Abstract
The development and application of neuroimaging methods offers powerful means to study brain functions, but the resulting knowledge is more likely to be beneficial when combined with conceptual analyses that decompose complex psychological constructs into component structures, representations, processes, and computations; converging measures that gauge neural events at different temporal and spatial scales; behavioral measures that permit fine-grain analyses of brain–behavior associations; and experimental (e.g., lesion studies and/or transcranial magnetic stimulation) and nonhuman animal studies that test the putative role of specific brain structures, circuits, or processes. In addition, quantitative meta-analyses are important to move beyond idiosyncrasies of individual studies, and neurodevelopmental investigations can contribute to our understanding of brain–behavior associations.
Keywords: social neuroscience, functional neuroimaging, neurodevelopment, meta-analyses, lesion studies, computational processes
Advances in technical and quantitative methods of neuroscience over the past two decades have caught the attention of the investigators in various fields of psychology and in related disciplines, but the advance that has captured the attention of the media and the imagination of the public is functional neuroimaging. The notion seems to be that if you can visualize putative changes in brain activation to specific tasks in the normal human brain, then you have captured something real. There are no more concerns about the validity of self-reports or behavior—if it can be seen in the brain then it must be true. The seductive appeal of neuroimaging is reminiscent of how most people feel about eye-witness evidence, and it is at least as fraught with error.
The articles in this special section represent four very different perspectives on neuroimaging. We agree with Beck (2010, this issue) that investigators should do their best to avoid hyperbole and to accurately convey the results of their scientific investigations, but we also recognize that journalists and nonexperts have different agendas and often place more stock in what they find to be intuitive than in what is empirically sound. The same can be said for administrators and scientists in other fields who have long been suspicious of a science built upon self-report (or even behavioral) data. There is a danger, however, that our nonexpert colleagues will grow weary of unsubstantiated claims and will dismiss the entire enterprise.
Miller (2010, this issue) argues that suspicions about a science built on verbal or behavioral data are logically indefensible and have been costly to the discipline. We are aware that the current models of brain functioning and psychopathology are limited, but we do not share his pessimism about the potential contributions of the neurosciences to our understanding of the mind and behavior. There are alternatives to the models of dualism and radical reductionism that Miller so thoughtfully reviews, such as integrative analyses across levels of organization that acknowledge the fact that we are biological, cognitive, and social animals with a lifespan that unfolds over time (e.g., Anderson, 1998; Cacioppo & Berntson, 1992; Cacioppo & Decety, 2009).
Human neuroscience generally, and neuroimaging in particular, is still in its infancy, and false-starts, mistakes, and dead-ends are to be expected. These should be minimized to the extent possible, of course, and both Poldrack (2010, this issue) and Gonsalves and Cohen (2010, this issue) offer suggestions for doing so. These recommended approaches must also prove their generative and discriminative value, something that will take time and effort by dedicated investigators who will undoubtedly encounter criticism, disappointment, and insights, which in time will lead to further advances in the field. Substantial progress has already been made in the field of neuroimaging, though. For instance, neophrenology, in which large regions of the brain are simply equated with complex psychological constructs, is growing less prevalent in the literature. Thus, we are generally optimistic about the future.
Scientific Progress and the Golden Triangle
Scientific theories in psychology seek to provide a mechanistic explanation for behavioral or brain function in terms of antecedents, structure and processes, and consequences. There are various forms of explanation that might be sought. For instance, one might seek a mechanistic account in terms of psychological (e.g., cognitive, social, affective) constructs, information processing components, or computations underlying a behavioral phenomenon. Evidence from neuroimaging is not necessary in such studies, but it may prove useful either as a source of hypotheses about what these constructs, components, or computations might be or as a means of performing a crucial test between theories, made possible by breaking down the component processes of the psychological construct of interest and showing how, based on the prior literature on the brain, different predictions about what regions should be activated can be derived from two or more theories. It is less useful to take a complex psychological construct and simply correlate it with regions in the brain to report neural correlates.
Neuroimaging in the psychological sciences can also be used to help us understand brain function. Science has solved the mysteries of cardiac function, pulmonary function, skeletomuscular function, and so forth, but brain function remains largely a mystery. Few doubt that the brain functions to orchestrate physiological processes, produce consciousness, and control adaptive behavior in a complex physical and social environment, but how these functions are performed remains largely a mystery. What is clear, as detailed in the other articles in this special section, is that neuroimaging is not sufficient to solve this mystery. However, it is equally clear that, because neuroimaging is noninvasive, it has an important role to play in the development, testing, and refinement of theories of complex psychological processes that are difficult to study in nonhuman animals.
The equilateral triangle depicted in Figure 1 represents the equal importance of three converging approaches that may help us to understand brain functions. First, behavioral analyses and assessments of complex psychological constructs are foundational (Cacioppo & Decety, 2009; Posner & Raichle, 1994). Psychological constructs need to be decomposed into component structures, representations, and processes that could plausibly be implemented by the brain. These, in turn, can be decomposed into the computations that are likely to be implemented by the complex neural machinery that constitutes the brain. What these might be will change with advances in theory, methods, and evidence. Tasks can then be defined that permit the isolation of one or more specific components, as verified by behavioral analyses, which in turn permit fine-grain analyses of brain–behavior associations. Work is still needed, as well, to determine how the neural components might be combined to produce distinct psychological processes. One metaphor is the Lego set, in which the computations performed in localized neural regions are fixed (like distinct pieces of a Lego set), but different pieces and configurations of these building blocks produce different psychological processes. An alternative metaphor is the periodic table in chemistry, in which different neural component processes may have properties and affinities with a function (computation) that depends on the network of areas with which they are combined. There is no evidence at present to favor either perspective, but the important point here is that they suggest there are very different ways of thinking about neural activity and psychological function that need yet to be sorted out.
Fig. 1.
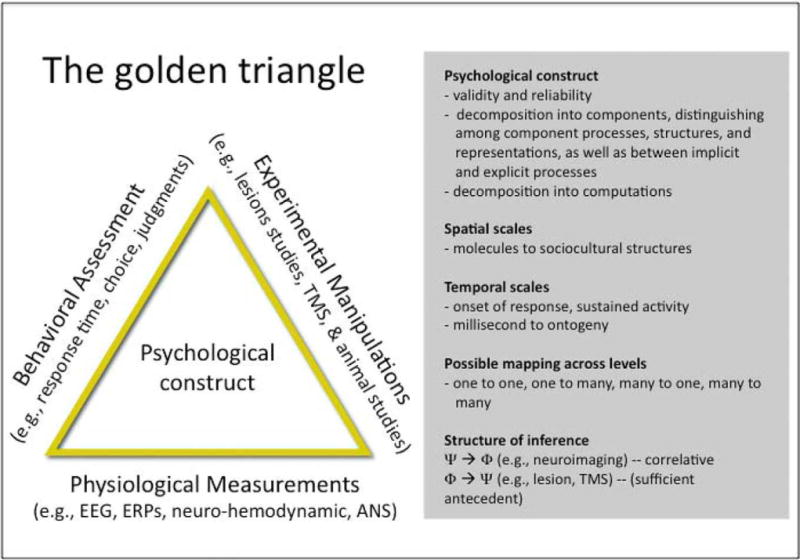
The Golden Triangle of human neuroscience research. This equilateral triangle represents the equal importance of three angles (correlative brain evidence, experimental brain evidence, and behavioral evidence) to perform sound research on brain–behavior relationships.
Second, correlative evidence from the normal waking brain using a variety of measurement techniques provides important information. The brain does not operate exclusively at the spatial level of molecules, cells, nuclei, regions, circuits, or systems, nor does it operate exclusively at the temporal level of milliseconds, seconds, minutes, hours, or days. Neuroimaging provides a partial view of brain activity within a very limited range of spatial and temporal levels. Therefore, converging measures that gauge neural events at different temporal and spatial scales can be used to provide a more complete picture of brain function.
Third, experimental evidence from animals and humans is important. Neuroimaging is a correlative measure, so experimental studies including lesion, transcranial magnetic stimulation, and pharmacological interventions (e.g., ligands, drugs) in human and nonhuman animal are essential to further elucidate the causal role of any given neural structure, circuit, or process in a given task.
Each of these angles has limitations, but the confluence of the three can facilitate advances in our understanding of brain function. The point here is that neuroimaging represents an important part of the armamentarium, but the resulting knowledge is more likely to be beneficial when combined with conceptual analyses that decompose complex psychological constructs into component structures, representations, processes, and computations; converging measures that gauge neural events at different temporal and spatial scales; behavioral measures that permit fine-grain analyses of brain–behavior associations; and experimental and nonhuman animal studies that test the putative role of specific brain structures, circuits, or processes.
In sum, neuroimaging work is leading to a rethinking of the parceling of psychological and neural functions. The presence of so many open questions in the field is both a daunting challenge and an exciting opportunity. We may well need a new lexicon of constructs as we usher in a new era of psychological theory in which the elements constituting elemental component processes (functional elements) are tied to specific neural mechanisms (structural elements) and in which the properties of interrelated networks of areas may sometimes produce more than the sum of the parts. Moreover, replications are an essential part of science. As we discuss next, this is especially the case in neuroimaging studies.
Meta-Analyses
Given the cost of MRI scanning, the vast majority of studies include small number of participants (usually around 12 to 24 individuals)—single studies are not sufficient. Meta-analytic methods applied to neuroimaging data can overcome these limitations. Such methods are critical to look beyond the idiosyncrasies of individual experiments and can also provide a starting point for evaluating competing theories. They highlight issues that will help make the results of future studies more cohesive and interpretable. Two recent meta-analyses illustrate their value in neuroimaging studies. One concerns the involvement of the mirror neuron system in imitation, and the other focuses on the role of the temporo-parietal junction (TPJ) in social cognition.
The discovery of mirror neurons, which discharge both during action execution and action observation and were first documented in the monkey premotor and parietal cortex, has led to the formulation of several theories about their function in humans, including suggestions that mirror neurons are involved in understanding the meaning and intentions of observed actions, learning by imitation, feeling empathy, formation of a “theory of mind” (ToM), and even the development of language. Yet, despite a decade of prolific research on these appealing theories, there is little evidence to support them (Distein, Thomas, Behrmann, & Heeger, 2008). A recent meta-analysis of 20 functional MRI studies of imitation of hand and finger movements reported that in the frontal lobe, the dorsal premotor cortex rather than the inferior frontal gyrus is consistently active, and in the parietal region the superior and inferior parietal lobules are equally activated during imitation (Molenberghs, Cunnington, & Mattingly, 2009). These results seriously question the crucial role of the inferior frontal gyrus during imitation (the region considered to be the homologue of the monkey ventral premotor cortex) and demonstrate the importance of conducting meta-analyses to look beyond the idiosyncrasies of individual experiment.
A second reason why meta-analyses are important in neuroimaging studies is that they contribute to accumulate consensus across tasks that involve putatively similar processes or in identifying hypotheses regarding the least common denominator. For instance, accumulative evidence from functional MRI studies indicates that a relatively restricted area at the TPJ is systematically associated with a variety of psychological constructs including perspective-taking, empathy, ToM, and moral reasoning. This has led to the speculation that the TPJ is specialized for the possibly uniquely human ability to reason about others’ affective and cognitive mental states.
However, it is important to note that the TPJ is not only activated during higher level psychological processes, but also when individuals must distinguish themselves from others and during attention reorienting. Indeed, a number of studies on the sense of agency (i.e., the feeling of being the cause of one’s own actions, desires, or thoughts), which relies on the comparison between self-generated and externally produced sensory signals, have consistently resulted in activation of the right TPJ. In addition, the TPJ region is also activated by the violations in expectation about external physical events, such as the presentation of visual stimuli in a noncued screen location (Corbetta & Shulman, 2002). Decety and Lamm (2007) conducted quantitative meta-analyses of 70 functional neuroimaging studies that reported activation of the TPJ during various social cognitive tasks. Results demonstrated that this area is also engaged in lower level (bottom-up) computational processes associated with the sense of agency and reorienting attention to salient stimuli. The authors argued that this domain-general computational mechanism is crucial for higher level social cognitive processing including moral reasoning. The analyses performed for this study are in line with the general heuristic of the meta-analytic approach. This heuristic consists of a dialectic loop that proceeds from meta-analyzing published or available data to generating new or revising old hypotheses that are then challenged in new experimental studies.
Brain Functions Unfold Over Time
Until recently, there was relatively limited research on the neuro-biological changes accompanying the affective and cognitive changes that occur during normal development from early childhood to adulthood. Nevertheless, advances in neuroimaging techniques over the past decade have allowed us to begin tracking changes in the structural and functional brain organization (Yurgelun-Todd, 2007).
The focus of studying subcomponents of more complex behaviors can be particularly useful from a developmental perspective when only some components of, or precursors to, more complex behaviors are observable. In addition, developmental studies can provide unique opportunities to see how the components of the system interact in ways not possible in adults—where all the components are fully mature and operational (De Haan & Gunnar, 2009). For example, a recent study used functional MRI to characterize developmental changes in brain activation in the neural circuits underpinning empathy and sympathy (Decety & Michalska, 2010). Fifty-seven individuals, ages ranging from 7 to 40 years old, were presented with short animated visual stimuli depicting painful and nonpainful situations. These situations involved either a person whose pain was accidentally caused or a person whose pain was intentionally inflicted by another individual to elicit empathic (feeling as the other) or sympathetic (feeling concern for the other) emotions, respectively. The stimuli were extensively validated prior to the MRI scanning with eye tracking and pupilary measures, as well as perceived intentionality and empathic concern judgments. Results demonstrated monotonic age-related changes in the amygdala, supplementary motor area, and posterior insula when participants were exposed to painful situations that were accidentally caused. When participants observed painful situations intentionally inflicted by another individual, age-related changes were detected in the dorsolateral prefrontal and ventro-medial prefrontal cortex, with a gradual shift in that latter region from its medial to its lateral portion. This pattern of activation seems to reflect a change from a visceral emotional response critical for the analysis of the affective significance of stimuli to a more evaluative function. Furthermore, these functional changes support the general notion that the development of affective processing from childhood to adulthood is accompanied by reduced activity within limbic affect processing systems and increased involvement of other prefrontal systems (Killgore & Yurgelun-Todd, 2007).
Nonhuman primate models are also critical for fully understanding brain–behavior relationships. For example, amygdala lesion studies in neonatal rhesus macaques have provided important information on the role of the amygdala and the development of social behavior (Amaral et al., 2004). Such research, in turn, helps understanding human development. For instance, Shaw and colleagues (2004) examined how lesions of the amygdala occurring at different stages of development affected this key aspect of social cognition. Individuals with early damage to the amygdala were found to be impaired relative to all other groups on more advanced tests of ToM reasoning. In contrast, subjects who acquired damage to the amygdala in adulthood (usually as part of an anterior temporal lobectomy) were not impaired in ToM reasoning relative to both clinical and healthy controls, supporting the position that the amygdala is not part of the neural circuitry mediating the “on-line” performance of ToM reasoning.
In sum, although neuroimaging is neither necessary nor sufficient to address all questions in psychology, it represents a technical advance that creates an opportunity to address old questions in new ways and uncover new questions about brain function and behavior. When neuroimaging data are combined with fine-grain conceptual analyses, effective and functional connectivity analyses, quantitative meta-analyses, lesion studies, and other sources of information (e.g., genetics, hormones, electrophysiology, autonomic nervous system), they can significantly advance our understanding of the human mind and psychopathological dysfunctions. We do not underestimate the magnitude of the challenges that lay ahead, but we are optimistic that iterative scientific progress can be made in the years to come, even if the progress may sometimes appear erratically incremental.
Acknowledgments
Preparation of this article was supported by National Science Foundation Grant No. BCS-0718480, National Institute on Aging Grant No. RO1 AG034052-01, and the John Templeton Foundation.
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
- Amaral DG, Capitanio JP, Jurdain M, Mason WA, Mendoza SP, Prather M. The amygdala: Is it an essential component of the neural network for social cognition? Neuropsychologia. 2003;41:235–240. doi: 10.1016/s0028-3932(02)00154-9. [DOI] [PubMed] [Google Scholar]
- Anderson NB. Levels of analysis in health science: A framework for integrating sociobehavioral and biomedical research. Annals of the New York Academy of Sciences. 1998;840:563–576. doi: 10.1111/j.1749-6632.1998.tb09595.x. [DOI] [PubMed] [Google Scholar]
- Beck DM. The appeal of the brain in the popular press. Perspectives on Psychological Science. 2010;5:762–766. doi: 10.1177/1745691610388779. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG. Social psychological contributions to the decade of the brain: Doctrine of multilevel analysis. American Psychologist. 1992;47:1019–1028. doi: 10.1037//0003-066x.47.8.1019. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Decety J. What are the brain mechanisms on which psychological processes are based? Perspectives on Psychological Science. 2009;4:10–18. doi: 10.1111/j.1745-6924.2009.01094.x. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Neuroscience Reviews. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: How low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13:580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ. Neurodevelopmental changes in the circuits underlying empathy and sympathy from childhood to adulthood. Developmental Science. 2010 doi: 10.1111/j.1467-7687.2009.00940.x. [DOI] [PubMed] [Google Scholar]
- De Haan M, Gunnar MR. The brain in a social environment. Why study development? In: De Haan M, Gunnar MR, editors. Handbook of developmental social neuroscience. New York: Guilford Press; 2009. pp. 3–10. [Google Scholar]
- Distein I, Thomas C, Behrmann M, Heeger DJ. A mirror up to nature. Current Biology. 2008;18:R13–R18. doi: 10.1016/j.cub.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves BD, Cohen NJ. Brain imaging, cognitive processes, and brain networks. Perspectives on Psychological Science. 2010;5:744–752. doi: 10.1177/1745691610388776. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun-Todd DA. Unconscious processing of facial affect in children and adolescents. Social Neuroscience. 2007;2:28–47. doi: 10.1080/17470910701214186. [DOI] [PubMed] [Google Scholar]
- Miller GA. Mistreating psychology in the Decade of the Brain. Perspectives on Psychological Science. 2010;5:716–743. doi: 10.1177/1745691610388774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P, Cunnington P, Mattingley JB. Is the mirror neuron system involved in imitation? A short review and meta-analysis. Neuroscience and Biobehavioral Reviews. 2009;33:975–980. doi: 10.1016/j.neubiorev.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Mapping mental function to brain structure: How can cognitive neuroimaging succeed? Perspectives on Psychological Science. 2010;5:753–761. doi: 10.1177/1745691610388777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Raichle ME. Images of mind. New York: Scientific American Library; 1994. [Google Scholar]
- Shaw P, Lawrence EJ, Radbourne C, Bramham J, Polkey CE, David AS. The impact of early and late damage to the human amygdala on theory of mind reasoning. Brain. 2004;127:1535–1548. doi: 10.1093/brain/awh168. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Current Opinion in Neurobiology. 2007;17:251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]


