Abstract
Background
Atrial fibrillation (AF) is an increasingly prevalent condition in the ageing population, with significantly associated morbidity and mortality. Surgical and catheter ablative strategies both aim to reduce mortality and morbidity through freedom from AF. This review consolidates all currently available comparative data to evaluate these two interventions.
Methods
A systematic search was conducted across MEDLINE, PubMed, Embase, Cochrane Central Register of Controlled Trials and the Cochrane Database of Systematic Reviews from January 2000 until August 2013. All studies were critically appraised and only those directly comparing surgical and catheter ablation were included.
Results
Seven studies were deemed suitable for analysis according to the inclusion criteria. Freedom from AF was significantly higher in the surgical ablation group versus the catheter ablation group at 6-month, 12-month and study endpoint follow-up periods. Subgroup analysis demonstrated similar trends, with higher freedom from AF in the surgical ablation group for paroxysmal AF patients. The incidence of pacemaker implantation was higher, while no difference in stroke or cardiac tamponade was demonstrated for the surgical versus catheter ablation groups.
Conclusions
Current evidence suggests that epicardial ablative strategies are associated with higher freedom from AF, higher pacemaker implantation rates and comparable neurological complications and cardiac tamponade incidence to catheter ablative treatment. Other complications and risks were poorly reported, which warrants further randomized controlled trials (RCTs) of adequate power and follow-up duration.
Keywords: Atrial fibrillation (AF), catheter ablation, surgical ablation, epicardial ablation, endocardial ablation
Introduction
Atrial fibrillation (AF) is a relatively common abnormal rhythm of the heart, occurring in 1-2% of the general population (1). The prevalence of AF increases with age and is responsible for 15-20% of ischemic strokes, thus representing a significant health care burden (2). It is present in two forms: paroxysmal or intermittent AF, characterized by episodes with varying frequency and periodicity, and persistent or chronic AF, a sustained rhythm. Consideration of both anti-thrombotic and anti-arrhythmic therapies informs the treatment approach for AF (1).
Pharmacological anti-arrhythmic agents are the first-line therapy for paroxysmal and persistent AF. However, these medications are often poorly tolerated or cause significant morbidity and mortality themselves (1). AF is associated with a decreased quality of life relative to sinus rhythm. Much controversy surrounds the use of rate- or rhythm-controlling agents as optimal therapy. Despite this, rate versus rhythm control trials, utilizing both types of medication, have not been able to conclusively demonstrate an advantage for rhythm control on a quality of life scale (1). As such, clinician preference is often a deciding factor.
Ablation strategies are used with the intent of “curing” AF, removing the requirement for ongoing antiarrhythmic medication. Both catheter and surgical ablation procedures rely on expert staff at specialized institutions for safety. Present guidelines recommend catheter ablation for patients with symptomatic paroxysmal AF resistant to at least one anti-arrhythmic medication (1). Meta-analyses comparing catheter ablation to anti-arrhythmic medication have demonstrated a clear superiority in favor of catheter ablation for rhythm outcomes and freedom from anti-arrhythmic medication (3,4). The reported efficacy of catheter ablation is 61-89% (5) with a complication rate of 6%. Around a third of patients require multiple ablations for successful treatment (5).
Surgical ablation was initially developed with cut and sew lesions and was first described by Cox et al. in 1991 (6). Since its inception, the Cox-Maze procedure has undergone many changes and refinements (7). The cut and sew Maze-III procedure has a reported efficacy of up to 90% when considering the outcome of freedom from symptomatic AF (8). However, the technical difficulty and risks associated with this on-pump, open heart procedure have led to the development of alternative techniques with various energy sources, including cryoablation, radiofrequency ablation and pulmonary vein isolation. Minimally invasive video-assisted thoracoscopic surgery (VATS) off-pump operations utilizing radiofrequency or cryoablation energy delivery devices have been employed to perform epicardial ablation (9). These operations have a reported rate of freedom from AF of 65-82% as reported in small case series (10-13).
While there is clear evidence for the superiority of catheter ablation compared to antiarrhythmic therapy, its relative efficacy compared with surgical ablation is not well established. Thus, this review aims to consolidate a number of smaller studies comparing transcatheter endocardial ablation with epicardial ablation and cut and sew techniques to assess their relative efficacy and safety in clinical practice.
Methods
Literature search strategy
A systematic review of studies comparing surgical ablation to catheter ablation for the treatment of AF was performed. Five electronic databases including MEDLINE, PubMed, Embase, Cochrane Central Register of Controlled Trials and the Cochrane Database of Systematic Reviews were searched from January 2000 until August 2013. Appropriate free text and MeSH terms were used to identify all studies: “cardiac catheterisation” OR “endocardial ablation” OR “pulmonary vein isolation” OR “catheter” OR “catheter ablation” and “surgical ablation” OR “epicardial ablation” OR “MAZE” OR “video assisted thoracic surgery” OR “videothoracoscopy” OR “thoracoscopy” and “AF”. Reference lists of all articles found were searched to further identify potentially relevant studies.
Outcome measures
The findings from initial scoping searches were used in deciding which outcomes to include in the present review. Freedom from AF was the primary endpoint identified. Secondary outcomes identified include adverse events such as hematoma pacemaker implantation, pneumothorax, myocardial infarction and cerebrovascular events.
Eligibility criteria
Studies eligible for this systematic review directly compared surgical ablation techniques to catheter ablation techniques in patients with AF. Experimental or observational studies were also included. Case reports, series with less than ten patients, abstracts, editorials and expert opinions were excluded. If more than one article had been published from the same center with the same dataset, only the article with the most complete dataset published was used. All studies selected were human trials and in English.
Data extraction and critical appraisal
Three reviewers (WYC, MYH, RS) independently appraised studies from January 2000 to August 2013, using a standard form and extracted data on methodology, quality criteria and outcome measures. All extracted and tabulated data were checked by an additional reviewer (KK). The quality of studies was assessed using assessment criteria recommended by the Centre for Evidence Based Medicine (University of Oxford) (14). Discrepancies between reviewers were resolved by discussion and consensus was reached.
Statistical analysis
The odds ratio (OR) was used as a summary statistic. In the present study, both fixed- and random-effect models were tested. In the fixed-effects model, it was assumed that treatment effect in each study was the same, whereas in a random-effects model, it was assumed that there were variations between studies. χ2 tests were used to study heterogeneity between trials. I2 statistic was used to estimate the percentage of total variation across studies, owing to heterogeneity rather than chance, with values greater than 50% considered as substantial heterogeneity. I2 can be calculated as: I2 =100% × (Q – df)/Q, with Q defined as Cochrane’s heterogeneity statistics and df defined as degree of freedom (15). If there was substantial heterogeneity, the possible clinical and methodological reasons for this were explored qualitatively. In the present meta-analysis, the results using the random-effects model were presented to take into account the possible clinical diversity and methodological variation between studies. Specific analyses considering confounding factors were not possible because raw data were not available. All P values were 2-sided. All statistical analysis was conducted with Review Manager Version 5.2.1 (Cochrane Collaboration, Software Update, Oxford, United Kingdom).
Results
Quantity of studies
A total of 406 studies were identified from the databases searched. Initial evaluation of the titles and abstracts of the articles found identified seven potentially relevant publications. When the inclusion criteria were applied to these studies, all seven articles remained relevant for assessment (Figure 1).
Figure 1.
PRISMA flow chart.
Quality of evidence
All studies appraised were from specialized tertiary referral centers. Two prospective randomized controlled trials were found (16,17), as well as 5 retrospective analyses (18-22). Six of the 7 were from single institutions (17-22), with one study deriving data from multiple institutions (16). Six of the 7 studies had 99 or more patients (range 99-291, Table 1), with one smaller study (20) presenting data from only 45 patients.
Table 1. Included studies.
| First author (year) | N | CA | SA | Study type | Mean follow-up | Outcome(s) | Inclusion criteria | Exclusion criteria |
|---|---|---|---|---|---|---|---|---|
| Stulak (18) (2011) | 291 | 194 | 97 | Case-control | 5.6 yrs (median) (SA); 3.1 yrs (CA) |
❖Freedom from atrial fibrillation; ❖Freedom from atrial fibrillation without anti arrhythmic. Measured by reviewing ECG and 24 h Holter monitor data |
SA: Cox-Maze III from lone AF between Jan 1993 and Dec 2007; CA: catheter ablation for AF during the same period. Patients matched 1:2 according to age, sex and AF type |
None specified |
| Boersma (16) (2011) | 124 | 63 | 61 | RCT | 2 yrs | Freedom from left atrial arrhythmia (lasting >30 secs) without anti-arrhythmic drugs | Antiarrhythmic refractory AF, with LA dilatation and hypertension or failed prior CA | Longstanding AF >1 yr, cardiac CA or a surgical cardiac procedure in last 3 months, previous stroke/TIA, LA thrombus, LA diameter >65 mm, LVEF <45%, MR or AR > grade 2, mod/severe AS or MS, active infection or sepsis, pregnancy, unstable angina, MI in last 3 months, AF secondary to electrolyte imbalance, thyroid disease or other reversible or non-cardiac AF cause, history of blood clotting abnormalities, known sensitivity to heparin or warfarin, life expectancy <12 months, involvement in another clinical study, pleural adhesions, prior thoracotomy, prior cardiac surgery and elevated hemidiaphragm |
| Wang (19) (2011) | 166 | 83 | 83 | Pair-matched case-control | 1 yr to 3.6 yrs | Freedom from AF lasting >30 secs; Freedom from anti-arrhythmic drugs |
SA: patients with long-standing persistent AF (>12 non-pharmacological intervention) treated with thoracoscopic surgical ablation. No prior CA. Cases: symptomatic AF refractory to at least 1 class 1 or 3 antiarrhythmic. No prior CA |
SA: LVEF <30%, sick sinus syndrome, severe pleural adhesions, prior attempts at CA. CA: LVEF <30%, LA thrombus on TOE, prior attempts to CA or SA |
| Mahapatra (20) (2011) | 45 | 30 | 15 | Case-control | 30 | Event-free survival of any documented AF recurrence or AAD use | Cases: patients with persistent or long-standing persistent atrial fibrillation who failed at least one catheter ablation and one anti-arrhythmic drug underwent SA Sequential patients were matched to 30 who had previously failed catheter ablation and underwent a repeat catheter ablation |
Any other indication for cardiac surgery, clot in LAA, significant pulmonary hypertension (RV systolic pressure >60 mmHg on TTE), lung disease, less than 18 yrs of age or had a reversible cause of AF |
| Gu (17) (2013) | 95 | 47 | 48 | RCT | 48 | Freedom from recurrence of atrial arrhythmia lasting >30 sec 12-48 months post ablation; Secondary endpoints: incidence of post procedural complications |
Patients with rheumatic heart disease and persistent AF for >1 yr who underwent valvular heart operation from Jan 2006 to Jun 2008 | Paroxysmal AF, non-rheumatic heart disease, left atrial thrombus on TOE, previous AF ablation and valve commissurotomy |
| Krakor (21) (2011) | 112 | 78 | 34 | Cohort | 6 | Freedom from atrial fibrillation at 6 months | Patients undergoing endoscopic mitral valve repair known to have atrial fibrillation; Patients with current AV block or insitu pacemaker were excluded | None specified |
| De Maat (21) (2014) | 99 | 66 | 33 | Case-control | 12 | Freedom from atrial fibrillation at 12 months as measured by Holter monitor | Patients with paroxysmal or short-lasting persistent AF who failed on at least 1 AAD but without prior ablation | Left atrial size >55 mm, prior CA, prior heart or lung surgery, significant coronary disease or previous MI, LVH >12 mm, previous hospitalization for heart failure, LVEF <50%, mod/severe mitral or aortic valve disease, COPD Gold class III-IV or prior TB |
AF, atrial fibrillation; SA, surgical ablation; CA, catheter ablation.
One study, Gu (2013) (17), reported data on a subgroup of patients with rheumatic heart disease undergoing a valvular heart operation concurrently. Similarly, Krakor and colleagues (21) reported on a patient population concurrently undergoing endoscopic mitral valve repair.
Follow-up duration varied between studies from 6 months to a median of 5.6 years for the surgical cases in Stulak and colleagues (18). Three studies reported data for the primary outcome at 12 months, with one at 6 months and the remaining three at 20 months or longer (Table 2).
Table 2. Summary of baseline patient characteristics.
| First author (year of publication) | Type of procedure | Age | Male (%) | Paroxysmal atrial fibrillation (%) | AF duration (years) | LVEF (%) | LA diameter (mm) | Rheumatic heart disease (%) | Previous catheter ablation (%) |
|---|---|---|---|---|---|---|---|---|---|
| Stulak (2011) | SA | 56M | 68.0 | 69.0 | NR | NR | NR | 0 | 6.2 |
| CA | 54M | 71.0 | 71.0 | NR | NR | NR | 0 | 11 | |
| Boersma (2011) | SA | 56.1 | 73.8 | 73.8 | 7.4 | 57.7 | 42.5 | 0 | Yes |
| CA | 56.0 | 87.3 | 58.8 | 6.8 | 55.5 | 43.2 | 0 | ||
| Wang (2011) | SA | 57 | 69.9 | 0 | 5.9 | 62.0 | 51.0 | 0 | No |
| CA | 55 | 62.7 | 0 | 5.8 | 61.0 | 53.0 | 0 | NR | |
| Mahapatra (2011) | SA | 59.5 | 53.3 | 0 | 5.4 | 47.0 | 52.3 | 0 | Yes |
| CA | 59.2 | 63.3 | 0 | 4.9 | 54.7 | 45.3 | 0 | ||
| Gu (2013) | SA | 48 | 42.0 | 0 | 5.6 | 65.1 | 61.7 | 100 | No |
| CA | 47 | 47.0 | 0 | 6.3 | 61.3 | 60.4 | 100 | ||
| Krakor (2011) | SA | 62 | 47.0 | 21.0 | 2.4 | 43.5 | 50.3 | 0 | No |
| CA | 61 | 58.0 | 23.0 | 2.2 | 48.5 | 48.1 | 0 | ||
| De Maat (2014) | SA | 51 | 82.0 | 85.0 | 3.4 | NR | 41.7 | 0 | No |
| CA | 53 | 82.0 | 73.0 | 4.8 | NR | 40.8 | 0 | ||
| Minimum | 47 | 42.0 | 0 | 2.2 | 43.5 | 40.8 | 0 | ||
| Maximum | 62 | 82.0 | 83.0 | 7.4 | 65.1 | 61.7 | 100 | ||
| Weighted average mean | 55.1 | 67.1 | 41.6 | 3.6 | 56.9 | 49.0 | 12.5 |
NR, not reported; SA, surgical ablation; CA, catheter ablation; M, median.
Differences in surgical intervention methods between studies are described in Table 3. Gu et al. (17) and Stulak et al. (18) both describe an open heart procedure requiring cardiopulmonary bypass and a sternotomy. Stulak is the only study to utilize a cut and sew procedure instead of ablation. The other five studies (16,19-22) use a minimally invasive thoracoscopic procedure not requiring cardiopulmonary bypass. The left atrial appendage was removed or excluded in four of the seven studies (16,17,19,20).
Table 3. Comparison of study protocols.
| Author (year) | Surgical ablation protocol | Left atrial appendage removal/excluded | Catheter ablation protocol | Blanking period | Postablative medical management | Post procedure amiodarone | Post procedure anticoagulation |
|---|---|---|---|---|---|---|---|
| Stulak (2011) | Biatrial cut and sew Cox Maze III procedure between 1993 and 2007 as described by Cox and colleagues (7) using cardiopulmonary bypass. During this time 2 minor modifications to the procedure were used that have been previously described (8) | No | Techniques and lesions sets varied during the study period. Three general approaches were used. All included invasive catheter mapping and then either focal radiofrequency ablation, segmental RF ablation or wide area AF ablation around pulmonary veins | 3 months for surgical patients | Surgical: Amiodarone used in those who went into an atrial arrhythmia in hospital and continued for 3 months with electrical cardioversion as needed. Warfarin used for 3 months post-operatively. Catheter: Warfarin anticoagulation usually initiated and continued for at least 3 months. Anticoagulation continued for ongoing AF or >50% narrowing in a pulmonary vein. No mention of amiodarone |
In surgical patients who had a recurrence in first 3 months |
3 months anticoagulation postoperatively and continued if clinically appropriate |
| Boersma (2011) | Video-assisted thoracoscopy technique described by Wolf (9) Epicardial PVI was performed with bipolar radiofrequency clamp. At least 2 overlapping applications were made around each vein, and isolation was confirmed. At one site (Barcelona) an additional application was made in the interatrial Waterson groove to isolate the ganglionic plexi. At the other site (St Antonius) the bilateral epicardial ganglia was ablated additionally. LA appendage was removed in all patients | Yes | Wide area linear antrum ablation with documented PV isolation as an end point | 3 months blanking period in which electrical or chemical cardioversion to sinus rhythm if clinically indicated | All patients were warfarinized or treated with aspirin for 3 months based on CHADS2 score which was continued or ceased based on the treating cardiologist | Not mentioned |
Anticoagulated for 3 months based on VHADS2 score |
| Wang (2011) | Video-assisted thoracoscopic surgical procedure performing bilateral pulmonary vein isolation. Bipolar radiofrequency clamp and generator system used (Atricure). Ligament of Marshall taken down in all patients as well as thoracoscopic left atrial appendage exclusion | Yes | Radiofrequency current ablation used to isolate pulmonary veins, contiguous lesions at least 20 mm from PV ostia, current delivered until local electrogram amplitude reduced by 80% | 200 mg amiodarone PO daily for 3 months. Anticoagulated according to AHA/ACC/European Society of Cardiology guidelines (11) based on CHADS score. AAD discontinued when SR with present. During follow up: if AF or AFL found, patient was cardioverted and underwent repeat CPVI |
3 months amiodarone | Anticoagulated based on CHADS2 score. | |
| Mahapatra (2011) | Thoracoscopic epicardial ablation was performed as described as the Dallas Lesion set (12) | Yes | Catheter ablation patients were chosen retrospectively but lesion set included at least antral ablation, roof line and CTI line. Mitral lines were made in 17 cases, CS ablation in 9, SVC isolation in 11 and CFAE performed in 12 cases | 3 months–electrical cardioversion within 72 hours if indicated | 3 months amiodarone (one month at 400 mg daily, 200 mg for remaining 2 months). If intolerant, placed on dofetilide. Anticoagulated based on CHADS2 score for 3 months and indefinitely if ≥2 |
3 months AAD (amiodarone or dofetilide) | For 3 months and indefinitely if CHADS2 ≥2 |
| Gu (2013) | Modified CryoMaze III was performed with sternotomy and cardiopulmonary bypass at the same time as valvular heart operation. LAA excluded. RF ablation used to make lesions | Yes | Underwent valvular heart operation and 6 months after, catheter ablation was performed. Circumferential PV ablation was performed at angiographically identified PV ostia, with an endpoint of PVI. For those with AF persistence, CFAEs in the PV area, roof, anterior wall, septum and poster-inferior wall of the left atria were mapped and ablated. If AF still persisted, linear ablation was carried out according to the method previously described by Knecht (13) | First 1 month after ablation–defined as early recurrence. After this period, any episode of AF was considered a recurrence. Reablation was performed at least 1 month after initial procedure. CT was used at 3 months post procedure to assess pulmonary vein stenosis | All cases received low molecular weight heparin and then warfarin anticoagulation with an INR between 2.5 and 3. Amiodarone was loaded with 600 mg IV daily for 3 days, 600 mg PO for 1 week and then 200-400 daily for 3 months. It was then withdrawn in those without AF recurrence but continued in those with recurrence | For 3 months | Indefinitely where indicated |
| Krakor (2011) | Endoscopic mitral valve repair with concomitant radiofrequency epicardial ablation accessed via right thoracotomy | Not specified | Endoscopic mitral valve repair with concomitant endocardial cryoablation (first 78 patients) | 90% of patients in both groups were on amiodarone for 6 to 12 months post operatively with the only determinant of withdrawal amiodarone intolerance | 6 to 12 months of amiodarone | Not specified | |
| De Maat (2014) | Minimally invasive off-pump VATS bipolar RF clamp used to isolate pulmonary veins and ablate active ganglionic plexi when found | No [2-4] | Right femoral vein access obtained, transseptal puncture made then endocardial wide area circumferential PVI ablation performed with a radiofrequency unipolar electrode | Catheter ablation patients were given LMWH and restarted oral anticoagulation as per CHADS2VASC score. In surgical patients, LMWH heparin was restarted then oral anticoagulation was restarted 1 month after surgery. Oral anticoagulation was restarted immediately in the CA group based on CHADS2VASC score. Antiarrhythmic drugs were continued for the first 3 months | 3 months | As clinically indicated based on CHADS2VASC score |
Techniques amongst studies for endocardial ablation also varied (Table 3). Radiofrequency ablation was used in all but one study which favored cryoablation (21). Lesion sets between studies were also variable and are presented in Table 3.
Baseline demographics
Three of the seven studies included only patients with persistent AF (17,19,20). The prevalence of paroxysmal AF varied from 21% to 85% amongst those who included that patient population (Table 2). Subgroup data analysis for this patient population was only performed for two studies (16,18). This data is included in Table 4.
Table 4. Results.
| First author (year) | Type of procedure | Freedom from AF at 6 months (%) | Freedom from AF at 12 months (%) | Freedom from AF at study endpoint (%) | Mean follow up at study endpoint (months) | Absolute increase in freedom from AF (%) | Overall % followed up (%) | Paroxysmal AF, free from arrhythmia at study endpoint | Persistent AF, free from arrhythmia at study endpoint | Prior failed CA, free from AF at 12 months | LA dilatation/HTN, free from AF at 12 months |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stulak (2011) | SA | NR | NR | 84.0 | 67.2 | 10.0 | NR | 84% | 83% | NR | NR |
| CA | NR | NR | 74.0 | 37.2 | 76% | 69% | NR | NR | |||
| Boersma (2011) | SA | 67.2 | 65.6 | 65.6 | 12.0 | 29.1 | 96.8 | 68.9%* | 58.8%* | 68.2% | 58.8% |
| CA | 44.4 | 36.5 | 36.5 | 12.0 | 95.5 | 35.1%* | 36%* | 36.8% | 36% | ||
| Wang (2011) | SA | NR | NR | 74.7 | 26.4 | 15.7 | 98.8 | NR | NR | NR | NR |
| CA | NR | NR | 59.0 | 26.4 | 97.6 | NR | NR | NR | NR | ||
| Mahapatra (2011) | SA | NR | NR | 86.7 | 20.7 | 33.4 | NR | NR | NR | NR | NR |
| CA | NR | NR | 53.3 | 20.7 | NR | NR | NR | NR | |||
| Gu (2013) | SA | NR | 79.2 | 62.5 | 48.0 | 17.0 | 96.0 | NR | NR | NR | NR |
| CA | NR | 53.2 | 34.0 | 48.0 | 94.0 | NR | NR | NR | NR | ||
| Krakor (2011) | SA | 82.0 | NR | NR | NR | 8.0 | NR | NR | NR | NR | NR |
| CA | 74.0 | NR | NR | NR | NR | NR | NR | NR | |||
| De Maat (2014) | SA | NR | 90.0 | NR | NR | 27.0 | 93.9 | NR | NR | NR | NR |
| CA | NR | 63.0 | NR | NR | 97.0 | NR | NR | NR | NR | ||
| Minimum | NA | NA | 34.0 | 12.0 | 8.0 | 93.9 | NA | NA | NA | NA | |
| Maximum | NA | NA | 86.7 | 67.2 | 33.4 | 98.8 | NA | NA | NA | NA | |
| Weighted average mean | SA | NA | NA | 74.5 | 39.7 | 17.0 | 96.9 | NA | NA | NA | NA |
| CA | NA | NA | 59.4 | 31.3 | 96.3 | NA | NA | NA | NA |
NA, not applicable; NR, not reported; AF, atrial fibrillation; SA, surgical ablation; CA, catheter ablation; *, at 12 months.
Duration of AF varied from 2.2 to 7.4 years, with data available for all but one study (18). Looked exclusively at patients suffering from rheumatic heart disease undergoing concomitant valvular surgery.
In five of 7 studies, patients had not previously undergone an endocardial ablation procedure. Stulak (18) and Mahapatra (20) studies both contained patients who had previously had a catheter ablation procedure but did not include data for individual results for these subgroups.
Assessment of efficacy
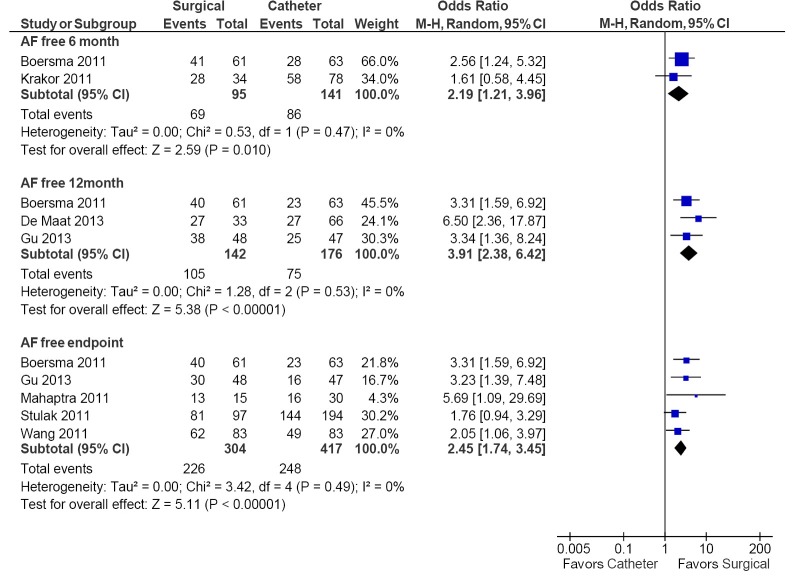
Seven studies reported the incidence of freedom from AF and demonstrated superior efficacy in the surgical ablation arm compared to catheter ablation at 6 months (73% vs. 61%; OR, 2.19; 95% CI, 1.21-3.96; P=0.01; I2=0%), 12 months (74% vs. 43%; OR, 3.91; 95% CI, 2.38-6.42; P<0.00001; I2=0%), and at the study endpoint (74% vs. 59%; OR, 2.45; 95% CI, 1.74-3.45; P<0.00001; I2=0%). These results are summarized in Figure 2. At study endpoint (1-5.6 years), absolute increase in freedom from AF varied from 8-45% between the studies included (Table 4).
Figure 2.
Forest plot of the odds ratio (OR) of freedom from AF at 6 months, 12 months and endpoint in AF patients undergoing surgical or catheter ablation. The estimate of the OR of each trial corresponds to the middle of the squares and the horizontal line shows the 95% confidence interval (CI). On each line, the number of events as a fraction of the total number randomized is shown for both treatment and control groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. AF, atrial fibrillation; M-H, Mantel-Haenszel.
Freedom from AF subgroup data for paroxysmal AF and persistent AF were available for two studies (16,18). In the paroxysmal subgroup, higher freedom from AF was reported in the surgical arm compared to catheter ablation (77% vs. 67%; OR, 2.47; 95% CI, 1.00-6.12; P=0.05; I2=57%). For the persistent AF subgroup, there was a non-significant trend towards higher freedom from AF outcomes in the surgical ablation arm (74% vs. 55%; OR, 2.34; 95% CI, 0.98-5.62; P=0.06; I2=0%). These results are summarized in Figure 3. Similar increases in freedom from AF were also observed for patients with prior failed catheter ablation and those with left atrial dilatation and hypertension, however this was only reported in one study (16).
Figure 3.
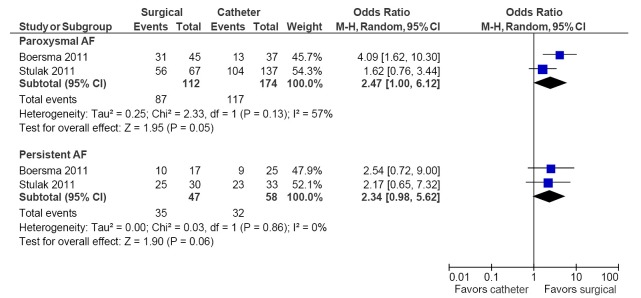
Forest plot of the odds ratio (OR) of freedom from AF for paroxysmal versus persistent AF patients undergoing surgical or catheter ablation. The estimate of the OR of each trial corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the number of events as a fraction of the total number randomized is shown for both treatment and control groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. AF, atrial fibrillation; M-H, Mantel-Haenszel.
Assessment of safety
Table 5 depicts all adverse events reported in these seven studies. The most common adverse event was the development of pulmonary vein stenosis, with an incidence of >50% in one study, in 19 of 194 catheter ablation arm patients (18). Pacemaker implantation rates were significantly higher in the surgical ablation arm compared to catheter ablation (5.4% vs. 1.5%; OR, 3.63; 95% CI, 1.30-10.13; P=0.01; I2=0%; Figure 4). No differences between surgery and catheter groups were observed in terms of incidence of stroke/TIA (1.9% vs. 0.7%; OR, 2.34; 95% CI, 0.69-7.91; P=0.17; I2=0%; Figure 5) and cardiac tamponade or pericardial effusion (2.0% vs. 3.0%; OR, 1.16; 95% CI, 0.25-5.41; P=0.85; I2=0%; Figure 6).
Table 5. Adverse events.
| First author (year published) | Type of procedure | MI/cardiac event (%) | Respiratory failure (%) | Renal failure (%) | Pericardial tamponade/effusion (%) | TIA/stroke (%) | Pneumothorax (%) | Hemothorax (%) | Rib fracture (%) | Sternotomy for bleeding (%) | Pneumonia (%) | Death | Pacemaker implant (%) | Minor groin haematoma/bleed (%) | Sternal wound infection (%) | Pulmonary vein stenosis >50% (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stulak (2011) | SA | 1 | 1 | 1 | 0 | 2.1 | NR | NR | NR | NR | NR | NR | 7.2 | 0 | 1 | 0 |
| CA | 0 | 0 | 0 | 4.6 | 1.0 | NR | NR | NR | NR | NR | NR | 2.6 | 3.1 | 0 | 9.8 | |
| Boersma (2011) | SA | NR | NR | NR | 1.6 | 1.6 | 9.8 | 1.6 | 1.6 | 1.6 | 1.6 | NR | 3.3 | 0 | NR | NR |
| CA | NR | NR | NR | 1.6 | 1.6 | 0 | 0 | 0 | 0 | 0 | NR | 0 | 6.3 | NR | NR | |
| Wang (2011) | SA | NR | NR | NR | NR | 1.2 | NR | NR | NR | NR | NR | NR | 4.8 | NR | NR | NR |
| CA | NR | NR | NR | NR | 0 | NR | NR | NR | NR | NR | NR | 0 | NR | NR | NR | |
| Mahapatra (2011) | SA | NR | NR | NR | 0 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| CA | NR | NR | NR | 3.3 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| Gu (2013) | SA | NR | NR | NR | 2.1 | 2.1 | NR | NR | NR | NR | 6.3 | NR | NR | NR | 8.3 | NR |
| CA | NR | NR | NR | 0 | 0 | NR | NR | NR | NR | 8.5 | NR | NR | NR | 6.4 | NR | |
| Krakor (2011) | SA | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| CA | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| De Maat (2014) | SA | NR | NR | NR | 9.1 | 3.0 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| CA | NR | NR | NR | 1.5 | 0 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
NR, not reported. SA, surgical ablation; CA, catheter ablation.
Figure 4.
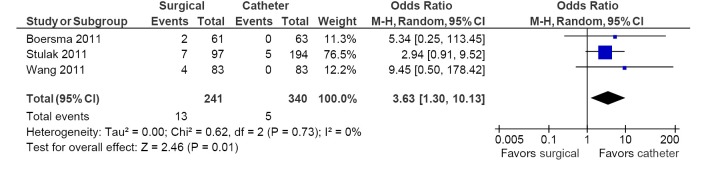
Forest plot of the odds ratio (OR) of pacemaker implantations in AF patients undergoing surgical versus catheter ablation. The estimate of the OR of each trial corresponds to the middle of the squares and the horizontal line shows the 95% confidence interval (CI). On each line, the number of events as a fraction of the total number randomized is shown for both treatment and control groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. AF, atrial fibrillation; M-H, Mantel-Haenszel.
Figure 5.
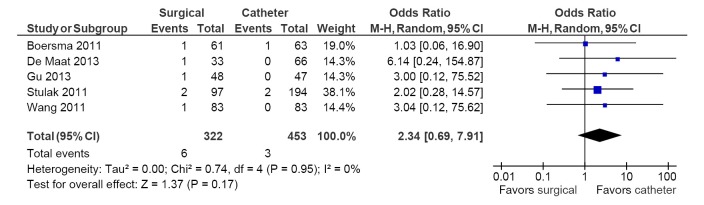
Forest plot of the odds ratio (OR) of strokes/TIA in AF patients undergoing surgical versus catheter ablation. The estimate of the OR of each trial corresponds to the middle of the squares and the horizontal line shows the 95% confidence interval (CI). On each line, the number of events as a fraction of the total number randomized is shown for both treatment and control groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. AF, atrial fibrillation; M-H, Mantel-Haenszel.
Figure 6.
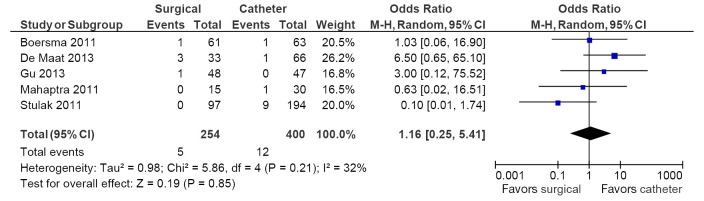
Forest plot of the odds ratio (OR) of cardiac tamponade or pericardial effusion in AF patients undergoing surgical versus catheter ablation. The estimate of the OR of each trial corresponds to the middle of the squares and the horizontal line shows the 95% confidence interval (CI). On each line, the number of events as a fraction of the total number randomized is shown for both treatment and control groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. AF, atrial fibrillation; M-H, Mantel-Haenszel.
Discussion
Catheter and surgical ablation techniques have been developed over the past 20 years as curative strategies for AF. From initial cut and sew techniques to energy delivery devices applied epicardially and endocardially, the complexity of AF ablation strategies is still evolving. This review covers a spectrum of surgical ablation techniques, from cut and sew techniques as performed by the initial pioneers, to innovative epicardial ablation delivered via a minimally invasive VATS procedure.
According to the European Society of Cardiology guidelines, surgical and catheter ablation procedures are reserved for those failing anti-arrhythmic drug therapy (1). The same guidelines in 2010 further recommended that surgical ablation should be reserved for patients failing catheter ablation. Since that time, six of the seven studies included in this review have been published (16,18-22). Epicardial ablation strategies have traditionally shown better efficacy relative to endocardial ablation (9-13) but have rarely been directly compared.
Freedom from AF is an important clinical outcome from AF treatment, and has been shown to be a predictor of quality of life and survival. The cut and sew procedure demonstrated increased freedom from AF relative to catheter ablation at short-term (6-month), mid-term (12-month) and long-term follow-up periods. Indeed, VATS and epicardial ablation procedures showed an 8-45% absolute increase in freedom from AF (Table 4). These results are consistent with previous studies comparing the efficacy of surgical ablation versus catheter treatment of AF. The lowest increase in efficacy was demonstrated by Krakor (21), however these results may have been influenced by the patient population undergoing concomitant mitral valve repair surgery. In this population, one could hypothesize that there is a different causative mechanism for AF.
Three studies looked exclusively at persistent AF patients: Wang (19), Mahapatra (20) and Gu (17). These studies showed an additional benefit for surgical ablation of 15.7-33.4% (Table 4). They also demonstrated excellent overall freedom from AF, with rates of persistent AF at study endpoint of 74.7-88% (Table 4).
Subgroup analysis demonstrated significantly higher freedom from AF with surgical ablation in paroxysmal AF patients. Boersma (16) had subgroup data for paroxysmal AF showing a 33.8% absolute increase in freedom from AF at 12 months. This is a greater overall benefit than that for persistent AF within this study, which was 22.8%. The surgical and catheter ablation techniques achieved the lowest rates of efficacy in this review (Table 4). We postulate that this may be in part due to the use of a patient population who had previously failed catheter ablation and had been proven to have refractory AF (Table 2). Only Mahapatra (20) also studied this population and while the overall results were better, the in-study procedures showed a greater degree of variation due to its retrospective case-control design. A similar but non-significant trend supporting the superior efficacy of surgical ablation in delivering freedom of AF is also apparent for persistent AF (P=0.06, n=67). Future studies of larger sample sizes with adequate power may potentially prove higher freedom of AF from surgical ablation in persistent AF patients as well.
Previous studies have reported a higher incidence of complications associated with surgical ablation versus catheter ablation. In the current meta-analysis, three studies reported the pacemaker implantation incidence, which was found to be significantly higher in the surgical ablation arm (5.4% vs. 1.5%). Others have suggested that the Cox Maze procedure is linked with sinus atrial node injury and dysfunction, which justifies the higher incidence of pacemaker insertion reported. In a recent meta-analysis by Phan et al. (23), similar pacemaker implantation rates were demonstrated in AF patients with and without surgical ablation. Collectively, these results suggest that catheter ablation may have lower pacemaker implantation rates and thus may be a more suitable treatment modality for patients with contraindications for pacemaker implantation. The incidence of stroke/TIA and of pericardial effusion were reported in 5 studies and were found to be comparable between the surgery and catheter intervention arms (2% vs. 0.7%, P=0.17; 2% vs. 3%, P=0.85, respectively). Previous meta-analyses have suggested that surgical ablation has a protective effect against stroke and thromboembolism, however this trend is not evident in our study.
Other complications, including incidence of respiratory failure, renal failure, haemothorax, rib fracture and wound infections were poorly reported in the current evidence (Table 5), supporting the notion that these adverse effects were generally rare across all studies. Stulak (18) reported 19/124 catheter ablation patients developing >50% pulmonary stenosis, 14 of which required intervention. This finding was unique to this study and may be explained in part by the variety of transcatheter techniques used across the study’s wide timeframe (January 1993—December 2007). While this may also explain the higher (9/124) rate of patients suffering from pericardial tamponade/effusion (18), these elevated morbidity rates were not consistent across the smaller studies. Overall, surgical ablation appears to be a viable treatment method for AF, given the superior efficacy in delivering freedom from AF, as well as the comparable incidence of complications to catheter ablation.
This review was limited by the heterogeneity of the studies included. Only 2 prospective RCTs were found: Boersma and Liu. One of these studies focused exclusively on patients with rheumatic heart disease (17). These 2 RCTs had 124 and 99 patients respectively. The largest study was a retrospective analysis spanning a time period from January 1993 until December 2007, during which time expert techniques developed and procedures were refined.
There was also significant heterogeneity in the study protocols used to compare surgical ablation with catheter ablation (Table 3). Two of seven studies used an on-pump surgical procedure, one of which employed the Cox Maze III procedure (Table 3). The rest of the studies utilized off-pump procedures and may have affected the adverse event profile of the review. Transcatheter ablation procedures also varied in lesions made between studies, which may have affected the efficacy and clinical outcomes of the tested interventions. Furthermore, no definitive conclusion regarding the relative operative risks and clinical outcomes of surgical and catheter ablation could be made due to the poor reporting and small sample sizes of inadequate power. Future RCTs should aim to investigate larger patient populations with a focus on the complication rates, in addition to outcomes of freedom from AF. While all included studies utilised objective measures, no investigator blinding was present. The marked heterogeneity of the included studies’ techniques, patient populations, analysis and designs mean that our results must be interpreted with care.
In conclusion, to best answer the question of surgical ablation versus catheter ablation, a blinded, large, multi-center RCT comparing the efficacy of existing techniques in patients with both paroxysmal and persistent AF is needed. Utilizing the existing evidence presented in this review, surgical ablative techniques appear to demonstrate greater efficacy when compared to catheter-based techniques. Epicardial ablation delivered by VATS showed a higher rate of pacemaker implantation than catheter ablation; stroke and tamponade incidence were comparable between the groups (9-13). This may represent greater technical skill with this new procedure and provides the rationale for further study to clarify these results.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.European Heart Rhythm Association , European Association for Cardio-Thoracic Surgery, Camm AJ, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369-429 [DOI] [PubMed] [Google Scholar]
- 2.Stewart S, Hart CL, Hole DJ, et al. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart 2001;86:516-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 2010;303:333-40 [DOI] [PubMed] [Google Scholar]
- 4.Calkins H, Reynolds MR, Spector P, et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol 2009;2:349-61 [DOI] [PubMed] [Google Scholar]
- 5.Weerasooriya R, Khairy P, Litalien J, et al. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol 2011;57:160-6 [DOI] [PubMed] [Google Scholar]
- 6.Cox JL, Schuessler RB, D’Agostino HJ, Jr, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991;101:569-83 [PubMed] [Google Scholar]
- 7.Stulak JM, Sundt TM, 3rd, Dearani JA, et al. Ten-year experience with the Cox-maze procedure for atrial fibrillation: how do we define success? Ann Thorac Surg 2007;83:1319-24 [DOI] [PubMed] [Google Scholar]
- 8.Hemels ME, Gu YL, Tuinenburg AE, et al. Favorable long-term outcome of Maze surgery in patients with lone atrial fibrillation. Ann Thorac Surg 2006;81:1773-9 [DOI] [PubMed] [Google Scholar]
- 9.Wolf RK, Schneeberger EW, Osterday R, et al. Video-assisted bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. J Thorac Cardiovasc Surg 2005;130:797-802 [DOI] [PubMed] [Google Scholar]
- 10.Santini M, Loiaconi V, Tocco MP, et al. Feasibility and efficacy of minimally invasive stand-alone surgical ablation of atrial fibrillation. A single-center experience. J Interv Card Electrophysiol 2012;34:79-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han FT, Kasirajan V, Kowalski M, et al. Results of a minimally invasive surgical pulmonary vein isolation and ganglionic plexi ablation for atrial fibrillation: single-center experience with 12-month follow-up. Circ Arrhythm Electrophysiol 2009;2:370-7 [DOI] [PubMed] [Google Scholar]
- 12.Edgerton JR, Brinkman WT, Weaver T, et al. Pulmonary vein isolation and autonomic denervation for the management of paroxysmal atrial fibrillation by a minimally invasive surgical approach. J Thorac Cardiovasc Surg 2010;140:823-8 [DOI] [PubMed] [Google Scholar]
- 13.Yilmaz A, Geuzebroek GS, Van Putte BP, et al. Completely thoracoscopic pulmonary vein isolation with ganglionic plexus ablation and left atrial appendage amputation for treatment of atrial fibrillation. Eur J Cardiothorac Surg 2010;38:356-60 [DOI] [PubMed] [Google Scholar]
- 14.Glasziou P, Irwig L, Bain C, et al. Systematic Reviews in Health Care: A Practical Guide. Cambridge University Press, 2001. [Google Scholar]
- 15.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58 [DOI] [PubMed] [Google Scholar]
- 16.Boersma LV, Castella M, van Boven W, et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation 2012;125:23-30 [DOI] [PubMed] [Google Scholar]
- 17.Gu J, Liu X, Jiang WF, et al. Comparison of catheter ablation and surgical ablation in patients with long-standing persistent atrial fibrillation and rheumatic heart disease: a four-year follow-up study. Int J Cardiol 2013;168:5372-7 [DOI] [PubMed] [Google Scholar]
- 18.Stulak JM, Dearani JA, Sundt TM 3rd, et al. Ablation of atrial fibrillation: comparison of catheter-based techniques and the Cox-Maze III operation. Ann Thorac Surg 2011;91:1882-8; discussion 1888-9. [DOI] [PubMed]
- 19.Wang J, Li Y, Shi J, et al. Minimally invasive surgical versus catheter ablation for the long-lasting persistent atrial fibrillation. PLoS One 2011;6:e22122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahapatra S, LaPar DJ, Kamath S, et al. Initial experience of sequential surgical epicardial-catheter endocardial ablation for persistent and long-standing persistent atrial fibrillation with long-term follow-up. Ann Thorac Surg 2011;91:1890-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krakor R, Chares M, Schneider J, et al. Mid-term results of endoscopic mitral valve repair in combination with endocardial or epicardial ablation. Eur J Cardiothorac Surg 2011;40:e125-9 [DOI] [PubMed] [Google Scholar]
- 22.De Maat GE, Van Gelder IC, Rienstra M, et al. Surgical vs. transcatheter pulmonary vein isolation as first invasive treatment in patients with atrial fibrillation: a matched group comparison. Europace 2014;16:33-9 [DOI] [PubMed] [Google Scholar]
- 23.Phan K, Xie A, Tian DH, et al. Systematic review protocol: surgical ablation for atrial fibrillation during mitral valve surgery. Ann Cardiothorac Surg 2013;2:855. [DOI] [PMC free article] [PubMed] [Google Scholar]