Abstract
Extramedullary manifestations of acute myeloid leukemia (AML) were described as early as the 19th century. However, the incidence, clinical significance, and pathobiology of extramedullary AML remain ill defined. We reviewed case reports, retrospective case series, pilot studies, and imaging studies of extramedullary leukemia (EML) to determine its frequency, characteristics, clinical presentation, and significance. EML precedes or accompanies development of AML and occurs during or following treatment, even during remission. Although imaging studies are rarely conducted and the true incidence of EML has yet to be verified, authors have reported several estimates based on retrospective and autopsy studies. The incidence of EML in patients with AML of all ages is estimated to be about 9% and EML in children with AML was detected in 40% of patients at diagnosis. The combination of positron emission tomography and computed tomography were the most sensitive and reliable techniques of detecting and monitoring EML. Based on our literature review, the frequency of EML is likely underreported. The well-documented nature of EML in AML patients suggests that AML can manifest as a solid tumor. The extent to which EML accompanies AML and whether EML is derived from bone marrow are unknown. Furthermore, questions remain regarding the role of the microenvironment, which may or may not facilitate the survival and proliferation of EML, and the implications of these interactions with regard to minimal residual disease, tumor cell quiescence, and relapse. Therefore, prospective studies of detection and characterization of EML in AML patients are warranted.
Keywords: Acute Myeloid Leukemia (AML), extramedullary leukemia (EML), myelodysplastic syndromes (MDS), chronic myeloid leukemia (CML), myeloproliferative neoplasms (MPN)
Introduction
Extramedullary leukemia (EML) is referred to as granulocytic sarcoma and myeloid sarcoma as well as chloroma, a term derived from the Greek word chloros (green) referring to the green color of myeloperoxidase. 1, 2 Although the British physician A. Burns first described this phenomenon in 1811, 3 the term chloroma did not appear in the literature until 1853.4 Despite Dock and Warthin’s recognition of the link between chloroma and acute leukemia in 1902,5 acute myeloid leukemia (AML) continues to be referred to as a “liquid tumor,” implying dissemination of bone marrow (BM)-derived cells in the blood. Remarkably, when EML manifests as a tumor, sites of resulting bone and tissue destruction resemble solid tumor metastases.6 Besides AML, EML occurs in association with acute promyelocytic leukemia (APL), myelodysplastic syndrome (MDS), chronic myeloid leukemia (CML), myeloproliferative neoplasms (MPNs), and chronic myelomonocytic leukemia (CMML).7–10 All extramedullary manifestations of AML, APL, MDS, CML, MPNs and CMML are referred to herein as EML.
CMML has been categorized as a separate entity in the WHO 2008 classification system categorized as an MDS/MPN disorder11. Like other monocytic leukemias, CMML is associated with extramedullary spread, and has a particular propensity for infiltration of the spleen, liver, skin and lymph nodes.7, 12–14 Characteristic features of CMML include persistent peripheral blood monocytosis (>1×10/L), absence of the Philadelphia (Ph) chromosome, less than 20% blasts in the peripheral blood and bone marrow (BM), and myelodysplasia involving one or more myeloid lineages.
No specific guidelines on the management of extramedullary CMML are available and systemic AML therapy has been used.15 In a recent retrospective study of 108 patients with CMML, 11 patients (10.2%) had cutaneous EML,16 suggesting that cutaneous EML predicts progression to AML. The overall survival in patients with cutaneous EML was 28.2 months, significantly shorter than that of CMML patients without cutaneous EML (44 months).16 The majority of CMML patients with cutaneous involvement progressed to AML in an average of 6.6 months. Most patients died within 2–24 months.16 This poor outcome suggests that leukemic skin infiltration represents a systemic disease for which chemotherapy is warranted.15
Because only case reports and small retrospective analyses of EML are available in the literature, the incidence and significance of EML are not well described. Also unclear is the extent to which extramedullary disease is present at diagnosis (of AML, APL, MDS, CML, and MPNs) and whether it is eliminated by conventional chemotherapy or remains intact, thereby serving as a reservoir of residual disease. In this review article we summarize the findings of case reports, retrospective case series, pilot studies, and imaging studies describing the frequency and clinical manifestations of EML.
General Features of EML
EML can manifest anywhere in the body. 17 Although the true incidence of EML in AML has yet to be verified prospectively, authors have reported several estimates based on retrospective and autopsy studies. Whereas the incidence rate of EML in AML patients of all ages is estimated to be about 9%,9, 18 it has reached 40% in children with AML at diagnosis. 19 Autopsy studies have indicated frequent occurrences of EML in myeloid leukemias. In one such study of 338 patients with myeloid leukemia in Hiroshima and Nagasaki, Japan, EML was not more frequent in individuals with heavier versus lower radiation exposure.6 Specifically, 8% of AML, 4% of CML, and 4% of CML in blast crisis patients had EML, with an overall EML incidence of 7%. A majority of the patients had multiple tumor sites, and EML was significantly more common in patients under the age of 15 years.6 In another large autopsy study, half of EML cases were clinically silent during the patients’ lifetimes.20
The French-American-British subtypes of AML most commonly associated with cutaneous (in up to 50% of cases) and noncutaneous EML are acute myelomonocytic (AML-M4) and acute monocytic (AML-M5) leukemia. 9, 21–26 In APL, EML is more likely to occur at relapse, with incidence estimated at approximately 3%. EML is rare at APL diagnosis, with half of cases affecting the skin. EML in APL can involve the pleura, gingiva, breast, spine, lymph nodes, spleen, and central nervous system (CNS).27, 28 Although rare, the CNS is a known site of relapse of APL.28
Presenting as isolated or multifocal lesions as well as in bodily fluids, EML manifests in any organ or tissue compartment. Furthermore, it develops at any point during the disease course 29 with or without BM involvement as an initial disease manifestation or evidence of relapse.
The WHO 2008 classifications system distinguishes myeloid sarcoma specifically as a tumor mass, arising in any anatomical location other than the BM, composed of myeloid blasts with or without maturation. Thus, according to WHO 2008, when myeloid blasts infiltrate any part of the body, the extramedullary involvement is not classified as myeloid sarcoma unless tumor masses are formed.7
EML may develop without symptoms, preceding BM involvement by several years, 30, 31 or manifest in AML patients considered to be in complete remission (CR) based on blood counts and BM analysis. 24 The clinical course of EML varies. 24 One study demonstrated that in APL, the durations from diagnosis of BM disease to development of EML ranged from 11 to 50 months. 28 In MDS, AML, and CML patients, EML preceded primary malignancies by 0.5–10.0 months, and the median time to develop EML after treatment was 16 months (range, 1–77 months). The median overall survival (OS) duration was 20 months (range, 1–75 months) in AML patients presenting with EML but no BM disease.32
In a retrospective study of 24 patients with EML, the median OS duration from the time of EML diagnosis to death was 9 months (range, 1–80 months). Treatments varied depending on the site of involvement and included surgery, chemotherapy, allogeneic stem cell transplantation (allo-SCT), and radiotherapy. Patients receiving chemotherapy had a median OS duration from the time of EML diagnosis to death of 13 months, whereas those who did not receive chemotherapy had a median OS duration of 3 months (P = 0.0009).17
In AML, authors have reported extramedullary relapse after allo-SCT in up to 20% of long-term survivors, with the incidence continuing to rise as patients live longer. The estimated median time to diagnosis of EML after allo-SCT ranges from 12 to 17 months.33 After allo-SCT, EML tends to occur more frequently in sanctuary sites such as the testes, ovaries, and CNS. Other EML sites include bone and the paranasal sinuses, breasts, gastrointestinal tract, skin, retroperitoneum, and kidneys. Because the incidence of graft-versus-host disease is increased in patients with extramedullary relapse, leukemia cells in extramedullary tissues are thought to evade immune surveillance. 33 Risk factors for EML after allo-SCT include age under 18 years, AML-M4, AML-M5, unfavorable cytogenetic abnormalities, EML before transplantation, and allo-SCT in relapse. Additionally, increased expression of the Wilms tumor (WT)-1 gene has predicted post-allo-SCT EML. A retrospective analysis of AML patients with post allo-SCT relapse demonstrated that monitoring peripheral blood or BM WT1 levels enabled early detection of extramedullary relapse. Compared to patients with BM relapse, patients with isolated extramedullary relapse had abnormally high WT1levels as early as 42 days before relapse. At relapse, WT1 levels were significantly lower in patients with isolated extramedullary relapse than in patients with BM relapses.33, 34
Ocular EML is a rare complication of AML.35 The choroid is most commonly affected in such cases, whereas conjunctival involvement occurs in only 2–4% of cases.24 Ocular EML is more common in children than in adults. 22, 24 Patients with ocular EML may present with proptosis, pain, chemosis, extraocular motion impairment, visual acuity defects 36, or exophthalmos (Table 1).30 Acute myeloblastic leukemia with granulocytic maturation (AML-M2), AML-M4, and AML-M5 were more often associated with ocular EML than were other French-American-British subtypes of AML. 21, 22, 24 In a large prospective case series of ocular EML, no patients had clinical evidence of CNS leukemia, whereas 53% had retinopathy. Other ocular EML findings include conjunctival hemorrhage and retinovascular abnormalities.21
Table 1.
Anatomical sites and clinical manifestations of EML
| Site | Potential manifestations |
|---|---|
| Bone, periosteum, soft tissue 6, 33 | Symptomatic osseous lesions, bone and tissue destruction |
| Lymphatic system 9, 17 | Lymphadenopathy |
| Cutaneous37–39, 65, 77 | Ecchymosis, rash, palpable purpura, ulcers, erythroderma, bullous lesions |
| Head and neck | |
| Scalp 28 | Lesions |
| Oral cavity: gingiva, salivary glands, and pharynx 5, 17, 44 | Sore throat, mouth pain, gingival mass |
| Nasal cavity 17, 44 | Nasal cavity mass |
| Skull 17 | Facial nerve palsy |
| Thyroid, parathyroid, pharyngeal, and thymic involvement 6 | Noted in autopsy studies but may present clinically as palpable masses or space-occupying lesions |
| Nasal septum involvement 17 | Epistaxis |
| Ocular 30, 36 | Pain, chemosis, extraocular motion impairment, visual acuity defect, exophthalmos |
| CNS | |
| Spinal cord compression 43, 59, 64 | Lower extremity pain, weakness, and numbness; urinary incontinence |
| Intracerebral parenchymal lesions 43, 46, 48, 59, 63, 64, 89–91 | Somnolence, seizures, headaches |
| Leptomeningeal involvement 64 | Cranial nerve palsies, increased intracranial pressure |
| Peripheral nervous system | |
| Nerve plexus infiltration 61, 65 | Neuropathy, pain, weakness |
| Mediastinum and intrathoracic | |
| Vena caval infiltration 10, 92 | Superior vena cava syndrome, plethora, upper extremity edema |
| Trachea and bronchi 42 | Tracheobroncheal tree occlusion or compression |
| Cardiac 92 | Endomyocardial densities on echocardiograms |
| Pericardium 92 | Pericardial effusion |
| Large mediastinal mass 10 | Mediastinal widening |
| Pulmonary parenchyma 10 | Pulmonary parenchymal masses |
| Pleura 27 | Pleural tumors, pleural effusion |
| Gastrointestinal tract | |
| Colorectal 48 | Hematochezia/rectal bleeding |
| Pancreas 47 | Epigastric pain |
| Small bowel 49, 54, 55 | Intussusception or obstruction |
| Gastric 67 | Abdominal pain |
| Duodenum 53 | Jaundice |
| Peritoneum 49 | Ascites, peritoneal masses, mesenteric and omental thickening |
| Urogenital tract | |
| Testes 93, 94 | Testicular masses |
| Uterus 95 | Abnormal uterine bleeding |
| Prostate 96 | Obstructive renal failure |
| Penis 97 | Dysuria, penile induration, penile skin thickening |
| Kidney 48, 98 | Kidney mass |
| Ovaries 52 | Ovarian mass |
| Cervix 50, 99 | Postmenopausal bleeding |
| Vagina 51 | Vaginal lump or pelvic masses |
| Breast 52 | Ipsilateral or bilateral breast masses, axillary adenopathy, fungating tumors eroding through breasts |
Cutaneous EML (“leukemia cutis”) is characterized by infiltration of the epidermis, dermis, or subcutis, causing single or multiple erythematous nodular and/or papular lesions 9, 37. Other cutaneous manifestations include ecchymosis, palpable purpura, ulcers, erythroderma, and bullous lesions (Table 1). 38 Cutaneous EML is most common in AML patients, occurring in up to 15% of cases, 50% of which are AML-M4 or -M5. As discussed above, cutaneous EML is also seen in CMML.37 It is frequently reported after the diagnosis of AML is established, but as with other types of EML, cutaneous EML lesions precede or occur concurrently with signs and symptoms of the disease.9 Cutaneous manifestations have been accompanied by EML in other sites, most frequently the meninges. 39 Keratinocytes produce a wide array of proinflammatory cytokines mediated by proteins in the inflammatory interleukin-1 family; 40, 41 whether this serves as a leukemogenic stimulus for cutaneous EML remains to be determined.
Clinical Presentations of EML
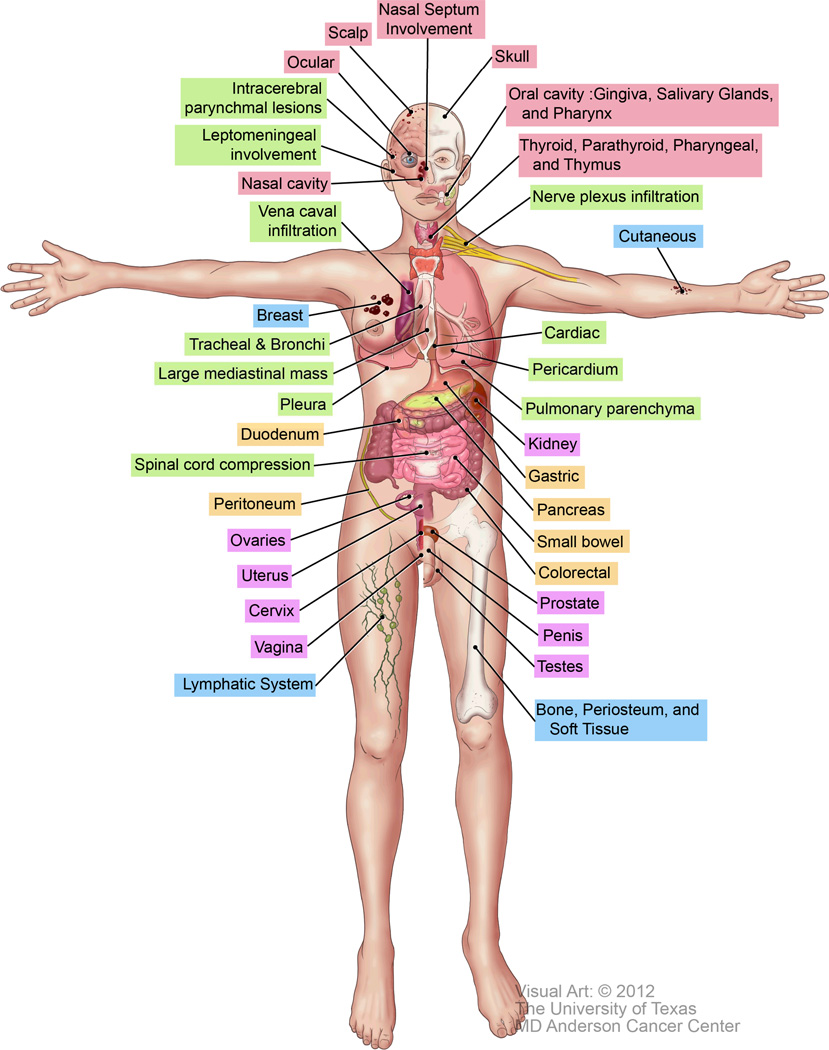
The clinical presentations of EML vary depending on the site and extent of involvement. The most commonly reported sites include bone, the periosteum, soft tissue, skin, and lymph nodes (Table 1; Figure 1).9, 17 However, these data are derived mostly from clinical presentation and could therefore only be an assessment of the accessible EML sites. Depending on the location, EML may present with life-threatening complications, including superior vena cava syndrome, tracheobronchial tree compression, pleural effusion,42 spinal cord compression,43 and extremity paresis.8 The sections below describe various clinical presentations of EML in major organ systems during all phases of AML, MDS, CML, and MPNs. The sites and clinical manifestations of EML are listed in Table 1.
Figure 1.
Anatomical sites of EML. The colors represent the EML sites depicted in Table 1.
EML concurrent with the diagnosis of AML, MDS, or MPNs
Features of EML diagnosed simultaneously with AML include abdominal masses, lymphadenopathy, soft tissue masses, and pleural effusions. 44 In particular, researchers noted pleural effusions in juvenile myelomonocytic leukemia and chronic myelomonocytic leukemia cases, in which pericardial effusions occurred, as well. In MDS-concurrent EML cases, affected sites included the orbits, lymph nodes, gingiva, and spine. 45 In childhood AML cases, EML at diagnosis involved the orbits, skin, and epidural sites. 22 In addition to manifestations of obviously emergent EML (e.g., superior vena cava syndrome, pleural effusions, cord compression), sites of EML involvement requiring immediate attention have included the cerebrospinal fluid10, 46 small bowel, peritoneum, liver, pancreas, and retroperitoneum.47–49 Authors reported cervical EML with postmenopausal bleeding50 and vaginal EML with urinary incontinence. 51 Furthermore, investigators observed asymptomatic ocular manifestations 24 and breast involvement 52 of EML at diagnosis of AML.
Isolated and multifocal EML in patients with no prior history of BM disease
Interestingly, both isolated and multifocal EML can manifest without concurrent or previous BM and/or systemic hematologic disease. However, systemic hematologic disease commonly ensues in EML cases. At our institution, researchers found isolated EML in 21 of 1520 AML and 402 high-risk MDS patients, 5 of whom experienced progression to AML at a median of 5 months after diagnosis of EML (range, 4–26 months) despite undergoing chemotherapy. Sites of EML involvement in these patients included consisted of the mesentery, pancreas, spleen, CNS, lymph nodes, heart, tongue, bones, uterus, ovaries, and fallopian tubes.32 Authors have reported pleural tumors as the sole manifestations of EML in patients with APL.27 Other isolated EML manifestations in patients without blood or BM involvement at initial presentation have included infiltration at gastric, duodenal, cecal, colorectal,53 ocular,30 gingival,7, 44 cutaneous, skeletal, neuromuscular, and other visceral sites, such as intussusception and small bowel obstruction owing to isolated small bowel EML masses.8, 54, 55 Although many EML cases are reported as solitary or isolated masses, very few case reports mention use of positron emission tomography (PET) or computed tomography (CT) to accurately verify the true extent of the disease.27, 54, 55
In one study, identification of isolated EML in the breast in 39 patients before BM diagnosis (probably because of routine breast cancer screening) provided an opportunity to assess the clinical course of extramedullary AML.52 Because breast tissue is hematopoietic during embryogenesis, researchers hypothesized that local myelopoiesis may recur. In the appropriate setting of leukemogenic cytokine and/or chemokine stimuli and under the influence of (or regardless of) the local microenvironment, circulating and/or local breast hematopoietic cells may undergo leukemic transformation. Alternatively, breast tissue is composed primarily of adipose tissue, and adipose-derived stromal cells are multipotent and possess stem-cell characteristics. One could speculate that neoplastic transformation of those cells may occur.56
Similarly, the eye is a reservoir of stem cells. Thus, triggering of ocular EML by a local leukemogenic stimulus is plausible. Corneal epithelial stem cells are confined to the limbus, which is the vascular outer rim separating the cornea from the conjunctiva.57 The heterogeneity of mesenchymal stem cell populations in the body may enable diverse tissues to adapt and respond to various microenvironmental stimuli.58
EML during the course of or at relapse of AML, APL, MDS, and MPNs
EML manifests during the course of treatment and at relapse of hematologic malignancies in unpredictable ways. For example, authors have reported clinical presentations of intracerebral EML mimicking infectious meningitis during the course of progressive CML.59 Intraparenchymal masses develop with disease progression.49 Furthermore, EML has occurred in patients who experienced transformation of hypereosinophilic syndrome, polycythemia vera, or myelofibrosis to AML. EML manifestations in these patients included destructive osseous lesions, lymphadenopathy, and spinal tumors.31 Sites of EML during progression of MDS to AML included the skin, colon, cecum, prostate, nasal cavity, breast, lymph nodes, brain, spine, nasal cavity, lip, foot, brain, and chest wall and endobronchial tissue.28, 60
In addition, authors have reported typical and unusual neurologic presentations of EML, such as compression neuropathy.61 Another study demonstrated conjunctival lesions with only minimal patchy BM involvement.62
Isolated EML at relapse
EML can present as isolated evidence of relapse without BM involvement. Usually, BM relapse ensues months to years after EML diagnosis.31 Researchers have detected isolated intracerebral masses in AML patients in CR.63, 64 Authors have reported isolated extramedullary relapse of APL with spinal cord compression and paraplegia 37 months into remission followed by systemic relapse, as well.28 Isolated peripheral nervous system EML manifestations, such as brachial plexus and lumbosacral plexus involvement 65 and multifocal nerve entrapment 66 have occurred. Additionally, patients in CR of AML have experienced isolated gastric,67 cardiac68 breast,52 and ocular 23, 25 EML.
Mechanisms of EML development
Various cytogenetic abnormalities are associated with increased incidence of EML and, as a result, alterations in homing molecules are implicated. Diverse patterns of AML blast migration to, invasion of, and accumulation in extramedullary tissue may be attributed to variable expression of and interactions among chemokines and chemokine receptors.19 Investigators have noted increased numbers of circulating CCR2-positive AML cells only in patients with cutaneous EML, suggesting that expression of the chemokine receptor CCR2 is involved in homing of EML to the skin. This study demonstrated that AML cells in the skin expressed a set of chemokine receptors different from that expressed in BM and circulating blasts, suggesting that homing of AML blasts to the skin and their retention there result from specific chemokine-chemokine receptor interactions.19 Similarly, researchers found that specific interactions between matrix metalloproteinase-9 and leukocyte β2 integrin are necessary for pericellular proteolysis and migration of AML cells.1 Mechanisms responsible for the attachment of leukemia cells to a specific organ and inducers of local proliferation of these cells in a nonhematopoietic organ rather than in the BM, the “natural” leukemiacell sanctuary, remain unclear. Integrin-mediated signaling and adhesion play a role in AML progression and chemosensitivity69 and are also involved in extramedullary infiltration. Specific binding of MMP-9 to leukocyte surface β2 integrin has been shown to be required for pericellular proteolysis and migration of AML-derived cells.1
Impaired adhesion of hematopoietic stem cells to BM niches are hypothesized to allow for their mobilization and migration into the peripheral blood, spleen and other extra-medullary sites. The impaired adhesion could be explained by altered expression of membrane adhesion molecules and integrins. For example, in vivo the hematopoietic stem cells of CML patients have reduced adhesion molecules expression including Lselectin, CD44 and N-cadherin. This decrease correlates with, in vitro, with reduced adhesive capacity of HSCs from CML patients.70
Authors have described a variety of proposed mechanisms of intracerebral and leptomeningeal disease. For example, investigators suggested that poor penetration of chemotherapeutic agents into the CNS results in intracerebral sanctuary deposits of leukemia cells that give rise to EML64 and that meningeal seeding involves hematogenous dissemination or extension from adjacent osseous lesions.71 These hypotheses warrant further studies because cytarabine, which readily penetrates the CNS, is usually administered at high doses to younger but not older patients with AML. Meningeal deposits of leukemia cells are implicated in cases of multiple cranial nerve palsies and elevated intracranial pressure. In cases of severe leukostasis, multiple small leukemic masses cause small vessel occlusion, hemorrhage, and leukemia cell extravasation into the intracerebral parenchyma. Another hypothesis regarding intraparenchymal invasion is that leukemia cells migrate from the skull through the dura into the subarachnoid and Virchow-Robin spaces.64 Enhanced CNS penetration has occurred with expression of neural cell adhesion molecule (CD56) on the surface of AML blasts.48, 72
Resemblance of EML to Solid Tumors
Researchers have observed striking similarities between breast EML and breast epithelial tumors. This is supported by reports of morphologic similarities between lobular carcinoma and EML in the breast.52 In one study, although the majority of patients with breast EML presented with unilateral breast involvement, 40% of them presented with bilateral breast disease. Similar to lobular breast carcinoma, breast EML can recur in the ipsilateral breast, contralateral breast, and CNS (Table 1). EML tumors may appear identical to epithelial breast tumors during surgery and mammography.52
The clinical course of isolated breast EML demonstrates similarities with breast epithelial tumors. For example, among 17 of 35 patients with isolated breast EML receiving only local therapy (surgery and/or radiotherapy), 2 experienced EML relapses at sites other than the breast without BM involvement at 12 and 72 months, respectively. Two patients remained disease free at 1 and 8 years, respectively. Long responses to local therapy in cases of isolated EML suggest that the tumors were initially confined to non-BM spaces. Fifteen patients underwent local therapy and chemotherapy, all with clinical responses. Subsequent EML without BM involvement occurred in three patients between 1 and 45 months. One had extramedullary and BM relapse at 4 years; 4 others had only BM relapse between 3 and 96 months. Among 3 patients with responses to chemotherapy alone, one remained disease free at 10 years. Given that many patients have EML involving multiple anatomical sites calls into question the current practice of BM-directed anti-leukemia therapy.52
Imaging Studies for Detection of EML
Despite a lack of large prospective studies of PET and CT in AML patients, clinical investigators have recognized and described the importance of these modalities in diagnosing and managing EML.52 For example, investigators found that retention of 18F-fluorothymidine (18-FLT-PET) was significantly higher in the spleen (P < 0.05) and BM (P < 0.05) in patients with AML than in healthy controls. 73 In addition, they identified EML involvement of the meninges, pericardium, abdomen, testes, and lymph nodes. Furthermore, in a retrospective study of patients with histologically proven EML, combined 18-F-fluorodeoxyglucose (18-F-FDG PET)/CT more accurately diagnosed EML than did PET alone, with the former modality avoiding five false-positive EML findings that were obtained with the latter. 74 In addition, compared to 18-F-FDG-PET/CT, CT alone resulted in 13 false-negative findings and 1 false-positive finding. 74 In another study, of 10 patients with de novo and/or relapsed AML and histologically confirmed EML, 18-F-FDG-PET/CT detected known EML lesions in 9 of them. 29 Moreover, 18-F-FDG PET/CT detected additional sites of EML in six of those patients.
Detection of EML using PET/CT is of potential therapeutic benefit because it facilitates changing and/or intensifying systemic therapy and extramedullary-directed therapy (e.g., surgery, radiotherapy), which may result in improved disease control and, likely, outcome. The combination of PET and CT enables monitoring of therapeutic response and identification of EML reservoirs, which may require increasingly individualized multidisciplinary care. Large prospective studies of PET/CT for detection of EML in AML patients would result in increased accuracy in determination of the true incidence of EML.
It is important to note that 18-FLT-PET and 18-FDG-PET are two different imaging procedures. Specifically, 18-F-FDG is not a tumor-specific tracer; therefore false-positive findings can occur in sites of inflammation29. Furthermore, leukemic involvement in areas such as the meninges and or pericardium which have high glucose metabolism may not be adequately detected by 18F-FDG-PET30. Conversely, the thymidine analogue 18F-FLT-PET is considered a stable tumor cell proliferation imaging agent.29 It is resistant to degradation in vivo and accumulates primarily in proliferating tissues.30
A prospective study of18F-FLT-PET in malignant lymphoma patients suggested it may be a superior PET tracer for detection of malignant lymphoma in organs with high physiologic fluorodeoxyglucose uptake. In one study of 18-FLT-PET vs. 18-FDG-PET in non-small cell lung cancer (NSCLC), 18-FLT-PET resulted in understaging of more patients but overstaging of fewer patients. For regional lymph node evaluation 18-FLT-PET demonstrated better specificity, accuracy and positive predictive value than 18-FDG PET/CT in NSCLC. 29 Recently in a preclinical study, Li et al demonstrated 18F-FLT-PET is superior to 18F-FDG-PET for very early response prediction in immune-deficient mice with xenotransplants of NPM-ALK-Positive Lymphoma. The mice were treated with targeted therapies. The authors showed that 18-FLT-PET but not 18-FDG-PET is able to predict response to targeted treatments very early in the course, enabling early prediction of treatment efficacy.31
Prognostic Significance of EML
Owing to a lack of prospective studies of EML and the limitations of retrospective studies, the prognostic significance of EML is not fully established. Interpretations of the prognostic implications of EML vary in the literature. Some authors reported that survival rates in AML patients with EML were similar to those for AML in general, ranging from 20% to 30%.9, 17 Others reported that EML was associated with superior survival rates.17 Data from our institution suggest that AML patients with EML undergoing systemic chemotherapy had better event-free survival and OS durations than did “classical” AML patients, suggesting that systemic therapy is required for EML in patients without BM disease.75 The need for AML therapy in this setting is further justified by studies demonstrating that the median durations of progression from nonleukemic EML to BM-confirmed AML ranged from 5 to 12 months 9, 32, 52 and by retrospective comparisons showing that 5-year OS rates in EML patients undergoing systemic chemotherapy were better than those in patients undergoing other treatments (P = 0.0009).17
Although the t(8;21) translocation is commonly associated with EML and generally considered a good prognostic feature of AML, whether EML confers a poor prognosis in AML remains unclear, as published results regarding this finding vary. One group reported that the presence of EML adversely affects survival, with a median OS duration of 5.4 months versus 59.5 months in those without EML (P = 0.002).5 The CR rate in EML patients was 50% versus 92% in those without EML (P = 0.006). Only one EML patient with a CR remained alive; this patient was the only EML patient who was administered high-dose cytarabine. The poor outcomes in these EML patients may be related to insufficient CNS-directed therapy in those with disease at that site and the fact that therapy directed at the primary EML site was not common. Only one patient in that study received intrathecal chemotherapy during induction; the patient’s cerebrospinal fluid was negative for leukemic blasts prior to initiating intensification. Likewise, only one patient received radiotherapy (9 Gy); the patient underwent this therapy at rapid onset of paraplegia.5 Another group studying the association between t(8;21) and EML in a previously described case series of 18 children reported that the presence of EML was not an adverse prognostic marker.22 Their protocol included intrathecal treatments and high-dose cytarabine use during the intensification phase.
Several retrospective studies have demonstrated that cutaneous EML is associated with an aggressive clinical course and poor survival rates.37 In one study of 26 patients with AML and CML along with cutaneous EML, 22 patients with adequate follow-up died of their leukemia within 24 months of development of cutaneous lesions.76 The mean survival durations were 7.6 months in AML patients and 9.4 months in CML patients. In another retrospective study of AML associated with cutaneous EML, the remission rates in patients with cutaneous EML did not differ significantly from those patients without it, but the investigators did note a trend of shorter remission durations in patients with cutaneous EML.77
Immunohistochemical, Cytogenetic, and Molecular Characteristics of EML
Biopsies of certain EML sites such as leukemic retinal infiltrates78,79 have not been conducted and not all subjects in retrospective EML studies had confirmed tissue diagnosis. The need for tissue diagnosis cannot be underestimated as a histopathologic diagnosis is essential for the management of EML. Establishing a diagnosis of EML using biopsy analysis of tissue samples is generally straightforward. For example, immunohistochemical and morphologic analyses using paraffin-embedded sections of EML specimens demonstrated that a panel of antibodies against CD20, CD43, CD68, and MPO successfully identified 96% of EML cases.80
EML is frequently associated with two core-binding factor variants of AML: cases with inv(16)9, 49 and t(8;21),5, 17 the latter of which is particularly associated with this leukemia. Whereas t(8;21) is associated with pediatric ocular EML,79, 9, 24 inv(16) is associated with intra-abdominal EML.9 Trisomy 8 and other chromosome 8 abnormalities are associated with EML.9, 29, 75 Other cytogenetic abnormalities detected in EML patients are t(15;17), t(9;11), t(1;11), t(8;17), t(8;16), del(16q), del(5q), del(20q), monosomy 7, and trisomy 4.9 Furthermore, researchers noted a significantly higher frequency of chromosome 8 abnormalities in patients with cutaneous EML than in patients without cutaneous involvement (P < 0.0001).77
While t(8;21) is commonly reported in association with EML, it seems to be more closely linked to pediatric populations.22, 24, 79 A retrospective analysis of 92 adult patients with myeloid sarcoma identified a higher incidence in patients with the following aberrations: t(8;21), 11q23 (MLL rearrangement), monosomy 7, and trisomy 8.81 In other studies, t(8;21) was not found to be associated with EML. For example Deeb et al. conducted genomic profiling of seven myeloid sarcoma cases by array comparative genomic hybridization, and no cases of t(8;21) were identified; however in their series chromosome 8 abnormalities occurred most frequently (3/7 cases).4 Stolzel et al. reported that clonal evolution, including partial loss of human leukocyte antigen genes, was associated with extramedullary relapse after allo-SCT.82
Fewer data are available on molecular abnormalities associated with EML than on classical characterization of leukemia.2 A well-documented molecular abnormality is a mutation in the nucleophosmin (NPM)-1 gene.3 In one study, cytoplasmic NPM, correlating with the NPM1 mutation, was the most common molecular lesion in leukemia cells of AML patients with EML.83 Accounting for approximately one-third of all AML, the NPM1 mutation is considered a founder genetic alteration,84 which is stable over the course of AML, as demonstrated by late relapses reported at 8 years85 and the occurrence of myeloid sarcoma after 20 years.86 The NPM1 mutation induces expansion of hematopoietic, mostly myeloid, cells in various animal models, including an accumulation in extramedullary sites. For example, the NPM1 mutation induces hematopoietic cell proliferation in the posterior blood island and the ventral aorta of the zebrafish87 as well as in the spleen of transgenic mice.88 At the present time, one can only speculate as to why the NPM mutation is associated with EML? NPM1 is usually mutually exclusive of other recurrent AML genetic abnormalities and is consistently recurrent at relapse. It has been considered a driver mutation as it is detected in approximately 60% of AML with normal karyotype.84 Subsequent cytogenetic abnormalities described above are likely secondary events. The NPM1 mutation results in aberrant cytoplasmic dislocation of the mutant protein which leads to leukemogenesis.84 Multiple cellular pathways are altered by increased nucleophosmin export into cytoplasm.84 Whether the cell-metabolic difference as a reaction to altered shuttling of the NPM1 protein between nucleus and cytoplasm sets the stage for EML warrants further exploration. The significance of the association between NPM1 mutations and altered microRNA signatures in EML is unknown, but may also provide a clue about the pathobiology of EML.84
Conclusions
Unlike in patients with lymphoma, imaging studies are not routinely conducted in those with AML, as it is considered a liquid tumor. Our review suggests that EML is more common in AML, as well as, MDS and MPN patients than currently thought. EML may manifest a reservoir of leukemia cells capable of contributing to disease progression or recurrence. Therefore, prospective imaging studies, with PET/CT imaging for patients with newly diagnosed AML are warranted and will help establish the true incidence of EML. In such a study, newly diagnosed patients would undergo pre-treatment baseline PET/CT imaging. If sites of EML are identified, the patients would continue to be monitored with imaging to assess treatment response. Identification of EML with PET/CT imaging may enable clinicians to intensify or modify therapy. At present, the clinical significance of this phenomenon or its association with peripheral blast count and clinical outcome are largely unknown.
Furthermore, because EML manifests as a relatively common presentation of relapsed AML following allo-SCT, routine monitoring for EML should be considered. In view of this, revision of the current approach to workup and follow-up in AML, MDS, CML, and MPN patients is needed.
Given that EML is detected in every organ, comprehensive workup with particular attention devoted to the head and neck, eyes, and skin is warranted for patients with AML. For women, physicians should not dismiss uterine bleeding caused by hormonal deregulation or thrombocytopenia at presentation or during chemotherapy and should always keep in mind the possibility of uterine, ovarian, cervical, or vaginal EML.
EML raises important biological questions, such as why does EML evolve in a certain organ but not in another? Why does EML precede or appear concurrently with BM disease in some patients but not in others? Furthermore, is the current understanding of the origin of leukemia valid? Is leukemia always BM-derived? Does leukemia emerge only from a BM-derived leukemia stem cell that resides in and depend on the BM niche?4, 19 Does EML arise from a circulating or tissue specific cell? Although authors have reported EML lesions to be isolated, results of autopsy and EML imaging studies and various retrospective series imply that EML tumors might involve more than one site.6, 29, 52 Therefore, given the reported frequency of EML in AML patients, one should question whether AML is a liquid tumor rather than a systemic disease with a broad array of clinical manifestations.
Translational Relevance.
Unlike the management of patients with lymphoma, imaging studies are not routinely conducted in patients with acute myeloid leukemia (AML). While AML is generally considered a “liquid tumor,” our review suggests that extramedullary manifestations of AML occur frequently, and are likely under-reported. The well-documented nature of extramedullary leukemia (EML) challenges the current practice of monitoring only blood and bone marrow response to chemotherapy and puts the current practice of BM-only-directed anti-leukemia therapy in to question. Furthermore, it should be asked whether a BM-directed approach in combination with local therapy (either surgical excision or local radiotherapy) could be the standard of care for all EML (with or without BM disease)? Unidentified EML may manifest a reservoir of leukemia cells capable of contributing to disease progression or recurrence. Therefore, prospective imaging studies for patients with AML are warranted.
Acknowledgements
We thank Don Norwood for editing our manuscript.
Grant Support: This research was supported in part by the MD Anderson Cancer Center Support Grant CA016672.
Footnotes
Disclosure: The authors have no conflicts of interest to disclose.
References
- 1.Stefanidakis M, Karjalainen K, Jaalouk DE, Gahmberg CG, O'Brien S, Pasqualini R, Arap W, Koivunen E. Role of leukemia cell invadosome in extramedullary infiltration. Blood. 2009;114:3008–3017. doi: 10.1182/blood-2008-04-148643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avni B, Koren-Michowitz M. Myeloid Sarcoma: Current Approach and Therapeutic Options. Ther Adv Hem. 2011;2:309–316. doi: 10.1177/2040620711410774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, La Starza R, Diverio D, Colombo E, Santucci A, Bigerna B, Pacini R, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352:254–266. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 4.Deeb G, Baer MR, Gaile DP, Sait SN, Barcos M, Wetzler M, Conroy JM, Nowak NJ, Cowell JK, Cheney RT. Genomic profiling of myeloid sarcoma by array comparative genomic hybridization. Genes Chromosomes Cancer. 2005;44:373–383. doi: 10.1002/gcc.20239. [DOI] [PubMed] [Google Scholar]
- 5.Byrd JC, Weiss RB, Arthur DC, Lawrence D, Baer MR, Davey F, Trikha ES, Carroll AJ, Tantravahi R, Qumsiyeh M, Patil SR, Moore JO, et al. Extramedullary leukemia adversely affects hematologic complete remission rate and overall survival in patients with t(8;21)(q22;q22): results from Cancer and Leukemia Group B 8461. J Clin Oncol. 1997;15:466–475. doi: 10.1200/JCO.1997.15.2.466. [DOI] [PubMed] [Google Scholar]
- 6.Liu PI, Ishimaru T, McGregor DH, Okada H, Steer A. Autopsy study of granulocytic sarcoma (chloroma) in patients with myelogenous leukemia, Hiroshima-Nagasaki 1949–1969. Cancer. 1973;31:948–955. doi: 10.1002/1097-0142(197304)31:4<948::aid-cncr2820310428>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 7.Swerdlow SH CE, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. 2008 [Google Scholar]
- 8.Glossmann JP, Staak JO, Wickenhauser C, Diehl V, Josting A. Extramedullary acute myeloid leukemia (granulocytic sarcoma) with arm paresis, maculopapular exanthema and organ involvement. Leuk Lymphoma. 2003;44:1619–1621. doi: 10.3109/10428190309178788. [DOI] [PubMed] [Google Scholar]
- 9.Bakst RL, Tallman MS, Douer D, Yahalom J. How I treat extramedullary acute myeloid leukemia. Blood. 2011;118:3785–3793. doi: 10.1182/blood-2011-04-347229. [DOI] [PubMed] [Google Scholar]
- 10.Ravandi-Kashani F, Cortes J, Giles FJ. Myelodysplasia presenting as granulocytic sarcoma of mediastinum causing superior vena cava syndrome. Leuk Lymphoma. 2000;36:631–637. doi: 10.3109/10428190009148412. [DOI] [PubMed] [Google Scholar]
- 11.Aribi A, Borthakur G, Ravandi F, Shan J, Davisson J, Cortes J, Kantarjian H. Activity of decitabine, a hypomethylating agent, in chronic myelomonocytic leukemia. Cancer. 2007;109:713–717. doi: 10.1002/cncr.22457. [DOI] [PubMed] [Google Scholar]
- 12.Campidelli C, Agostinelli C, Stitson R, Pileri SA. Myeloid sarcoma: extramedullary manifestation of myeloid disorders. Am J Clin Pathol. 2009;132:426–437. doi: 10.1309/AJCP1ZA7HYZKAZHS. [DOI] [PubMed] [Google Scholar]
- 13.Habens F, Srinivasan N, Oakley F, Mann DA, Ganesan A, Packham G. Novel sulfasalazine analogues with enhanced NF-kB inhibitory and apoptosis promoting activity. Apoptosis. 2005;10:481–491. doi: 10.1007/s10495-005-1877-0. [DOI] [PubMed] [Google Scholar]
- 14.Azoulay E, Fieux F, Moreau D, Thiery G, Rousselot P, Parrot A, Le Gall JR, Dombret H, Schlemmer B. Acute monocytic leukemia presenting as acute respiratory failure. Am J Respir Crit Care Med. 2003;167:1329–1333. doi: 10.1164/rccm.200206-554OC. [DOI] [PubMed] [Google Scholar]
- 15.Slomowitz SJ, Shami PJ. Management of extramedullary leukemia as a presentation of acute myeloid leukemia. J Natl Compr Canc Netw. 2012;10:1165–1169. doi: 10.6004/jnccn.2012.0120. [DOI] [PubMed] [Google Scholar]
- 16.Mathew RA, Bennett JM, Liu JJ, Komrokji RS, Lancet JE, Naghashpour M, Messina JL, List AF, Moscinski LC, Zhang L. Cutaneous manifestations in CMML: Indication of disease acceleration or transformation to AML and review of the literature. Leuk Res. 2012;36:72–80. doi: 10.1016/j.leukres.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Lan TY, Lin DT, Tien HF, Yang RS, Chen CY, Wu K. Prognostic factors of treatment outcomes in patients with granulocytic sarcoma. Acta Haematol. 2009;122:238–246. doi: 10.1159/000253592. [DOI] [PubMed] [Google Scholar]
- 18.Avni B, Rund D, Levin M, Grisariu S, Ben-Yehuda D, Bar-Cohen S, Paltiel O. Clinical implications of acute myeloid leukemia presenting as myeloid sarcoma. Hematol Oncol. 2012;30:34–40. doi: 10.1002/hon.994. [DOI] [PubMed] [Google Scholar]
- 19.Yang W, Zhang Y, Fu Z, Yu J, Sun X, Mu D, Han A. Imaging of proliferation with 18F-FLT PET/CT versus 18F-FDG PET/CT in non-small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2010;37:1291–1299. doi: 10.1007/s00259-010-1412-6. [DOI] [PubMed] [Google Scholar]
- 20.Muss HB, Moloney WC. Chloroma and other myeloblastic tumors. Blood. 1973;42:721–8. [PubMed] [Google Scholar]
- 21.Karesh JW, Goldman, Edward J, et al. Shalom, Kelman, et al. A prospective Opthalmic Evaluation of Patients With Acute Myeloid Leukemia: Correlation of Ocular and Hematologic Findings. Journal of Clinical Oncology. 1989;7:1528–1532. doi: 10.1200/JCO.1989.7.10.1528. [DOI] [PubMed] [Google Scholar]
- 22.Felice MSea. Good outcome of Children with Acute Myeloid Leukemia and t(8;21)(q22;q22) Even When Associated with Granulocytic Sarcoma A Report from a Single Institution in Argentina. Cancer. 2000;88:1939–1944. [PubMed] [Google Scholar]
- 23.Murray JA, Mehrotra PK, Brown MJ, Slater DN. Monocytic sarcoma of the sclera. Acta Haematol. 1984;71:407–409. doi: 10.1159/000206627. [DOI] [PubMed] [Google Scholar]
- 24.Fleckenstein K, Geinitz H, Grosu A, Goetze K, Werner M, Molls M. Irradiation for conjunctival granulocytic sarcoma. Strahlenther Onkol. 2003;179:187–190. doi: 10.1007/s00066-003-1002-7. [DOI] [PubMed] [Google Scholar]
- 25.Kiratli H, Demiroglu H, Emec S. Ocular relapse in acute myeloid leukemia (M4) with normal bone marrow. Int Ophthalmol. 2009;29:243–245. doi: 10.1007/s10792-008-9207-5. [DOI] [PubMed] [Google Scholar]
- 26.Rootman JG, Gedy Treatment of Ocular Leukemia With Local Chemotherapy. Cancer Treatment Reports. 1985;69:119–122. [PubMed] [Google Scholar]
- 27.Mueller BU, Buerger AU, Solenthaler M, Garamvoelgyi EM, Oppliger-Leibundgut E, Fey MF, Pabst T. Pleural tumors as sole primary manifestation of acute promyelocytic leukemia. Ann Oncol. 2006;17:722–723. doi: 10.1093/annonc/mdj062. [DOI] [PubMed] [Google Scholar]
- 28.Vega-Ruiz A, Faderl S, Estrov Z, Pierce S, Cortes J, Kantarjian H, Ravandi F. Incidence of extramedullary disease in patients with acute promyelocytic leukemia: a single-institution experience. Int J Hematol. 2009;89:489–496. doi: 10.1007/s12185-009-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stolzel F, Rollig C, Radke J, Mohr B, Platzbecker U, Bornhauser M, Paulus T, Ehninger G, Zophel K, Schaich M. (1)F-FDG-PET/CT for detection of extramedullary acute myeloid leukemia. Haematologica. 2011;96:1552–1556. doi: 10.3324/haematol.2011.045047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Veen S, Kluin PM, de Keizer RJ, Kluin-Nelemans HC. Granulocytic sarcoma(chloroma)Presentation of an unusual case. Am J Clin Pathol. 1991;95:567–571. doi: 10.1093/ajcp/95.4.567. [DOI] [PubMed] [Google Scholar]
- 31.Neiman RS, Barcos M, Berard C, Bonner H, Mann R, Rydell RE, Bennett JM. Granulocytic sarcoma: a clinicopathologic study of 61 biopsied cases. Cancer. 1981;48:1426–1437. doi: 10.1002/1097-0142(19810915)48:6<1426::aid-cncr2820480626>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 32.Tsimberidou AM, Kantarjian HM, Estey E, Cortes JE, Verstovsek S, Faderl S, Thomas DA, Garcia-Manero G, Ferrajoli A, Manning JT, Keating MJ, Albitar M, et al. Outcome in patients with nonleukemic granulocytic sarcoma treated with chemotherapy with or without radiotherapy. Leukemia. 2003;17:1100–1103. doi: 10.1038/sj.leu.2402958. [DOI] [PubMed] [Google Scholar]
- 33.Clark WB, Strickland SA, Barrett AJ, Savani BN. Extramedullary relapses after allogeneic stem cell transplantation for acute myeloid leukemia and myelodysplastic syndrome. Haematologica. 2010;95:860–863. doi: 10.3324/haematol.2010.025890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshihara STH, Ikegame K, et al. Early prediction of extramedullary relapse of leukemia following allogeneic stem cell transplantation using the WT1 transcript assay. Biol Blood Marrow Transplant. 2006;12(2 supplement 1):86. [Google Scholar]
- 35.Smiddy WE, Graham ML, Cheo DL, Schachat AP. Intraocular penetration of cytosine arabinoside following intravenous administration in primates. J Ocul Pharmacol. 1988;4:133–136. doi: 10.1089/jop.1988.4.133. [DOI] [PubMed] [Google Scholar]
- 36.Watkins LM, Remulla HD, Rubin PA. Orbital granulocytic sarcoma in an elderly patient. Am J Ophthalmol. 1997;123:854–856. doi: 10.1016/s0002-9394(14)71146-8. [DOI] [PubMed] [Google Scholar]
- 37.Cho-Vega JH, Medeiros LJ, Prieto VG, Vega F. Leukemia cutis. Am J Clin Pathol. 2008;129:130–142. doi: 10.1309/WYACYWF6NGM3WBRT. [DOI] [PubMed] [Google Scholar]
- 38.Su WP, Buechner SA, Li CY. Clinicopathologic correlations in leukemia cutis. J Am Acad Dermatol. 1984;11:121–128. doi: 10.1016/s0190-9622(84)70145-9. [DOI] [PubMed] [Google Scholar]
- 39.Baer MR, Barcos M, Farrell H, Raza A, Preisler HD. Acute myelogenous leukemia with leukemia cutis. Eighteen cases seen between 1969 and 1986. . Cancer. 1989;63:2192–2200. doi: 10.1002/1097-0142(19890601)63:11<2192::aid-cncr2820631122>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 40.Nasti TH, Timares L. Inflammasome activation of IL-1 family mediators in response to cutaneous photodamage(dagger) Photochem Photobiol. 2012 doi: 10.1111/j.1751-1097.2012.01182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Contassot E, Beer H, French L. Interleukin-1, inflammasomes, autoinflammation and the skin. Swiss Med Wkly. 2012;142:0. doi: 10.4414/smw.2012.13590. [DOI] [PubMed] [Google Scholar]
- 42.de Corla-Souza A, Kolitz J, Shah R, Stannek A, Talwar A. Intrathoracic Myeloid Sarcoma. Journal of Bronchology. 2005;12:119–122. [Google Scholar]
- 43.Sönmez G, Görür A, Mutlu H, ÖZTÜRK E, Sıldıroğlu O, Karagöz B. Spinal Cord Compression Due To Epidural Extramedullary Haematopoiesis In Acute Myeloid Leukemia: Mri Findings. European Journal of General Medicine. Vol 5 [Google Scholar]
- 44.Paydas S, Zorludemir S, Ergin M. Granulocytic sarcoma: 32 cases and review of the literature. Leuk Lymphoma. 2006;47:2527–2541. doi: 10.1080/10428190600967196. [DOI] [PubMed] [Google Scholar]
- 45.Hicsonmez G, Cetin M, Yenicesu I, Olcay L, Koc A, Aktas D, Tuncbilek E, Tuncer M. Evaluation of children with myelodysplastic syndrome: importance of extramedullary disease as a presenting symptom. Leuk Lymphoma. 2001;42:665–674. doi: 10.3109/10428190109099328. [DOI] [PubMed] [Google Scholar]
- 46.Chitragar S, Agarwal S, Iyer VK, Mathur SR, Karak AK, Chharchhodawala T, Sharma A, Bakhshi S. Cyto-morphological features of extramedullary acute megakaryoblastic leukemia on fine needle aspiration and cerebrospinal fluid cytology: A case report. Cytojournal. 2011;8:17. doi: 10.4103/1742-6413.85496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ravandi-Kashani F, Estey E, Cortes J, Medeiros LJ, Giles FJ. Granulocytic sarcoma of the pancreas: a report of two cases and literature review. Clin Lab Haematol. 1999;21:219–224. doi: 10.1046/j.1365-2257.1999.00205.x. [DOI] [PubMed] [Google Scholar]
- 48.Best-Aguilera CR, Vazquez-Del Mercado M, Munoz-Valle JF, Herrera-Zarate L, Navarro-Hernandez RE, Martin-Marquez BT, Oregon-Romero E, Ruiz-Quezada S, Bonilla GM, Lomeli-Guerrero A. Massive myeloid sarcoma affecting the central nervous system, mediastinum, retroperitoneum, liver, and rectum associated with acute myeloblastic leukaemia: a case report. J Clin Pathol. 2005;58:325–327. doi: 10.1136/jcp.2003.015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alvarez P, Navascues CA, Ordieres C, Pipa M, Vega IF, Granero P, Alvarez JA, Rodriguez M. Granulocytic sarcoma of the small bowel, greater omentum and peritoneum associated with a CBFbeta/MYH11 fusion and inv(16) (p13q22): a case report. Int Arch Med. 2011;4:3. doi: 10.1186/1755-7682-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henes M, Nauth A, Staebler A, Becker S, Henes JC. Postmenopausal bleeding as first sign of an acute myelogenous leukaemia: A case report and review of the literature. Med Oncol. 2010;27:815–819. doi: 10.1007/s12032-009-9291-z. [DOI] [PubMed] [Google Scholar]
- 51.Skeete DH, Cesar-Rittenberg P, Jong R, Murray SK, Colgan TJ. Myeloid sarcoma of the vagina: a report of 2 cases. J Low Genit Tract Dis. 2010;14:136–141. doi: 10.1097/LGT.0b013e3181be2999. [DOI] [PubMed] [Google Scholar]
- 52.Cunningham I. A basis for updating our approach to resistant acute leukemia. Am J Hematol. 2012;87:251–257. doi: 10.1002/ajh.22256. [DOI] [PubMed] [Google Scholar]
- 53.Antic D, Elezovic I, Bogdanovic A, Vukovic NS, Pavlovic A, Jovanovic MP, Jakovic L, Kraguljac N. Isolated myeloid sarcoma of the gastrointestinal tract. Intern Med. 2010;49:853–856. doi: 10.2169/internalmedicine.49.2874. [DOI] [PubMed] [Google Scholar]
- 54.Lee SY, Park SJ, Kim YH, Lee JH. Nonleukemic granulocytic sarcoma presenting as intussusception of small bowel. Int J Clin Oncol. 2008;13:467–470. doi: 10.1007/s10147-008-0774-2. [DOI] [PubMed] [Google Scholar]
- 55.Jung SH, Kim HC, Yu CS, Kim JC. Solitary preleukemic granulocytic sarcoma as a cause of small bowel obstruction. Gut Liver. 2007;1:82–86. doi: 10.5009/gnl.2007.1.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sachs PC, Francis MP, Zhao M, Brumelle J, Rao RR, Elmore LW, Holt SE. Defining essential stem cell characteristics in adipose-derived stromal cells extracted from distinct anatomical sites. Cell Tissue Res. 2012 doi: 10.1007/s00441-012-1423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolosin JM, Budak MT, Akinci MA. Ocular surface epithelial and stem cell development. Int J Dev Biol. 2004;48:981–991. doi: 10.1387/ijdb.041876jw. [DOI] [PubMed] [Google Scholar]
- 58.Pevsner-Fischer M, Levin S, Zipori D. The origins of mesenchymal stromal cell heterogeneity. Stem Cell Rev. 2011;7:560–568. doi: 10.1007/s12015-011-9229-7. [DOI] [PubMed] [Google Scholar]
- 59.Shah RS, Shin RK, Castellani RJ. Granulocytic sarcoma mimicking HSV encephalitis. Neurologist. 2010;16:319–321. doi: 10.1097/NRL.0b013e3181b0be8d. [DOI] [PubMed] [Google Scholar]
- 60.Byrd JC, Edenfield WJ, Dow NS, Aylesworth C, Dawson N. Extramedullary myeloid cell tumors in myelodysplastic-syndromes: not a true indication of impending acute myeloid leukemia. Leuk Lymphoma. 1996;21:153–159. doi: 10.3109/10428199609067593. [DOI] [PubMed] [Google Scholar]
- 61.Warme B, Sullivan J, Tigrani DY, Fred DM. Chloroma of the forearm: a case report of leukemia recurrence presenting with compression neuropathy and tenosynovitis. Iowa Orthop J. 2009;29:114–116. [PMC free article] [PubMed] [Google Scholar]
- 62.Hon C, Shek TW, Liang R. Conjunctival chloroma (granulocytic sarcoma) Lancet. 2002;359:2247. doi: 10.1016/s0140-6736(02)09294-2. [DOI] [PubMed] [Google Scholar]
- 63.Suzer T, Colakoglu N, Cirak B, Keskin A, Coskun E, Tahta K. Intracerebellar granulocytic sarcoma complicating acute myelogenous leukemia: a case report and review of the literature. J Clin Neurosci. 2004;11:914–917. doi: 10.1016/j.jocn.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 64.Woo E, Yue CP, Mann KS, Cheung FM, Chan TK, Todd DIntracerebral chloromas. Report of a case and review of the literature. Clin Neurol Neurosurg. 1986;88:135–139. doi: 10.1016/s0303-8467(86)80010-5. [DOI] [PubMed] [Google Scholar]
- 65.Bakst R, Jakubowski A, Yahalom J. Recurrent neurotropic chloroma: report of a case and review of the literature. Adv Hematol. 2011:854240. doi: 10.1155/2011/854240. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Verra WC, Snijders TJ, Seute T, Han KS, Nieuwenhuis HK, Rutten GJ. Myeloid sarcoma presenting as a recurrent, multifocal nerve root entrapment syndrome. J Neurooncol. 2009;91:59–62. doi: 10.1007/s11060-008-9679-1. [DOI] [PubMed] [Google Scholar]
- 67.Suga K, Kawakami Y, Hiyama A, Takeda K, Tanizawa Y, Matsunaga N. F-18 FDG PET/CT findings in a case of gastric relapse of acute myeloblastic leukemia. Clin Nucl Med. 2009;34:788–790. doi: 10.1097/RLU.0b013e3181b81d80. [DOI] [PubMed] [Google Scholar]
- 68.Cash T, Becton D, Mian A. Cardiac myeloid sarcoma: a case report and review of literature. J Pediatr Hematol Oncol. 2011;33:e330–e332. doi: 10.1097/MPH.0b013e3182329b6b. [DOI] [PubMed] [Google Scholar]
- 69.De Toni F, Racaud-Sultan C, Chicanne G, Mas VM, Cariven C, Mesange F, Salles JP, Demur C, Allouche M, Payrastre B, Manenti S, Ysebaert L. A crosstalk between the Wnt and the adhesion-dependent signaling pathways governs the chemosensitivity of acute myeloid leukemia. Oncogene. 2006;25:3113–3122. doi: 10.1038/sj.onc.1209346. [DOI] [PubMed] [Google Scholar]
- 70.Lamorte Sara RL. Dias Sergio. Communication between bone marrow niches in normal bone marrow function and during hemopathies progression. Hematol Rev. 2009 Jul 1;:1. 1. [Google Scholar]
- 71.Ohanian M, Alaly J, S S, Cable C, Halka K. Leptomeningeal myelomatosis in previously treated high-risk kappa light chain multiple myeloma: case report and literature review. Blood and Lymphatic Cancer: Targets and Therapy. 2011;2011:9–18. [Google Scholar]
- 72.Ravandi F, Cortes J, Estrov Z, Thomas D, Giles FJ, Huh YO, Pierce S, O’Brien S, Faderl S, Kantarjian HM. CD56 expression predicts occurrence of CNS disease in acute lymphoblastic leukemia. Leuk Res. 2002;26:643–649. doi: 10.1016/s0145-2126(01)00188-6. [DOI] [PubMed] [Google Scholar]
- 73.Buck AK, Bommer M, Juweid ME, Glatting G, Stilgenbauer S, Mottaghy FM, Schulz M, Kull T, Bunjes D, Moller P, Dohner H, Reske SN. First demonstration of leukemia imaging with the proliferation marker 18F-fluorodeoxythymidine. J Nucl Med. 2008;49:1756–1762. doi: 10.2967/jnumed.108.055335. [DOI] [PubMed] [Google Scholar]
- 74.Aschoff P, Hantschel M, Oksuz M, Werner MK, Lichy M, Vogel W, Pfannenberg C. Integrated FDG-PET/CT for detection, therapy monitoring follow-up of granulocytic sarcoma Initial results. Nuklearmedizin. 2009;48:185–191. doi: 10.3413/nukmed-0236. [DOI] [PubMed] [Google Scholar]
- 75.Tsimberidou AM, Kantarjian HM, Wen S, Keating MJ, O’Brien S, Brandt M, Pierce S, Freireich EJ, Medeiros LJ, Estey E. Myeloid sarcoma is associated with superior event-free survival and overall survival compared with acute myeloid leukemia. Cancer. 2008;113:1370–1378. doi: 10.1002/cncr.23691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaddu S, Zenahlik P, Beham-Schmid C, Kerl H, Cerroni L. Specific cutaneous infiltrates in patients with myelogenous leukemia: a clinicopathologic study of 26 patients with assessment of diagnostic criteria. J Am Acad Dermatol. 1999;40:966–978. doi: 10.1016/s0190-9622(99)70086-1. [DOI] [PubMed] [Google Scholar]
- 77.Agis H, Weltermann A, Fonatsch C, Haas O, Mitterbauer G, Mullauer L, Schreiber S, Schwarzinger I, Juretzka W, Valent P, Jager U, Lechner K, et al. A comparative study on demographic, hematological, and cytogenetic findings and prognosis in acute myeloid leukemia with and without leukemia cutis. Ann Hematol. 2002;81:90–95. doi: 10.1007/s00277-001-0412-9. [DOI] [PubMed] [Google Scholar]
- 78.Ohanian M, Alattar M, Estrov Z, Quintas-Cardama A, Manning J, Ravandi F, Abruzzo L, Cortes J, Pemmaraju N. Myeloid Sarcoma of the Orbit and Ocular Adnexae. The 2012 ASH Annual Meeting abstracts. 2012 https://ash.confex.com/ash/2012/webprogram/Paper52328.html. [Google Scholar]
- 79.Felice M, Zubizarreta P, Alfaro E, Gallego M, Cygler A, Rosso D, Rossi J. Good outcome of Children with Acute Myeloid Leukemia and t(8;21)(q22;q22) Even When Associated with Granulocytic Sarcoma A Report from a Single Institution in Argentina. Cancer. 2000;88:1939–1944. [PubMed] [Google Scholar]
- 80.Traweek ST, Arber DA, Rappaport H, Brynes RK. Extramedullary myeloid cell tumors An immunohistochemical and morphologic study of 28 cases. Am J Surg Pathol. 1993;17:1011–1019. doi: 10.1097/00000478-199310000-00006. [DOI] [PubMed] [Google Scholar]
- 81.Pileri SA, Ascani S, Cox MC, Campidelli C, Bacci F, Piccioli M, Piccaluga PP, Agostinelli C, Asioli S, Novero D, Bisceglia M, Ponzoni M, et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Vol. 21. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, U.K: 2007. pp. 340–350. [DOI] [PubMed] [Google Scholar]
- 82.Stolzel F, Hackmann K, Kuithan F, Mohr B, Fussel M, Oelschlagel U, Thiede C, Rollig C, Platzbecker U, Schetelig J, Illmer T, Schaich M, et al. Clonal evolution including partial loss of human leukocyte antigen genes favoring extramedullary acute myeloid leukemia relapse after matched related allogeneic hematopoietic stem cell transplantation. Transplantation. 2012;93:744–749. doi: 10.1097/TP.0b013e3182481113. [DOI] [PubMed] [Google Scholar]
- 83.Falini B, Lenze D, Hasserjian R, Coupland S, Jaehne D, Soupir C, Liso A, Martelli MP, Bolli N, Bacci F, Pettirossi V, Santucci A, et al. Cytoplasmic mutated nucleophosmin (NPM) defines the molecular status of a significant fraction of myeloid sarcomas. Leukemia. 2007;21:1566–1570. doi: 10.1038/sj.leu.2404699. [DOI] [PubMed] [Google Scholar]
- 84.Falini B, Martelli MP, Bolli N, Sportoletti P, Liso A, Tiacci E, Haferlach T. Acute myeloid leukemia with mutated nucleophosmin (NPM1): is it a distinct entity? Blood. 2011;117:1109–1120. doi: 10.1182/blood-2010-08-299990. [DOI] [PubMed] [Google Scholar]
- 85.Meloni G, Mancini M, Gianfelici V, Martelli MP, Foa R, Falini B. Late relapse of acute myeloid leukemia with mutated NPM1 after eight years: evidence of NPM1 mutation stability. Haematologica. 2009;94:298–300. doi: 10.3324/haematol.2008.000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bolli N, Galimberti S, Martelli MP, Tabarrini A, Roti G, Mecucci C, Martelli MF, Petrini M, Falini B. Cytoplasmic nucleophosmin in myeloid sarcoma occurring 20 years after diagnosis of acute myeloid leukaemia. Lancet Oncol. 2006;7:350–352. doi: 10.1016/S1470-2045(06)70661-1. [DOI] [PubMed] [Google Scholar]
- 87.Bolli N, Payne EM, Grabher C, Lee JS, Johnston AB, Falini B, Kanki JP, Look AT. Expression of the cytoplasmic NPM1 mutant (NPMc+) causes the expansion of hematopoietic cells in zebrafish. Blood. 2010;115:3329–3340. doi: 10.1182/blood-2009-02-207225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cheng K, Sportoletti P, Ito K, Clohessy JG, Teruya-Feldstein J, Kutok JL, Pandolfi PP. The cytoplasmic NPM mutant induces myeloproliferation in a transgenic mouse model. Blood. 2010;115:3341–3345. doi: 10.1182/blood-2009-03-208587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee HS, Jeon JW, Kim JH, Park TE, Park SK, Won JH, Baick SH, Hong DS, Park HS. A case of granulocytic sarcoma of the brain in a patient with myelodysplastic syndrome. Korean J Intern Med. 1995;10:160–163. doi: 10.3904/kjim.1995.10.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alvarez M. Intracerebral granulocytic sarcoma. J Neurosci Nurs. 2007;39:297–304. doi: 10.1097/01376517-200710000-00007. [DOI] [PubMed] [Google Scholar]
- 91.Obara H, Nishimura S, Hayashi N, Numagami Y, Inoue T, Kubo K, Kaimori M, Nishijima M. [Intracranial granulocytic sarcoma in a patient with acute myeloid leukemia] No To Shinkei. 2006;58:797–801. [PubMed] [Google Scholar]
- 92.Makaryus AN, Tung F, Liu W, Mangion J, Kort S. Extensive neoplastic cardiac infiltration in a patient with acute myelogenous leukemia: role of echocardiography. Echocardiography. 2003;20:539–544. doi: 10.1046/j.1540-8175.2003.03092.x. [DOI] [PubMed] [Google Scholar]
- 93.Economopoulos T, Alexopoulos C, Anagnostou D, Stathakis N, Constantinidou M, Papageorgiou E. Primary granulocytic sarcoma of the testis. Vol. 8. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, U.K: 1994. pp. 199–200. [PubMed] [Google Scholar]
- 94.Valbuena JR, Admirand JH, Lin P, Medeiros LJ. Myeloid sarcoma involving the testis. Am J Clin Pathol. 2005;124:445–452. doi: 10.1309/NXLC-J1B1-6YDF-QWND. [DOI] [PubMed] [Google Scholar]
- 95.Kilic G, Boruban MC, Bueco-Ramos C, Konoplev SN. Granulocytic sarcoma involving the uterus and right fallopian tube with negative endometrial biopsy. Eur J Gynaecol Oncol. 2007;28:270–272. [PubMed] [Google Scholar]
- 96.Thalhammer F, Gisslinger H, Chott A, Haas O, Etele-Hainz A, Helbich T, Kusec R, Linkesch W, Simonitsch I, Strobl H, et al. Granulocytic sarcoma of the prostate as the first manifestation of a late relapse of acute myelogenous leukemia. Ann Hematol. 1994;68:97–99. doi: 10.1007/BF01715141. [DOI] [PubMed] [Google Scholar]
- 97.Hsiao LT, Yang CF, Tzeng CH. Penis: a ‘sanctuary’ site of extramedullary leukemia relapse. Int J Hematol. 2009;90:125–126. doi: 10.1007/s12185-009-0369-3. [DOI] [PubMed] [Google Scholar]
- 98.Bagg MD, Wettlaufer JN, Willadsen DS, Ho V, Lane D, Thrasher JB. Granulocytic sarcoma presenting as a diffuse renal mass before hematological manifestations of acute myelogenous leukemia. J Urol. 1994;152:2092–2093. doi: 10.1016/s0022-5347(17)32318-2. [DOI] [PubMed] [Google Scholar]
- 99.Abeler V, Kjorstad KE, Langholm R, Marton PF. Granulocytic sarcoma (chloroma) of the uterine cervix: report of two cases. Int J Gynecol Pathol. 1983;2:88–92. doi: 10.1097/00004347-198301000-00008. [DOI] [PubMed] [Google Scholar]