Abstract
Background
Surgical ablation has emerged as an acceptable treatment modality for patients with atrial fibrillation (AF) undertaking concomitant cardiac surgery. However, the efficacy of surgical ablation in patient populations undergoing mitral valve surgery is not well established. The present meta-analysis aims to establish the current randomized evidence on clinical outcomes of surgical ablation versus no ablative treatment in patients with AF undergoing mitral valve surgery.
Methods
Electronic searches were performed using six databases from their inception to September 2013, identifying all relevant randomized controlled trials (RCTs) comparing surgical ablation versus no ablation in AF patients undergoing mitral valve surgery. Data were extracted and analyzed according to predefined clinical endpoints.
Results
Nine relevant RCTs were identified for inclusion in the present analysis. The number of patients in sinus rhythm (SR) was significantly improved in the surgical ablation group compared to the non-ablation group at discharge. This effect on SR remained at all follow-up periods until >1 year. Results indicated that there was no significant difference between surgical ablation and no ablation in terms of 30-day mortality, all-cause mortality, pacemaker implantation, stroke, thromboembolism, cardiac tamponade, reoperation for bleeding and myocardial infarction.
Conclusions
Results from the present meta-analysis demonstrate that the addition of surgical ablation for AF leads to a significantly higher rate of sinus rhythm in mitral valve surgical patients, with no increase in the rates of mortality, pacemaker implantation, stroke and thromboembolism. Further research should be directed at correlating different surgical ablation subtypes to cardiac and cerebrovascular events at long-term follow-up.
Keywords: Surgical ablation, atrial fibrillation, mitral valve surgery, Maze, meta-analysis
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia observed in clinical practice and confers a high incidence of thromboembolic events and deaths. Although anti-arrhythmic drugs are the first-line treatment for some AF patients, controversy exists regarding their limited efficacy and adverse outcomes (1-3). Surgical ablation represents a non-pharmacological treatment for AF in patients undergoing concomitant cardiac surgery for valvular repair or replacement or coronary artery bypass.
The Cox-Maze III procedure was the first curative option for AF patients and remains the gold-standard treatment today (4). Following animal and human mapping studies, the Cox-Maze III procedure that evolved in 1992 enabled efficacious elimination of abnormal re-entry circuits (5). A series of endocardial incisions through the walls of both atria via median sternotomy and cardiopulmonary bypass (CBP) led to the advent of the pioneering “cut and sew” method. Although this technique is effective in maintaining sinus rhythm (SR) and atrial mechanical function, its widespread application is limited by an associated increase in operative times, morbidity and technical complexity (6-10).
Recent technological advances and an improved understanding of AF etiology have given rise to newer surgical ablation techniques. The discovery by Haïssaguerre and colleagues that the pulmonary veins are the source of ectopic activity in paroxysmal AF led to the development of the pulmonary vein isolation approach, which employs a minimal left atrial lesion set (11). Furthermore, the advent of alternative energy sources has greatly simplified the “cut and sew” technique. Because radiofrequency, microwave and cryothermal energy can be used to create lines of transmural necrosis, surgical incisions used in the Maze procedure are no longer required (12-15). Such developments have transformed surgical ablation into an easier and faster procedure with reduced morbidity.
Previous meta-analyses and systematic reviews have assessed diverse populations undergoing multiple types of surgery, including mitral valve surgery, coronary artery bypass grafting and aortic valve replacement (16-19). However, there are high levels of heterogeneity in some of the outcomes reported, likely derived from such mixed surgical populations. The efficacy of surgical ablation in patient populations undergoing only mitral valve surgery is not well established. Thus, the present meta-analysis aims to provide randomized evidence to evaluate clinical outcomes of surgical ablation in AF patients undergoing mitral valve surgery.
Methods
Literature search strategy
Electronic searches were performed using Ovid Medline, PubMed, Cochrane Central Register of Controlled Trials (CCTR), Cochrane Database of Systematic Reviews (CDSR), ACP Journal Club and Database of Abstracts of Review of Effectiveness (DARE) from their dates of inception to September 2013. To achieve maximum sensitivity of the search strategy and identify all studies, we combined the terms: “AF” and “ablation” or “pulmonary vein” or “maze” and “RCT” as either keywords or MeSH terms. The reference lists of all retrieved articles were reviewed for further identification of potentially relevant studies. All identified articles were systematically assessed using the inclusion and exclusion criteria.
Selection criteria
Eligible randomized controlled trials (RCTs) for the present systematic review and meta-analysis included those in which patient cohorts underwent mitral valve surgery concomitantly with treatment of AF, which utilized surgical ablation techniques including Cox-Maze, radiofrequency ablation, cryoablation and microwave ablation. Studies that did not include SR or AF-free survival as endpoints were excluded. When institutions published duplicate studies with accumulating numbers of patients or increased lengths of follow-up, only the most complete reports were included for quantitative assessment at each time interval. All publications were limited to those involving human subjects and in the English language. Abstracts, case reports, conference presentations, editorials and expert opinions were excluded. Review articles were omitted because of potential publication bias and duplication of results.
Data extraction and critical appraisal
All data were extracted from article texts, tables and figures. Two investigators independently reviewed each retrieved article (K.P. and A.X.). Discrepancies between the two reviewers were resolved by discussion and consensus with a third reviewer (D.H.T.). Assessment of risk of bias for each selected study was performed according to the most updated Cochrane statement. Discrepancies between the two reviewers were resolved by discussion and consensus. The final results were reviewed by the senior investigator (T.D.Y.).
Statistical analysis
The odds ratio (OR) was used as a summary statistic. In the present study, both fixed- and random-effect models were tested. In the fixed-effects model, it was assumed that treatment effect in each study was the same, whereas in a random-effects model, it was assumed that there were variations between studies. χ2 tests were used to study heterogeneity between trials. I2 statistic was used to estimate the percentage of total variation across studies, owing to heterogeneity rather than chance, with values greater than 50% considered as substantial heterogeneity. I2 can be calculated as: I2 = 100% × (Q – df)/Q, with Q defined as Cochrane’s heterogeneity statistics and df defined as degree of freedom (20). If there was substantial heterogeneity, the possible clinical and methodological reasons for this were explored qualitatively. In the present meta-analysis, the results using the random-effects model were presented to take into account the possible clinical diversity and methodological variation between studies. Specific analyses considering confounding factors were not possible because raw data were not available. All P values were 2-sided. All statistical analysis was conducted with Review Manager Version 5.2.1 (Cochrane Collaboration, Software Update, Oxford, United Kingdom).
Results
Quality of studies
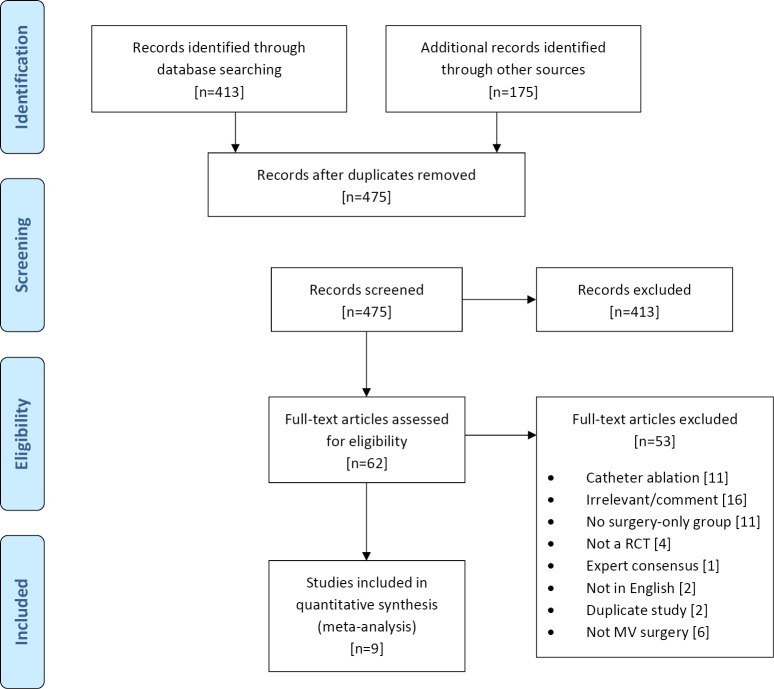
A total of 588 references were identified through six electronic database searches (Figure 1). After exclusion of duplicate or irrelevant references, 474 potentially relevant articles were retrieved. After detailed evaluation of these articles, 62 studies remained for assessment. Manual search of reference lists yielded one new study. After applying the selection criteria, nine RCTs were selected for analysis. The study characteristics of these trials are summarized in Table 1. In these nine studies, 496 patients underwent procedures that involved mitral valve surgery with surgical ablation (MV + SA group; n=270) or without surgical ablation (MV group; n=226). Baseline patient characteristics and risk factors are summarized in Table 2.
Figure 1.
Search strategy of meta-analysis on aortic arch surgery utilizing surgical ablation with mitral valve surgery (MV + SA) versus mitral valve surgery alone (MV) in atrial fibrillation (AF) patients.
Table 1. Summary of RCTs comparing MV + SA versus MV only surgical treatment in AF patients.
| First author (reference) | Year | Institution | Study period | MV + SA | MV | SA type | Primary endpoint |
|---|---|---|---|---|---|---|---|
| Deneke (21) | 2002 | Bergmannsheil University Hospital (Bochum, Germany) | 1998-1999 | 15 | 15 | RF | SR |
| Akpinar (22) | 2003 | Florence Nightingale Hospital (Istanbul, Turkey) | NR | 33 | 34 | RF | AF-free survival |
| Jessurun (23) | 2003 | St Antonius Hospital (Nieuwegein, The Netherlands) | 1996-1999 | 25 | 10 | CS | SR |
| Vasconcelos (24) | 2004 | Instituto do Coracao (São Paulo, Brazil) | 2000-2002 | 15 | 14 | CS | AF-free survival |
| Abreu Filho (25) | 2005 | University of São Paulo Medical School (São Paulo, Brazil) | 2002-2003 | 42 | 28 | RF | SR |
| Doukas (26) | 2005 | Glenfield Hospital (Leicester, England) | 2001-2003 | 49 | 48 | RF | SR |
| Blomström-Lundqvist (27) | 2007 | Uppsala University Hospital (Uppsala, Sweden) | 2003-2005 | 30 | 35 | CY | SR |
| Albrecht (28) | 2009 | Fundação Universitária de Cardiologia (Porto Alegre, Brazil) | 1999-2004 | 40 | 20 | PVI/CS | SR |
| Chevalier (29) | 2009 | Hôpital Louis Pradel (Louis-Pradel, France) | 2002-2005 | 21 | 22 | RF | SR |
NR, not reported; RF, radiofrequency; CS, cut and sew; CY, cryoablation; PVI, pulmonary vein isolation; MV, mitral valve surgery; SA, surgical ablation; AF, atrial fibrillation; SR, sinus rhythm.
Table 2. Summary of baseline patient characteristics and risk factors in studies comparing MV + SA with MV alone in surgical treatment for AF.
| First author | Age |
Male (%) |
LVEF (%) |
LAD (mm) |
AF duration (mo) |
NYHA III-IV (%) |
Mitral stenosis (%) |
Mitral regurgitation (%) |
Mitral mixed (%) |
Permanent or persistent AF (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MV + SA | MV | MV + SA | MV | MV + SA |
MV | MV + SA | MV | MV + SA | MV | MV + SA | MV | MV + SA | MV | MV + SA | MV | MV + SA | MV | MV + SA | MV | |
| Deneke | 65.0 | 70.0 | 40.0 | 20.0 | 64±11 | 61±9 | 60±5 | 58±6 | 44.4 | 43.2 | NR | NR | 13.0 | 20.0 | 53.0 | 66.0 | 33.0 | 13.0 | 100.0 | 100.0 |
| Akpinar | 53±10 | 50±8 | 39.0 | 26.0 | 55±6 | 55±8 | 62±10 | 67±9 | 20±11 | 22±14 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Jessurun | 64±12 | 64±9 | 56.0 | 50.0 | 40±8 | 45±6 | 53±9 | 56±7 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 48.0 | 80.0 |
| Vasconcelos | 50±10 | 51±10 | 27.0 | 43.0 | 69±9 | 66±11 | 55±5 | 56±5 | 24±20 | 34±29 | NR | NR | NR | NR | NR | NR | NR | NR | 100.0 | 100.0 |
| Abreu Filho | 55±13 | 51±10 | 33.0 | 43.0 | 63±9 | 66±11 | 61±11 | 59±5 | 66±58 | 44±29 | 100.0 | 100.0 | 61.9 | 64.0 | 38.1 | 35.8 | 0 | 0 | 100.0 | 100.0 |
| Doukas | 67±9 | 67±8 | 63.0 | 50.0 | 57±6 | 58±7 | 58±7 | 60±11 | 57±55 | 47±64 | NR | NR | NR | NR | 83.6 | 83.6 | 16.4 | 16.7 | NR | NR |
| Blomström-Lundqvist | 66±9 | 66±8 | 83.0 | 74.0 | 54±9 | 57±12 | 61±11 | 58±7 | 26±33 | 33±54 | 66.7 | 68.6 | 3.3 | 0 | 97.7 | 100.0 | 0 | 0 | 100.0 | 100.0 |
| Albrecht | 53±11 | 51±14 | 28.0 | 50.0 | 63±9 | 64±7 | 56±12 | 62±12 | 37±35 | 25±32 | 70.0 | 80.0 | 50.0 | 25.0 | 40.0 | 55.0 | 10.0 | 20.0 | 100.0 | 100.0 |
| Chevalier | 70±6 | 66±10 | 24.0 | 50.0 | 60±9 | 61±9 | 55±11 | 53±11 | 161.0 | 89.2 | NR | NR | 19.1 | 23.0 | 57.1 | 63.6 | NR | NR | 100.0 | 100.0 |
| Minimum | 50.0 | 50.0 | 24.0 | 20.0 | 40.0 | 45.0 | 53.0 | 53.0 | 20.0 | 22.0 | 66.7 | 68.6 | 3.3 | 0 | 38.1 | 35.8 | 10.0 | 13.0 | 48.0 | 80.0 |
| Maximum | 70.0 | 70.0 | 83.0 | 74.0 | 69.0 | 66.0 | 62.0 | 67.0 | 161.0 | 89.2 | 100.0 | 100.0 | 61.9 | 64.0 | 97.7 | 100.0 | 33.0 | 20.0 | 100.0 | 100.0 |
| Weighted average | 60.1 | 59.9 | 45.5 | 46.8 | 58.0 | 59.3 | 58.2 | 59.6 | 52.6 | 39.5 | 80.4 | 81.9 | 35.8 | 25.8 | 62.0 | 71.5 | 9.7 | 9.6 | 93.1 | 98.6 |
LVEF, left ventricular ejection fraction; LAD, left atrial diameter; AF, atrial fibrillation; NYHA, New York Heart Association Functional Classification; NR, not reported.
All of the included studies were RCTs (Level 1 evidence) (21-29). Four studies had >50 patients (range, 29-97 patients) (22,25-27), while the remaining studies had less than 50 patients (range, 29-49 patients) (21,23,24,30,31). Four studies used radiofrequency ablation (21,25,26,29), one study used radiofrequency with port-access (22), three studies used Cox-Maze cut-and-sew (23,24,28), one study used cryoablation (27) and one study reported patients undergoing pulmonary vein isolation (28). Permanent AF, persistent AF and a mixture of permanent, persistent and paroxysmal AF populations were evaluated by six studies (21,23,25,27-29), one study (24) and two studies (23,26), respectively.
One study reported follow-up of greater than 3 years (56 months) (28). Two studies had follow-up between 2-3 years (range, 26-29 months) (21,24), while six studies had follow-up <2 years (12-18 months) (22,23,25-27,29). SR was the primary endpoint in seven studies (21,23,25-29), while AF-free survival was the primary endpoint in two studies (22,24). 1-, 3-, 6-, 12-month and >1 year SR outcomes were reported in one study (24), seven studies (21-26,29), six studies (21,22,24-27), nine studies (21-29) and three studies (22,24,28), respectively. 30-day mortality was reported by all nine studies (21-29).
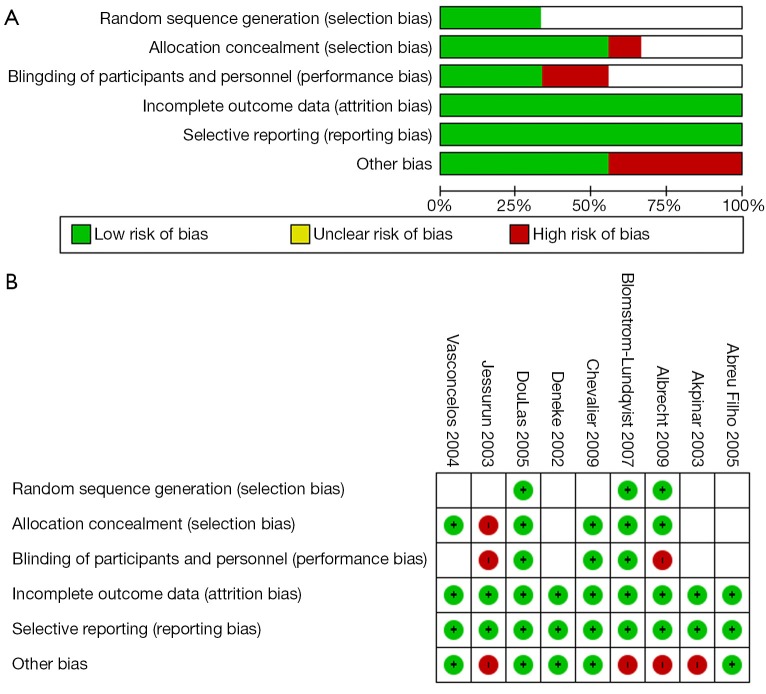
The 9 RCTs were also assessed qualitatively using tools recommended by the Cochrane Collaboration for the risk of bias. A graph and summary of selection bias, performance bias, detection bias, attrition bias, reporting bias and other bias identified in each individual RCT is shown in Figure 2.
Figure 2.
(A) Methodological quality graph and (B) Methodological quality summary for the risk of bias from randomized controlled trials comparing concomitant surgical ablation and mitral valve surgery versus mitral valve surgery alone.
Demographic and operative characteristics
Similar baseline characteristics were observed in both comparison arms. Males accounted for 24-83% of patients undergoing MV + SA and 20-74% undergoing MV alone (weighted mean: 46% vs. 47%; P=0.85). The average age ranged between 50-70 years for both MV + SA and MV groups (weighted mean: 60.1 vs. 59.9; P=0.60) for MV + SA and MV groups respectively. A greater number of patients in each study presented with mitral regurgitation compared to stenosis. However, no significant difference was found between MV + SA and MV groups in the proportion of patients with mitral valve stenosis (weighted mean: 35.8% vs. 25.8%; P=0.29), mitral valve regurgitation (weighted mean: 62.0% vs. 71.5%; P=0.47) or mixed mitral pathology (weighted mean: 9.7% vs. 9.6%; P=0.89). Furthermore, most patients recruited presented with persistent or permanent AF, with no significant differences between the treated and control groups (weighted mean: 93.1% vs. 98.6%; P=0.89). All other comparative preoperative characteristics were infrequently reported, including chest/sternal infection, mediastinitis, endocarditis, pneumonia and sepsis.
CBP time was significantly longer when MV surgery was performed concomitantly with surgical ablation. With the exception of two studies (21,29) which did not report CBP, all other studies reported CBP times which demonstrated longer average CBP for the MV + SA group (weighted mean: 144.4 vs. 101.3 minutes; P=0.02). Similarly, cross-clamp time was significantly longer for the MV + SA group compared to MV (weighted mean: 76.4 vs. 63.8 minutes; P<0.00001). For both cohorts, the frequency of the different subtypes of MV surgery was comparable. Mitral valve repair ranged from 0-77% and 0-80% for MV + SA and MV (weighted mean: 45.4% vs. 46.3%; P=0.92) respectively. Mitral valve replacement ranged from 23-100% and 20-100% for MV + SA and MV (weighted mean: 52.2% vs. 57.4%; P=0.33) respectively.
Assessment of safety
Mortality
Mortality outcomes at 30 days were reported in all studies. The risk of 30-day all-cause mortality was not significantly different between MV + SA and MV groups at 30 days [4.4% vs. 2.7%; OR, 1.45; 95% confidence interval (CI), 0.55-3.83; P=0.46; I2=0%]. Furthermore, all-cause mortality was also not significantly different (6.3% vs. 4.0%; OR, 1.40; 95% CI, 0.30-3.26; P=0.43; I2=0%; Figure 3). No significant heterogeneity was observed in these two comparisons.
Figure 3.
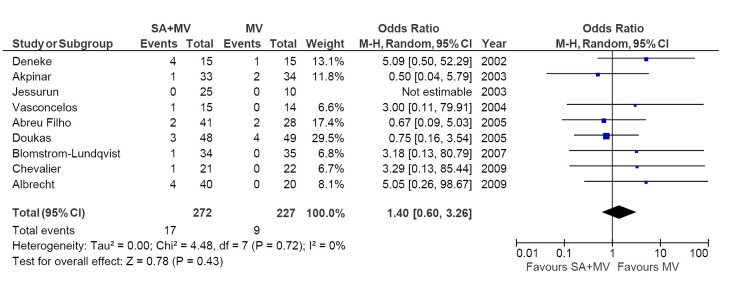
Forest plot of the odds ratio (OR) of all-cause mortality in AF patients with surgical ablation (SA + MV) or without ablation (MV). The estimate of the OR of each trial corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the number of events as a fraction of the total number randomized is shown for both treatment and control groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. M-H, Mantel-Haenszel.
Pacemaker implants
All but one study reported outcomes for pacemaker implantation. Overall, there was no difference in pacemaker implantations whether surgical ablation was performed or not (7.0% vs. 7.5%; OR, 1.00; 95% CI, 0.47-2.13; P=1.00; I2=0%; Figure 4).
Figure 4.
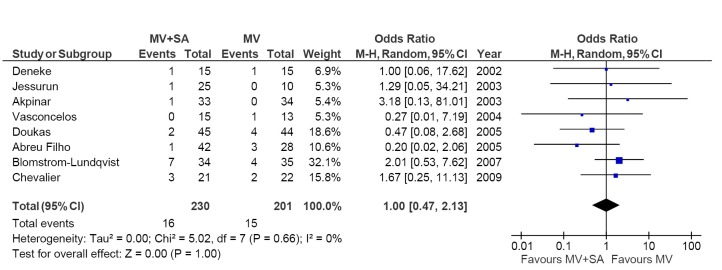
Forest plot of the odds ratio (OR) of permanent pacemaker implantation in AF patients with surgical ablation (MV + SA) or without ablation (MV). The estimate of the OR of each trial corresponds to the middle of the squares and the horizontal line shows the 95% confidence interval (CI). On each line, the number of events as a fraction of the total number randomized is shown for both treatment and control groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. M-H, Mantel-Haenszel.
Stroke
Stroke outcomes were reported in eight out of nine included RCTs, with comparable results between MV + SA and MV groups (5.5% vs. 3.9%; OR, 1.43; 95% CI, 0.56-3.65; P=0.45; I2=0%; Figure 5).
Figure 5.
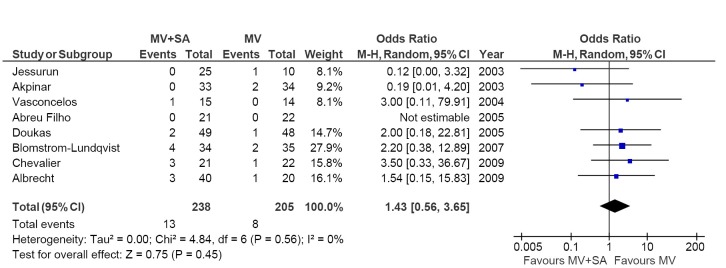
Forest plot of the odds ratio (OR) of stroke and thromboembolic events in AF patients with surgical ablation (MV + SA) or without ablation (MV). The estimate of the OR of each trial corresponds to the middle of the squares and the horizontal line shows the 95% confidence interval (CI). On each line, the number of events as a fraction of the total number randomized is shown for both treatment and control groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. M-H, Mantel-Haenszel.
Other morbidities
The frequency of cardiac tamponade and pericardial effusion as perioperative complications was favorable for MV + SA compared to MV groups (2.4% vs. 9.0%; OR, 0.27; 95% CI, 0.08-0.94; P=0.04; I2=0%). This study showed no difference between the treated and control groups with regards to reoperative bleeding (6.8% vs. 5.8%; OR, 1.19; 95% CI, 0.46-3.06; P=0.71; I2=0%). Additionally, the incidence of myocardial infarction was comparable between both groups (35% vs. 29%; OR, 1.31; 95% CI, 0.70-2.48; P=0.40; I2=0%). Other clinical outcomes including low cardiac output syndrome, hypertension and heart failure were not reported in more than two studies (Table 3).
Table 3. Summary of perioperative characteristics and complications.
| First author | CBP time (min) |
Cross-clamp time (min) |
Mitral valve repair (%) |
Mitral valve replacement (%) |
30-day mortality (%) |
Cardiac tamponade (%) |
Reoperation for bleeding (%) |
Antiarrhythmics at 1-year follow-up (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MV + SA | MV | MV + SA | MV | MV + SA | MV | MV + SA | MV | MV + SA | MV | MV + SA | MV | MV+ SA | MV | MV + SA | MV | |
| Deneke | 188M | 127M | 103M | 85M | 0 | 0 | 100.0 | 100.0 | 13.0 | 0 | NR | NR | NR | NR | NR | NR |
| Akpinar | 141±34 | 128±28 | 89±13 | 79±9 | 36 | 38.0 | 64.0 | 62.0 | 3.0 | 2.9 | 3.0 | 6.0 | 3.0 | 2.9 | 0 | 8.9 |
| Jessurun | 155±27 | 97±27 | 90±24 | 60±18 | 40 | 40.0 | 60.0 | 60.0 | 0 | 0 | NR | NR | NR | NR | 52.0 | 90.0 |
| Vasconcelos | 106±17 | 78±24 | NR | NR | 6.7 | 0 | 93.0 | 100.0 | 6.7 | 7.1 | 6.7 | 28.6 | 6.7 | 0 | NR | NR |
| Abreu Filho | 107±21 | 78±24 | 68±14 | 47±16 | 30.9 | 3.5 | 69.1 | 96.5 | 2.3 | 0 | 3.6 | 7.1 | NR | NR | NR | NR |
| Doukas | 106±34 | 99±37 | 70±26 | 64±28 | 77 | 71.0 | 23.0 | 29.0 | 6.1 | 8.3 | NR | NR | NR | NR | NR | NR |
| Blomström-Lundqvist | 147±28 | 119±33 | 87±95 | 84±23 | 70 | 80.0 | 30.0 | 20.0 | 3.3 | 0 | 0.0 | 2.9 | 5.9 | 5.7 | 26.7 | 31.4 |
| Albrecht | 223±22 | 62±24 | 76±18 | 45±21 | 60 | 57.5 | 42.5 | 40.0 | 15.0 | 0 | NR | NR | 5.0 | 0 | NR | NR |
| Chevalier | NR | NR | 93±32 | 74±19 | 19 | 59.9 | 45.5 | 80.9 | 4.8 | 0 | NR | NR | NR | NR | NR | NR |
| Minimum | 106.0 | 62.0 | 68.0 | 45.0 | 0 | 0 | 23.0 | 20.0 | 0 | 0 | 0 | 2.9 | 3.0 | 0 | 0 | 8.9 |
| Maximum | 223.0 | 128.0 | 103.0 | 85.0 | 77.0 | 80.0 | 100 | 100.0 | 15.0 | 8.3 | 6.7 | 28.6 | 6.7 | 5.7 | 52.0 | 90.0 |
| Weighted average | 144.4 | 101.3 | 76.4 | 63.8 | 45.4 | 46.3 | 52.2 | 57.4 | 5.9 | 2.6 | 2.9 | 8.2 | 4.9 | 2.9 | 23.9 | 29.1 |
CBP, cardiopulmonary bypass time; NR, not reported; M, mean.
Assessment of efficacy
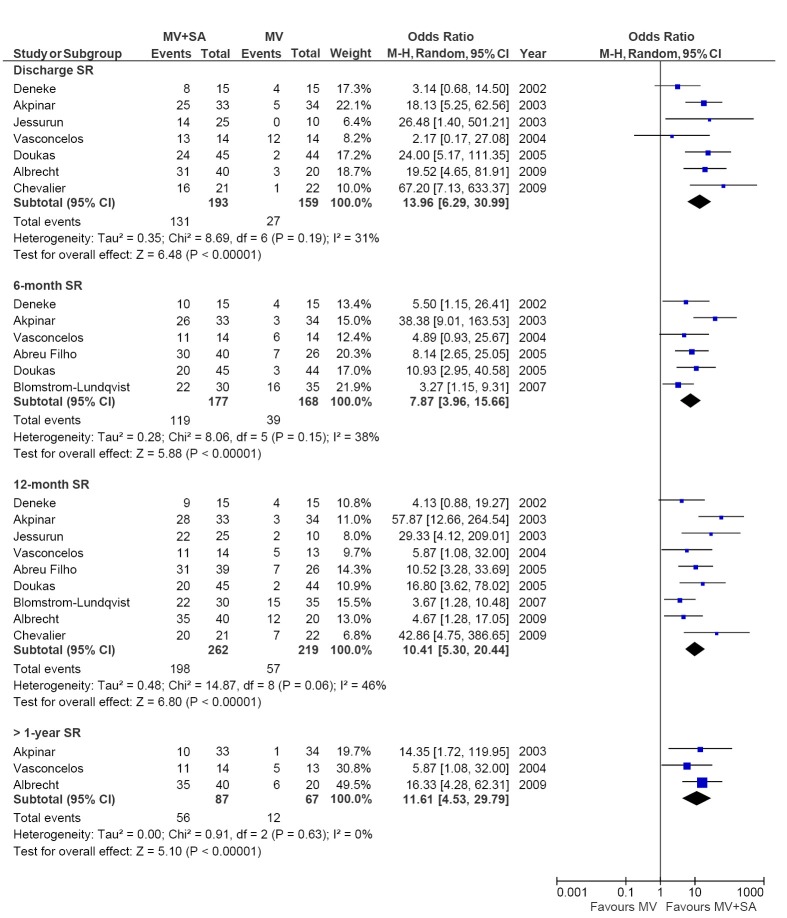
The number of patients in SR at discharge was significantly higher in the MV + SA group compared to the MV group (67.9% vs. 17.0%; OR, 13.96; 95% CI, 6.29-30.99; P<0.00001; I2=31%). The MV + SA group also had a significantly higher proportion of patients in SR compared to MV only at 3-month (65% vs. 21.2%; OR, 7.46; 95% CI, 4.16-13.37; P<0.00001; I2=0%), 6-month (67.2% vs. 23.2%; OR, 7.87; 95% CI, 3.96-15.66; P<0.00001; I2=38%), 12-month (75.5% vs. 26%; OR, 10.41; 95% CI, 5.30-20.44; P<0.00001; I2=46%) and >12-month (64.4% vs. 17.9%; OR, 11.61; 95% CI, 4.53-29.79; P<0.00001; I2=0%) follow-up periods. The results are summarized in Figure 6. Subgroup analysis of the different surgical ablation techniques including radiofrequency ablation, cut and sew, pulmonary vein isolation and cryoablation demonstrated no significant difference affecting SR outcomes any all follow-up periods.
Figure 6.
Forest plot of the odds ratio (OR) of discharge, 6-month, 12-month and >1-year SR in AF patients with surgical ablation (MV + SA) or without ablation (MV). The estimate of the OR of each trial corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the number of events as a fraction of the total number randomized is shown for both treatment and control groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. M-H, Mantel-Haenszel.
Discussion
A substantial increase in the incidence of AF has been reported in patients with indications for cardiac surgery, which has also been demonstrated to be a profound risk factor for mortality in multiple studies (32-35). The weight of this evidence has provided the impetus for the combination of surgical AF treatment and core cardiac surgical intervention, with the hope of synergistic improvements in both SR prevalence and risk of morbidity and mortality (36). A recent prospective RCT revealed differing outcomes between various types of cardiac surgery, with MV surgery demonstrating superior benefit from surgical ablation compared to coronary artery bypass and aortic valve procedures (37). Despite this, robust evidence regarding the efficacy of surgical ablation in MV surgery is still lacking. The present meta-analysis is the first comprehensive review, to our knowledge, of all published RCTs reporting the clinical outcomes of MV + SA versus MV alone.
Given that AF has consistently been shown to be an independent predictor of mortality (38-40), the maintenance of SR is vital for quality of life and survival. For example, the AFFIRM study demonstrated that the prevalence of SR was a profound, independent predictor of survival, even after adjustment for all other clinical variables including age and various comorbidities (41). Patients in SR were almost half as likely to die compared to patients who did not improve from AF (adjusted hazard ratio, 0.53; 99% CI, 0.39 to 0.72; P<0.0001). As such, the prevalence of SR at both short- and long-term follow-up is the most important endpoint considered for AF patients. Our results demonstrate significant improvement in restoration of SR in the ablation group compared to the control group at discharge (67.9% vs. 17.0%), 3-month (65% vs. 21.2%), 12-month (75.5% vs. 26%), and >1-year (64.4% vs. 17.9%) follow-up periods. These results are consistent with previous meta-analyses involving mixed surgical populations including MV and CABG. For example, Cheng et al. published a meta-analysis, which included ten RCTs and 23 non-RCTs with an overall study population of 4,647 patients (18). They found SR prevalence at >1-year follow-up to be 74.6%, which is very similar to that reported in the current meta-analysis. However, this result had significant heterogeneity (I2=84%), with only one RCT included in the analysis. Their meta-analysis also grouped RCTs with retrospective observational studies, some of which had controversial study designs and outcomes.
With the increased incidence of AF, the short- and long-term mortality following surgical ablative treatment is of key clinical interest. The present review found acceptable overall mortality rates at 30-day (range: 0-15%) follow-up periods with no difference between MV + SA and MV groups. Similar results were attained by Boller et al. at both short- and long-term follow-up (mean: 16.2% vs. 17.4%; P=0.80) in their large multicenter, prospective RCT (n=224) (30). However, the patient cohort for this RCT predominantly underwent cryoablation, a less invasive thoracoscopic procedure, which involves creation of transmural lesions and scarring via tissue freezing. This highly specific cohort means that their results cannot be widely applied to all surgical ablation techniques, thus justifying the importance and necessity of the current study. While a previous meta-analysis of non-RCT studies suggested that all-cause mortality is reduced in surgical ablation groups compared to control cardiac surgery groups, such a trend was not reflected in the present study, with no difference in all-cause mortality found at the latest follow-up ≥1 year (overall: 0-17.4%). This can be attributed to the susceptibility of non-RCT studies to bias in patient selection and incomplete ascertainment of outcomes. The resultant higher level of heterogeneity (I2=44%) warrants caution when interpreting their study outcomes.
The current study revealed no difference in permanent pacemaker implantation in the surgical ablation group compared to the control group (mean: 7.0% vs. 7.5%; P=1.00). In contrast, a previous meta-analysis by Khargi et al. argued that there was a small difference in post-operative pacemaker implantation in favor of the ablation group (mean: 4.9% vs. 5.8%), albeit this difference was not found to be significant (19). While it is plausible that surgical ablation may reduce the incidence of pacemaker implantation due to enhanced SR outcomes, this interpretation is brought into question once the variability of indications for pacemaker implantation is considered. Some groups have aggressively reported atypical bradycardic arrhythmia (24,25) as indications for pacemaker implantations, while other groups are more conservative in their approach, reporting atrioventricular block or bundle branch block as indications (22,27,29).
In previous studies, surgical ablation using the Cox-Maze technique in AF patients undergoing concomitant surgery was demonstrated to have a potential protective effect from stroke and thromboembolism in the long term (4,10,18,42). However, it is not clear whether this effect is due to the nature of the Maze operation, resumption of SR, removal of the left atrial appendage, or even continued anticoagulation with warfarin. The results of the present meta-analysis also indicate no significant difference in the incidence of stroke and thromboembolic events in favor of the surgical ablation group compared with the control MV surgery group (mean: 5.5% vs. 3.9%; P=0.45). In contrast, Boersma et al. published a RCT comparing surgical ablation with catheter ablation for treatment of AF, with fewer incidents of stroke and thrombus formation reported in the surgical ablation group at 12-month follow-up (43). These superior outcomes can be explained by the nature of the surgical procedure, where any thrombus formation or occlusion of the left atrial appendage can be easily resolved by intraoperative resection of the left auricle. For the same reason, our lack of difference in stroke incidence between MV + SA and MV groups is expected, considering both procedures involve left atrial incisions. Thus, our meta-analysis demonstrates that superior long-term SR prevalence can be achieved via surgical ablation with no additional risk of stroke or thromboembolism.
Other clinical outcomes were poorly reported in the RCTs included in this meta-analysis. There was a significantly lower incidence of cardiac tamponade in the MV + SA group compared to the MV group. No significant difference between MV + SA and MV groups was found in terms of incidence of re-operative bleeding and myocardial infarction. However, it is likely, although it cannot be proven, that the incidence of these perioperative complications is underestimated in these studies as some patients did not survive the operation and thus such events were not diagnosed. This emphasizes the importance of consistent reporting of composite clinical complications, including low cardiac output syndrome, hypertension and heart failure. Some groups have posited that the lack of improvement in clinical outcomes supports the argument that surgical ablation should be re-evaluated as an option for AF treatment (31,44). In contrast, an alternative interpretation of such outcomes is that surgical ablation can effectively maintain SR rhythm for long follow-up periods without significant increases in morbidity and mortality. For these reasons, surgical ablation should still be considered an effective and safe treatment option that addresses AF at its electrophysiological origin and offers a reasonable chance of an enduring cure.
The present findings are limited by a number of key constraints. Firstly, although the RCTs included in the present meta-analysis include a highly selected cohort who underwent isolated MV surgery, the greater proportion of patients had undergone MV replacement compared to mitral valve repair. Future subgroup analysis of the types of MV surgery will elucidate whether this factor influences the efficacy and mortality outcomes of surgical ablation. Secondly, subgroup analysis of the outcomes according to the type of surgical ablation technique was not feasible, due to the low number of RCTs in each subgroup. Indeed, only 4 radiofrequency and 3 cut-and-sew studies, and one cryoablation and one pulmonary vein isolation study were identified in this meta-analysis, which highlights the need for future RCTs or large registries which evaluate surgical ablation techniques according to subgroup. Thirdly, although >1-year follow-up SR is significantly more favorable for patients who underwent surgical ablation, this was based only on three studies. The shortage of long-term data beyond one year limits the provision of evidence-based guidelines and recommendations and thus more long-term data are required. Long-term studies are also required to compare perioperative complications between MV + SA and MV groups, because very few complications were consistently reported in the RCTs included in this meta-analysis. However, owing to the randomization process, confounding factors were reduced within each individual study, and the present meta-analysis represents a summary of the available randomized evidence in the current literature.
In summary, we conclude that concomitant surgical ablation and mitral valve surgery for AF offers better short- and mid-term SR outcomes than for patients who do not undergo surgical ablation. No differences were found between the two groups in terms of 30-day mortality, all-cause mortality, pacemaker implantation, stroke and thromboembolic events. Thus, this meta-analysis indicates that surgical ablation can be performed in AF patients without increased risk of morbidity and mortality.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Boriani G, Diemberger I, Biffi M, et al. Pharmacological cardioversion of atrial fibrillation: current management and treatment options. Drugs 2004;64:2741-62 [DOI] [PubMed] [Google Scholar]
- 2.Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002;347:1834-40 [DOI] [PubMed] [Google Scholar]
- 3.Hohnloser SH, Kuck KH. Randomized trial of rhythm or rate control in atrial fibrillation: the Pharmacological Intervention in Atrial Fibrillation Trial (PIAF). Eur Heart J 2001;22:801-2 [DOI] [PubMed] [Google Scholar]
- 4.Prasad SM, Maniar HS, Camillo CJ, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg 2003;126:1822-8 [DOI] [PubMed] [Google Scholar]
- 5.Cox JL, Schuessler RB, D’Agostino HJ, Jr, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991;101:569-83 [PubMed] [Google Scholar]
- 6.Chiappini B, Martìn-Suàrez S, LoForte A, et al. Cox/Maze III operation versus radiofrequency ablation for the surgical treatment of atrial fibrillation: a comparative study. Ann Thorac Surg 2004;77:87-92 [DOI] [PubMed] [Google Scholar]
- 7.Izumoto H, Kawase T, Ishihara K, et al. Survival and sinus rhythm maintenance after modified Cox/maze procedure and mitral valve operation in patients with chronic atrial fibrillation. Jpn J Thorac Cardiovasc Surg 2001;49:58-61 [DOI] [PubMed] [Google Scholar]
- 8.Raanani E, Albage A, David TE, et al. The efficacy of the Cox/maze procedure combined with mitral valve surgery: a matched control study. Eur J Cardiothorac Surg 2001;19:438-42 [DOI] [PubMed] [Google Scholar]
- 9.Bando K, Kobayashi J, Kosakai Y, et al. Impact of Cox maze procedure on outcome in patients with atrial fibrillation and mitral valve disease. J Thorac Cardiovasc Surg 2002;124:575-83 [DOI] [PubMed] [Google Scholar]
- 10.Schaff HV, Dearani JA, Daly RC, et al. Cox-Maze procedure for atrial fibrillation: Mayo Clinic experience. Semin Thorac Cardiovasc Surg 2000;12:30-7 [DOI] [PubMed] [Google Scholar]
- 11.Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659-66 [DOI] [PubMed] [Google Scholar]
- 12.Ad N, Henry L, Massimiano P, et al. The state of surgical ablation for atrial fibrillation in patients with mitral valve disease. Curr Opin Cardiol 2013;28:170-80 [DOI] [PubMed] [Google Scholar]
- 13.MacDonald DR, Maruthappu M, Nagendran M. How effective is microwave ablation for atrial fibrillation during concomitant cardiac surgery? Interact Cardiovasc Thorac Surg 2012;15:122-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edgerton ZJ, Edgerton JR. A review of current surgical treatment of patients with atrial fibrillation. Proc (Bayl Univ Med Cent) 2012;25:218-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams MR, Garrido M, Oz MC, et al. Alternative energy sources for surgical atrial ablation. J Card Surg 2004;19:201-6 [DOI] [PubMed] [Google Scholar]
- 16.Reston JT, Shuhaiber JH. Meta-analysis of clinical outcomes of maze-related surgical procedures for medically refractory atrial fibrillation. Eur J Cardiothorac Surg 2005;28:724-30 [DOI] [PubMed] [Google Scholar]
- 17.Basu S, Nagendran M, Maruthappu M.How effective is bipolar radiofrequency ablation for atrial fibrillation during concomitant cardiac surgery? Interact Cardiovasc Thorac Surg 2012;15:741-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng DC, Ad N, Martin J, et al. Surgical ablation for atrial fibrillation in cardiac surgery: a meta-analysis and systematic review. Innovations (Phila) 2010;5:84-96 [DOI] [PubMed] [Google Scholar]
- 19.Khargi K, Hutten BA, Lemke B, et al. Surgical treatment of atrial fibrillation; a systematic review. Eur J Cardiothorac Surg 2005;27:258-65 [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58 [DOI] [PubMed] [Google Scholar]
- 21.Deneke T, Khargi K, Grewe PH, et al. Efficacy of an additional MAZE procedure using cooled-tip radiofrequency ablation in patients with chronic atrial fibrillation and mitral valve disease. A randomized, prospective trial. Eur Heart J 2002;23:558-66 [DOI] [PubMed] [Google Scholar]
- 22.Akpinar B, Guden M, Sagbas E, et al. Combined radiofrequency modified maze and mitral valve procedure through a port access approach: early and mid-term results. Eur J Cardiothorac Surg 2003;24:223-30 [DOI] [PubMed] [Google Scholar]
- 23.Jessurun ER, van Hemel NM, Defauw JJ, et al. A randomized study of combining maze surgery for atrial fibrillation with mitral valve surgery. J Cardiovasc Surg (Torino) 2003;44:9-18 [PubMed] [Google Scholar]
- 24.Vasconcelos JT, Scanavacca MI, Sampaio RO, et al. Surgical treatment of atrial fibrillation through isolation of the left atrial posterior wall in patients with chronic rheumatic mitral valve disease. A randomized study with control group. Arq Bras Cardiol 2004;83:211-8; 203-10. [DOI] [PubMed]
- 25.Abreu Filho CA, Lisboa LA, Dallan LA, et al. Effectiveness of the maze procedure using cooled-tip radiofrequency ablation in patients with permanent atrial fibrillation and rheumatic mitral valve disease. Circulation 2005;112:I20-5 [DOI] [PubMed] [Google Scholar]
- 26.Doukas G, Samani NJ, Alexiou C, et al. Left atrial radiofrequency ablation during mitral valve surgery for continuous atrial fibrillation: a randomized controlled trial. JAMA 2005;294:2323-9 [DOI] [PubMed] [Google Scholar]
- 27.Blomström-Lundqvist C, Johansson B, Berglin E, et al. A randomized double-blind study of epicardial left atrial cryoablation for permanent atrial fibrillation in patients undergoing mitral valve surgery: the SWEDish Multicentre Atrial Fibrillation study (SWEDMAF). Eur Heart J 2007;28:2902-8 [DOI] [PubMed] [Google Scholar]
- 28.Albrecht A, Kalil RA, Schuch L, et al. Randomized study of surgical isolation of the pulmonary veins for correction of permanent atrial fibrillation associated with mitral valve disease. J Thorac Cardiovasc Surg 2009;138:454-9 [DOI] [PubMed] [Google Scholar]
- 29.Chevalier P, Leizorovicz A, Maureira P, et al. Left atrial radiofrequency ablation during mitral valve surgery: a prospective randomized multicentre study (SAFIR). Arch Cardiovasc Dis 2009;102:769-75 [DOI] [PubMed] [Google Scholar]
- 30.Boller C, Buderath M.Fatigue in aerostructures--where structural health monitoring can contribute to a complex subject. Philos Trans A Math Phys Eng Sci 2007;365:561-87 [DOI] [PubMed] [Google Scholar]
- 31.Whitlock RP, Healey JS, Connolly SJ. Left atrial appendage occlusion does not eliminate the need for warfarin. Circulation 2009;120:1927-32; discussion 1932. [DOI] [PubMed]
- 32.Saxena A, Dinh D, Dimitriou J, et al. Preoperative atrial fibrillation is an independent risk factor for mid-term mortality after concomitant aortic valve replacement and coronary artery bypass graft surgery. Interact Cardiovasc Thorac Surg 2013;16:488-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wyse DG, Love JC, Yao Q, et al. Atrial fibrillation: a risk factor for increased mortality--an AVID registry analysis. J Interv Card Electrophysiol 2001;5:267-73 [DOI] [PubMed] [Google Scholar]
- 34.Crandall MA, Horne BD, Day JD, et al. Atrial fibrillation significantly increases total mortality and stroke risk beyond that conveyed by the CHADS2 risk factors. Pacing Clin Electrophysiol 2009;32:981-6 [DOI] [PubMed] [Google Scholar]
- 35.Naccarelli GV, Johnston SS, Lin J, et al. Cost burden of cardiovascular hospitalization and mortality in ATHENA-like patients with atrial fibrillation/atrial flutter in the United States. Clin Cardiol 2010;33:270-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edgerton ZJ, Edgerton JR. Rationale for minimally invasive pulmonary vein isolation and partial autonomic denervation for surgical treatment of atrial fibrillation. Innovations (Phila) 2008;3:121-4 [DOI] [PubMed] [Google Scholar]
- 37.Budera P, Straka Z, Osmančík P, et al. Comparison of cardiac surgery with left atrial surgical ablation vs. cardiac surgery without atrial ablation in patients with coronary and/or valvular heart disease plus atrial fibrillation: final results of the PRAGUE-12 randomized multicentre study. Eur Heart J 2012;33:2644-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villareal RP, Hariharan R, Liu BC, et al. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol 2004;43:742-8 [DOI] [PubMed] [Google Scholar]
- 39.Glotzer TV, Hellkamp AS, Zimmerman J, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation 2003;107:1614-9 [DOI] [PubMed] [Google Scholar]
- 40.Wolf PA, Mitchell JB, Baker CS, et al. Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch Intern Med 1998;158:229-34 [DOI] [PubMed] [Google Scholar]
- 41.Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation 2004;109:1509-13 [DOI] [PubMed] [Google Scholar]
- 42.McCarthy PM, Gillinov AM, Castle L, et al. The Cox-Maze procedure: the Cleveland Clinic experience. Semin Thorac Cardiovasc Surg 2000;12:25-9 [DOI] [PubMed] [Google Scholar]
- 43.Boersma LV, Castella M, van Boven W, et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation 2012;125:23-30 [DOI] [PubMed] [Google Scholar]
- 44.Hindricks G, Piorkowski C.Surgical ablation of atrial fibrillation after the PRAGUE-12 study: more questions than answers. Eur Heart J 2012;33:2636-8 [DOI] [PubMed] [Google Scholar]