Abstract
Background and Purpose: Cyclophosphamide induces urotoxicity characterized by the development of cystitis, which involves bladder overactivity and inflammation. Here, we investigated the roles of chemokine receptor 2 (CXCR2) and transient receptor potential vanilloid 1 (TRPV1) channels in a rat model of cyclophosphamide-induced cystitis.
Experimental Approach: Cystitis induced by cyclophosphamide in rats was assessed by gross morphology, histology and immunohistochemistry of bladder tissue. mRNA for CXCR2 and TRPV1 channels were measured by RT-PCR. Nociceptive responses in paw and abdomen, along with cystometric measures were recorded.
Key Results: Cyclophosphamide, i.p., induced pain behaviour, bladder inflammation and voiding dysfunction. The CXCR2 antagonist, SB225002, the TRPV1 channel antagonist, SB366791 or their combination reduced the mechanical hypersensitivity of paw and abdominal area and nociceptive behaviour after cyclophosphamide. Cyclophosphamide-induced cystitis was characterized by haemorrhage, oedema, neutrophil infiltration and other inflammatory changes, which were markedly decreased by the antagonists. Up-regulation of CXCR2 and TRPV1 mRNA in the bladder after cyclophosphamide was inhibited by SB225002, SB366791 or their combination. Expression of CXCR2 and TRPV1 channels was increased in the urothelium after cyclophosphamide. Bladder dysfunction was shown by increased number of non-voiding contractions (NVCs) and bladder pressures and a reduction in bladder capacity (BC), voided volume (VV) and voiding efficiency (VE). SB225002 or its combination with SB366791 reduced bladder pressures, whereas SB225002, SB366791 or their combination increased BC, VV and VE, and also reduced the number of NVCs.
Conclusions and Implications: CXCR2 and TRPV1 channels play important roles in cyclophosphamide-induced cystitis in rats and could provide potential therapeutic targets for cystitis.
Keywords: Cyclophosphamide-induced cystitis, CXCR2 receptors, TRPV1 channels, bladder inflammation, voiding dysfunction, pain behaviour
Introduction
Inflammatory conditions of the urinary bladder (cystitis) are characterized by complex symptoms, such as increased urinary frequency and urgency, nocturia and intense pelvic pain (Sakthivel et al., 2008). These symptoms can seriously affect the quality of life of patients and generate high costs to healthcare systems. Evidence suggests that cystitis can be caused by multiple mechanisms related to inflammatory responses to infection, stress or exposure to chemicals (Arms et al., 2010; Everaerts et al., 2010). Cyclophosphamide is an alkylating agent frequently used for treating breast cancer, B-cell lymphoma and leukaemia (Korkmaz et al., 2007; Abraham and Rabi, 2009). Clinical use of cyclophosphamide is associated with serious urological side effects, including voiding disturbances, urothelial damage, bladder oedema, neovascularization and haemorrhage. Cyclophosphamide-induced cystitis is characterized by neurochemical and electrophysiological changes, as well as by functional alterations of micturition reflex and visceral pain behaviour (Corrow and Vizzard, 2009).
Particular attention has been given to the role of chemotactic cytokines, known as chemokines, in inflammatory conditions and these chemokines induce the migration of leukocytes to inflamed tissues. Potential roles of chemokine receptors in the physiopathological processes of cystitis are emerging. Notably, the expression of chemokines, such as CX3CL1 (fractalkine), CXCL12, CXCL10, and their corresponding receptors CXCR1, CXCR4 and CXCR3 (receptor nomenclature follows Alexander et al., 2013), are up-regulated after cyclophosphamide-induced cystitis and this up-regulation may contribute to some functional alterations in the bladder of mice, rats and humans (Yuridullah et al., 2006; Sakthivel et al., 2008; Vera et al., 2008; Arms et al., 2010).
CXCR2 is a chemokine receptor expressed on the cellular surface of leukocytes, notably neutrophils, endothelial cells and a range of other cells throughout the human body. CXCR2 binds several chemokines with high affinity, including IL-8 (Murphy and Tiffany, 1991) and this receptor plays a critical role in the development of numerous inflammatory disorders, such as wound healing, tumour progression and painful conditions (Reiland et al., 1999; Boisvert et al., 2000; Souza et al., 2004; Coelho et al., 2008; Zaja-Milatovic and Richmond, 2008; Manjavachi et al., 2010). Although it can act as an angiogenic factor (Sukkar et al., 2008), CXCR2 mainly mediates cell migration and its functional blockade prevented neutrophil chemotaxis and tissue damage in experimental colitis in mice (Bento et al., 2008). The potential of the CXCR2 selective antagonist, SB225002 [N-(2-hydroxy-4-nitrophenyl)-N9-(2-bromophenyl) urea], has been evaluated pre-clinically for the management of rheumatoid arthritis, atherosclerosis and painful conditions (Reutershan, 2006; Barsante et al., 2008; Manjavachi et al., 2010).
Cyclophosphamide-induced haemorrhagic cystitis is characterized by intense inflammatory changes, accompanied by marked pain symptoms. Tissue damage caused by cyclophosphamide administration leads to the release of several inflammatory and hyperalgesic mediators, including chemokines (Wong and Gavva, 2009). Moreover, the activation of ion channels, such as those of the transient receptor potential vanilloid 1 (TRPV1) type, could contribute to the development and maintenance of pain (Wang et al., 2008). The TRPV1 channel is expressed in afferent nerve fibres that innervate the bladder, but also appears to be present in the urothelial cells, smooth muscle cells and interstitial cells (Ost et al., 2002; Charrua et al., 2009; Everaerts et al., 2009). Different studies have already confirmed that functional TRPV1 channels are present in urothelial cells of mice, rats (Birder et al., 2001; Kullmann et al., 2009) and humans (Charrua et al., 2009). In addition, some studies suggest a role of TRPV1 channels in visceral mechanosensation. In this context, TRPV1 channels can modulate the sensory functions of the bladder, affecting its voiding frequency and pressure, contributing to the development of bladder overactivity and mechanical hyperalgesia caused by cystitis (Daly et al., 2007; Wang et al., 2008; Frias et al., 2012; Lei and Malykhina, 2012).
The activation of chemokine receptors during inflammation appears to potentiate the sensitivity of TRPV1 channels, eventually promoting hyperalgesia (Zhang et al., 2005; Kao et al., 2012). In this context, the chemokine CCL3 sensitized TRPV1 channels, increasing pain sensation (Zhang et al., 2005). Recently, it was demonstrated that CCL2 induced hyperalgesia by up-regulating the function and expression of TRPV1 channels in DRG sensory neurons (Kao et al., 2012). Therefore, chemokines released during the inflammatory process seem to be involved in the increased perception of pain through sensitization of TRPV1 channels.
In the present study, we evaluated, for the first time, the role of CXCR2 in the bladder function and visceral hypersensitivity following cyclophosphamide-induced cystitis. Moreover, we aimed to confirm the possible correlation between expression and activation of CXCR2 and TRPV1 channels in cystitis, and whether the blockade of both receptors would improve cyclophosphamide-induced cystitis.
Methods
Animals
All animal care and experimental procedures followed the National Institutes of Health Animal Care Guidelines (NIH Publications No. 80-23) and ethical guidelines for investigation of experimental pain in conscious animals (Zimmermann, 2002); they were approved by the ethics committee of the Federal University of Santa Catarina (PP00607). The results of all studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). About 100 animals were used in this study. Non-fasted female Wistar Hannover rats (six to eight per group, 150–220 g) from the animal housing unit of the Laboratory of Experimental Pharmacology at the Federal University of Santa Catarina (LAFEX/UFSC) were used in this study. The animals were housed in groups of five and maintained in controlled temperature (22 ± 2°C) and humidity (60–80%), under a 12:12 h light–dark cycle with food and water available ad libitum.
Cyclophosphamide-induced cystitis
Female rats were anaesthetized with isoflurane (2%) inhalation and cystitis was induced by a single i.p. injection of cyclophosphamide (200 mg·kg−1) (Boucher et al., 2000; Chuang et al., 2011; Kyung et al., 2011; Pan et al., 2012). Control animals received saline injections by the same route. In order to evaluate the effect of CXCR2 and TRPV1 channel blockade on cyclophosphamide-induced cystitis, different groups of animals were injected i.p., 30 min before cyclophosphamide, with the CXCR2 selective antagonist, SB225002 (0.3 mg·kg−1), the selective TRPV1 channel antagonist, SB366791 (4′-chloro-3-methoxycinnamanilide) (0.3 mg·kg−1), or a combination of both antagonists (0.3 mg·kg−1 each).
Nociceptive scoring
The experimental design used in the present study was similar to that described by Boucher et al. (2000), with minor modifications. The rats were placed individually in observation boxes and were acclimatized for 30 min prior to behavioural testing. A single i.p. administration of cyclophosphamide (200 mg·kg−1) produced changes in three behavioural parameters – breathing rate, opening of the eyes and posture – reflecting the nociceptive alterations associated with haemorrhagic cystitis. Therefore, these parameters were adopted as nociceptive indexes and scored at 1, 4, 8, 24 and 48 h after administration of CYP. All the experiments were performed without knowledge of the treatments. For the breathing rate, every 10 cycles·min−1 was scored as one, with control values of about 140 cycles·min−1. For the opening or closing of the eyes, the following scores were assumed: 0 for complete opening, corresponding to normal eyes; 10 for complete closing; 5 for half-closed eyes; and 2 and 7 for the two intermediate positions (between open and half-closed and between half-closed and closed respectively). Finally, regarding the posture changes, when either the rounded-back with the whole body aligned or complete limpness was observed, the score was 10. When no specific posture was seen over the observation period, the score was 0. In order to determine the inhibitory action of the antagonists on nociceptive responses, we calculated the area under nociception-time curve (AUC). The AUC values of the experimental groups were compared and the inhibition percentages were calculated.
Cyclophosphamide-induced mechanical hypersensitivity
Referred hyperalgesia was measured by testing the frequency of withdrawal responses to the application of von Frey hairs (VFH) (von Frey Hairs; Stoelting, Chicago, IL, USA) on the abdomen and the right hindpaw. The nociceptive responses were evaluated at different time points (1, 4, 8, 24 and 48 h) following cyclophosphamide injection. For that purpose, the rats were placed individually in clear Plexiglas boxes (10 × 10 × 8 cm), on elevated wire mesh platforms to allow access to the ventral surface of the right hindpaw and to the abdominal area. The animals were acclimatized for 1 h prior to behavioural testing. The withdrawal response frequency (%) was measured following 10 applications (3 s each application, and an interval of 10 s among the applications) of VFH. Stimuli were delivered below to the plantar surface of the right hindpaw and to the abdomen area. The 8.0 g VFH filament applied in the hindpaw and the 26.0 g VFH applied in the abdomen area produces a mean withdrawal frequency of about 20%, which is considered an adequate value for the measurement of mechanical hypersensitivity. The appearance of any of the following behaviours on application of a filament to the abdomen was considered a withdrawal response: retraction of the abdomen, licking or scratching of site of VHF application, or jumping. All of the groups were evaluated before cyclophosphamide injection, to establish the basal nociceptive threshold to mechanical stimuli. In order to determinate the inhibitory action of the antagonists on nociception, we calculated the AUC. The AUC values of the experimental groups were compared and the inhibition percentages were calculated.
Gross evaluation and wet weight determination of the bladder
The gross evaluation was based on criteria established by Gray et al. (1986). All bladders were dissected free from connecting tissues and transected at the bladder neck 24 h after cyclophosphamide injection. The wet weight of each bladder was recorded and expressed as mg per 100 g body weight. Each bladder was additionally examined macroscopically for oedema formation, which was categorized as (0) none, (1) mild, (2) moderate or (3) severe. Oedema was considered severe when fluid was seen externally and internally in the walls of the bladder. When the oedema was confined to the internal mucosa, it was reported as moderate; when it was between normal and moderate, the oedema was defined as mild. The bladders were also examined for bleeding in the walls and categorized as (0) normal, (1) telangiectasia or dilatation of the bladder vessels, (2) mucosal hematomas and (3) intravesical clots.
Histopathological analysis
Animals were killed and bladders were carefully removed 24 h after cyclophosphamide to assess the bladder histopathology. Tissue samples were fixed in a PBS solution containing 4% paraformaldehyde for 24 h at room temperature. Following fixation, tissue samples were embedded in paraffin, sectioned (5 μm slices) and stained with haematoxylin and eosin. Histopathological analyses were conducted based on the criteria established by Gray et al. (1986).
Neutrophil myeloperoxidase (MPO) assay
Neutrophil recruitment to the rat bladder was assessed indirectly by means of tissue MPO activity. The bladder tissues were removed 8 h after cyclophosphamide injection. Then, samples were homogenized at 5% (w/v) in EDTA/NaCl buffer (pH 4.7) and centrifuged at 10 621 x g for 15 min at 4°C. The pellet was re-suspended in 0.5% hexadecyltrimethyl ammonium bromide buffer (pH 5.4), and the samples were frozen in liquid nitrogen. Upon thawing, the samples were re-centrifuged, and 25 μL of the supernatant was used for MPO assay. The enzymic reaction was assessed with 1.6 mM tetramethylbenzidine, 80 mM NaPO4 and 0.3 mM hydrogen peroxide. The absorbance was measured with a spectrophotometer at 690 nm, and the results were expressed as OD per mg tissue.
Determination of cytokine concentrations
For determination of cytokine concentrations, whole bladders were removed 4 h (IL-1β) or 8 h (TNF-α) after cyclophosphamide injection and homogenized in phosphate buffer containing 0.05% Tween 20, 0.1 mmol·L−1 PMSF, 0.1 mmol·L−1 benzethonium chloride, 10 mmol·L−1 EDTA and 20 UI aprotinin A. The homogenate was centrifuged at 5000× g for 10 min, and the supernatants were stored at 70°C for further analysis. Levels of TNF-α and IL-1β were evaluated using elisa kits from R&D Systems (Minneapolis, MN, USA), according to the manufacturer's instructions. The amount of protein in each sample was measured using the Bradford method (Bradford, 1976).
Real-time quantitative PCR
Total RNA was extracted from bladder samples collected 1, 4, 8, 24 and 48 h after the administration of cyclophosphamide using TRizol® reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturers' protocol and its concentration was determined by NanoDrop™ 1100 (NanoDrop Technologies, Wilmington, DE, USA). A reverse transcription assay was performed as described in the M-MLV Reverse Transcriptase protocol according to the manufacturer's instructions. cDNA (300 ng) was amplified in triplicate using TaqMan Universal PCR Master Mix Kit with specific TaqMan Gene Expression target genes, the 3′ quencher MGB and FAM-labelled probes for rat CXCR2, TRPV1 and β-actin (which was used as an endogenous control for normalization). The PCRs were performed in a 96-well Optical Reaction Plate (Applied Biosystems, Foster City, CA, USA). The thermocycler parameters were as follows: 50°C for 2 min, 95°C for 10 min, 50 cycles of 95°C for 15 s and 60°C for 1 min. Expression of the target genes was calibrated against conditions found in control animals, that is, those that received i.p. vehicle (saline 0.9% NaCl).
Immunolabelling protocol
In another set of experiments, the animals were killed (24 h after cyclophosphamide treatment); the bladders removed and fixed in 4% paraformaldehyde for 15 min. Following embedding in Tissue-Tek® (Sakura Finetek, Tokyo, Japan), frozen slices of bladder (6 μm) were obtained using Cryostat (Leica Microsystems, Wetzlar, Germany). After three washes in PBS, the slides were incubated for 30 min with a blocking buffer of 1% BSA dissolved in PBS. Antibodies were diluted in blocking buffer. A solution of mixed primary antibodies was applied: monoclonal rabbit anti-CXCR2 (1:100) and polyclonal mouse anti-TRPV1 (1:200) following overnight incubation at 4°C. After washing, secondary antibodies were incubated in a mix solution. In order to target CXCR2, we used chicken anti-rabbit Alexa Fluor® 488 (green), and for TRPV1 immunolabelling, we used goat anti-mouse Alexa Fluor 568 (red) both at the concentration of 1:250. Images were obtained by using a Fluorescence Bx41 Model Microscopy (Olympus America Inc., Center Valley, PA, USA).
Cystometric parameters
The urodynamic studies were carried out 24 h after cyclophosphamide injection. A PE-60 polyethylene catheter (Clay Adams, Parsippany, NJ, USA) was inserted via a midline abdominal incision into the bladder through the bladder dome, under anaesthesia (i.p. urethane, 0.9–1.2 g·kg−1). The intravesical catheter was connected via a three-way stopcock to a pressure transducer (ADInstruments, Castle Hill, Australia) and to an infusion pump (Insight Scientific Equipments, São Paulo, Brazil) to record intravesical pressure and to infuse saline into the bladder respectively. Intravesical pressure was recorded continuously using data-acquisition software (PowerLab 8/30; ADInstruments). After catheter implantation, rats were left for 30 min for bladder stabilization. After this period, the animals received a continuous infusion of saline (0.9% NaCl; 37°C) at a rate of 0.1 mL·min−1. We assessed the micturition pressure (MP; maximum bladder pressure during micturition), basal pressure (BP; the lowest bladder pressure between micturitions), threshold pressure (TP; bladder pressure immediately before micturition) and the intercontraction interval (ICI). Non-voiding contractions (NVCs) were defined as rhythmic intravesical pressure increases greater than 5 mmHg from baseline pressure without release of saline from the urethra. Fluid voided from urethra was collected and the voided volume (VV) was measured. To determine residual volume (RV), saline infusion was stopped at the beginning of the voiding contraction and the catheter was disconnected from the system (pressure transducer and infusion pump). The animal was then positioned vertically and the RV was measured by withdrawing saline through the intravesical catheter and then manually expressing the remaining intravesical contents by exerting pressure on the bladder abdominal wall. The BC was calculated as VV plus RV. The voiding efficiency (VE) was estimated as a percentage using the following equation: VE = [(VV/BC) × 100]. The cystometric parameters were calculated from voiding cycles obtained over 45 min. When investigating the effects of antagonists, animals were treated with vehicle (saline), SB225002 (0.3 mg·kg−1), SB366791 (0.3 mg·kg−1) or the combination of the antagonists (0.3 mg·kg−1 each), 30 min before cyclophosphamide injection.
Data analysis
Results are shown as means ± SEM. Statistical analysis of the data was performed by two-way anova followed by Bonferroni's post hoc test or one-way anova followed by the Student–Newman–Keuls test. P-values less than 0.05 were considered significant.
Materials
Cyclophosphamide (Genuxal®) was purchased from Baxter Oncology GmbH (Frankfurt, Germany). SB225002 was synthesized as described by White et al., 1998 and was dissolved in 1% Tween 80 in 0.9% NaCl solution. SB366791 was purchased from Tocris Cookson Inc. (Ellisville, MO, USA) and a solution prepared in 2% absolute ethanol in 0.9% NaCl solution. Tween 80 and EDTA were purchased from Sigma Chemical Co. (St. Louis, MO, USA). IL-1β and TNF-α DuoSet kits were obtained from R&D Systems. The monoclonal mouse anti-VR1 and the polyclonal rabbit anti-CXCR2 were obtained from Abcam (Cambridge, MA, USA). The secondary antibodies – chicken anti-rabbit Alexa Fluor 488 and goat anti-mouse Alexa Fluor 568 – were obtained from Invitrogen Life Technologies (Grand Island, NY, USA).
Results
Cyclophosphamide-induced nociceptive behaviour
As shown in Figure 1, a single injection of cyclophosphamide (200 mg·kg−1, i.p.) induced marked behavioural changes in rats. These effects were observed as early as 1 h and lasted for up to 24 h. Pretreatment of animals with SB225002 (0.3 mg·kg−1, i.p.) or SB366791 (0.3 mg·kg−1, i.p.) inhibited the nociceptive responses induced by cyclophosphamide (Figure 1A and B) (20.9 ± 0.4 and 40.6 ± 0.4% respectively). Furthermore, the co-injection of SB225002 (0.3 mg·kg−1, i.p.) with SB366791 (0.3 mg·kg−1, i.p.) led to a marked decrease of the nociceptive behaviour induced by cyclophosphamide (80.9 ± 0.4%) (Figure 1A and B). Significant differences between single treatments with SB225002 or SB366791 and their combination were observed (Figure 1A and B: 75.8 ± 0.5 and 67.8 ± 0.7% respectively). SB225002 and SB366791 did not elicit any significant effects per se on locomotor activity, according to assessment in the open-field apparatus (data not shown).
Figure 1.
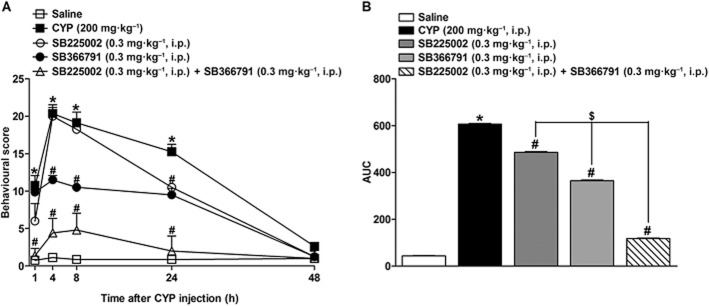
(A) Effect of pretreatment with SB225002 (0.3 mg·kg−1, i.p.), SB366791 (0.3 mg·kg−1, i.p.) or SB225002 co-injected with SB366791 (0.3 mg·kg−1, i.p., each) on behavioural score induced by cyclophosphamide (CYP; 200 mg·kg−1, i.p.) at different time points (1–48 h) after cyclophosphamide administration. (B) represents the area under the curves of panel (A). Each point on the curve represents the (mean + SEM) of six to eight animals. *P < 0.05, significantly different from control (saline) values; #P < 0.05, significantly different from cyclophosphamide values; $P < 0.05, significantly different from SB225002 or SB366791 values (two-way anova followed by Bonferroni's post hoc test).
Mechanical hypersensitivity
Previous studies demonstrate increased peripheral mechanical sensitivity after cyclophosphamide-induced cystitis (Guerios et al., 2008; Cheppudira et al., 2009). In our experiments, cyclophosphamide (200 mg·kg−1, i.p.) produced a prominent mechanical hypersensitivity in the rat paw (Figure 2A and B) and abdominal area (Figure 2C and D), an effect that lasted for up to 24 h, in response to 8.0 and 26.0 g VFH stimulation respectively. Treatment with SB225002 (0.3 mg·kg−1, i.p.) reduced the mechanical hypersensitivity either in the paw (50.0 ± 2.0%) or the abdominal area (44.0 ± 1.0%). Treatment with SB366791 (0.3 mg·kg−1, i.p.) also inhibited the mechanical hypersensitivity, in both the paw (45 ± = 1%), and the abdominal area (36 ± 2%). Co-treatment with both antagonists produced a significant decrease in paw and abdomen responses (62.0 ± 1.0 and 67.0 ± 2.0% respectively).
Figure 2.
Effect of pretreatment with SB225002 (0.3 mg·kg−1, i.p.), SB366791 (0.3 mg·kg−1, i.p.) or SB225002 co-injected with SB366791 (0.3 mg·kg−1, i.p., each) on the mechanical hypersensitivity induced by cyclophosphamide (CYP; 200 mg·kg−1, i.p.) assessed in the paw (A) or abdomen (C) at different time points (1–48 h) after cyclophosphamide administration. (B, D) represent the area under the curves of panel (A) and (C) respectively. Data are shown as mean percentage of response frequency. Each column represents the mean + SEM of six to eight animals. *P < 0.05, significantly different from control (saline) values; #P < 0.05, significantly different from cyclophosphamide values; $P < 0.05, significantly different from SB225002 or SB366791 values (two-way anova followed by Bonferroni's post hoc test).
Gross evaluation
The macroscopic damage score of oedema and haemorrhage in the cyclophosphamide group was significantly higher (scores 2.7 ± 0.2 and 2.7± 0.2, respectively) than that in the control group (score 0) (Figure 3A). Treatment with SB225002 or SB366791, given singly, did not show any significant inhibition of oedema and haemorrhage scores but the combination of antagonists significantly reduced oedema (45.4 ± 10.5%) and haemorrhage (54.4 ± 9.1%) induced by cyclophosphamide (Figure 3A). Furthermore, significant differences were also observed between the antagonists and the combination of both, in the gross evaluation (40.0 ± 12.0 and 44.4 ± 11.0% respectively). Representative bladder images are shown in panel (B).
Figure 3.
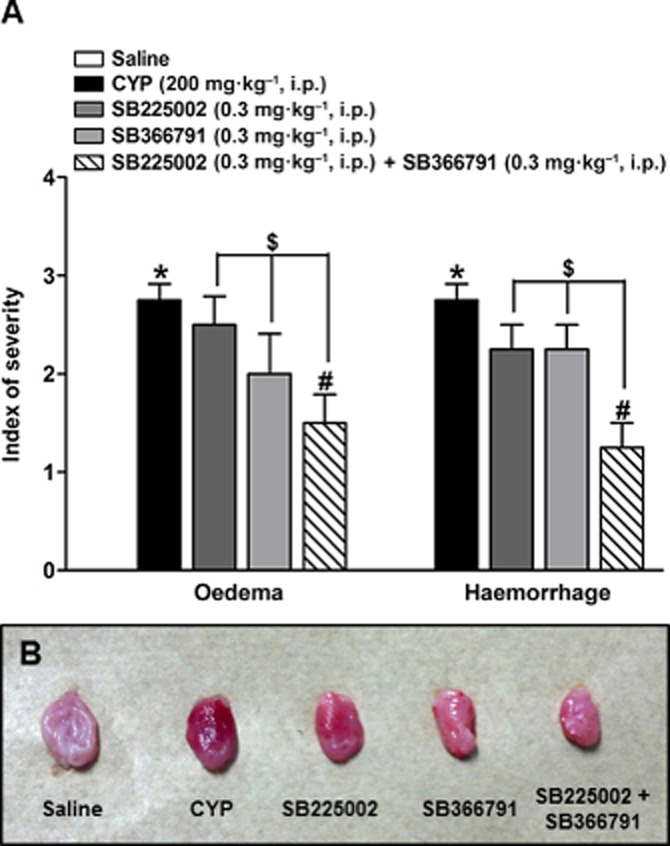
(A) Effect of pretreatment with SB225002 (0.3 mg·kg−1, i.p.), SB366791 (0.3 mg·kg−1, i.p.) or SB225002 co-injected with SB366791 (0.3 mg·kg−1, i.p., each) on macroscopic haemorrhage and oedema evaluation in cystitis induced by cyclophosphamide (CYP; 200 mg·kg−1, i.p., 24 h). (B) Bladder representative images of gross evaluation. Each column represents the mean + SEM of six to eight animals *P < 0.05, significantly different from control (saline) values; #P < 0.05, significantly different from cyclophosphamide values $P < 0.05, significantly different from SB225002 or SB366791 values (one-way anova followed by Bonferroni's post hoc test).
Analysis of microscopic damage
To detect pathological changes resulting from cyclophosphamide-induced cystitis, we conducted a qualitative histological analysis of the urinary bladder (Figure 4). In accordance with the histopathological criteria described by Gray (1986), microscopical analysis of the bladder of the animals treated with cyclophosphamide (24 h) (Figure 4B) when compared with control animals (Figure 4A) revealed extensive cystitis characterized by acute inflammation with vascular congestion, pronounced oedema, severe haemorrhage, infiltration of neutrophils and reduction in the number of layers of urothelial cells. These changes were attenuated by pretreatment of animals with the CXCR2 (SB225002, 0.3 mg·kg−1, i.p.) (Figure 4C) and TRPV1 channel (SB366791, 0.3 mg·kg−1, i.p.) antagonists (Figure 4D) and their combination (Figure 4E). The microscopic damage score is shown in panel (F).
Figure 4.
Effect of pre-treatment with SB225002 (0.3 mg·kg−1, i.p.), SB366791 (0.3 mg·kg−1, i.p.), or SB225002 co-injected with SB366791 (0.3 mg·kg−1, i.p., each) on changes in bladder histopathology 24 h after cyclophosphamide (CYP; 200 mg·kg−1, i.p.) administration. Bladder representative images of control (saline) (A), cyclophosphamide group (B), SB225002 (C), SB366791 (D) and SB225002 + SB366791 (E). Cyclophosphamide-induced cystitis indicate urothelial damage (black arrowhead), leukocyte infiltration (arrow, higher magnification), blood vessel congestion and dilation (open arrow), and oedema (asterisk), (haematoxylin and eosin staining, scale bar 100 μm). Gray's macroscopic score of bladder histopathological alterations (F). Each column represents the mean + SEM of six to eight animals. *P < 0.05, significantly different from control (saline) values; #P < 0.05, significantly different from cyclophosphamide values; $P < 0.05, significantly different from SB225002 or SB366791 values (one-way anova followed by Bonferroni's post hoc test).
Bladder wet weight
Bladder wet weight was used as an additional indicator of bladder oedema. Cyclophosphamide-treated animals showed thickened bladder walls with an obviously decreased lumen capacity. As demonstrated in Figure 5A, mean bladder wet weight of the cyclophosphamide group was significantly greater than that of the control group (4.3-fold increase). This increase in bladder wet weight in cyclophosphamide-treated rats was significantly inhibited by SB225002 (0.3 mg·kg−1, i.p.) and SB366791 (0.3 mg·kg−1, i.p.) (27.2 ± 2.4 and 32.6 ± 3.3% respectively). Likewise, the co-administration of the antagonists decreased the bladder wet weight in cyclophosphamide-treated rats in a significant manner (47.7 ± 1.4%) and it was significantly different from the effects of SB225002 or SB366791 injected singly (25.4 ± 6.2 and 22.7 ± 8.0% respectively).
Figure 5.
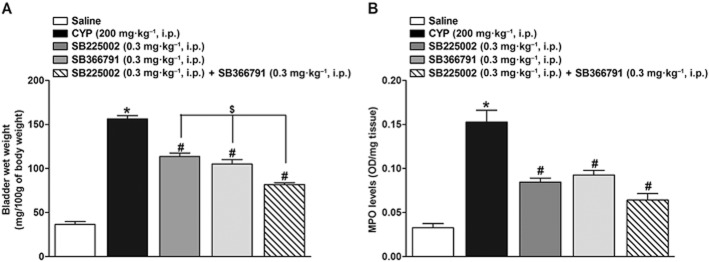
Effect of pretreatment with SB225002 (0.3 mg·kg−1, i.p.), SB366791 (0.3 mg·kg−1, i.p.) or SB225002 co-injected with SB366791 (0.3 mg·kg−1, i.p., each) on bladder wet weight, 24 h (A) or MPO activity, 8 h (B) in cystitis induced by cyclophosphamide (CYP; 200 mg·kg−1, i.p.). Each column represents the mean + SEM of six to eight animals *P < 0.05, significantly different from control (saline) values; #P < 0.05, significantly different from cyclophosphamide values; $P < 0.05, significantly different from SB225002 or SB366791 values (one-way anova followed by Bonferroni's post hoc test).
MPO assay
Cystitis induced by cyclophosphamide was also characterized by a significant increase in MPO activity in the bladder, compared with that in the control group (5.3-fold increase; Figure 5B). As previously demonstrated by Santos et al. (2010), the increase in MPO activity peaked 8 h following cyclophosphamide administration (data not shown). Therefore, the 8 h time point was adopted to evaluate the effect of CXCR2 and TRPV1 channel blockade on MPO levels. Treatment with SB225002 (0.3 mg·kg−1, i.p.), SB366791 (0.3 mg·kg−1, i.p.) or the co-administration of the antagonists resulted in a marked inhibition of MPO activity in the bladder (39.4 ± 4.5, 31.8 ± 6.8 and 47.0 ± 9.0%, respectively) in comparison to the cyclophosphamide group. No significant differences were observed between the antagonists and their combination (Figure 5B).
Cytokine levels
TNF-α and IL-1β are crucial mediators involved in the inflammatory events occurring in cyclophosphamide-induced haemorrhagic cystitis (Malley and Vizzard, 2002). Earlier, we carried out a time course of the effect of cyclophosphamide on IL-1β and TNF-α levels and found that the levels of IL-1β and TNF-α in the bladder were increased 4 and 8 h after cyclophosphamide injection respectively (data not shown). Therefore, these time points were adopted to evaluate the effect of CXCR2 and TRPV1 channel blockade on cytokine levels. As shown in Figure 6A and B, there was a significant increase in the levels of IL-1β and TNF-α in the bladder, after cyclophosphamide treatment and this increase was significantly reduced by treatment with SB225002 (0.3 mg·kg−1, i.p.) (48.2 ± 3.2 and 44.2 ± 2.6% respectively). Likewise, treatment with SB366791 significantly inhibited IL-1β and TNF-α production (40.7 ± 6.3 and 40 ± 4.6% respectively). Combination of the antagonists was also effective in reducing cytokine levels, by 57.0 ± 2.3 and 60.6 ± 3.2% for IL-1β and TNF-α respectively.
Figure 6.

Effect of pretreatment with SB225002 (0.3 mg·kg−1, i.p.), SB366791 (0.3 mg·kg−1, i.p.) or SB225002 co-injected with SB366791 (0.3 mg·kg−1, i.p., each) on IL-1β (4 h after CYP, A) or TNF-α (8 h after CYP, B) bladder levels in cystitis induced by cyclophosphamide (CYP; 200 mg·kg−1, i.p.). Each column represents the mean + SEM of six to eight animals. *P < 0.05, significantly different from control (saline) values; #P < 0.05, significantly different from cyclophosphamide values (one-way anova followed by Bonferroni's post hoc test).
Expression of CXCR2 and TRPV1 channels in the rat bladder with cyclophosphamide-induced cystitis
Figure 7A shows the CXCR2 expression in the rat whole bladder. Cyclophosphamide produced a marked and time-related increase of CXCR2 receptor mRNA expression in the rat bladder, which was evident as early as 1 h (5.5-fold increase) and peaked at 24 h (13.2-fold increase) after cyclophosphamide administration. Therefore, to assess the CXCR2 receptor expression in response to the antagonists, we adopted the 24 h time point (Figure 7B). Treatment with SB225002 (0.3 mg·kg−1, i.p.), but not with SB366791, produced a significant reduction (41.7 ± 5.6%) in CXCR2 mRNA expression in the bladder of animals with cystitis induced by cyclophosphamide. Interestingly, the combination of the antagonists as well as SB225002 alone significantly reduced CXCR2 mRNA expression in the bladder, compared with the cyclophosphamide group (76.0 ±2.8 and 58.4 ± 9.5%, respectively) (Figure 7A and B).
Figure 7.
Time-dependent changes in CXCR2 (A) and TRPV1 channel (C) mRNA expression in whole bladder following cyclophosphamide (CYP; 200 mg·kg−1, i.p.) administration and effect of the pre-treatment with SB225002 (0.3 mg·kg−1, i.p.), SB366791 (0.3 mg·kg−1, i.p.), or SB225002 co-injected with SB366791 (0.3 mg·kg−1, i.p., each) on CXCR2 (B) and TRPV1 (D) mRNA expression. Each column represents the mean + SEM of six animals. *P < 0.05, significantly different from control (saline) values; #P < 0.05, significantly different from cyclophosphamide values;$P < 0.05, significantly different fromSB225002 and SB366791 values (one-way anova followed by Bonferroni's post hoc test).
Similarly, cyclophosphamide significantly increased expression of mRNA for TRPV1 channels in the bladder (Figure 7C). This increase was evident 1 and 24 h (5.6-fold increase) after cyclophosphamide. Thus, to assess the receptor expression in response to antagonists, we choose the time point of 24 h as there was no significant difference between 1 and 24 h time points (Figure 7C). Pretreatment with the CXCR2 antagonist, SB225002 (0.3 mg·kg−1, i.p.) or with the TRPV1 antagonist, SB366791 (0.3 mg·kg−1, i.p.), resulted in a significant decrease in the expression of TRPV1 channel mRNA in the bladder of these animals (49.9 ± 3.8 and 74.4 ± 3.9% respectively). The combination of both antagonists also significantly reduced (92.1 ± 3.2%) the mRNA for TRPV1 channels in the bladder, in comparison to the cyclophosphamide and SB225002 group, but not compared with animals treated with SB366791 alone (Figure 7C and D).
CXCR2 and TRPV1 localization in the bladder and effect of treatment with cyclophosphamide
We next examined the localization of CXCR2 and TRPV1 channels in the bladder using dual immunofluorescence. Figure 8 shows representative bladder sections from each group immunostained for CXCR2 (green), TRPV1 channels (red) and an overlay of those panels (co-localization indicated by orange colour). In saline-treated rats (control group), both CXCR2 and TRPV1 channels could be poorly localized in the nerve fibres innervating the detrusor muscle, lamina propria region and superficial cells of the urothelium (Figure 8A and B respectively). After cyclophosphamide treatment, CXCR2 and TRPV1 channels were readily localized throughout the urothelium, vascular epithelium and nerve fibres innervating detrusor muscle. CXCR2 immunostaining seemed to be more pronounced in superficial cells of urothelium, whereas the immunostaining for TRPV1 channels was on basal cells of urothelium and lamina propria region (Figure 8D and E). We examined the co-localization of CXCR2 and TRPV1 channels in the bladder using dual immunofluorescence (Figure 8C and F). Interestingly, CXCR2 and TRPV1 channels were co-localized in the nerve fibres innervating detrusor muscle and vascular epithelium after cyclophosphamide treatment (Figure 8F).
Figure 8.
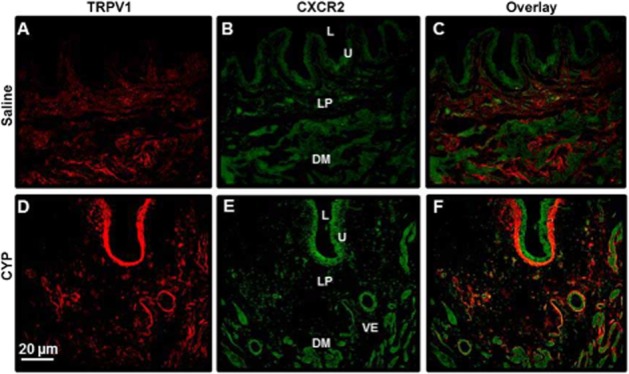
Bladder localization of TRPV1 channels and CXCR2 receptors. Representative sections from rats treated with saline (control) or cyclophosphamide (200 mg·kg−1, i.p.) are shown (scale bar = 20 μm). The figure shows TRPV1 channel (A, D) immunostaining (red immunofluorescence), CXCR2 (B, E) immunostaining (green immunofluorescence) and an overlay panel (C, F) combining both immunostaining (orange). L: lumen; U: urothelium; LP: lamina propria; DM: detrusor muscle; VE: vascular epithelium.
Cystometric parameters
We next investigated the bladder activity by measuring the intravesical pressure and volume. The cyclophosphamide-treated group showed a significant increase in BP (124.5 ± 5.5%), TP (123.8 ± 4.3%) and maximum pressure (MP: 118.3 ± 3.3%) (Figure 9A and B, Table 2009). Additionally, cyclophosphamide-treated animals displayed marked functional alterations, such as reduction in BC (50 ± 2%) (Figure 9B, Table 2009), ICI (73.4 ± 2.4%) (Figure 9B, Table 2009), VV (73.6 ± 3.1%) and VE (90.4 ± 2.0%) (Figure 9, Table 2009). During the bladder filling phase, cyclophosphamide-treated animals showed more NVCs than control animals (15.3 ± 0.5 vs. 3.6 ± 0.5) (Table 2009). Treatment with SB225002, but not with SB366791 (Figure 9C and D, respectively), significantly reduced the BP (36.1 ± 5.5%) and TP (33.7 ± 7.8%). Treatment with SB225002 and SB366791 administered alone significantly reduced MP (31.7 ± 1.5 and 29.8 ± 2.3%) (Figure 9C and D, Table 2009) and the number of NVC (32.6 ± 2.7 and 28.2 ± 2.4%) (Table 2009). The same treatment, increased BC (52 ± 4 and 42 ± 6%), VV (102.2 ± 13 and 97.7 ± 15.4%), VE (388.3 ± 66 and 341.8 ± 66%). The co-injection of the antagonists reduced the number of NVCs (60.9 ± 2.4%) (Table 2009), BP (60.7 ± 2.7%), TP (61.1 ± 1.2) and MP (53.2 ± 1.2%), and increased significantly ICI (213.7 ± 10.3%), BC (76 ± 6%), VV (194.4 ± 7.8%) and VE (644.2 ± 29.3%) (Figure 9E, Table 2009).
Figure 9.

Representative cystometrogram recordings of control group (A), cyclophosphamide (CYP; 200 mg·kg−1, i.p.) (B), SB225002 (0.3 mg·kg−1, i.p.) (C), SB366791 (0.3 mg·kg−1, i.p.) (D) and SB225002 co-injected with SB366791 (0.3 mg·kg−1, i.p., each) (E) using continuous intravesical infusion of saline. Intercontraction interval:dotted arrow; basal pressure: asterisk; threshold pressure: open arrow; maximum voiding pressure: arrowhead.
Table 1.
Changes in urodynamic parameters induced by cyclophosphamide (CYP; 200 mg·kg−1, i.p.) and effect of the pretreatment with SB225002 (0.3 mg·kg−1, i.p.), SB366791 (0.3 mg·kg−1, i.p.) or SB225002 co-injected with SB366791 (0.3 mg·kg−1, i.p., each)
| Saline | CYP | SB225002 | SB366791 | SB225002 + SB366791 | |
|---|---|---|---|---|---|
| Number of NVCs | 3.6 ± 0.5 | 15.3 ± 0.5*2010 | 10.3 ± 0.4# | 11 ± 0.3# | 6 ± 0.3$ |
| Basal pressure (BP; mmHg) | 8.2 ± 0.3 | 18.5 ± 0.4*2010 | 11.8 ± 1.0# | 16.1 ± 1.1# | 7.2 ± 0.5$ |
| Threshold pressure (TP; mmHg) | 8.5 ± 0.9 | 19.1 ± 0.3*2010 | 12.7 ± 1.5# | 15.4 ± 1.1 | 7.4 ± 0.3$ |
| Maximum voiding pressure (MP; mmHg) | 11.3 ± 0.5 | 24.6 ± 0.3*2010 | 18.7 ± 0.4# | 17.3 ± 0.5# | 11.5 ± 0.3$ |
| Intercontraction interval (ICI; min) | 3.8 ± 0.1 | 1.2 ± 0.1*2010 | 2.3 ± 0.2# | 2.4 ± 0.2# | 3.3 ± 0.2$ |
| Voided volume (VV; mL) | 0.14 ± 0.01 | 0.037 ± 0.004*2010 | 0.074 ± 0.005# | 0.072 ± 0.006# | 0.11 ± 0.003$ |
| Bladder capacity (BC; mL) | 1.0s1 ± 0.06 | 0.5 ± 0.02*2010 | 0.76 ± 0.02# | 0.71 ± 0.03# | 0.88 ± 0.03$ |
| Voiding efficiency (%) | 75 ± 1.8 | 7.2 ± 1.5*2010 | 35 ± 4.8# | 31.6 ± 4.7# | 53.3 ± 2.1$ |
Values represent the mean ± SEM of six animals
P < 0.05, significantly different from control (saline) values.
P < 0.05, significantly different from cyclophosphamide values.
P < 0.05, significantly different from SB225002 or SB366791 values; one-way anova followed by Bonferroni's post hoc test.
NVCs, non-voiding contractions.
Discussion
CYP is an anti-tumour agent that is metabolized in the liver to the urotoxic metabolite acrolein. The most important side effect of cyclophosphamide is haemorrhagic cystitis that is characterized by urothelial damage, oedema, haemorrhage, ulceration, neovascularization, leukocyte infiltration and pain (Assreuy et al., 1999; Korkmaz et al., 2007).
As demonstrated previously, cyclophosphamide induced marked alterations in the pain-like behaviour of rats (Boucher et al., 2000). These modifications included closing of the eyes, decreasing breath rate and specific postures as rounded-back and limpness that lasted for up 24 h period. Previous studies have also shown that CXCR2 antagonist, SB225002, and the TRPV1 channel antagonist, SB366791, produced antinociceptive effects in different models of spontaneous pain (Marotta et al., 2009; Niiyama et al., 2009; Manjavachi et al., 2010). In this context, we evaluated the effects of these antagonists on nociceptive behaviour elicited by cyclophosphamide. Although visceral pain is difficult to evaluate directly, antagonists of CXCR2 and of the TRPV1 channel and their combination, significantly inhibited cyclophosphamide-induced pain-like behaviour. Notably, the doses of the CXCR2 (0.3 mg·kg−1, i.p.) and TRPV1 (0.3 mg·kg−1, i.p.) antagonists used in the present study did not significantly change the locomotor activity of the animals when assessed in the open-field test, indicating that the observed antinociception caused by the antagonists seems to be not a consequence of motor abnormality.
Visceral pain is one of the main symptoms of haemorrhagic cystitis and is a limiting factor for the quality of life of patients receiving chemotherapy with cyclophosphamide (Morais et al., 1999). Bladder mechanical sensitivity in cystitis is considered an indicator of visceral pain (Cervero and Laird, 2004). One important feature of visceral sensitivity is the referred pain that is the perception of pain in sites different from the area of actual visceral injury (Wang et al., 2008). Increased mechanical sensitivity in the hindpaw and abdomen area following cyclophosphamide treatment has been previously demonstrated (Guerios et al., 2008; Studeny et al., 2008; Cheppudira et al., 2009). In this context, we have analysed here the mechanical sensitivity responses in abdomen area and in the rat hindpaw, in cyclophosphamide-injected rats. The results of these experiments showed that haemorrhagic cystitis is related to a prolonged peripheral nociceptive sensitivity to mechanical stimuli, an effect that persisted for up to 24 h after cyclophosphamide injection. In agreement with previous findings (Studeny et al., 2008), we also demonstrated a return to baseline sensitivity in the hindpaw and abdomen 48 h after cyclophosphamide. Similar to the results observed in behavioural scores, the nociceptive effect was significantly attenuated by pretreatment with CXCR2 and TRPV1 channel antagonists, as well as by their combination. The contribution of cytokines, chemokines and the TRPV1 channels to visceral pain is well known (Meyer-Siegler et al., 2004; Daly et al., 2007; Wang et al., 2008; Foster et al., 2011; Frias et al., 2012; Lei and Malykhina, 2012). In this regard, TRPV1 channels participate in the development of visceral pain in the presence of bladder inflammation, as reflected by increased referred mechanical sensitivity in peripheral tissues (Wang et al., 2008). Therefore, changes in bladder sensitivity detected in cyclophosphamide-induced cystitis might be mediated, at least in part, by an increase in expression of chemokine receptors and TRPV1 channels and cytokine production in the bladder. In fact, we have observed a significant increase in TNF-α and IL-1 β levels and also of CXCR2 and TRPV1 channels mRNA following cyclophosphamide administration. Notably, the combination of both antagonists was able to significantly reduce the up-regulation of CXCR2 and TRPV1 channels mRNA, as well as the levels of TNF-α and IL-1 β in the bladder, an event that seems to correlate with attenuation of the nociceptive process.
It is now well established that cyclophosphamide-induced cystitis is characterized by marked bladder oedema and haemorrhage and urothelial damage (Korkmaz et al., 2007). There is good evidence that cytokines, such as TNF-α and IL-1β, are critical mediators of the inflammatory process in cyclophosphamide-induced cystitis (Hu et al., 2003; Kiuchi et al., 2009; Smaldone et al., 2009). Our data showed increased TNF-α and IL-1β in the bladder, agreeing with earlier reports of Malley and Vizzard (2002), who found a rapid and significant increase in cytokines expression such as IL-1β, IL-2, IL-4, IL-6 and TNF-α in the bladder after cyclophosphamide administration. In the present study, we observed marked bladder inflammation after cyclophosphamide, characterized by increased bladder wet weight, presence of vascular congestion, pronounced oedema, severe haemorrhage, fibrin deposition, infiltration of neutrophils and reduction in the number of layers urothelial cells. Systemic treatment with the CXCR2 and TRPV1 antagonists alone or in combination significantly attenuated this bladder inflammation induced by cyclophosphamide treatment.
Because we noted the establishment of pronounced bladder inflammation, we evaluated the MPO activity in this tissue as an indirect measure of neutrophil migration. As previously reported (Linares-Fernández and Alfieri, 2007), cyclophosphamide administration significantly enhanced the MPO activity in the bladder, which correlates well with increased leukocyte migration to the bladder. Our data showed that MPO activity induced by cyclophosphamide was significantly inhibited by pretreatment of rats with the selective antagonists of CXCR2 and TRPV1 channels, alone or in combination. These findings confirm and also extended previous studies, which showed that CXCR2 blockade with SB225002 reduced neutrophil influx and MPO activity in the colon of mice with colitis induced by 2,4,6-trinitrobenzene sulfonic acid (TNBS). Moreover, TRPV1 channel antagonism with capsazepine reduced pancreatic MPO activity of mice with cerulein-induced pancreatitis (Nathan et al., 2001) and the lack of TRPV1 channels was reported to reduce the neutrophil infiltration in the colon of mice with dextran sulfate sodium-induced colitis (Bento et al., 2008). However, stimulation of TRPV1 channels during an inflammatory process may constitute a protective mechanism. In this regard, gene ablation or blockade of TRPV1 channels exacerbated LPS-induced renal inflammation including aggravated renal neutrophil/macrophage infiltration and increased chemokine/cytokine levels (Wang and Wang, 2013). In addition, neutrophils and macrophage infiltration and MPO activity were significantly greater in TRPV1 channel knockout mice with LPS-induced airways inflammation (Helyes et al., 2007). Therefore, activation of TRPV1 channels during an inflammatory process may constitute a protective or a damaging event, depending on the inflammatory model investigated.
Receptors for chemotactic cytokines, as CXCR4 and CX3CR1, as well as TRPV1 channels are found up-regulated following cyclophosphamide treatment (Yuridullah et al., 2006; Vera et al., 2008; Arms et al., 2010). Whereas up-regulation of TRPV1 channels is well described in cyclophosphamide-induced cystitis (Dattilio and Vizzard, 2013; Dang et al., 2013), alterations in CXCR2 expression have not yet been reported in the cystitis. Therefore, CXCR2 mRNA levels were determined in the bladder after cyclophosphamide injection, using real-time PCR assay. Our results demonstrated that CXCR2 mRNA was greatly up-regulated after cyclophosphamide, evident as early 1 h, reaching its peak at 24 h and decreasing by 48 h. In contrast to that observed with CXCR2 mRNA, the mRNA for TRPV1 channels was increased only at 1 and 24 h time points, decreasing 48 h after cyclophosphamide administration. Interestingly, blockade of CXCR2 with a selective antagonist significantly reduced the expression of CXCR2 mRNA in the bladder. Moreover, treatment of rats with the combination of CXCR2 and TRPV1 channel antagonists, but not with TRPV1 channel antagonist alone, significantly attenuated CXCR2 mRNA expression following cyclophosphamide administration. Therefore, CXCR2 inhibition seems to interfere in post-transcriptional regulation of CXCR2 gene expression and this effect seems to involve TRPV1 channels. It is possible that the inhibition of CXCR2 might reduce the expression of CXCR2 mRNA via modulation of downstream pathways after receptor activation, which results in the increased expression of different inflammatory components, including the receptor itself. Indeed, Qu et al. (2009) reported that CXCR2 mRNA was significantly reduced following SB225002 treatment in a model of choroidal neovascularization. Furthermore, Brait et al. (2011) demonstrated that the administration of SB225002 markedly reduces CXCR2 mRNA expression as well as neutrophil infiltration in the brain following cerebral ischaemia–reperfusion. Of great interest was the finding that the blockade of TRPV1 channels with CXCR2 and TRPV1 antagonists as well as their combination was able to reduce expression of mRNA for TRPV1 channels in the bladder. Therefore, we reported here, for the first time, the regulation of TRPV1 channels by selective TRPV1 and CXCR2 antagonists. However, additional studies are still necessary to clarify the mechanisms involved in this process.
TRPV1 channels are localized and function in different bladder structures. Thus the TRPV1 channels are expressed in urothelial cells (Birder et al., 2001; Charrua et al., 2009; Kullmann et al., 2009) and in nerve fibres (Avelino et al., 2002) of mice, rats and humans. Nevertheless, the expression of these channels in urothelial cells remains controversial (Everaerts et al., 2010). Interestingly, chemokine receptors up-regulate in the urothelium after bladder inflammation (Yuridullah et al., 2006; Vera et al., 2008; Arms et al., 2010), but the pattern of expression of CXCR2 in different structures of the bladder has not been described. In our study, TRPV1 channels and CXCR2 receptors were localized in the urothelium, lamina propria region and nerve fibres innervating detrusor muscle of saline-treated rats. After cyclophosphamide treatment, CXCR2 and TRPV1 channels are markedly localized throughout the urothelium, vascular epithelium and nerve fibres innervating detrusor muscle. CXCR2 immunostaining seems to be more pronounced in superficial cells of urothelium, whereas the TRPV1 channel immunostaining is mainly localized on basal cells of urothelium and lamina propria region. Interestingly, CXCR2 and TRPV1 channels were co-localized in nerve fibres innervating the detrusor muscle and vascular epithelium after cyclophosphamide treatment. Thus, our data show that both receptors are expressed in different levels in bladder and are up-regulated in the same structures after cystitis. These findings could imply interactions between these receptors in bladder function. In this context, it has been reported that urothelial cells can express a number of receptors and ion channels similar to those found in sensory neurons, and that urothelium could be a target for transmitters released from bladder nerves or that chemical mediators released by urothelial cells may alter afferent excitability (Birder et al., 2001; Birder, 2005). Therefore, up-regulation and interplay between CXCR2 and TRPV1 channels could be important in the development of pain and inflammatory symptoms reported in cystitis in the present and previous studies.
Next, we hypothesized that nociception detected in cyclophosphamide-induced cystitis might be associated with changes in the sensory physiology of the bladder. Thus, we assessed the participation of the receptors as well as their interaction in bladder function through urodynamic tests (cystometry). Confirming and extending previous studies (Hu et al., 2003; Klinger and Vizzard, 2008), we found that rats with cyclophosphamide-induced cystitis presented important changes in voiding behaviour evaluated as alterations in cystometric parameters, such as increased voiding frequency and number of NVCs, decreased VV and also altered bladder pressures. Evidence now suggests that TRPV1 channels are expressed in C-fibres that innervate the bladder and could contribute to the development of bladder overactivity acting as a sensor of bladder distention and chemical irritation (Everaerts et al., 2008). Block of TRPV1 channels abolished bladder overactivity after cyclophosphamide-induced cystitis (Charrua et al., 2009). Additionally, earlier studies have shown that the blockade of CXCR4 with a selective antagonist reduced the bladder overactivity induced by cyclophosphamide, as demonstrated by increased VV, BC and ICI (Arms et al., 2010). Corroborating and also extending these previous data, we also observed that the SB225002 and SB366791 alone or their combination greatly reduced bladder overactivity induced by cyclophosphamide. These effects could be observed as increased BC, ICI, VE, VE and diminished bladder pressures. Other important functional alteration observed in bladder overactivity induced by cyclophosphamide was the increased number of NVCs (Studeny et al., 2008). The NVCs are represented by increased spontaneous activity of the detrusor smooth muscle cells during the filling phase. Our results demonstrate that the separate blockade of CXCR2 and TRPV1 channels, as well as their combination, reduced the number of NVCs. Therefore, both receptors seem to contribute to the emergence of NVCs and consequently to the establishment of bladder overactivity. Again, these data suggest that the establishment of bladder hyperexcitability could be caused, at least in part, by the up-regulation and interplay between CXCR2 and TRPV1 channels.
Notably, the proposed relationship between these receptors in the physiopathology of cyclophosphamide-induced cystitis is evident in most of our findings. The combination of the antagonists showed either a synergistic interaction, in which the effect of the antagonist combination is greater than the sum of their separate effect at same doses such as observed in behavioural scores, histological and TRPV1 mRNA analysis or an additive interaction, in which this effect was equal to the sum of effects of the drugs taken separately, noted in other results. However, the dual action of the combination of the antagonists in different inflammatory and nociception parameters does not allow us to define adequately the relationship between CXCR2 and TRPV1 channels in cyclophosphamide-induced cystitis.
Another interesting aspect of the present study was the demonstration that even 24 h after administration of the CXCR2 and TRPV1 channel antagonists, effects of these compounds on cyclophosphamide-induced bladder inflammation and pain were observed. Our findings agree with earlier studies from our group that have demonstrated the long-term effect of CXCR2 and TRPV1 channel antagonists in different inflammatory and pain animal models (Marotta et al., 2009; Manjavachi et al., 2010). Marotta et al. (2009) showed that pretreatment with SB225002 or SB366791 prevented PAF-induced nociception and inflammation, an effect that lasted for up to 8 h. Moreover, treatment of mice with SB225002 produced antinociceptive and anti-inflammatory effects that lasted for up to 6 h, in the carrageenan and for up to 24 h in the CFA model of pain and inflammation (Manjavachi et al. (2010). Therefore, the long-lasting effect of SB225002 and SB366791 suggests that these antagonists might have a long biological half-life. Pharmacokinetic studies will be necessary to confirm this possibility.
In summary, the current study suggested that nociceptive behaviour, bladder inflammation and overactivity in cyclophosphamide-induced cystitis depended on CXCR2 and TRPV1 up-regulation and activation, as well as interactions between these receptors. Our work indicates that the blockade of both receptors could be an interesting therapeutic option for the clinical treatment of cystitis.
Acknowledgments
This study was supported by Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (Brazil). F. N. D. received a fellowship from CAPES; E. L. A. received a fellowship post-doctoral from CNPq.
Glossary
- BC
bladder capacity
- BP
basal pressure
- ICI
intercontraction interval
- MP
micturition pressure
- MPO
myeloperoxidase
- NVCs
non-voiding contractions
- RV
residual volume
- TP
threshold pressure
- VE
voiding efficiency
- VV
voided volume
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Abraham P, Rabi S. Protein nitration, PARP activation and NAD+ depletion may play a critical role in the pathogenesis of cyclophosphamide-induced hemorrhagic cystitis in the rat. Cancer Chemother Pharmacol. 2009;64:279–285. doi: 10.1007/s00280-008-0868-6. [DOI] [PubMed] [Google Scholar]
- 2.Alexander SPH, et al. The Concise Guide to PHARMACOLOGY 2013/14: Overview. Br J Pharmacol. 2013;170:1449–1867. doi: 10.1111/bph.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arms L, Girard BM, Vizzard MA. Expression and function of CXCL12/CXCR4 in rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol. 2010;298:589–600. doi: 10.1152/ajprenal.00628.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assreuy AM, Martins GJ, Moreira ME, Brito GA, Cavada BS, Ribeiro RA, et al. Prevention of cyclophosphamide-induced hemorrhagic cystitis by glucose-mannose binding plant lectins. J Urol. 1999;161:1988–1993. [PubMed] [Google Scholar]
- 5.Avelino A, Cruz C, Nagy I, Cruz F. Vanilloid receptor 1 expression in the rat urinary tract. Neuroscience. 2002;109:787–798. doi: 10.1016/s0306-4522(01)00496-1. [DOI] [PubMed] [Google Scholar]
- 6.Barsante MM, Cunha TM, Allegretti M, Cattani F, Policani F, Bizzarri C, et al. Blockade of the chemokine receptor CXCR2 ameliorates adjuvant-induced arthritis in rats. Br J Pharmacol. 2008;153:992–1002. doi: 10.1038/sj.bjp.0707462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bento AF, Leite DF, Claudino RF, Hara DB, Leal PC, Calixto JB. The selective nonpeptide CXCR2 antagonist SB225002 ameliorates acute experimental colitis in mice. J Leukoc Biol. 2008;84:121312–121321. doi: 10.1189/jlb.0408231. [DOI] [PubMed] [Google Scholar]
- 8.Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, et al. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci U S A. 2001;98:13396–13401. doi: 10.1073/pnas.231243698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boisvert WA, Curtiss LK, Terkeltaub RA. Interleukin-8 and its receptor CXCR2 in atherosclerosis. Immunol Res. 2000;21:129–137. doi: 10.1385/ir:21:2-3:129. [DOI] [PubMed] [Google Scholar]
- 10.Boucher M, Meen M, Codron JP, Coudore F, Kemeny JL, Eschalier A. Cyclophosphamide-induced cystitis in freely-moving conscious rats: behavioral approach to a new model of visceral pain. J Urol. 2000;164:203–208. [PubMed] [Google Scholar]
- 11.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 12.Brait VH, Rivera J, Broughton BR, Lee S, Drummond GR, Sobey CG. Chemokine-related gene expression in the brain following ischemic stroke: no role for CXCR2 in outcome. Brain Res. 2011;4:169–179. doi: 10.1016/j.brainres.2010.11.087. [DOI] [PubMed] [Google Scholar]
- 13.Cervero F, Laird JM. Understanding the signaling and transmission of visceral nociceptive events. J Neurobiol. 2004;61:45–54. doi: 10.1002/neu.20084. [DOI] [PubMed] [Google Scholar]
- 14.Charrua A, Reguenga C, Cordeiro JM, Correiade-Sá P, Paule C, Nagy I, et al. Functional transient receptor potential vanilloid 1 is expressed in human urothelial cells. J Urol. 2009;182:2944–2950. doi: 10.1016/j.juro.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 15.Cheppudira BP, Girard BM, Malley SE, Dattilio A, Schutz KC, May V, et al. Involvement of JAK-STAT signaling/function after cyclophosphamide-induced bladder inflammation in female rats. Am J Physiol Renal Physiol. 2009;297:F1038–F1044. doi: 10.1152/ajprenal.00110.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuang YC, Tyagi P, Huang HY, Yoshimura N, Wu M, Kaufman J, et al. Intravesical immune suppression by liposomal tacrolimus in cyclophosphamide-induced inflammatory cystitis. Neurourol Urodyn. 2011;30:421–427. doi: 10.1002/nau.20981. [DOI] [PubMed] [Google Scholar]
- 17.Coelho FM, Pinho V, Amaral FA, Sachs D, Costa VV, Rodrigues DH, et al. The chemokine receptors CXCR1/CXCR2 modulate antigen-induced arthritis by regulating adhesion of neutrophils to the synovial microvasculature. Arthritis Rheum. 2008;58:2329–2337. doi: 10.1002/art.23622. [DOI] [PubMed] [Google Scholar]
- 18.Corrow KA, Vizzard MA. Phosphorylation of extracellular signal-regulated kinases in bladder afferent pathways with cyclophosphamide-induced cystitis. Neuroscience. 2009;163:1353–1362. doi: 10.1016/j.neuroscience.2009.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daly D, Rong W, Chess-Williams R, Chapple C, Grundy D. Bladder afferent sensitivity in wild-type and TRPV1 knockout mice. J Physiol. 2007;583:663–674. doi: 10.1113/jphysiol.2007.139147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dang K, Bielefeldt K, Gebhart GF. Cyclophosphamide-induced cystitis reduces asic channel, but enhances TRPV1 receptor function in rat bladder sensory neurons. J Neurophysiol. 110:408–417. doi: 10.1152/jn.00945.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dattilio A, Vizzard MA. Up-regulation of protease activated receptors in bladder after cyclophosphamide induced cystitis and colocalization with capsaicin receptor (VR1) in bladder nerve fibers. J Urol. 173:635–639. doi: 10.1097/01.ju.0000143191.55468.1d. 2013-2005. [DOI] [PubMed] [Google Scholar]
- 22.Everaerts W, Gevaert T, Nilius B, De Ridder D. On the origin of bladder sensing: tr(i)ps in urology. Neurourol Urodyn. 2008;27:264–273. doi: 10.1002/nau.20511. [DOI] [PubMed] [Google Scholar]
- 23.Everaerts W, Sepúlveda MR, Gevaert T, Roskams T, Nilius B, De Ridder D. Where is TRPV1 expressed in the bladder, do we see the real channel? Naunyn Schmiedebergs Arch Pharmacol. 2009;379:421–425. doi: 10.1007/s00210-008-0391-7. [DOI] [PubMed] [Google Scholar]
- 24.Everaerts W, Vriens J, Owsianik G, Appendino G, Voets T, De Ridder D, et al. Functional characterization of transient receptor potential channels in mouse urothelial cells. Am J Physiol Renal Physiol. 2010;298:F692–F701. doi: 10.1152/ajprenal.00599.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foster R, Jung J, Farooq A, McClung C, Ripsch MS, Fitzgerald MP, et al. Sciatic nerve injury induces functional pro-nociceptive chemokine receptors in bladder-associated primary afferent neurons in the rat. Neuroscience. 2011;183:230–237. doi: 10.1016/j.neuroscience.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frias B, Charrua A, Avelino A, Michel MC, Cruz F, Cruz CD. Transient receptor potential vanilloid 1 mediates nerve growth factor-induced bladder hyperactivity and noxious input. BJU Int. 2012;110:E422–E428. doi: 10.1111/j.1464-410X.2012.11187.x. [DOI] [PubMed] [Google Scholar]
- 27.Gray KJ, Engelmann UH, Johnson EH, Fishman IJ. Evaluation of misoprostol cytoprotection of the bladder with cyclophosphamide (Cytoxan) therapy. J Urol. 1986;136:497–500. doi: 10.1016/s0022-5347(17)44929-9. [DOI] [PubMed] [Google Scholar]
- 28.Guerios SD, Wang ZY, Boldon K, Bushman W, Bjorling DE. Blockade of NGF and trk receptors inhibits increased peripheral mechanical sensitivity accompanying cystitis in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R111–R122. doi: 10.1152/ajpregu.00728.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helyes Z, Elekes K, Németh J, Pozsgai G, Sándor K, Kereskai L, et al. Role of transient receptor potential vanilloid 1 receptors in endotoxin-induced airway inflammation in the mouse. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1173–L1181. doi: 10.1152/ajplung.00406.2006. [DOI] [PubMed] [Google Scholar]
- 30.Hu VY, Malley S, Dattilio A, Folsom JB, Zvara P, Vizzard MA. COX-2 and prostanoid expression in micturition pathways after cyclophosphamide-induced cystitis in the rat. Am J Physiol Regul Integr Comp Physiol. 2003;284:574–585. doi: 10.1152/ajpregu.00465.2002. [DOI] [PubMed] [Google Scholar]
- 31.Kao DJ, Li AH, Chen JC, Luo RS, Chen YL, Lu JC, et al. CC chemokine ligand 2 upregulates the current density and expression of TRPV1 channels and Nav1.8 sodium channels in dorsal root ganglion neurons. J Neuroinflammation. 2012;9:189. doi: 10.1186/1742-2094-9-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiuchi H, Takao T, Yamamoto K, Nakayama J, Miyagawa Y, Tsujimura A, et al. Sesquiterpene lactone parthenolide ameliorates bladder inflammation and bladder overactivity in cyclophosphamide induced rat cystitis model by inhibiting nuclear factor-kappaB phosphorylation. J Urol. 2009;181:2339–2348. doi: 10.1016/j.juro.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Klinger MB, Vizzard MA. Role of p75NTR in female rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol. 2008;295:F1778–F1789. doi: 10.1152/ajprenal.90501.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korkmaz A, Topal T, Oter S. Pathophysiological aspects of cyclophosphamide and ifosfamide induced hemorrhagic cystitis; implication of reactive oxygen and nitrogen species as well as PARP activation. Cell Biol Toxicol. 2007;5:303–312. doi: 10.1007/s10565-006-0078-0. [DOI] [PubMed] [Google Scholar]
- 36.Kullmann FA, Shah MA, Birder LA, de Groat WC. Functional TRP and ASIC-like channels in cultured urothelial cells from the rat. Am J Physiol Renal Physiol. 2009;296:F892–F901. doi: 10.1152/ajprenal.90718.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kyung YS, Park HY, Lee G. Preservation of uroplakins by 2-mercaptoethanesulfonate in cyclophosphamide-induced rat cystitis. Arch Toxicol. 2011;85:51–57. doi: 10.1007/s00204-010-0523-y. [DOI] [PubMed] [Google Scholar]
- 38.Lei Q, Malykhina AP. Colonic inflammation up-regulates voltage-gated sodium channels in bladder sensory neurons via activation of peripheral transient potential vanilloid 1 receptors. Neurogastroenterol Motil. 2012;24:575–585. doi: 10.1111/j.1365-2982.2012.01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linares-Fernández BE, Alfieri AB. Cyclophosphamide induced cystitis: role of nitric oxide synthase, cyclooxygenase-1 and 2, and NK(1) receptors. J Urol. 2007;177:1531–1536. doi: 10.1016/j.juro.2006.11.072. [DOI] [PubMed] [Google Scholar]
- 40.Malley SE, Vizzard MA. Changes in urinary bladder cytokine mRNA and protein after cyclophosphamide-induced cystitis. Physiol Genomics. 2002;9:5–13. doi: 10.1152/physiolgenomics.00117.2001. [DOI] [PubMed] [Google Scholar]
- 41.Manjavachi MN, Quintão NL, Campos MM, Deschamps IK, Yunes RA, Nunes RJ. The effects of the selective and non-peptide CXCR2 receptor antagonist SB225002 on acute and long-lasting models of nociception in mice. Eur J Pain. 2010;14:23–31. doi: 10.1016/j.ejpain.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Marotta DM, Costa R, Motta EM, Fernandes ES, Medeiros R, Quintão NL, et al. Mechanisms underlying the nociceptive responses induced by platelet-activating factor (PAF) in the rat paw. Biochem Pharmacol. 2009;77:1223–1235. doi: 10.1016/j.bcp.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 43.Meyer-Siegler KL, Ordorica RC, Vera PL. Macrophage migration inhibitory factor is upregulated in an endotoxin-induced model of bladder inflammation in rats. J Interferon Cytokine Res. 2004;24:55–63. doi: 10.1089/107999004772719918. [DOI] [PubMed] [Google Scholar]
- 44.Morais MM, Belarmino-Filho JN, Brito GAC, Ribeiro RA. Pharmacological and histopathological study of cyclophosphamide-induced hemorrhagic cystitis - comparison of the effects of dexamethasone and Mesna. Braz J Med Biol Res. 1999;32:1211–1215. doi: 10.1590/s0100-879x1999001000006. [DOI] [PubMed] [Google Scholar]
- 45.Murphy PM, Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991;253:1280–1283. [PubMed] [Google Scholar]
- 46.Nathan JD, Patel AA, McVey DC, Thomas JE, Prpic V, Vigna SR, et al. Capsaicin vanilloid receptor-1 mediates substance P release in experimental pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2001;5:G1322–G1328. doi: 10.1152/ajpgi.2001.281.5.G1322. [DOI] [PubMed] [Google Scholar]
- 47.Niiyama Y, Kawamata T, Yamamoto J, Furuse S, Namiki A. SB366791, a TRPV1 antagonist, potentiates analgesic effects of systemic morphine in a murine model of bone cancer pain. Br J Anaesth. 2009;102:251–258. doi: 10.1093/bja/aen347. [DOI] [PubMed] [Google Scholar]
- 48.Ost D, Roskams T, Van Der Aa F, De Ridder D. Topography of the vanilloid receptor in the human bladder: more than just the nerve fibers. J Urol. 2002;168:293–297. [PubMed] [Google Scholar]
- 49.Pan F, Liu D, Han XM, Li WC, Pang ZL, Li B, et al. Urodynamic investigation of cyclophosphamide-induced overactive bladder in conscious rats. Chin Med J (Engl) 2012;125:321–325. [PubMed] [Google Scholar]
- 50.Qu Y, Zhou F, Xu XY. Selective non-peptide CXCR2 antagonist SB225002 inhibits choroidal neovascularization in rat model. Zhonghua Yan Ke Za Zhi. 2009;45:742–745. [PubMed] [Google Scholar]
- 51.Reiland J, Furcht LT, McCarthy JB. CXC-chemokines stimulate invasion and chemotaxis in prostate carcinoma cells through the CXCR2 receptor. Prostate. 1999;41:78–88. doi: 10.1002/(sici)1097-0045(19991001)41:2<78::aid-pros2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 52.Reutershan J. CXCR2 – the receptor to hit? Drug News Perspect. 2006;19:615–623. doi: 10.1358/dnp.2006.19.10.1068009. [DOI] [PubMed] [Google Scholar]
- 53.Sakthivel SK, Singh UP, Singh S, Taub DD, Novakovic KR, Lillard JW., Jr CXCL10 blockade protects mice from cyclophosphamide-induced cystitis. J Immune Based Ther Vaccines. 2008;6:6. doi: 10.1186/1476-8518-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santos AA, Jr, Leal PC, Edelweiss MI, Lopes TG, Calixto JB, Morrone FB, et al. Effects of the compounds MV8608 and MV8612 obtained from Mandevilla velutina in the model of hemorrhagic cystitis induced by cyclophosphamide in rats. Naunyn Schmiedebergs Arch Pharmacol. 2010;382:399–407. doi: 10.1007/s00210-010-0555-0. [DOI] [PubMed] [Google Scholar]
- 55.Smaldone MC, Vodovotz Y, Tyagi V, Barclay D, Philips BJ, Yoshimura N, et al. Multiplex analysis of urinary cytokine levels in rat model of cyclophosphamide-induced cystitis. Urology. 2009;73:421–426. doi: 10.1016/j.urology.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Souza DG, Bertini R, Vieira AT, Cunha FQ, Poole S, Allegretti M, et al. Repertaxin, a novel inhibitor of rat CXCR2 function, inhibits inflammatory responses that follow intestinal ischaemia and reperfusion injury. Br J Pharmacol. 2004;43:132–142. doi: 10.1038/sj.bjp.0705862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Studeny S, Cheppudira BP, Meyers S, Balestreire EM, Apodaca G, Birder LA, et al. Urinary bladder function and somatic sensitivity in vasoactive intestinal polypeptide (VIP)-/- mice. J Mol Neurosci. 2008;36:175–187. doi: 10.1007/s12031-008-9100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sukkar A, Jenkins J, Sánchez J, Wagner EM. Inhibition of CXCR2 attenuates bronchial angiogenesis in the ischemic rat lung. J Appl Physiol. 2008;104:1470–1475. doi: 10.1152/japplphysiol.00974.2007. [DOI] [PubMed] [Google Scholar]
- 59.Vera PL, Iczkowski KA, Wang X, Meyer-Siegler KL. Cyclophosphamide-induced cystitis increases bladder CXCR4 expression and CXCR4-macrophage migration inhibitory factor association. Plos ONE. 2008;3:e3898. doi: 10.1371/journal.pone.0003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y, Wang DH. TRPV1 ablation aggravates inflammatory responses and organ damage during endotoxic shock. Clin Vaccine Immunol. 2013;20:1008–1015. doi: 10.1128/CVI.00674-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang ZY, Wang P, Merriam FV, Bjorling DE. Lack of TRPV1 inhibits cystitis-induced increased mechanical sensitivity in mice. Pain. 2008;139:158–167. doi: 10.1016/j.pain.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 62.White JR, Lee JM, Young PR, Hertzberg RP, Jurewicz AJ, Chaikin MA, et al. Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration. J Biol Chem. 1998;273:10095–10098. doi: 10.1074/jbc.273.17.10095. [DOI] [PubMed] [Google Scholar]
- 63.Wong GY, Gavva NR. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: recent advances and setbacks. Brain Res Rev. 2009;60:267–277. doi: 10.1016/j.brainresrev.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 64.Yuridullah R, Corrow KA, Malley SE, Vizzard MA. Expression of fractalkine and fractalkine receptor in urinary bladder after cyclophosphamide (CYP)-induced cystitis. Auton Neurosci. 2006;126–127:380–389. doi: 10.1016/j.autneu.2006.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaja-Milatovic S, Richmond A. CXC chemokines and their receptors: a case for a significant biological role in cutaneous wound healing. Histol Histopathol. 2008;23:1399–1407. doi: 10.14670/hh-23.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang N, Inan S, Cowan A, Sun R, Wang JM, Rogers TJ, et al. A proinflammatory chemokine, CCL3, sensitizes the heat- and capsaicin-gated ion channel TRPV1. Proc Natl Acad Sci U S A. 2005;102:4536–4541. doi: 10.1073/pnas.0406030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 2002;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 1001.Birder LA. More than just a barrier: urothelium as a drug target for urinary bladder pain. Am J Physiol Renal Physiol. 2005;289:F489–F495. doi: 10.1152/ajprenal.00467.2004. [DOI] [PubMed] [Google Scholar]
- 5001.McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]





