Abstract
Treatment of atrial fibrillation (AF) in concomitant surgery is not unanimously agreed upon in the cardiac surgical community. The reason for this lack of consensus is threefold. Firstly, there is an absence of large multicenter randomized controlled trials (RCT) proving the benefit of restoring sinus rhythm in a patient population which we encounter almost daily (about 10% of cardiac surgery patients are diagnosed with AF). Secondly, for patients undergoing cardiac surgery without the need for an atriotomy, the Maze procedure is not widely accepted. In these patients, many surgeons do not think that the increased complexity outweighs the potential future benefits of sinus rhythm. Thirdly, due to our limited understanding of this pathology, we are confronted with many choices of ablation tools and lesion sets. In this perspective these issues are reviewed. As a possible solution, a total epicardial lesion set without any incisions is proposed.
Keywords: Atrial fibrillation (AF), concomitant surgery, epicardial
Introduction
A recent meta-analysis and systematic review of surgical ablation for atrial fibrillation (AF) as a concomitant procedure demonstrated a significantly higher rate of sinus rhythm in randomized controlled trials (RCT) and non-RCT studies compared with cardiac surgery alone (1). The effect of sinus rhythm in reducing risk of stroke and death is suggested with non-RCTs, but this remains to be proven in prospective RCTs with adequate power and follow-up.
The 2012 Heart Rhythm Society (HRS)/European Heart Rhythm Association (EHRA)/European Cardiac Arrhythmia Society (ECAS) Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation recommended that, based on the results of clinical trials and clinical experience, it is appropriate to consider all patients with symptomatic AF undergoing other cardiac surgery for AF ablation (2). This strong statement is rephrased immediately by adding the following assumptions: that there is a reasonable chance for success and that the surgery is performed by an experienced surgeon. The authors suggest certain lesion sets. A left atrial procedure should at least consist of pulmonary vein (PV) isolation, ideally with a connecting lesion to the mitral valve annulus. For patients with persistent and longstanding persistent AF, a bi-atrial procedure should be considered and, when it can be safely performed, complete occlusion of the left atrial appendage (LAA) should be considered. The indications for concomitant surgery according to this consensus statement are based on Class IIa Level C evidence. The assumptions make these recommendations somewhat vague, therefore leaving all treatment options available.
Development of an epicardial lesion set: rationale
For the patient population with mitral valve disease and AF, most surgeons will agree that since the left atrium is opened, a left-sided or bi-atrial Maze procedure is the therapy of choice. But what about patients that are having cardiac surgery without the need for atriotomies?
A more elaborated consensus statement on surgical ablation for AF in cardiac surgery is therefore needed. The 2009 consensus statement that has been provided by the International Society of Minimally Invasive Cardiothoracic Surgery (ISMICS) can help us to better understand which patients could benefit (3). The panel stated that in patients with persistent and permanent AF undergoing cardiac surgery, concomitant surgical ablation is recommended to increase the incidence of sinus rhythm at short- and long-term follow-up (Class 1, Level A); to reduce the risk of stroke and thromboembolic events (Class 2A, Level 1); to improve EF (Class 2A, Level A); and to improve exercise tolerance (Class 2A, Level A) and long-term survival (Class 2A, Level B). Importantly, in their conclusion the panel stated that the different lesion sets, the management of the LAA and the various energy sources in use (cryotherapy, radiofrequency, cut-and-sew) still need to be compared prospectively.
Despite the growing data in favor of concomitant treatment of AF, a recent publication from the Society of Thoracic Surgeons Adult Cardiac Surgery Database [2005-2010] showed an unusual trend in concomitant surgical ablation (4). On average, 61.5% of patients with preoperative AF undergoing mitral valve repair (MVR) underwent ablation compared with only 27.5% of patients undergoing coronary artery bypass grafting (CABG). Patients undergoing isolated aortic valve replacement (AVR) with AF underwent ablation 33.9% of the time. Similar trends were documented with combined AVR and CABG patients with AF (32.1%) and the combined CABG and MVR (51.8%). The authors concluded that the addition of left atrial atriotomy is a major negative factor in the decision-making of the surgeon, because of the perception that added atriotomies could be associated with increased operative risk. This data teaches us that for the patient population with AF not related to mitral valve disease, who typically have smaller left atria, normal right atria, and therefore probably a higher ablation success rate, surgeons are less motivated to treat this pathology.
The strategy for successfully treating this patient population has been studied by Ad et al. (5) The authors matched 95 patients undergoing an AVR or coronary bypass procedure who had a Cox-Maze III procedure with patients who did not undergo the Cox-Maze III. They demonstrated that the addition of the Cox-Maze III procedure did not increase major morbidity and perioperative risk. The Maze group had similar survival over time and improvement in health-related quality of life. The authors concluded that the Cox-Maze III should not be denied to patients in whom the cardiac surgical procedure does not include atriotomies because of the perceived increased operative risk.
Having solutions for non-mitral valve concomitant AF, but realizing that only a limited number of surgeons will perform them, it could be worthwhile to consider a lesion set without the need for an atriotomy. This is regardless of extracorporeal support: on-pump (on the beating or non-beating heart), or off-pump. If we want to design such a procedure, we must first understand the current limitations of energy delivery in the left and right atria and the necessity of a continuous and transmural lesion. Noncontinuous or nontransmural lines, as a result of incomplete ablation, can result in recurrence of AF and induction of atrial tachycardia.
Alternative energy sources and transmurality
The use of alternative energy sources for an epicardial ablation on the beating heart is challenging because the atrial wall muscle thickness is variable and can be covered with an epicardial layer of fat. Moreover, transmural lesions are difficult to obtain since the circulating intracavitary blood acts as a potential heat sink. Schuessler et al. tested the effect of epicardial ablation on the beating heart in domestic pigs using 9 different unidirectional devices (6). With these devices, all the energy is applied by a single transducer on a single heart surface. The authors concluded that the percentage of transmural lesions varied for a minimum of 5% up to 100% for the different devices tested. Furthermore, none of the devices tested demonstrated the ability to penetrate the atrial wall at its thickest dimensions. This study demonstrated in an animal model the potential risk of incomplete lesions using these devices.
To prevent recurrences of AF, it is necessary for a device to produce a transmural continuous lesion, because even small gaps in lesions increase the likelihood of recurrence (7). The most consistent devices for creating transmural lesions have been bipolar radiofrequency clamps (8,9). Damiano Jr et al. introduced the Cox-Maze IV procedure by replacing the incisions of the traditional Cox-Maze III procedure with less invasive linear lesions of ablation using bipolar radiofrequency (10). This procedure requires cardiopulmonary bypass and atriotomies, but the freedom from AF recurrence was 84% at two years for patients off antiarrhythmic drugs.
It is technically challenging to reproduce the Maze procedure epicardially on the beating heart. With current devices, a linear lesion across the left and right isthmus towards the tricuspid and/or mitral valve annulus is difficult to make. On the other hand, bipolar radiofrequency clamps can be used from the epicardium on the beating or non-beating heart to isolate pulmonary veins (PVs). Clamping of the PVs between the two jaws excludes the effect of circulating blood on delivery of power, thereby eliminating the heat-sink cooling effect to the tissue, which will increase the degree of transmurality. Although these bipolar clamps are probably the most reliable ablation tools available to the cardiac surgeons, failure to chronically isolate the PVs has been described (11). The reason for failure of a long-lasting block after an initial proven exit and entrance block is probably related to a transient clamp-induced mechanical block of the PVs (12).
Bipolar clamping devices can also be used to create linear lesions on the roof and floor of the left atrium by inserting one of the arms of the device into the atrium. A surgical technique using multi-purse strings to perform the full maze procedure without atriotomies has been studied by Ad (13). The right atrial lesion set was done without cardiopulmonary bypass using a bipolar clamp and a cryo device. After clamping of the aorta, the left lesion set was created.
Based on this idea, a modified Cox-Maze IV in off-pump CABG using only a bipolar clamp has been studied by Mariani et al. (14). The authors concluded that on the beating heart, bipolar radiofrequency clamps have advantages over other energy sources to provide transmurality of the lesions. The limitation of this technique is that the correct positioning of the jaws of the bipolar clamp cannot be visualized, therefore creating potentially incomplete line. A similar technique of a right isthmus lesion with a bipolar clamp on the arrested heart was described by Benussi et al. (15).
The feasibility of left and right isthmus ablation with a bipolar device has been challenged by a study performed by Castellá et al. in fresh explanted human hearts (16). In this paper, the anatomic structure of the mitral and tricuspid annuli, their relationship with the coronary arteries and veins, and the effect of atrial ablation with bipolar radiofrequency clamps were studied. The authors found that in all hearts studied, the coronary arteries and veins within the adipose tissue of the right or left atrioventricular groove lay in the atrial side, 3 to 18 mm away from the mitral or tricuspid annuli. When the bipolar radiofrequency clamp was closed toward the mitral annulus, the coronary sinus was always included between the jaws, and in left coronary dominant hearts, the circumflex artery was also included. The clamp never reached the annulus owing to the increase in thickness of the adipose tissue around the groove and the ventricular mass, leaving 5 to 10 mm of atrial myocardium free from the radiofrequency electrodes. In the right atrium, clamp placement toward the tricuspid annulus, excluding the right coronary, left 8 to 18 mm of atrial muscle free from the bipolar electrodes. Therefore, bipolar radiofrequency clamps are not sufficient to complete a Cox-Maze IV procedure. Moreover, they may compromise coronary arteries in patients with left coronary dominance.
Taking into account all the above limitations, how could we improve patient outcomes for concomitant AF surgery? Do we have to stick to the basic principle of the critical mass and only promote a Maze procedure by experienced surgeons, realizing that this will significantly limit the number of patients treated for AF? Or should we go for a less invasive, limited lesion set that could be more easily performed by most cardiac surgeons? In other words, how can we obtain an optimal patient outcome, an outcome that should be measured not only by the success rate of the AF ablation procedure but also by the complication rate? Should we promote the general adoption of this procedure not only by experts in the field of AF, but rather by every surgeon who is confronted with this problem? Could we propose a lesion set as a concomitant procedure to non-mitral valve surgery that is based upon an epicardial approach using a bipolar clamp, without the need for atriotomies or purse strings? Which lesion set could be accepted in this patient population with limited left atrial dilatation and often no right atrial dilatation?
Epicardial lesion set
We propose a strategy that is tailored to an off-pump and an on-pump procedure.
After sternotomy and opening of the pericardium, the pericardial reflections of the superior vena cava (SVC) and inferior vena cava (IVC) are bluntly dissected, by hand or by suction. A braid or rubber catheter is placed around the veins. This dissection will open a passage from the right side to the left side of the pericardial cavity, giving access to an anatomical space, the oblique sinus and the transverse sinus. The oblique sinus is a recess located behind the left atrium, which is formed by the reflection of the serous pericardium around the large veins. The transverse sinus is located superiorly to the heart between the arterial mesocardium, which envelopes the ascending aorta and pulmonary trunk anteriorly, and the venous mesocardium, which covers the SVC, left atrium and PVs posteriorly and inferiorly. The right PVs are then accessed. A blunt dissection is performed between the right superior pulmonary vein and the right pulmonary artery, lateral to the SVC, to gently separate these structures. This can easily be done with suction or by putting the index finger of the right hand posterior to the IVC into the oblique sinus until it reaches the superior border of the posterior left atrium. The thumb of the right hand is introduced into the area between the right pulmonary artery and the right superior pulmonary vein. Both fingers will touch each other, only separated by a thin layer of fatty tissue, which can easily be opened by rubbing both fingers. A braid or rubber catheter is passed. This maneuver is similar to the blunt dissection of the pericardial reflection of the IVC we often do during cardiac surgery. The technique is repeated on the left PVs. Importantly, this dissection is more easily performed medial to the ligament of Marshall (LM) since the tissues are less resistant in that area. Furthermore, this will also isolate the LM, a potential trigger of AF (Figure 1). The lower jaw of the clamp is guided behind the left atrial cuff adjacent to the right PVs. The braid or rubber catheter is then removed, and correct positioning of the clamp on the atrium and not on the PVs is verified by means of direct inspection of the device after closing the jaws of the clamp. Several ablations are performed, then the technique is repeated on the left side (Figure 2). The endpoint of PV isolation is entrance and exit blocks. Entrance block is defined as the absence of atrial potentials inside the area of the lesions, regardless of the rhythm. Exit block is defined as failure to capture the remaining left atrium when pacing from the PV, which can only be done if the patient is in sinus rhythm.
Figure 1.
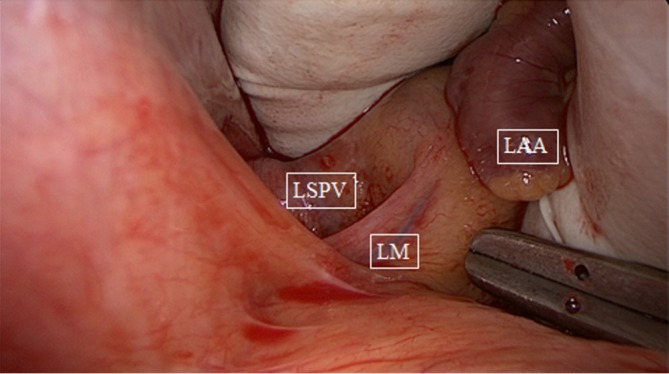
Blunt dissection of left pulmonary veins, medial to ligament of Marshall (LM). LSPV, left superior pulmonary vein; LAA, left atrial appendage.
Figure 2.
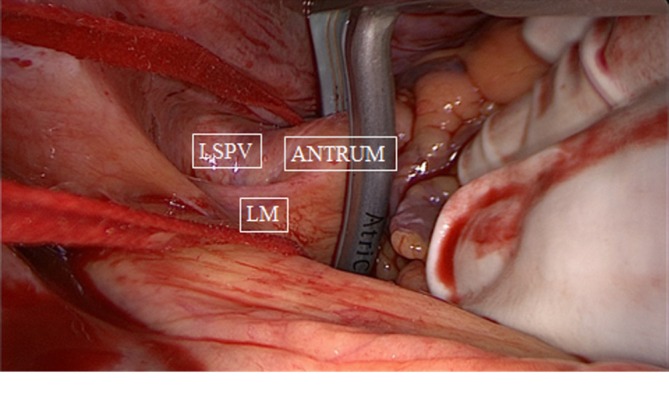
Bipolar clamping of left pulmonary veins antrum, medial to ligament of Marshall (LM). LSPV, left superior pulmonary vein.
The next step is a connecting lesion between the right PVs and left PVs. This can be achieved by inserting the tip of the upper jaw of the clamp in the created anatomical space between the right superior pulmonary vein and the right pulmonary artery, posterior to the SVC. The tip of the lower jaw is inserted in the created anatomical space between the right inferior pulmonary vein and the IVC (Figure 3). The clamp is then gently moved forward with the upper jaw crossing the transverse sinus (posterior to the great arteries) and the lower jaw crossing the oblique sinus. The clamp is closed just prior to the left PVs to avoid preload reduction and hemodynamic instability. Several ablations are performed. The technique is repeated coming from the left side. It is crucial that both lines (coming from the right and from the left) are connected. This will create a bipolar roof and inferior line in a similar way as when performing PV isolation. Clamping of the posterior left atrium between the two jaws excludes the effect of circulating blood on delivery of power, eliminating the heat-sink cooling effect to the tissue, thereby increasing the degree of transmurality. This maneuver is facilitated if the aorta is clamped and the left atrium is not filled. In this situation, it is almost always possible to position the clamp from the right PVs to the left PVs in a single step, avoiding the risk of an incomplete lesion if this ablation has to be done from the right and left side (Figure 4).
Figure 3.
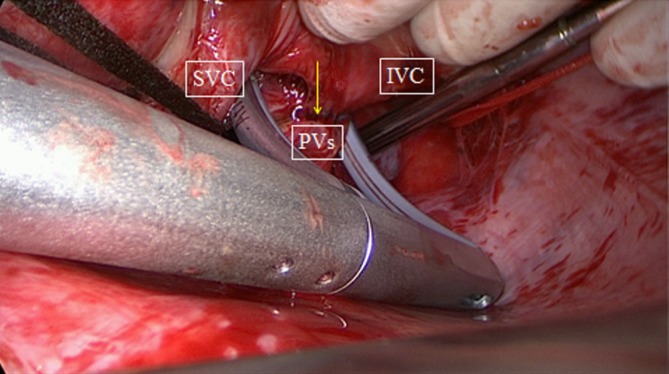
Bipolar clamping of posterior wall of the left atrium as seen from the right. Arrow points to antral ablation line of right PVs. SVC, superior caval vein; IVC, inferior caval vein; PVs, pulmonary veins.
Figure 4.
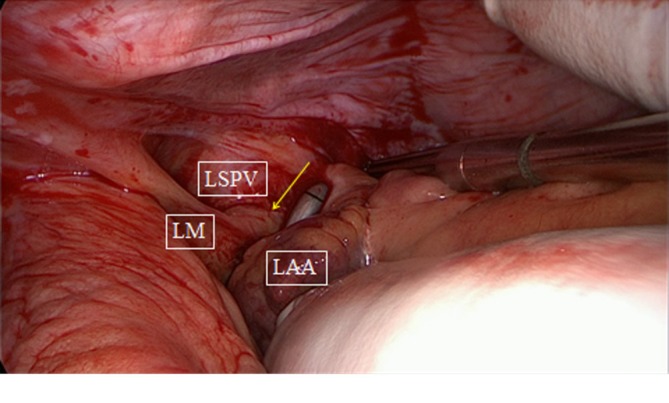
Bipolar clamping of posterior wall of the left atrium as seen from the left. Arrow points to antral ablation line of left pulmonary veins. LM, ligament of Marshal; LSPV, left superior pulmonary vein; LAA, left atrial appendage.
Excision or exclusion of the LAA is recommended in the American College of Cardiology/American Heart Association (ACC/AHA) guidelines and is often performed during the surgical ablation of AF (17). Most surgeons will avoid suturing or ligation of the LAA during an epicardial off-pump procedure. Since there is a growing concern regarding the safety of this maneuver and the risk for incomplete closure, we prefer to use a clip or sometimes a stapler to excise the appendage.
Mapping of active ganglionated plexi and their ablation may improve the outcome of the procedure. This can be added to the epicardial procedure, but the long-term effect on AF and autonomic nerve activities is still not clear.
Circumferential caval lesions and linear SVC-to-IVC lesions represent possible strategies during an epicardial ablation, since AF is sometimes triggered from arrhythmogenic foci originating from the SCV. The SVC is clamped distal to the opening into the right atrium to avoid the sinus node. The fat in the intra-atrial groove is dissected to facilitate the clamping of the SVC-to-IVC lesion. It is important to make this line inferior to the sinus node.
Conclusions
There is a role for both epicardial and endocardial ablation in the treatment of AF. The challenge with concomitant AF surgery in patients who do not need an atriotomy, is the development and establishment of an epicardial transmural lesion set that is safe, reliable and reproducible. If we can provide such a procedure, decisions regarding this lesion set should consider not only its success rate but also its complication rate, as well as the general adoption of this procedure, not only by experts in the field of AF, but by every surgeon.
Acknowledgements
Disclosure: Mark La Meir is a consultant for Atricure. Other co-authors declare no conflict of interest.
References
- 1.Cheng DC, Ad N, Martin J, et al. Surgical ablation for atrial fibrillation in cardiac surgery: a meta-analysis and systematic review. Innovations (Phila) 2010;5:84-96 [DOI] [PubMed] [Google Scholar]
- 2.Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012;14:528-606 [DOI] [PubMed] [Google Scholar]
- 3.Ad N, Cheng DC, Martin J, et al. Surgical ablation for atrial fibrillation in cardiac surgery: a consensus statement of the International Society of Minimally Invasive Cardiothoracic Surgery (ISMICS) 2009. Innovations (Phila) 2010;5:74-83 [DOI] [PubMed] [Google Scholar]
- 4.Ad N, Suri RM, Gammie JS, et al. Surgical ablation of atrial fibrillation trends and outcomes in North America. J Thorac Cardiovasc Surg 2012;144:1051-60 [DOI] [PubMed] [Google Scholar]
- 5.Ad N, Henry L, Hunt S, et al. Do we increase the operative risk by adding the Cox Maze III procedure to aortic valve replacement and coronary artery bypass surgery? J Thorac Cardiovasc Surg 2012;143:936-44 [DOI] [PubMed] [Google Scholar]
- 6.Schuessler RB, Lee AM, Melby SJ, et al. Animal studies of epicardial atrial ablation. Heart Rhythm 2009;6:S41-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melby SJ, Lee AM, Zierer A, et al. Atrial fibrillation propagates through gaps in ablation lines: implications for ablative treatment of atrial fibrillation. Heart Rhythm 2008;5:1296-301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melby SJ, Gaynor SL, Lubahn JG, et al. Efficacy and safety of right and left atrial ablations on the beating heart with irrigated bipolar radiofrequency energy: a long-term animal study. J Thorac Cardiovasc Surg 2006;132:853-60 [DOI] [PubMed] [Google Scholar]
- 9.Prasad SM, Maniar HS, Schuessler RB, et al. Chronic transmural atrial ablation by using bipolar radiofrequency energy on the beating heart. J Thorac Cardiovasc Surg 2002;124:708-13 [DOI] [PubMed] [Google Scholar]
- 10.Weimar T, Schena S, Bailey MS, et al. The cox-maze procedure for lone atrial fibrillation: a single-center experience over 2 decades. Circ Arrhythm Electrophysiol 2012;5:8-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kron J, Kasirajan V, Wood MA, et al. Management of recurrent atrial arrhythmias after minimally invasive surgical pulmonary vein isolation and ganglionic plexi ablation for atrial fibrillation. Heart Rhythm 2010;7:445-51 [DOI] [PubMed] [Google Scholar]
- 12.Pison L, La Meir M, van Opstal J, et al. Transient clamp-induced mechanical block of pulmonary vein potentials. J Thorac Cardiovasc Surg 2011;141:e15-6 [DOI] [PubMed] [Google Scholar]
- 13.Ad N.The multi-purse string maze procedure: a new surgical technique to perform the full maze procedure without atriotomies. J Thorac Cardiovasc Surg 2007;134:717-22 [DOI] [PubMed] [Google Scholar]
- 14.Mariani MA, Stoker T, Scholten MF, et al. Concomitant off-pump modified maze and coronary surgery. Ann Thorac Surg 2011;91:e96-8 [DOI] [PubMed] [Google Scholar]
- 15.Benussi S, Galanti A, Nascimbene S, et al. Complete right atrial ablation with bipolar radiofrequency. Ann Thorac Surg 2009;87:1573-6 [DOI] [PubMed] [Google Scholar]
- 16.Castellá M, García-Valentín A, Pereda D, et al. Anatomic aspects of the atrioventricular junction influencing radiofrequency Cox maze IV procedures. J Thorac Cardiovasc Surg 2008;136:419-23 [DOI] [PubMed] [Google Scholar]
- 17.Fuster V, Rydén LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol 2011;57:e101-98 [DOI] [PubMed] [Google Scholar]


