguished. Enhanced activation of VGCCs has also been observed in other excitable cells (Xu et al., 2002; Larsson et al., 2003; Squecco et al., 2011). It is noteworthy that while activation of Ca2+ release from intracellular stores is commonly observed in orexin receptor expression systems, there is only one suggestion of this based on direct measurements in neurons (Muroya et al., 2004), although two additional reports find orexin excitation depends on elevation of intracellular Ca2+ (Korotkova et al., 2002; Burdakov et al., 2003), suggesting that release may be involved. In other neurons, orexin-mediated Ca2+ elevation is insensitive to store depletion (van den Pol et al., 1998; Kohlmeier et al., 2004; 2008), although these Ca2+ measurements have focused mainly on the bulk somatic compartment, leaving the dendrites and other cellular compartments virtually unexplored.
In summary, stimulation of Ca2+ fluxes appears to be a central orexin response, but several mechanisms are likely involved (PLC-dependent Ca2+ release or influx mediated by NSCCs, VGCCs or possibly NCX). In addition, Ca2+ transients have been investigated in only a subset of orexin-responding tissues (including neurons). The physiological actions mediated by these Ca2+ fluxes are also largely unknown, although they are likely involved in the ability of orexin to promote transmitter release, postsynaptic plasticity and endocannabinoid production (see below). Future experiments should utilize high-resolution microscopy methods to examine dendrites and terminals and additional neuronal cell types to help clarify the relative importance of store release versus influx in orexin-mediated responses.
Orexin signalling in depolarization and synaptic function
Orexins produce direct postsynaptic depolarization in neurons. This depolarization has been studied extensively in brain slices and has been attributed to three mechanisms, which may co-exist in individual neurons: inhibition of K+ channels, stimulation of electrogenic NCX transport and activation of NSCCs (Figure 4). However, in most cases, the effector proteins underlying these excitatory actions of orexin have not yet been identified due to the lack of experiments with molecular precision (e.g. knockouts or knockdowns) and the general lack of specific pharmacological blockers.
Figure 4.
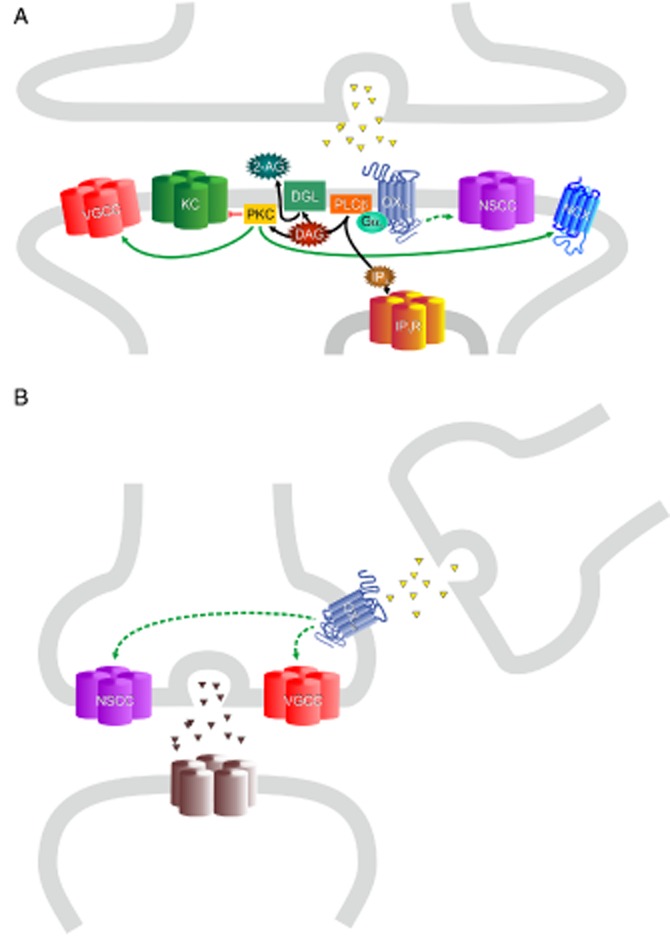
Schematic representation of possible orexinergic mechanisms in synaptic signalling. (A) Postsynaptic orexin signalling mechanisms. Ion fluxes are not shown. The PLC pathway may be active as suggested by some PKC-dependent effects as well as release of the endocannabinoid 2-AG, although there is little direct evidence for IP3-mediated Ca2+ release from ER in neurons. Inhibition of leak/inward rectifier/voltage-gated K+ channels (2P/Kir/Kv) (‘KC’) at least sometimes utilizes the Gq−PLCβ−PKC pathway. PKC could also be involved in the activation of NCX, but NCX may also act passively, driven by Ca2+ elevation (influx or release from ER; forward mode) or Na+ elevation (reverse mode). There also is some evidence for PKC-dependent activation of postsynaptic L-type VGCCs. The putative role of PIP2 is not depicted in the figure: PIP2 is required by NCX, some K+ and other channels, and PLD and cPLAα, and thus hydrolysis of PIP2 (by PLC) would lead to inhibition of these while elevated PIP2 (via, e.g. the PLD pathway) would stimulate these. Different NSCCs may be positively or negatively regulated by PIP2. (B) Presynaptic orexinergic modulation of glutamate- or GABAergic terminal signalling by orexinergic enhancement of VGCC-mediated Ca2+ influx. Indirect evidence suggests that orexin stimulation may be able to trigger transmitter release by depolarization or other means (depicted as NSCC, although not directly identified in this response).
K+ channel closure is typically identified by an increase in input resistance and current reversal at the K+ equilibrium potential. However, K+ channel subtype identities have not been widely scrutinized; at present, there is evidence that orexin turns off Kir and TASK family channels (inward rectifier and TWIK-related acid-sensitive K+ channels, respectively) (Hoang et al., 2003; 2004,; Doroshenko and Renaud, 2009). Kir3 channels are targets for GPCR inhibition via the Gq pathway both by PKC activation and by PIP2 degradation (Sadja et al., 2003; Luscher and Slesinger, 2010), while GPCR regulation of TASK channels may differ from this (Chen et al., 2006; Lindner et al., 2011; reviewed in Mathie, 2007).
Evidence for involvement of NCX (as distinguished from NSCCs) (Eriksson et al., 2001; Wu et al., 2002; Burdakov et al., 2003; Wu et al., 2004; Acuna-Goycolea and van den Pol, 2009; Zhang et al., 2011), in most cases, has relied on the sensitivity to NCX inhibitors. However, these inhibitors (Ni2+ and KB-R7943) may significantly inhibit other targets including TRPC channels (Kraft, 2007), while the Li+ permeability of TRP channels is generally not known. Additional evidence sometimes presented in support of NCX activation, such as no change of input resistance or a sensitivity to strong buffering of intracellular Ca2+, also have alternative explanations [e.g. channels both opening and closing, rectification and Ca2+ stimulation of channels (like TRPC3; Nilius et al., 2007)]. We therefore suggest involvement of NCX currents in orexin receptor-mediated depolarization in CNS neurons be considered provisional until more definitive analyses (e.g. more selective NCX blockers, knockouts or knockdowns) have been performed.
Evidence for NSCCs has come mainly from decreased membrane resistance, I–V curves, that reverse positive to resting membrane potential (some near 0 mV), a sensitivity to changing extracellular [Na+] and a large increase in membrane current noise, which is thought to represent current fluctuations from channels opening and closing (e.g. Brown et al., 2002; Burlet et al., 2002; Liu et al., 2002; Kohlmeier et al., 2008). Nevertheless, the underlying NSCC identities or their activation mechanisms have not been determined in central neurons. An important next step will be to test whether TRPC channels, which contribute to currents in recombinant cells (see Ca2+), are involved here. If TRPC channels correspond to the orexin-activated NSCCs, multiple known orexin receptor signals, including Ca2+, DAG, DAG → PKC, free fatty acids or their metabolites, lysophosphatidylcholine, or PIP2 decrease, could mediate their activation (reviewed in Nilius et al., 2007; Venkatachalam and Montell, 2007; Leonard and Kukkonen, 2013; Kukkonen, 2013a). However, many other TRP channel types are also activated by these messengers, suggesting that messenger identification alone will not unequivocally identify the effectors.
In some neurons, orexins increase the miniature excitatory and/or inhibitory postsynaptic current frequency (van den Pol et al., 1998; Li et al., 2002; Smith et al., 2002; Davis et al., 2003; Borgland et al., 2008; Ono et al., 2008; Acuna-Goycolea and van den Pol, 2009; Borgland et al., 2009; Dergacheva et al., 2012), which is measured after blocking action potentials with TTx. This is usually interpreted to mean that activation of presynaptic terminal orexin receptors increases the probability of vesicle release. It should be noted that orexins can also promote TTx-sensitive transmitter release through receptors at or near presynaptic terminals by stimulating terminal (aka ectopic) action potentials (Lambe and Aghajanian, 2003) or by modulating presynaptic VGCCs (see above) (Burlet et al., 2002).
Thus, orexins have diverse actions on central neurons. The most obvious response is postsynaptic stimulation and it is exciting that several mechanisms are utilized. However, there are also other prominent orexin responses in neurons including modulation of presynaptic transmitter release and changes in synaptic plasticity (Selbach et al., 2004; Borgland et al., 2006; Selbach et al., 2010; see also Leonard and Kukkonen, 2013). Nevertheless, the mechanisms underlying orexin receptor signalling in the CNS are largely unknown, especially with respect to the NSCC activation, which is an important class of responses for many GPCRs. Identification of both signalling mechanisms and channel targets in the CNS will be important future advances. As selective pharmacological inhibitors are scarce, the use of knockouts, knockdowns and more reduced culture systems should be exploited.
The evidence for the different mechanisms by which orexins may impact the electrical activity of neurons and other excitable cells is discussed in detail in Leonard and Kukkonen (2013).
Phosphoinositide-3-kinase
PI3K pathway is one of the central pathways regulating cell growth and survival. GPCRs can regulate PI3K of class Ia and b − phosphorylating PIP2 to PIP3 − via, for example, Ras or Gβγ (reviewed in Wymann et al., 2003). PIP3 activates phosphoinositide-dependent kinase 1 (PDK1), which helps activate other target kinases like protein kinase B (PKB). In glucagon-secreting InR1-G9 cells from Syrian golden hamster insulinoma cells, orexin-A (unknown receptor subtype) activates PI3K, leading to PIP3-regulated kinase cascades and inhibition of proglucagon mRNA production (Göncz et al., 2008; see Endocrine and exocrine pancreas in Leonard and Kukkonen, 2013). PI3K is also activated by orexin-A in differentiated mouse white adipocyte-like 3T3-L1 cells (OX1 and OX2 mRNA), leading to glucose uptake and triglyceride synthesis (Skrzypski et al., 2011; see White and brown adipose tissue in Leonard and Kukkonen, 2013). The mechanism utilized by orexin receptors to activate PI3K is unknown.
Cell death
Persistent orexin receptor stimulation induces programmed cell death. This occurs both in recombinant CHO cells and in cancer cell lines and primary cancer cells (Ammoun et al., 2003; Rouet-Benzineb et al., 2004; Ammoun et al., 2006b; Voisin et al., 2006; Voisin et al., 2011; see also Cell lines natively expressing orexin receptors and Recombinant cells in Leonard and Kukkonen, 2013). A cell death response is manifested via molecular markers such as loss of plasma membrane polarity, caspase activation, mitochondrial cytochrome C leakage into the cytosol, and nuclear fragmentation and condensation. The details of this process have mainly been investigated in the recombinant CHO cells. Two different pathways for cell death in human OX1-expressing CHO cells have been suggested. It has to be pointed out that the clones used are different and it is possible that the background cells, too, are different, namely one group is using regular CHO-K1 cells (Ammoun et al., 2006b) and the other CHO-S suspension-adapted subclones (Rouet-Benzineb et al., 2004; Voisin et al., 2008; El Firar et al., 2009); this may be reflected in the fact that cell death is blocked by serum in the former but not in the latter cells. In the detailed studies of Laburthe and co-workers, the signal cascade was suggested to involve Gq, Src (or a related kinase), phosphorylation of OX1 and recruitment of the protein phosphatase SHP-2 (Figure 5A). The studies identify two known phosphorylation/SHP-2 interaction motifs in orexin receptors, called ITIM (immunoreceptor tyrosine-based inhibitory motif; Thr-Asn-Tyr-Phe-Ile-Val) and ITSM (immunoreceptor tyrosine-based switch motif; Ile-Ile-Tyr-Asn-Phe-Leu) (Voisin et al., 2008; El Firar et al., 2009). As discussed in detail in Kukkonen (2013b), we believe that ITSM, in the transmembrane helix 2, is likely to be the correct site, as the putative ITIM sequence overlaps with the classical Asn-Pro-X-X-Tyr-motif, required for structural integrity of any GPCR. Hence, any exchange of this Tyr for another amino acid, which constitutes essential evidence in Voisin et al. (2008), disrupts the receptor structure, as is also indicated in these results upon careful reading. To firmly link the phosphorylation to Tyr-83 of the ITSM sequence, a phosphopeptide analysis of orexin receptor would be required. In the studies of Kukkonen and co-workers, p38 MAP kinase was identified as the carrier of the cell death response (Ammoun et al., 2003; 2006b,) (Figure 5B). Cell death also required mRNA and protein synthesis and caspase activity. Upon inhibition of the caspases, however, the cell death takes a ‘shortcut’ (Figure 5C). p38 MAPK has been associated with orexin responses in some other cell types, as well (see Cell plasticity).
Figure 5.
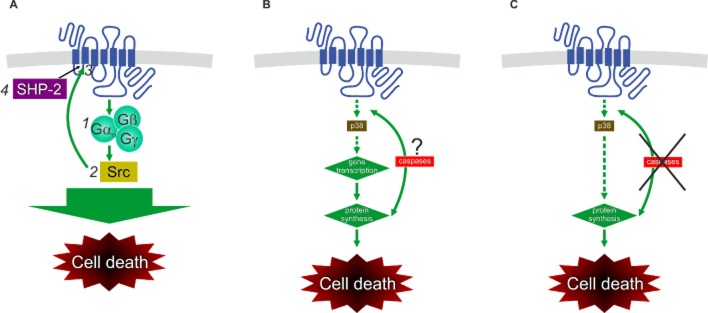
The OX1 receptor activation-mediated cell death pathways mapped in CHO cells. (A) the SHP-2-dependent cascade (Voisin et al., 2008; El Firar et al., 2009); (B) the p38-mediated cascade (see Ammoun et al., 2006b). Please note that the site of action of caspases in the cascade has not been determined. (C) The altered cell death response upon caspase inhibition (Ammoun et al., 2006b).
Both orexin receptor subtypes are capable of activating cell death as observed in recombinant CHO-S cells and in native cancer cell lines of colon carcinoma, neuroblastoma and pancreatic acinar tumour (Rouet-Benzineb et al., 2004; Voisin et al., 2006). Primary colorectal tumours (but not normal colon epithelium) also express OX1 receptors, and orexin receptor activation has been suggested to hold promise for treating chemotherapy-resistant carcinoma (Voisin et al., 2011).
There are several open questions relating to orexin-triggered cell death. Is this a physiological response? Cell death seems to require not extremely high but still rather significant orexin levels over a long time. Could such exposure take place in a normal physiological context or would ‘orexin overflow’ represent a pathological mechanism? Would only some cell types display the cell death response or is this a general mechanism for orexin receptors? While the former questions are currently rather difficult to answer, the latter one could be easily assessed using cultures of native orexin receptor-expressing cells (e.g. neurons).
Cell plasticity
Orexin receptors are in principle able to activate some classical plasticity-regulating cascades such as PKC, PI3K and ERK, and p38 MAPK. PKC activation has been described above (see Phospholipase C). ERK is activated in OX1- and OX2-expressing CHO cells and OX1-expressing neuro-2a cells (Ekholm et al., 2007). GPCRs can regulate ERK and other MAPK pathways via multiple mechanisms (reviewed in Rozengurt, 2007). In OX1-expressing CHO cells, ERK activation can be partially inhibited by inhibiting PLC, cPKC, Src and PI3K, which appear to converge at Ras; however, Src, PIK or Ras activity has not been directly assessed in these cells (Ammoun et al., 2006a). ERK signalling is also observed in recombinant HEK-293 cells (Milasta et al., 2005; Tang et al., 2008). In these cells, OX2 receptors activate AC and PLC; both protein kinase A (PKA) and PKC are suggested to contribute to ERK activation (Tang et al., 2008). p38 MAPK is activated in OX1-expressing CHO cells (Ammoun et al., 2003; 2006b), OX2-expressing HEK-293 cells (Tang et al., 2008) and native H295R cells (Ramanjaneya et al., 2009); the activation cascades are not known. In recombinant cells, no long-range effects have been assessed except that ERK may partially inhibit p38-induced cell death (see above) in CHO-hOX1 cells (Ammoun et al., 2003).
ERK and PKC have also been associated with synaptic plasticity. Orexin-A stimulated AMPA receptor trafficking to the plasma membrane in co-cultures of rat prefrontal cortex and striatum and the response was fully inhibited by removal of extracellular Ca2+ or by a MAPK/ERK kinase 1 (MEK1) inhibitor (Shin et al., 2009). Similar findings were obtained in a striatal slice preparation, but MEK1 inhibition was not tested (Shin et al., 2009). In contrast, PKC mediates the increased NMDA receptor trafficking to the plasma membrane in rat ventral tegmental area slices (Borgland et al., 2006). Both neuronal responses are observed within 10−20 min and therefore cannot represent new protein synthesis but likely result from plasma membrane fusion of pre-formed channel-harbouring vesicles.
Some long-term plastic effects have also been reported for orexin receptor activation. ERK is activated in native H295R cells (mainly OX2 mRNA), mediated entirely via PKC (Ramanjaneya et al., 2009). This cascade may contribute to differentiation towards steroid-producing cells (Ramanjaneya et al., 2008; Ramanjaneya et al., 2009; Wenzel et al., 2009; see Adrenal gland in Leonard and Kukkonen, 2013). Plancentally produced orexins were recently identified to pivotally contribute to the prenatal development of brown adipose tissue in mice via OX1 receptors (Sellayah et al., 2011; see White and brown adipose tissue in Leonard and Kukkonen, 2013). In contrast, differentiation of 3T3-L1 cells or rat primary adipocytes to mature adipocytes was not stimulated by orexin-A despite the presence of other orexin-mediated responses (Skrzypski et al., 2012; see Phosphoinositide-3-kinase and White and brown adipose tissue in Leonard and Kukkonen, 2013).
In the CNS, there is also evidence that the absence of one or both orexin receptors leads to cellular phenotypic differences compared with wild types in markers of cholinergic transmission (Kilduff et al., 1986; Kalogiannis et al., 2010). Moreover, there is new evidence that the numbers of histaminergic neurons in human narcoleptic brains and orexin peptide null mice are increased [Dr. T. E. Scammell, pers. comm.]. Whether these changes depend directly on the loss of orexin receptor signalling or other receptor properties or whether they are mediated by compensatory alterations is unknown.
Orexins and endocannabinoids
In recombinant CHO cells expressing human OX1 receptors, ERK phosphorylation is observed following orexin receptor stimulation at intermediate potency (EC50 ≈ 10−30 nM) (Hilairet et al., 2003; Ammoun et al., 2006a). When human CB1 cannabinoid receptors (also a GPCR) were co-expressed in these cells, the potency of orexin-A increased by 100-fold in a manner dependent on CB1 receptor activity (i.e. inhibited by a CB1 antagonist) (Hilairet et al., 2003). In contrast, CB1 signalling to ERK was not affected by OX1 co-expression, and neither was OX1 signalling to PLC by CB1 co-expression. The result was interpreted to mean OX1-CB1 heterodimerization (Figure 6A). Further studies in recombinant HEK-293 cells elaborated this view, and indeed, FRET and BRET techniques and co-immunoprecipitation have supported it (Ellis et al., 2006; Ward et al., 2011a; 2011b). However, we have recently shown that OX1 receptor stimulation in CHO cells (as well as in similarly recombinant neuro-2a and HEK-293 cells) induces production of the endocannabinoid 2-AG via the PLC−DGL cascade (see Phospholipase C) (Turunen et al., 2012). Furthermore, we have been able to show that blocking of the 2-AG production fully abolishes the CB1 receptor-mediated potentiation of OX1 signalling to ERK (Jäntti et al., 2013). We therefore believe that OX1 receptor stimulation produces DAG via the PLC pathway, DAG is hydrolyzed by DGL to 2-AG, and 2-AG stimulates CB1 receptors, which, by being able to more strongly couple to Gi/o proteins, co-stimulate ERK activation (Figure 6B). This does not necessarily exclude receptor heterodimerization, but 2-AG production seems to be the key to the process. A currently unresolved question is whether orexin receptors have a special propensity to stimulate 2-AG release in addition to the likely activation of PLCβ. Orexin receptors mediate receptor-operated Ca2+ influx and that could be an important factor in either stimulating DGL or boosting the activity of some PLC species.
Figure 6.
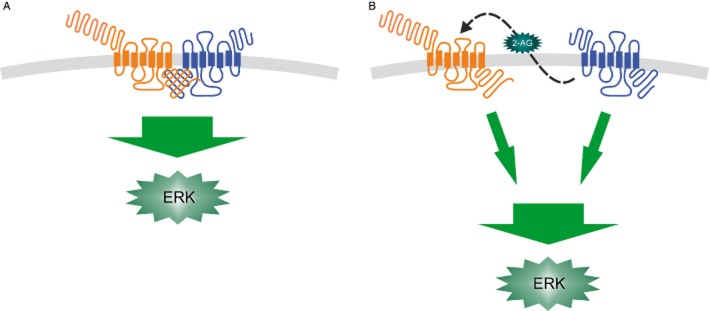
Suggested interaction schemes in ERK signalling upon OX1 orexin receptor (blue) and CB1 cannabinoid receptor (orange) co-expression in the same cells. (A) Dimerization of OX1 and CB1 receptors enhances the signalling/ligand potency (Hilairet et al., 2003; Ellis et al., 2006). (B) Enhanced signalling is obtained by OX1 receptor-mediated production of the CB1 ligand 2-AG and subsequent co-signalling of these two receptors (Turunen et al., 2012; Jäntti et al., 2013). See Orexins and endocannabinoids for details.
Endocannabinoid signalling has great importance for modulating synaptic transmission in the brain. Endocannabinoids are produced postsynaptically and mediate retrograde inhibitory actions on presynaptic terminals via CB1 receptors (reviewed in Kano et al., 2009). The gross response does not, however, need to be feedback inhibition as endocannabinoids also can inhibit the release of GABA from inhibitory terminals leading to postsynaptic disinhibition. Endocannabinoid responses in the brain are usually analyzed utilizing pharmacological inhibitors of the receptors or endocannabinoid generation or breakdown. Two studies nicely illustrate endocannabinoid actions in orexin signalling. In the dorsal raphé nucleus, orexin receptor signalling inhibits glutamate release onto serotonergic neurons. This appears mediated by orexin-induced production of 2-AG, which then acts on presynaptic CB1 receptors on glutamatergic terminals to inhibit glutamate release (Haj-Dahmane and Shen, 2005). The analgesic effects of orexin on the ventrolateral periaqueductal gray matter also takes place by endocannabinoid production and release, followed by action on presynaptic CB1 receptors on GABAergic terminals (Ho et al., 2011). In both cases, the endocannabinoid involved is 2-AG; involvement of 2-AG is easier to determine than that of the other important endocannabinoid, N-arachidonoylethanolamine (anandamide) as there are good inhibitors for 2-AG generation.
Conclusions
A wide variety of signals originate from orexin receptors. Orexins are able to trigger responses in many tissues in addition to the CNS, and different responses are observed at different sites. What determines the choice of specific signal cascades is unknown. To explain this, it is most logical to suggest that GPCRs would participate in preformed signal complexes with their effectors and the necessary elements of the signalling cascades. There is strong circumstantial evidence for such complexes (see, e.g. Kleuss, 1995; Brown, 2010), but very little direct molecular proof for any GPCR.
For orexin receptors, it is, in most cases, difficult to pinpoint signal cascades carrying the responses observed due to the limited set of tools available and sometimes even lack of analytical approach. It is also currently difficult to suggest the physiological significance of responses to exogenous orexins observed in sites other than CNS as the production of orexin peptides outside the CNS is not firmly proven.
We further explore these issues in the context of tissue responses to orexins in the sister review (Leonard and Kukkonen, 2013). Final conclusions concerning orexin receptor signalling are presented there.
Acknowledgments
This study was supported by the Academy of Finland (J. P. K.), the Magnus Ehrnrooth Foundation (J. P. K.), the Liv & Hälsa Foundation (J. P. K.) and grants NS027881 and HL064150 from the NIH of the USPHS (C. S. L.).
Glossary
- 2-AG
2-arachidonoylglycerol
- AA
arachidonic acid
- AC
adenylyl cyclase
- BRET and FRET
bioluminescence and Förster/fluorescence energy transfer, respectively
- CB1 and CB2
CB1 and CB2 cannabinoid receptors, respectively
- CHO
Chinese hamster ovary-K1 (cells)
- CNS
central nervous system
- cPLA2
cytosolic (Ca2+-sensitive) PLA2
- DAG
diacylglycerol
- DGL
DAG lipase
- Dynlt1
dynein light chain Tctex-type 1
- ER
endoplasmic reticulum
- ERK
extracellular signal-regulated kinase
- GPCR
G-protein-coupled receptor
- GRK
GPCR kinase
- HEK-293
Human embryonic kidney (cells)
- IP3
inositol-1,4,5-trisphosphate
- iPLA2
intracellular (Ca2+-independent) PLA2
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- ITSM
immunoreceptor tyrosine-based switch motif
- Kir channels
inward rectifier K+ channels
- LPA
lysophosphatidic acid
- MAPK
mitogen-activated protein kinase
- MEK1
MAPK/ERK kinase 1
- NCX
Na+/Ca2+-exchanger
- neuro-2a
a mouse neuroblastoma cell line
- nPKC
novel PKC
- NSCC
non-selective cation channel
- OX1 and OX2
OX1 and OX2 orexin receptors, respectively
- PA
phosphatidic acid
- PC12
a rat pheochromocytoma cell line
- PI3K
phosphoinositide-3-kinase
- PI
phosphatidylinositol
- PIP
phosphatidylinositol-4/5-phosphate
- PIP2
phosphatidylinositol-4,5-bisphoshate
- PIP3
phosphatidylinositol-3,4,5-trisphosphate
- PKA, PKB, PKC and PKD
protein kinase A, B, C and D, respectively
- PLA2, PLC and PLD
phospholipase A2, C and D, respectively
- PTx
pertussis toxin
- pyrrophenone
N-[[(2S,4R)-1-[2-(2,4-difluorobenzoyl)benzoyl]-4-[(triphenylmethyl)thio]-2-pyrrolidinyl]methyl]-4-[(Z)-(2,4-dioxo-5-thiazolidinylidene)methyl]-benzamide
- RNAi
RNA interference
- Src
a protein tyrosine kinase
- SHP-2
a protein tyrosine phosphatase
- TASK
TWIK-related acid-sensitive K+ (channel)
- TRP
transient receptor potential (channel)
- TRPC
TRP (channel) of the canonical subfamily
- VGCC
voltage-gated Ca2+ channel
- Y2H
yeast-2-hybrid
Conflicts of interest
None.
References
- 1.Acuna-Goycolea C, van den Pol AN. Neuroendocrine proopiomelanocortin neurons are excited by hypocretin/orexin. J Neurosci. 2009;29:1503–1513. doi: 10.1523/JNEUROSCI.5147-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander SPH, et al. The Concise Guide to PHARMACOLOGY 2013/14: Overview. Br J Pharmacol. 2013;170:1449–1867. doi: 10.1111/bph.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ammoun S, Johansson L, Ekholm ME, Holmqvist T, Danis AS, Korhonen L, et al. OX1 orexin receptors activate extracellular signal-regulated kinase (ERK) in CHO cells via multiple mechanisms: the role of Ca2+ influx in OX1 receptor signaling. Mol Endocrinol. 2006a;20:80–99. doi: 10.1210/me.2004-0389. [DOI] [PubMed] [Google Scholar]
- 4.Ammoun S, Johansson L, Åkerman KEO, Korhonen L, Lindholm D, Kukkonen JP. Regulation of MAP/SAP-kinase cascades by orexin receptors. 2003. Neuroscience 2003. New Orleans, LA. Society for Neuroscience: Washington, DC. Program No. 161.14.
- 5.Ammoun S, Lindholm D, Wootz H, Åkerman KE, Kukkonen JP. G-protein-coupled OX1 orexin/hcrtr-1 hypocretin receptors induce caspase-dependent and -independent cell death through p38 mitogen-/stress-activated protein kinase. J Biol Chem. 2006b;281:834–842. doi: 10.1074/jbc.M508603200. [DOI] [PubMed] [Google Scholar]
- 6.Balsinde J, Balboa MA. Cellular regulation and proposed biological functions of group VIA calcium-independent phospholipase A2 in activated cells. Cell Signal. 2005;17:1052–1062. doi: 10.1016/j.cellsig.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Bernard R, Lydic R, Baghdoyan HA. Hypocretin-1 activates G proteins in arousal-related brainstem nuclei of rat. Neuroreport. 2002;13:447–450. doi: 10.1097/00001756-200203250-00017. [DOI] [PubMed] [Google Scholar]
- 8.Bernard R, Lydic R, Baghdoyan HA. Hypocretin-1 causes G protein activation and increases ACh release in rat pons. Eur J Neurosci. 2003;18:1775–1785. doi: 10.1046/j.1460-9568.2003.02905.x. [DOI] [PubMed] [Google Scholar]
- 9.Borgland SL. Orexin system as a mediator of addiction & compulsion. Br J Pharmacol. 2013 (in press) [Google Scholar]
- 10.Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borgland SL, Storm E, Bonci A. Orexin B/hypocretin 2 increases glutamatergic transmission to ventral tegmental area neurons. Eur J Neurosci. 2008;28:1545–1556. doi: 10.1111/j.1460-9568.2008.06397.x. [DOI] [PubMed] [Google Scholar]
- 12.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Brown DA. Muscarinic acetylcholine receptors (mAChRs) in the nervous system: some functions and mechanisms. J Mol Neurosci. 2010;41:340–346. doi: 10.1007/s12031-010-9377-2. [DOI] [PubMed] [Google Scholar]
- 14.Brown RE, Sergeeva OA, Eriksson KS, Haas HL. Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline) J Neurosci. 2002;22:8850–8859. doi: 10.1523/JNEUROSCI.22-20-08850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bulenger S, Marullo S, Bouvier M. Emerging role of homo- and heterodimerization in G-protein-coupled receptor biosynthesis and maturation. Trends Pharmacol Sci. 2005;26:131–137. doi: 10.1016/j.tips.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Burdakov D, Liss B, Ashcroft FM. Orexin excites GABAergic neurons of the arcuate nucleus by activating the sodium–calcium exchanger. J Neurosci. 2003;23:4951–4957. doi: 10.1523/JNEUROSCI.23-12-04951.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burlet S, Tyler CJ, Leonard CS. Direct and indirect excitation of laterodorsal tegmental neurons by hypocretin/orexin peptides: implications for wakefulness and narcolepsy. J Neurosci. 2002;22:2862–2872. doi: 10.1523/JNEUROSCI.22-07-02862.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X, Talley EM, Patel N, Gomis A, McIntire WE, Dong B, et al. Inhibition of a background potassium channel by Gq protein alpha-subunits. Proc Natl Acad Sci U S A. 2006;103:3422–3427. doi: 10.1073/pnas.0507710103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis SF, Williams KW, Xu W, Glatzer NR, Smith BN. Selective enhancement of synaptic inhibition by hypocretin (orexin) in rat vagal motor neurons: implications for autonomic regulation. J Neurosci. 2003;23:3844–3854. doi: 10.1523/JNEUROSCI.23-09-03844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev. 2011;111:6130–6185. doi: 10.1021/cr200085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dergacheva O, Bateman R, Byrne P, Mendelowitz D. Orexinergic modulation of GABAergic neurotransmission to cardiac vagal neurons in the brain stem nucleus ambiguus changes during development. Neuroscience. 2012;209:12–20. doi: 10.1016/j.neuroscience.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doroshenko P, Renaud LP. Acid-sensitive TASK-like K+ conductances contribute to resting membrane potential and to orexin-induced membrane depolarization in rat thalamic paraventricular nucleus neurons. Neuroscience. 2009;158:1560–1570. doi: 10.1016/j.neuroscience.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Duguay D, Belanger-Nelson E, Mongrain V, Beben A, Khatchadourian A, Cermakian N. Dynein light chain Tctex-type 1 modulates orexin signaling through its interaction with orexin 1 receptor. PLoS ONE. 2011;6:e26430. doi: 10.1371/journal.pone.0026430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ekholm ME, Johansson L, Kukkonen JP. IP(3)-independent signalling of OX(1) orexin/hypocretin receptors to Ca(2+) influx and ERK. Biochem Biophys Res Commun. 2007;353:475–480. doi: 10.1016/j.bbrc.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 26.Ekholm ME, Johansson L, Kukkonen JP. Rapid and easy semi-quantitative evaluation method for diacylglycerol and inositol-1,4,5-trisphosphate generation in orexin receptor signalling. Acta Physiol (Oxf) 2010;198:387–392. doi: 10.1111/j.1748-1716.2009.02017.x. [DOI] [PubMed] [Google Scholar]
- 27.El Firar A, Voisin T, Rouyer-Fessard C, Ostuni MA, Couvineau A, Laburthe M. Discovery of a functional immunoreceptor tyrosine-based switch motif in a 7-transmembrane-spanning receptor: role in the orexin receptor OX1R-driven apoptosis. FASEB J. 2009;23:4069–4080. doi: 10.1096/fj.09-131367. [DOI] [PubMed] [Google Scholar]
- 28.Ellis J, Pediani JD, Canals M, Milasta S, Milligan G. Orexin-1 receptor-cannabinoid CB1 receptor heterodimerization results in both ligand-dependent and -independent coordinated alterations of receptor localization and function. J Biol Chem. 2006;281:38812–38824. doi: 10.1074/jbc.M602494200. [DOI] [PubMed] [Google Scholar]
- 29.Eriksson KS, Sergeeva O, Brown RE, Haas HL. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci. 2001;21:9273–9279. doi: 10.1523/JNEUROSCI.21-23-09273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans NA, Groarke DA, Warrack J, Greenwood CJ, Dodgson K, Milligan G, et al. Visualizing differences in ligand-induced beta-arrestin-GFP interactions and trafficking between three recently characterized G protein-coupled receptors. J Neurochem. 2001;77:476–485. doi: 10.1046/j.1471-4159.2001.00269.x. [DOI] [PubMed] [Google Scholar]
- 31.Gamper N, Shapiro MS. Regulation of ion transport proteins by membrane phosphoinositides. Nat Rev Neurosci. 2007;8:921–934. doi: 10.1038/nrn2257. [DOI] [PubMed] [Google Scholar]
- 32.Gees M, Colsoul B, Nilius B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb Perspect Biol. 2010;2:a003962. doi: 10.1101/cshperspect.a003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Göncz E, Strowski MZ, Grotzinger C, Nowak KW, Kaczmarek P, Sassek M, et al. Orexin-A inhibits glucagon secretion and gene expression through a Foxo1-dependent pathway. Endocrinology. 2008;149:1618–1626. doi: 10.1210/en.2007-1257. [DOI] [PubMed] [Google Scholar]
- 34.Haj-Dahmane S, Shen RY. The wake-promoting peptide orexin-B inhibits glutamatergic transmission to dorsal raphe nucleus serotonin neurons through retrograde endocannabinoid signaling. J Neurosci. 2005;25:896–905. doi: 10.1523/JNEUROSCI.3258-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hilairet S, Bouaboula M, Carriere D, Le Fur G, Casellas P. Hypersensitization of the Orexin 1 receptor by the CB1 receptor: evidence for cross-talk blocked by the specific CB1 antagonist, SR141716. J Biol Chem. 2003;278:23731–23737. doi: 10.1074/jbc.M212369200. [DOI] [PubMed] [Google Scholar]
- 36.Ho YC, Lee HJ, Tung LW, Liao YY, Fu SY, Teng SF, et al. Activation of orexin 1 receptors in the periaqueductal gray of male rats leads to antinociception via retrograde endocannabinoid (2-arachidonoylglycerol)-induced disinhibition. J Neurosci. 2011;31:14600–14610. doi: 10.1523/JNEUROSCI.2671-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoang QV, Bajic D, Yanagisawa M, Nakajima S, Nakajima Y. Effects of orexin (hypocretin) on GIRK channels. J Neurophysiol. 2003;90:693–702. doi: 10.1152/jn.00001.2003. [DOI] [PubMed] [Google Scholar]
- 38.Hoang QV, Zhao P, Nakajima S, Nakajima Y. Orexin (hypocretin) effects on constitutively active inward rectifier K+ channels in cultured nucleus basalis neurons. J Neurophysiol. 2004;92:3183–3191. doi: 10.1152/jn.01222.2003. [DOI] [PubMed] [Google Scholar]
- 39.Holmqvist T, Johansson L, Östman M, Ammoun S, Åkerman KE, Kukkonen JP. OX1 orexin receptors couple to adenylyl cyclase regulation via multiple mechanisms. J Biol Chem. 2005;280:6570–6579. doi: 10.1074/jbc.M407397200. [DOI] [PubMed] [Google Scholar]
- 40.Holmqvist T, Åkerman KEO, Kukkonen JP. Orexin signaling in recombinant neuron-like cells. FEBS Lett. 2002;526:11–14. doi: 10.1016/s0014-5793(02)03101-0. [DOI] [PubMed] [Google Scholar]
- 41.Ishibashi M, Takano S, Yanagida H, Takatsuna M, Nakajima K, Oomura Y, et al. Effects of orexins/hypocretins on neuronal activity in the paraventricular nucleus of the thalamus in rats in vitro. Peptides. 2005;26:471–481. doi: 10.1016/j.peptides.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Johansson L, Ekholm ME, Kukkonen JP. Multiple phospholipase activation by OX(1) orexin/hypocretin receptors. Cell Mol Life Sci. 2008;65:1948–1956. doi: 10.1007/s00018-008-8206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johansson L, Ekholm ME, Kukkonen JP. Regulation of OX(1) orexin/hypocretin receptor-coupling to phospholipase C by Ca(2+) influx. Br J Pharmacol. 2007;150:97–104. doi: 10.1038/sj.bjp.0706959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jäntti MH, Putula J, Somerharju P, Frohman MA, Kukkonen JP. OX(1) orexin/hypocretin receptor activation of phospholipase D. Br J Pharmacol. 2012;165:1109–1123. doi: 10.1111/j.1476-5381.2011.01565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jäntti MH, Putula J, Turunen PM, Näsman J, Reijonen S, Kukkonen JP. Autocrine endocannabinoid signaling potentiates orexin receptor signaling upon CB1 cannabinoid-OX1 orexin receptor coexpression. Mol Pharmacol. 2013;83:621–632. doi: 10.1124/mol.112.080523. [DOI] [PubMed] [Google Scholar]
- 46.Kalogiannis M, Grupke SL, Potter PE, Edwards JG, Chemelli RM, Kisanuki YY, et al. Narcoleptic orexin receptor knockout mice express enhanced cholinergic properties in laterodorsal tegmental neurons. Eur J Neurosci. 2010;32:130–142. doi: 10.1111/j.1460-9568.2010.07259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- 48.Karteris E, Chen J, Randeva HS. Expression of human prepro-orexin and signaling characteristics of orexin receptors in the male reproductive system. J Clin Endocrinol Metab. 2004;89:1957–1962. doi: 10.1210/jc.2003-031778. [DOI] [PubMed] [Google Scholar]
- 49.Karteris E, Machado RJ, Chen J, Zervou S, Hillhouse EW, Randeva HS. Food deprivation differentially modulates orexin receptor expression and signalling in the rat hypothalamus and adrenal cortex. Am J Physiol Endocrinol Metab. 2005;288:E1089–E1100. doi: 10.1152/ajpendo.00351.2004. [DOI] [PubMed] [Google Scholar]
- 50.Karteris E, Randeva HS, Grammatopoulos DK, Jaffe RB, Hillhouse EW. Expression and coupling characteristics of the crh and orexin type 2 receptors in human fetal adrenals. J Clin Endocrinol Metab. 2001;86:4512–4519. doi: 10.1210/jcem.86.9.7849. [DOI] [PubMed] [Google Scholar]
- 51.Kilduff TS, Bowersox SS, Kaitin KI, Baker TL, Ciaranello RD, Dement WC. Muscarinic cholinergic receptors and the canine model of narcolepsy. Sleep. 1986;9:102–106. doi: 10.1093/sleep/9.1.102. [DOI] [PubMed] [Google Scholar]
- 52.Kleuss C. Somatostatin modulates voltage-dependent Ca2+ channels in GH3 cells via a specific G(o) splice variant. Ciba Found Symp. 1995;190:171–182. doi: 10.1002/9780470514733.ch11. . discussion 182–176. [DOI] [PubMed] [Google Scholar]
- 53.Kohlmeier KA, Inoue T, Leonard CS. Hypocretin/orexin peptide signalling in the ascending arousal system: elevation of intracellular calcium in the mouse dorsal raphe and laterodorsal tegmentum. J Neurophysiol. 2004;92:221–235. doi: 10.1152/jn.00076.2004. [DOI] [PubMed] [Google Scholar]
- 54.Kohlmeier KA, Watanabe S, Tyler CJ, Burlet S, Leonard CS. Dual orexin actions on dorsal raphe and laterodorsal tegmentum neurons: noisy cation current activation and selective enhancement of Ca2+ transients mediated by L-type calcium channels. J Neurophysiol. 2008;100:2265–2281. doi: 10.1152/jn.01388.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Konieczny V, Keebler MV, Taylor CW. Spatial organization of intracellular Ca2+ signals. Semin Cell Dev Biol. 2012;23:172–180. doi: 10.1016/j.semcdb.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 56.Korotkova TM, Eriksson KS, Haas HL, Brown RE. Selective excitation of GABAergic neurons in the substantia nigra of the rat by orexin/hypocretin in vitro. Regul Pept. 2002;104:83–89. doi: 10.1016/s0167-0115(01)00323-8. [DOI] [PubMed] [Google Scholar]
- 57.Kraft R. The Na+/Ca2+ exchange inhibitor KB-R7943 potently blocks TRPC channels. Biochem Biophys Res Commun. 2007;361:230–236. doi: 10.1016/j.bbrc.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 59.Kukkonen JP. Lipid signaling cascades of orexin/hypocretin receptors. Biochimie. 2013a doi: 10.1016/j.biochi.2013.06.015. doi: 10.1016/j.biochi.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 60.Kukkonen JP. A ménage à trois made in heaven: G-protein-coupled receptors, lipids and TRP channels. Cell Calcium. 2011;50:9–26. doi: 10.1016/j.ceca.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 61.Kukkonen JP. Physiology of the orexinergic/hypocretinergic system: a revisit in 2012. Am J Physiol Cell Physiol. 2013b;301:C2–C32. doi: 10.1152/ajpcell.00227.2012. [DOI] [PubMed] [Google Scholar]
- 62.Kukkonen JP. Regulation of receptor-coupling to (multiple) G-proteins. A challenge for basic research and drug discovery. Receptors Channels. 2004;10:167–183. doi: 10.3109/10606820490926151. [DOI] [PubMed] [Google Scholar]
- 64.Kukkonen JP, Åkerman KEO. Orexin receptors couple to Ca2+ channels different from store-operated Ca2+ channels. Neuroreport. 2001;12:2017–2020. doi: 10.1097/00001756-200107030-00046. [DOI] [PubMed] [Google Scholar]
- 65.Laitinen JT. [35S]GTPgS autoradiography: a powerful functional approach with expanding potential for neuropharmacological studies on receptors coupled to Gi family of G proteins. Current Neuropharmacol. 2004;2:191–206. [Google Scholar]
- 66.Lambe EK, Aghajanian GK. Hypocretin (orexin) induces calcium transients in single spines postsynaptic to identified thalamocortical boutons in prefrontal slice. Neuron. 2003;40:139–150. doi: 10.1016/s0896-6273(03)00598-1. [DOI] [PubMed] [Google Scholar]
- 67.Larsson KP, Peltonen HM, Bart G, Louhivuori LM, Penttonen A, Antikainen M, et al. Orexin-A-induced Ca2+ entry: evidence for involvement of TRPC channels and protein kinase C regulation. J Biol Chem. 2005;280:1771–1781. doi: 10.1074/jbc.M406073200. [DOI] [PubMed] [Google Scholar]
- 68.Larsson KP, Åkerman KEO, Magga J, Uotila S, Kukkonen JP, Näsman J, et al. Orexin-A stimulates cholecystokinin release from intestinal neuroendocrine STC-1 cells by a Ca2+-dependent mechanism. Biochem Biophys Res Commun. 2003;309:209–216. doi: 10.1016/s0006-291x(03)01563-8. [DOI] [PubMed] [Google Scholar]
- 69.Leonard CS, Kukkonen JP. Orexin/hypocretin receptor signalling: a functional perspective. Br J Pharmacol. 2013;171:294–313. doi: 10.1111/bph.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li H, de Lecea L. The orexins as integrators of multiple physiological functions. Br J Pharmacol. 2013;171:332–350. doi: 10.1111/bph.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Y, Gao XB, Sakurai T, van den Pol AN. Hypocretin/orexin excites hypocretin neurons via a local glutamate neuron – a potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36:1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- 72.Lindner M, Leitner MG, Halaszovich CR, Hammond GR, Oliver D. Probing the regulation of TASK potassium channels by PI4,5P(2) with switchable phosphoinositide phosphatases. J Physiol. 2011;589:3149–3162. doi: 10.1113/jphysiol.2011.208983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu RJ, van den Pol AN, Aghajanian GK. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J Neurosci. 2002;22:9453–9464. doi: 10.1523/JNEUROSCI.22-21-09453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Louhivuori LM, Jansson L, Nordstrom T, Bart G, Nasman J, Akerman KE. Selective interference with TRPC3/6 channels disrupts OX1 receptor signalling via NCX and reveals a distinct calcium influx pathway. Cell Calcium. 2010;48:114–123. doi: 10.1016/j.ceca.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 75.Lund PE, Shariatmadari R, Uustare A, Detheux M, Parmentier M, Kukkonen JP, et al. The orexin OX1 receptor activates a novel Ca2+ influx pathway necessary for coupling to phospholipase C. J Biol Chem. 2000;275:30806–30812. doi: 10.1074/jbc.M002603200. [DOI] [PubMed] [Google Scholar]
- 76.Luscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci. 2010;11:301–315. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lytton J. Na+/Ca2+ exchangers: three mammalian gene families control Ca2+ transport. Biochem J. 2007;406:365–382. doi: 10.1042/BJ20070619. [DOI] [PubMed] [Google Scholar]
- 78.Magga J, Bart G, Oker-Blom C, Kukkonen JP, Åkerman KE, Näsman J. Agonist potency differentiates G protein activation and Ca2+ signalling by the orexin receptor type 1. Biochem Pharmacol. 2006;71:827–836. doi: 10.1016/j.bcp.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 79.Malendowicz LK, Tortorella C, Nussdorfer GG. Orexins stimulate corticosterone secretion of rat adrenocortical cells, through the activation of the adenylate cyclase-dependent signaling cascade. J Steroid Biochem Mol Biol. 1999;70:185–188. doi: 10.1016/s0960-0760(99)00110-7. [DOI] [PubMed] [Google Scholar]
- 80.Mangmool S, Kurose H. G(i/o) protein-dependent and -independent actions of pertussis toxin (PTX) Toxins (Basel) 2011;3:884–899. doi: 10.3390/toxins3070884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mathie A. Neuronal two-pore-domain potassium channels and their regulation by G protein-coupled receptors. J Physiol. 2007;578:377–385. doi: 10.1113/jphysiol.2006.121582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mazzocchi G, Malendowicz LK, Aragona F, Rebuffat P, Gottardo L, Nussdorfer GG. Human pheochromocytomas express orexin receptor type 2 gene and display an in vitro secretory response to orexins A and B. J Clin Endocrinol Metab. 2001a;86:4818–4821. doi: 10.1210/jcem.86.10.7929. [DOI] [PubMed] [Google Scholar]
- 83.Mazzocchi G, Malendowicz LK, Gottardo L, Aragona F, Nussdorfer GG. Orexin A stimulates cortisol secretion from human adrenocortical cells through activation of the adenylate cyclase-dependent signaling cascade. J Clin Endocrinol Metab. 2001b;86:778–782. doi: 10.1210/jcem.86.2.7233. [DOI] [PubMed] [Google Scholar]
- 84.Milasta S, Evans NA, Ormiston L, Wilson S, Lefkowitz RJ, Milligan G. The sustainability of interactions between the orexin-1 receptor and beta-arrestin-2 is defined by a single C-terminal cluster of hydroxy amino acids and modulates the kinetics of ERK MAPK regulation. Biochem J. 2005;387:573–584. doi: 10.1042/BJ20041745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Milligan G. G protein-coupled receptor hetero-dimerization: contribution to pharmacology and function. Br J Pharmacol. 2009;158:5–14. doi: 10.1111/j.1476-5381.2009.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Monovich L, Mugrage B, Quadros E, Toscano K, Tommasi R, LaVoie S, et al. Optimization of halopemide for phospholipase D2 inhibition. Bioorg Med Chem Lett. 2007;17:2310–2311. doi: 10.1016/j.bmcl.2007.01.059. [DOI] [PubMed] [Google Scholar]
- 87.Muroya S, Funahashi H, Yamanaka A, Kohno D, Uramura K, Nambu T, et al. Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca2+ signaling in a reciprocal manner to leptin: orexigenic neuronal pathways in the mediobasal hypothalamus. Eur J Neurosci. 2004;19:1524–1534. doi: 10.1111/j.1460-9568.2004.03255.x. [DOI] [PubMed] [Google Scholar]
- 88.Newton AC. Protein kinase C: poised to signal. Am J Physiol Endocrinol Metab. 2010;298:E395–E402. doi: 10.1152/ajpendo.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 90.Näsman J, Bart G, Larsson K, Louhivuori L, Peltonen H, Åkerman KE. The orexin OX1 receptor regulates Ca2+ entry via diacylglycerol-activated channels in differentiated neuroblastoma cells. J Neurosci. 2006;26:10658–10666. doi: 10.1523/JNEUROSCI.2609-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ono K, Kai A, Honda E, Inenaga K. Hypocretin-1/orexin-A activates subfornical organ neurons of rats. Neuroreport. 2008;19:69–73. doi: 10.1097/WNR.0b013e3282f32d64. [DOI] [PubMed] [Google Scholar]
- 92.Ono T, Yamada K, Chikazawa Y, Ueno M, Nakamoto S, Okuno T, et al. Characterization of a novel inhibitor of cytosolic phospholipase A2alpha, pyrrophenone. Biochem J. 2002;363:727–735. doi: 10.1042/0264-6021:3630727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peltonen HM, Magga JM, Bart G, Turunen PM, Antikainen MS, Kukkonen JP, et al. Involvement of TRPC3 channels in calcium oscillations mediated by OX(1) orexin receptors. Biochem Biophys Res Commun. 2009;385:408–412. doi: 10.1016/j.bbrc.2009.05.077. [DOI] [PubMed] [Google Scholar]
- 94.Peltonen HM, Åkerman KE, Bart G. A role for PKD1 and PKD3 activation in modulation of calcium oscillations induced by orexin receptor 1 stimulation. Biochim Biophys Acta. 2010;1803:1206–1212. doi: 10.1016/j.bbamcr.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 95.Putula J, Kukkonen JP. Mapping of the binding sites for the OX(1) orexin receptor antagonist, SB-334867, using orexin/hypocretin receptor chimaeras. Neurosci Lett. 2012;506:111–115. doi: 10.1016/j.neulet.2011.10.061. [DOI] [PubMed] [Google Scholar]
- 96.Putula J, Turunen PM, Jäntti MH, Ekholm ME, Kukkonen JP. Agonist ligand discrimination by the two orexin receptors depends on the expression system. Neurosci Lett. 2011;494:57–60. doi: 10.1016/j.neulet.2011.02.055. [DOI] [PubMed] [Google Scholar]
- 97.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ramanjaneya M, Conner AC, Chen J, Kumar P, Brown JE, Johren O, et al. Orexin-stimulated MAP kinase cascades are activated through multiple G-protein signalling pathways in human H295R adrenocortical cells: diverse roles for orexins A and B. J Endocrinol. 2009;202:249–261. doi: 10.1677/JOE-08-0536. [DOI] [PubMed] [Google Scholar]
- 99.Ramanjaneya M, Conner AC, Chen J, Stanfield PR, Randeva HS. Orexins stimulate steroidogenic acute regulatory protein expression through multiple signaling pathways in human adrenal H295R cells. Endocrinology. 2008;149:4106–4115. doi: 10.1210/en.2007-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Randeva HS, Karteris E, Grammatopoulos D, Hillhouse EW. Expression of orexin-A and functional orexin type 2 receptors in the human adult adrenals: implications for adrenal function and energy homeostasis. J Clin Endocrinol Metab. 2001;86:4808–4813. doi: 10.1210/jcem.86.10.7921. [DOI] [PubMed] [Google Scholar]
- 101.Ritter SL, Hall RA. Fine-tuning of GPCR activity by receptor-interacting proteins. Nat Rev Mol Cell Biol. 2009;10:819–830. doi: 10.1038/nrm2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rouet-Benzineb P, Rouyer-Fessard C, Jarry A, Avondo V, Pouzet C, Yanagisawa M, et al. Orexins acting at native OX(1) receptor in colon cancer and neuroblastoma cells or at recombinant OX(1) receptor suppress cell growth by inducing apoptosis. J Biol Chem. 2004;279:45875–45886. doi: 10.1074/jbc.M404136200. [DOI] [PubMed] [Google Scholar]
- 103.Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol. 2007;213:589–602. doi: 10.1002/jcp.21246. [DOI] [PubMed] [Google Scholar]
- 104.Sadja R, Alagem N, Reuveny E. Gating of GIRK channels: details of an intricate, membrane-delimited signaling complex. Neuron. 2003;39:9–12. doi: 10.1016/s0896-6273(03)00402-1. [DOI] [PubMed] [Google Scholar]
- 105.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 106.Schneider OD, Weiss AA, Miller WE. Pertussis toxin utilizes proximal components of the T-cell receptor complex to initiate signal transduction events in T cells. Infect Immun. 2007;75:4040–4049. doi: 10.1128/IAI.00414-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Scott SA, Selvy PE, Buck JR, Cho HP, Criswell TL, Thomas AL, et al. Design of isoform-selective phospholipase D inhibitors that modulate cancer cell invasiveness. Nat Chem Biol. 2009;5:108–117. doi: 10.1038/nchembio.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Selbach O, Doreulee N, Bohla C, Eriksson KS, Sergeeva OA, Poelchen W, et al. Orexins/hypocretins cause sharp wave- and theta-related synaptic plasticity in the hippocampus via glutamatergic, gabaergic, noradrenergic, and cholinergic signaling. Neuroscience. 2004;127:519–528. doi: 10.1016/j.neuroscience.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 108.Selbach O, Bohla C, Barbara A, Doreulee N, Eriksson KS, Sergeeva OA, et al. Orexins/hypocretins control bistability of hippocampal long-term synaptic plasticity through co-activation of multiple kinases. Acta Physiol (Oxf) 2010;198:277–285. doi: 10.1111/j.1748-1716.2009.02021.x. [DOI] [PubMed] [Google Scholar]
- 110.Sellayah D, Bharaj P, Sikder D. Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab. 2011;14:478–490. doi: 10.1016/j.cmet.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 111.Shenoy SK, Lefkowitz RJ. β-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shin HS, Cho HS, Sung KW, Yoon BJ. Orexin-A increases cell surface expression of AMPA receptors in the striatum. Biochem Biophys Res Commun. 2009;378:409–413. doi: 10.1016/j.bbrc.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 113.Shuttleworth TJ. Arachidonic acid, ARC channels, and Orai proteins. Cell Calcium. 2009;45:602–610. doi: 10.1016/j.ceca.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Skrzypski M, Le TT, Kaczmarek P, Pruszynska-Oszmalek E, Pietrzak P, Szczepankiewicz D, et al. Orexin A stimulates glucose uptake, lipid accumulation and adiponectin secretion from 3T3-L1 adipocytes and isolated primary rat adipocytes. Diabetologia. 2011;54:1841–1852. doi: 10.1007/s00125-011-2152-2. [DOI] [PubMed] [Google Scholar]
- 114.Skrzypski M, Kaczmarek P, Le TT, Wojciechowicz T, Pruszynska-Oszmalek E, Szczepankiewicz D, et al. Effects of orexin A on proliferation, survival, apoptosis and differentiation of 3T3-L1 preadipocytes into mature adipocytes. FEBS Lett. 2012;586:4157–4164. doi: 10.1016/j.febslet.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 116.Smart D, Jerman JC, Brough SJ, Rushton SL, Murdock PR, Jewitt F, et al. Characterization of recombinant human orexin receptor pharmacology in a Chinese hamster ovary cell-line using FLIPR. Br J Pharmacol. 1999;128:1–3. doi: 10.1038/sj.bjp.0702780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Smith BN, Davis SF, Van Den Pol AN, Xu W. Selective enhancement of excitatory synaptic activity in the rat nucleus tractus solitarius by hypocretin 2. Neuroscience. 2002;115:707–714. doi: 10.1016/s0306-4522(02)00488-8. [DOI] [PubMed] [Google Scholar]
- 118.Squecco R, Garella R, Luciani G, Francini F, Baccari MC. Muscular effects of orexin A on the mouse duodenum: mechanical and electrophysiological studies. J Physiol. 2011;589:5231–5246. doi: 10.1113/jphysiol.2011.214940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Su W, Yeku O, Olepu S, Genna A, Park JS, Ren H, et al. 5-Fluoro-2-indolyl des-chlorohalopemide (FIPI), a phospholipase D pharmacological inhibitor that alters cell spreading and inhibits chemotaxis. Mol Pharmacol. 2009;75:437–446. doi: 10.1124/mol.108.053298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys. 2008;37:175–195. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sunahara RK, Taussig R. Isoforms of mammalian adenylyl cyclase: multiplicities of signaling. Mol Interv. 2002;2:168–184. doi: 10.1124/mi.2.3.168. [DOI] [PubMed] [Google Scholar]
- 122.Tang J, Chen J, Ramanjaneya M, Punn A, Conner AC, Randeva HS. The signalling profile of recombinant human orexin-2 receptor. Cell Signal. 2008;20:1651–1661. doi: 10.1016/j.cellsig.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 123.Taylor CW, Broad LM. Pharmacological analysis of intracellular Ca2+ signalling: problems and pitfalls. Trends Pharmacol Sci. 1998;19:370–375. doi: 10.1016/s0165-6147(98)01243-7. [DOI] [PubMed] [Google Scholar]
- 124.Tsujino N, Yamanaka A, Ichiki K, Muraki Y, Kilduff TS, Yagami K, et al. Cholecystokinin activates orexin/hypocretin neurons through the cholecystokinin A receptor. J Neurosci. 2005;25:7459–7469. doi: 10.1523/JNEUROSCI.1193-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Turunen PM, Ekholm ME, Somerharju P, Kukkonen JP. Arachidonic acid release mediated by OX(1) orexin receptors. Br J Pharmacol. 2010;159:212–221. doi: 10.1111/j.1476-5381.2009.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Turunen PM, Jäntti MH, Kukkonen JP. OX1 orexin/hypocretin receptor signaling via arachidonic acid and endocannabinoid release. Mol Pharmacol. 2012;82:156–167. doi: 10.1124/mol.112.078063. [DOI] [PubMed] [Google Scholar]
- 127.Uramura K, Funahashi H, Muroya S, Shioda S, Takigawa M, Yada T. Orexin-a activates phospholipase C- and protein kinase C-mediated Ca2+ signaling in dopamine neurons of the ventral tegmental area. Neuroreport. 2001;12:1885–1889. doi: 10.1097/00001756-200107030-00024. [DOI] [PubMed] [Google Scholar]
- 128.van den Pol AN. Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J Neurosci. 1999;19:3171–3182. doi: 10.1523/JNEUROSCI.19-08-03171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van den Pol AN, Patrylo PR, Ghosh PK, Gao XB. Lateral hypothalamus: early developmental expression and response to hypocretin (orexin) J Comp Neurol. 2001;433:349–363. doi: 10.1002/cne.1144. [DOI] [PubMed] [Google Scholar]
- 131.Ward RJ, Pediani JD, Milligan G. Hetero-multimerization of the cannabinoid CB1 receptor and the orexin OX1 receptor generates a unique complex in which both protomers are regulated by orexin A. J Biol Chem. 2011a;286:37414–37428. doi: 10.1074/jbc.M111.287649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ward RJ, Pediani JD, Milligan G. Ligand-induced internalization of the orexin OX(1) and cannabinoid CB(1) receptors assessed via N-terminal SNAP and CLIP-tagging. Br J Pharmacol. 2011b;162:1439–1452. doi: 10.1111/j.1476-5381.2010.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wenzel J, Grabinski N, Knopp CA, Dendorfer A, Ramanjaneya M, Randeva HS, et al. Hypocretin/orexin increases the expression of steroidogenic enzymes in human adrenocortical NCI H295R cells. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1601–R1609. doi: 10.1152/ajpregu.91034.2008. [DOI] [PubMed] [Google Scholar]
- 135.Winrow CJ, Renger JJ. Discovery and development of orexin receptor antagonists as therapeutics for insomnia. Br J Pharmacol. 2013;171:283–293. doi: 10.1111/bph.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Voisin T, El Firar A, Fasseu M, Rouyer-Fessard C, Descatoire V, Walker F, et al. Aberrant expression of OX1 receptors for orexins in colon cancers and liver metastases: an openable gate to apoptosis. Cancer Res. 2011;71:3341–3351. doi: 10.1158/0008-5472.CAN-10-3473. [DOI] [PubMed] [Google Scholar]
- 137.Voisin T, El Firar A, Rouyer-Fessard C, Gratio V, Laburthe M. A hallmark of immunoreceptor, the tyrosine-based inhibitory motif ITIM, is present in the G protein-coupled receptor OX1R for orexins and drives apoptosis: a novel mechanism. FASEB J. 2008;22:1993–2002. doi: 10.1096/fj.07-098723. [DOI] [PubMed] [Google Scholar]
- 138.Voisin T, Firar AE, Avondo V, Laburthe M. Orexin-induced apoptosis: the key role of the seven-transmembrane domain orexin type 2 receptor. Endocrinology. 2006;147:4977–4984. doi: 10.1210/en.2006-0201. [DOI] [PubMed] [Google Scholar]
- 139.Wu M, Zaborszky L, Hajszan T, van den Pol AN, Alreja M. Hypocretin/orexin innervation and excitation of identified septohippocampal cholinergic neurons. J Neurosci. 2004;24:3527–3536. doi: 10.1523/JNEUROSCI.5364-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu M, Zhang Z, Leranth C, Xu C, van den Pol AN, Alreja M. Hypocretin increases impulse flow in the septohippocampal GABAergic pathway: implications for arousal via a mechanism of hippocampal disinhibition. J Neurosci. 2002;22:7754–7765. doi: 10.1523/JNEUROSCI.22-17-07754.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wymann MP, Zvelebil M, Laffargue M. Phosphoinositide 3-kinase signalling – which way to target? Trends Pharmacol Sci. 2003;24:366–376. doi: 10.1016/S0165-6147(03)00163-9. [DOI] [PubMed] [Google Scholar]
- 142.Xu R, Wang Q, Yan M, Hernandez M, Gong C, Boon WC, et al. Orexin-A augments voltage-gated Ca(2+) currents and synergistically increases growth hormone (GH) secretion with GH-releasing hormone in primary cultured ovine somatotropes. Endocrinology. 2002;143:4609–4619. doi: 10.1210/en.2002-220506. [DOI] [PubMed] [Google Scholar]
- 143.Xu TR, Ward RJ, Pediani JD, Milligan G. The orexin OX1 receptor exists predominantly as a homodimer in the basal state: potential regulation of receptor organization by both agonist and antagonist ligands. Biochem J. 2011;439:171–183. doi: 10.1042/BJ20110230. [DOI] [PubMed] [Google Scholar]
- 144.Yang B, Samson WK, Ferguson AV. Excitatory effects of orexin-A on nucleus tractus solitarius neurons are mediated by phospholipase C and protein kinase C. J Neurosci. 2003;23:6215–6222. doi: 10.1523/JNEUROSCI.23-15-06215.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yuan JP, Lee KP, Hong JH, Muallem S. The closing and opening of TRPC channels by Homer1 and STIM1. Acta Physiol (Oxf) 2012;204:238–247. doi: 10.1111/j.1748-1716.2011.02319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang J, Li B, Yu L, He YC, Li HZ, Zhu JN, et al. A role for orexin in central vestibular motor control. Neuron. 2011;69:793–804. doi: 10.1016/j.neuron.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 147.Zhang L, Kolaj M, Renaud LP. Ca2+-dependent and Na+-dependent K+ conductances contribute to a slow AHP in thalamic paraventricular nucleus neurons: a novel target for orexin receptors. J Neurophysiol. 2010;104:2052–2062. doi: 10.1152/jn.00320.2010. [DOI] [PubMed] [Google Scholar]