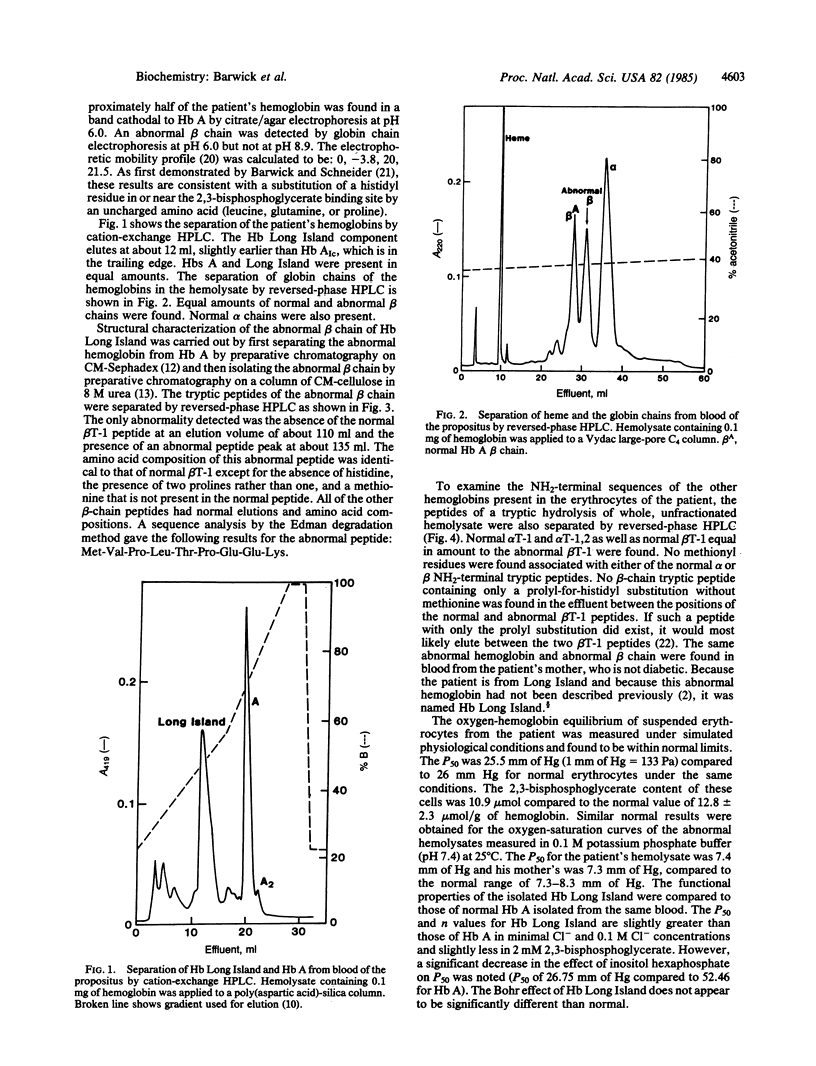
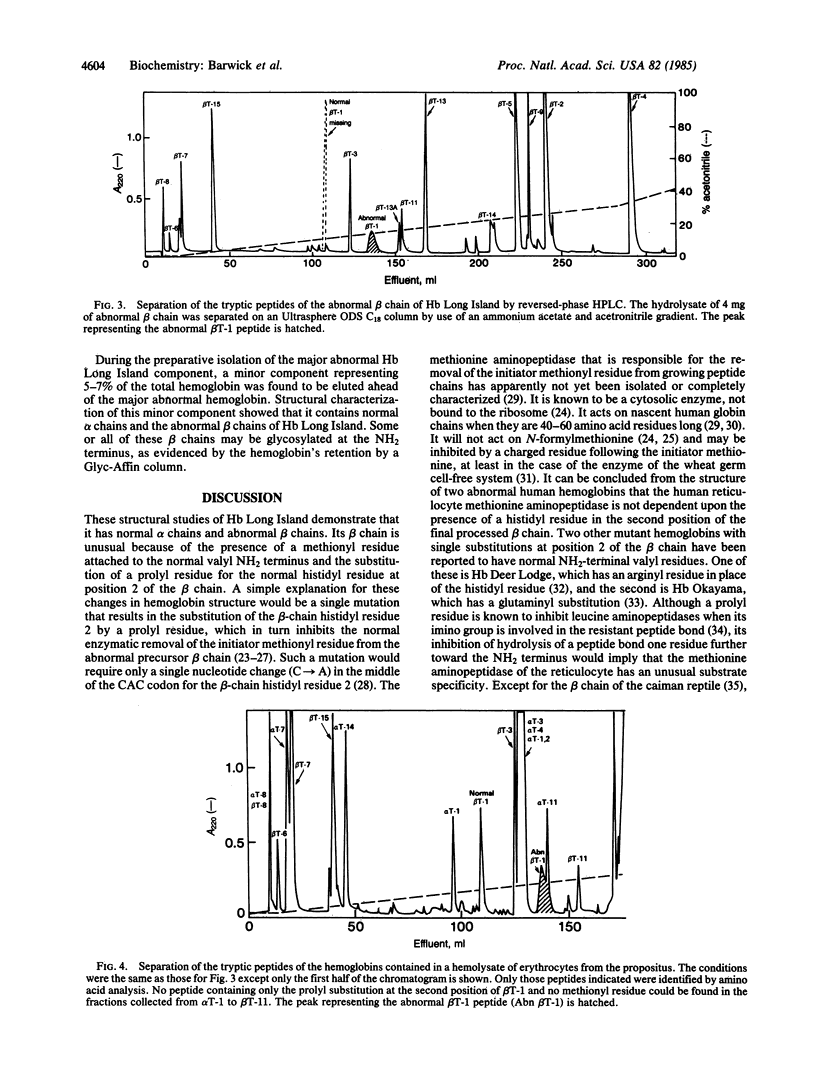
Abstract
Hb Long Island was found in a diabetic man and his nondiabetic mother as the result of a routine clinical measurement of Hb AIc. It is present in amounts approximately equal to Hb A. Its alpha chains are normal but its beta chains have two alterations compared to the normal. A methionyl residue is attached to the usual NH2-terminal valyl residue. This valyl residue is followed by prolyl residue in place of the usual histidyl residue 2. The remaining sequence of the beta chain is normal. No hemoglobin or abnormal beta chain containing only the prolyl substitution could be detected by several different electrophoretic and HPLC procedures. We postulate that Hb Long Island is the result of a mutation in which a single nucleotide change causes the substitution of a prolyl residue for the normal histidyl residue at position 2 of the beta chain. We further postulate that this abnormal prolyl residue inhibits enzymatic cleavage of the initiator methionyl residue from the abnormal beta chain during posttranslational processing. Although the oxygen affinities of the whole blood, suspended cells, and hemolysate are normal, the affinity of the isolated Hb Long Island is slightly decreased and the effects of organic phosphates are reduced compared to normal. These changes are consistent with the loss of the normal histidyl residue 2 and the extension of the NH2-terminal end of the beta-chain molecule.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham E. C., Perry R. E., Stallings M. Application of affinity chromatography for separation and quantitation of glycosylated hemoglobins. J Lab Clin Med. 1983 Aug;102(2):187–197. [PubMed] [Google Scholar]
- Barwick R. C., Schneider R. G. The computer-assisted differentiation of hemoglobin variants. Tex Rep Biol Med. 1980;40:143–156. [PubMed] [Google Scholar]
- Barwick R., Schneider R. G. Correlation of mobilities of mutant hemoglobins in citrate agar electrophoresis with specific molecular locations of affected residues. Biochim Biophys Acta. 1981 May 29;668(3):485–489. doi: 10.1016/0005-2795(81)90183-5. [DOI] [PubMed] [Google Scholar]
- Burstein Y., Schechter I. Primary structures of N-terminal extra peptide segments linked to the variable and constant regions of immunoglobulin light chain precursors: implications on the organization and controlled expression of immunoglobulin genes. Biochemistry. 1978 Jun 13;17(12):2392–2400. doi: 10.1021/bi00605a022. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- Harano T., Harano K., Shibata S., Ueda S., Mori H., Arimasa N. Hemoglobin Okayama [beta 2 (NA 2) His replaced by Gln]: a new 'silent' hemoglobin variant with substituted amino acid residue at the 2,3-diphosphoglycerate binding site. FEBS Lett. 1983 May 30;156(1):20–22. doi: 10.1016/0014-5793(83)80239-7. [DOI] [PubMed] [Google Scholar]
- Housman D., Jacobs-Lorena M., Rajbhandary U. L., Lodish H. F. Initiation of haemoglobin synthesis by methionyl-tRNA. Nature. 1970 Aug 29;227(5261):913–918. doi: 10.1038/227913a0. [DOI] [PubMed] [Google Scholar]
- Imai K. Analysis of ligand binding equilibria. Methods Enzymol. 1981;76:470–486. doi: 10.1016/0076-6879(81)76137-8. [DOI] [PubMed] [Google Scholar]
- Imanishi H., Suzuki H., Itano H. A. The initiation of fetal hemoglobin biosynthesis. Biochim Biophys Acta. 1979 Oct 25;564(3):488–494. doi: 10.1016/0005-2787(79)90038-8. [DOI] [PubMed] [Google Scholar]
- Jackson R., Hunter T. Role of methionine in the initiation of haemoglobin synthesis. Nature. 1970 Aug 15;227(5259):672–676. doi: 10.1038/227672a0. [DOI] [PubMed] [Google Scholar]
- Jones M. B., Koler R. D., Jones R. T. Micro-column method for the determination of hemoglobin minor fractions A I a+b and A I c. Hemoglobin. 1978;2(1):53–58. doi: 10.3109/03630267808999188. [DOI] [PubMed] [Google Scholar]
- Labossiere A., Vella F., Hiebert J., Galbraith P. Hemoglobin Deer Lodge: 2 2 2 His leads to Arg . Clin Biochem. 1972 Mar;5(1):46–50. doi: 10.1016/s0009-9120(72)80007-9. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Efstratiadis A., O'Connell C., Maniatis T. The nucleotide sequence of the human beta-globin gene. Cell. 1980 Oct;21(3):647–651. doi: 10.1016/0092-8674(80)90428-6. [DOI] [PubMed] [Google Scholar]
- Leclercq F., Schnek A. G., Braunitzer G., Stangl A., Schrank B. Direct reciprocal allosteric interaction of oxygen and hydrogen carbonate sequence of the haemoglobins of the Caiman (Caiman crocodylus), the Nile crocodile (Crocodylus niloticus) and the Mississippi crocodile (Alligator mississippiensis). Hoppe Seylers Z Physiol Chem. 1981 Aug;362(8):1151–1158. [PubMed] [Google Scholar]
- Moo-Penn W. F., Jue D. L., Bechtel K. C., Johnson M. H., Schmidt R. M. Hemoglobin Providence. A human hemoglobin variant occurring in two forms in vivo. J Biol Chem. 1976 Dec 10;251(23):7557–7562. [PubMed] [Google Scholar]
- Nakatsuji I., Wilson J. B., Lam H., Huisman T. H. Identification and quantitation of Hb Olympia [beta 20(B2)Val leads to Met] and Hb San Diego [beta 109(G11)Val leads to Met] by high-performance liquid chromatography. J Chromatogr. 1983 Apr 15;259(3):511–514. doi: 10.1016/s0021-9673(01)88043-2. [DOI] [PubMed] [Google Scholar]
- Ou C. N., Buffone G. J., Reimer G. L., Alpert A. J. High-performance liquid chromatography of human hemoglobins on a new cation exchanger. J Chromatogr. 1983 Aug 26;266:197–205. doi: 10.1016/s0021-9673(01)90893-3. [DOI] [PubMed] [Google Scholar]
- PAULING L., ITANO H. A. Sickle cell anemia a molecular disease. Science. 1949 Nov 25;110(2865):543–548. doi: 10.1126/science.110.2865.543. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Bauer C., Gros G., Leclercq F., Vandecasserie C., Schnek A. G., Braunitzer G., Friday A. E., Joysey K. A. Allosteric regulation of crocodilian haemoglobin. Nature. 1981 Jun 25;291(5817):682–684. doi: 10.1038/291682a0. [DOI] [PubMed] [Google Scholar]
- Randhawat Z. I., Jones R. T., Lie-Injo L. E. Separation of the tryptic peptides and cyanogen bromide fragments of the human embryonic zeta chains of hemoglobin in Portland I and II by reverse phase high performance liquid chromatography. Hemoglobin. 1984;8(5):463–482. doi: 10.3109/03630268408991732. [DOI] [PubMed] [Google Scholar]
- SCHROEDER W. A., SHELTON J. R., SHELTON J. B., CORMICK J., JONES R. T. THE AMINO ACID SEQUENCE OF THE GAMMA CHAIN OF HUMAN FETAL HEMOGLOBIN. Biochemistry. 1963 Sep-Oct;2:992–1008. doi: 10.1021/bi00905a016. [DOI] [PubMed] [Google Scholar]
- Schroeder W. A., Shelton J. B., Shelton J. R. High performance liquid chromatography in the identification of human hemoglobin variants. Prog Clin Biol Res. 1981;60:1–22. [PubMed] [Google Scholar]
- Shafritz D. A., Anderson W. F. Factor dependent binding of methionyl-tRNAs to reticulocyte ribosomes. Nature. 1970 Aug 29;227(5261):918–920. doi: 10.1038/227918a0. [DOI] [PubMed] [Google Scholar]
- Shih T., Jones R. T., Bonaventura J., Bonaventura C., Schneider R. G. Involvement of His HC3 (146) beta in the Bohr effect of human hemoglobin. Studies of native and N-ethylmaleimide-treated hemoglobin A and hemoglobin Cowtown (beta 146 His replaced by Leu). J Biol Chem. 1984 Jan 25;259(2):967–974. [PubMed] [Google Scholar]
- Suzuki H., Itano H. A. Quantitative differences between N-terminal methionyl nascent globin chains of human and rabbit reticulocytes. Nat New Biol. 1973 Nov 28;246(152):107–109. doi: 10.1038/newbio246107a0. [DOI] [PubMed] [Google Scholar]
- Tarr G. E. Improved manual sequencing methods. Methods Enzymol. 1977;47:335–357. doi: 10.1016/0076-6879(77)47036-8. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Dintzis H. M. Protein chain initiation in rabbit reticulocytes. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1282–1289. doi: 10.1073/pnas.66.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A., Lin M. NH 2 -terminal formylmethionine- and NH 2 -terminal methionine-cleaving enzymes in rabbits. J Biol Chem. 1972 Feb 10;247(3):952–957. [PubMed] [Google Scholar]



