Abstract
Aromatic amines and structurally related heterocyclic aromatic amines (HAAs) are produced during the combustion of tobacco or during the high-temperature cooking of meat. Exposure to some of these chemicals may contribute to the etiology of several common types of human cancers. 2-Amino-9H-pyrido[2,3-b]indole (AαC) is the most abundant HAA formed in mainstream tobacco smoke: it arises in amounts that are 25–100 times greater than the levels of the arylamine, 4-aminobiphenyl (4-ABP), a human carcinogen. 2-Amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) is a prevalent HAA formed in cooked meats. AαC and MeIQx are rodent carcinogens; however, their carcinogenic potency in humans is unknown. A preliminary assessment of the carcinogenic potential of these HAAs in humans was conducted by examining the capacity of primary human hepatocytes to form DNA adducts of AαC and MeIQx, in comparison to 4-ABP, followed by the kinetics of DNA adduct removal by cellular enzyme repair systems. The principal DNA adducts formed were N-(deoxyguanosin-8-yl) (dG-C8) adducts. Comparable levels of DNA adducts were formed with AαC and 4-ABP, whereas adduct formation was ~5-fold lower for MeIQx. dG-C8-AαC and dG-C8-4-ABP were formed at comparable levels in a concentration-dependent manner in human hepatocytes treated with procarcinogens over a ten thousand-fold concentration range (1 nM – 10 µM). Pretreatment of hepatocytes with furafylline, a selective inhibitor of cytochrome P450 1A2, resulted in a strong diminution of DNA adducts signifying that P450 1A2 is a major P450 isoform involved in bioactivation of these procarcinogens. The kinetics of adduct removal varied for each hepatocyte donor. Approximately half of the DNA adducts were removed within 24 h of treatment; however, the remaining lesions persisted over 5 days. The high levels of AαC present in tobacco smoke and its propensity to form persistent DNA adducts in human hepatocytes, suggests that AαC can contribute to DNA damage and the risk of hepatocellular cancer in smokers.
Keywords: Heterocyclic aromatic amines, DNA adducts, DNA repair
INTRODUCTION
Certain aromatic amines present in tobacco smoke, including 4-aminobiphenyl (4-ABP) and 2-naphthylamine (2-NA), are classified as human carcinogens (Group 1), and several prevalent heterocyclic aromatic amines (HAAs) formed in tobacco smoke or cooked meats are classified as probable or possible human carcinogens (Group 2A and 2B), based on toxicity data reviewed by the International Agency for Research on Cancer.1,2 There are extensive epidemiological data reported in the literature on 4-ABP and 2-NA, which are human urinary bladder carcinogens.1,2 Many epidemiological studies have implicated frequent consumption of well-done cooked meats containing HAAs with an increased risk in the development of cancers of the digestive tract, prostate gland, or mammary gland of women.3–6
The HAA 2-amino-9H-pyrido[2,3-b]indole (AαC) is an experimental animal carcinogen.7 AαC was discovered in a pyrolysate of soy bean globulin,8 and subsequently detected in well-done cooked meat,9 and in environmental fumes.10 AαC also arises in mainstream cigarette smoke at levels ranging from 37 to 258 ng/cigarette.11,12 These levels are 25 to 100-fold greater than the amounts of 4-ABP present in tobacco smoke.13 4-ABP has been implicated in the pathogenesis of bladder cancer in smokers.1 Recently, we detected AαC in the urine of individuals of the Chinese cohort study.14 The number of cigarettes smoked per day was positively and significantly related to urinary levels of AαC in study subjects. This data signifies that cigarette smoking is a major point source of exposure to AαC. Apart from the endocyclic nitrogen atoms, AαC has the same chemical structure as 2-aminofluorene, one of the most well-studied carcinogenic aromatic amines.15 2-Amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx), is another prevalent HAA, which is formed in well-done cooked meats and fish,16 and it induces cancer of the liver and multiple extrahepatic organs of rodents.7 MeIQx has been identified in urine of meat-eaters.17
Arylamines and HAAs are bioactivated via a cytochrome P450 (P450) mediated N-oxidation of the exocyclic amine group.18 The N-hydroxylated metabolites undergo conjugation by phase II enzymes to form unstable esters, which hydrolyze to form the presumed nitrenium ions, reactive electrophiles that covalently bind to DNA.17,19 The bladder carcinogenicity of 4-ABP is thought to be attributed to the ability of 4-ABP to form DNA adducts in bladder cells, probably by its P450-mediated bioactivation in the liver and transport to the bladder.19 The major DNA adduct of 4-ABP, N-(deoxyguanosin-8-yl)-4-aminobiphenyl (dG-C8-ABP), has been detected in human urinary bladder, by 32P-postlabeling20 and LC/MS methods.21 4-ABP is also a hepatic carcinogen in rodents.22 One case-control study measured 4-ABP-DNA adducts, by immunohistochemistry methods, in hepatocytes of subjects with hepatocellular carcinoma. A statistically significant increase in risk for hepatocellular carcinoma was reported with increasing levels of adducts.23 Because cigarette smoking is a major source of exposure to 4-ABP in humans, this molecular epidemiologic study strengthened the notion that tobacco smoke is a hepatic carcinogen in humans.24,25
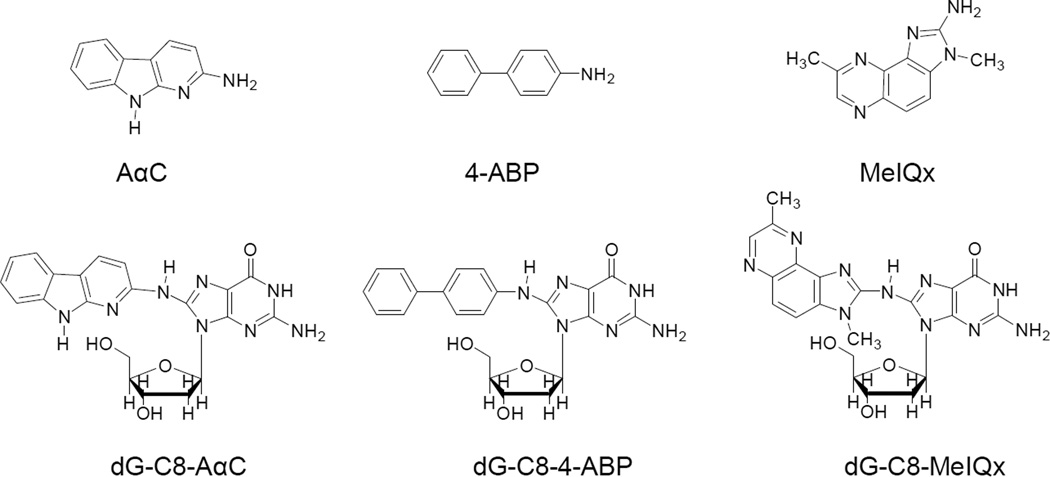
AαC and MeIQx also form a DNA adduct at the C8 atom of deoxyguanosine (Figure 1). N-(Deoxyguanosin-8-yl)-dG-C8-MeIQx and N-(Deoxyguanosin-8-yl)-dG-C8-AαC occur in vitro by reaction of 2-hydroxyamino-3,8-dimethylimidazo[4,5-f]quinoxaline (HONH-MeIQx) or 2-hydroxyamino-9H-pyrido[2,3-b]indole (HONH-AαC) with calf thymus DNA. These adducts are also formed in rat and human primary hepatocytes exposed to AαC and MeIQx26–28 and have been detected in salivary DNA of smokers or meat-eaters.29 Liver tissue of human donors catalyze the formation of AαC–DNA adducts, and a positive correlation was observed among the exogenously formed levels of AαC–DNA adducts, endogenous levels of hepatic DNA adducts, and P450 1A2 activities in microsomes prepared from the liver tissue.30 The genotoxic potentials of AαC and MeIQx in humans are not known; however, both AαC and MeIQx are liver carcinogens in mice, transgene colon mutagens and inducers of aberrant crypt foci, early biomarkers of neoplasms, in the colon of mice.31,32
Figure 1.
Chemical structures of AαC, 4-ABP, MeIQx, and their dG-C8 adducts.
Epidemiologic studies conducted over the past two decades have consistently shown that tobacco smoking is a risk factor for cancers of the digestive tract,33,34 and there is increasing evidence that tobacco smoke is an independent risk factor for hepatocellular carcinoma, the predominant form of human liver cancer.35,36 However, the causal agents of these cancers in tobacco smoke are uncertain. The ability of chemical carcinogens to form DNA adducts is regarded as one important factor in their carcinogenic potential.37,38 In vivo animal models have been employed to measure DNA adduct formation and the level of covalent binding of genotoxicants to DNA has been correlated to cancer risk.39–41
Freshly cultured human hepatocytes are also an excellent system to investigate different pathways of carcinogen metabolism and can be employed to measure DNA adduct formation within the cell, where cofactors are present at physiological concentrations and biotransformation pathways may closely simulate those which occur in vivo.42 We recently reported that human hepatocytes in primary culture bioactivate AαC, 4-ABP, and the cooked meat carcinogens 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and MeIQx to reactive species that bind to DNA.28 The levels of AαC-DNA adducts were higher than the adduct levels formed with PhIP or MeIQx, and were comparable to the levels of adducts formed with 4-ABP in hepatocytes exposed to an elevated concentration (10 µM) of procarcinogens. However, the relationships between DNA adduct formation and procarcinogen exposure was not investigated. The objective of our current study was to examine DNA adduct formation of AαC in primary human hepatocytes as a function of exposure to AαC over wide concentration range (1 nM – 10 µM); the lowest concentration (1 nM) may approach the level of exposure to AαC in tobacco smokers.11 The ability of AαC to form DNA adducts in different hepatocyte donors was compared to those levels of adducts formed by MeIQx, a rodent hepatocarcinogen, and to 4-ABP, a known human carcinogen. A second objective was to assess the role of cytochrome P450 1A2 in DNA adduct formation in hepatocytes. P450 1A2 is thought to be a major hepatic P450 isoform involved in the bioactivation and DNA adduct formation of HAAs and 4-ABP, on the basis of metabolism studies with human liver microsomes and recombinant human P450s.43–45 As a third objective, we examined the capacity of human hepatocytes to repair the principal dG-C8 adducts of these structurally related genotoxicants (Figure 1). These data provide a first assessment of the DNA binding and genotoxic potential of AαC and MeIQx, in comparison to 4-ABP, in a human liver cell model where enzyme biotransformation and DNA repair pathways closely simulate those which occur in humans.42,46,47
MATERIALS AND METHODS
Caution
AαC, 4-ABP, and MeIQx are carcinogens, and they should only be handled in a well-ventilated fume hood with the appropriate protective clothing.
Chemicals
MeIQx, 3-[2H3C]-MeIQx, and AαC were purchased from Toronto Research Chemicals (Toronto, ON, Canada). [4b,5,6,7,8,8a-13C6]-AαC was a kind gift from Dr. Daniel Doerge, National Center for Toxicological Research (Jefferson, AR). The isotopic purity of all compounds exceeded 99.5% except for 3-[2H3C]-MeIQx, which was 96.5% isotopically pure. 4-ABP was purchased from Aldrich (Milwaukee, WI). 2-Nitro-9H-pyrido[4,5-b]indole was a kind gift from Dr. D. Miller, National Center for Toxicological Research (Jefferson, AR).. [2H9]-4-Nitrobiphenyl (99.3% isotopically pure) was purchased from C/D/N Isotopes, Inc. (Pointe-Claire, Quebec, Canada). [2H9]-4-ABP was prepared by reduction of [2H9]-4-nitrobiphenyl with Zn in C2H5OH/HCl 60 °C for 2 h as previously described.48 [13C10]-dG (99.9% isotopically pure) was purchased from Cambridge Isotopes (Andover, MA). Alkaline phosphatase (from E. coli) and nuclease P1 (from Penicillium citrinum) were purchased from Sigma (St. Louis, MO). Phosphodiesterase I (from Crotalus adamanteus venom) was from Worthington Biochemical Corp. (Lakewood, NJ). All solvents used were high-purity B & J Brand from Honeywell Burdick and Jackson (Muskegon, MI). ACS reagent-grade formic acid (88%) was purchased from J.T. Baker (Phillipsburg, NJ).
Synthesis of the DNA Adducts
N-(Deoxyguanosin-8-yl)-AαC (dG-C8-AαC) and N-(deoxyguanosin-8-yl)-MeIQx (dG-C8-MeIQx) was prepared by a reaction of their N-acetoxy-HAA derivatives with dG or [13C10]-dG (5 mg) in 100 mM potassium phosphate buffer (pH 8.0). N-(Deoxyguanosin-8-yl)-4-aminobiphenyl (dG-C8-4-ABP) was prepared by the reaction of N-hydroxy-4-ABP with pyruvonitrile with dG or [13C10]-dG.49 For the case of dG-C8-MeIQx, the internal standard was prepared by a reaction of its N-acetoxy derivative of 3-[2H3C]-MeIQx with dG.26
Cell Isolation, Culture and Carcinogen Treatment
Human liver samples were obtained from eight patients undergoing liver resection for primary or secondary hepatomas through the Biological Ressource Center (CHRU Pontchaillou, Rennes, France). The research protocol was conducted under French legal guidelines and fulfilled the requirements of the local institutional ethics committee. This study was approved by the Institutional Review Board at the Wadsworth Center. Due to limited number of cells, all experiments (DNA adducts and enzymatic activities) were performed in duplicate to quadruplicate in at least three different donors. Hepatocytes were isolated by a two-step collagenase perfusion procedure and parenchymal cells were seeded in Petri dishes at a density of 3 × 106 viable cells/19.5 cm2 dish, in Williams’ modified medium during the 36 h prior to incubation with AαC, 4-ABP, and MeIQx in DMSO (0.1% v/v) at the different (0 – 10 µM) concentrations and times, as previously described.28 The media of the control and treated cells were renewed every 24 h.
Dose Response Study of DNA Adduct Formation
Cells were treated with 4-ABP or AαC at a concentration of 0.001, 0.003, 0.010, 0.10, 1.0 or 10.0 µM for 8 h. Thereafter, the culture media were collected and immediately stored at −80 °C. The cells were then washed wish PBS, before being scraped from the Petri Dishes. The cells were centrifuged and the pellets containing DNA were also stored at −80°C. Cellular pellets were then assayed for DNA adducts. These concentrations did not produce toxicity on the basis of the methylthiazoltetrazolium test.28
DNA Adduct Repair Study
The cells were treated with AαC, MeIQx or 4-ABP at a concentration of 1 µM for 8 h. Thereafter, the medium containing the carcinogens was removed, cells were washed twice with PBS, and fresh medium was renewed without carcinogen. The medium was then changed every 24 h. Cells and the media were collected every 12 h for 5 days and immediately stored at −80 °C. When the number of cells was limited, the time points investigated were 24, 48 or 120 h after removal of the carcinogens (donors 7 and 8). The incubation was terminated as described above. Cellular pellets were assayed for DNA adducts, and the media were assayed for the extent of metabolism of the carcinogens.
Measurement of P450 1A Activities
Ethoxyresorufin O-deethylase (EROD) and methoxyresorufin O-demethylase (MROD) activity associated with P450 1A2 in liver50 were measured in all eight primary cultured hepatocytes used in this study as described previously.51 The reaction rates were linear with time and proportional to protein concentration. Cellular protein content was estimated by the Bradford procedure.52 Values (pmol/min/mg protein) are the mean ± SD of quadruplicate measurements.
Role of P450 1A2 in the formation of DNA adducts of AαC, MeIQx, and 4-ABP
The cells were pre-treated with furafylline (5 µM) or 0.1% DMSO (v/v) for 24 h. Then, the medium was renewed with or without furafylline, and the cells were incubated with 4-ABP or HAA (1 or 10 µM) for an additional 8 or 24 h. At the end of the treatment, the cells were washed with PBS, before being scraped from the Petri Dishes. Cellular pellets were assayed for DNA adducts.
Isolation and Digestion of DNA for Adduct Measurements
Cellular pellets were homogenized in 400 µL TE buffer pH 8.0 (50 mM Tris-HCl, 10 mM EDTA) and incubated with RNase T1 (318.75 U) and RNase A (2 µL of a 10 mg/mL solution) for 30 min at 37 °C. Thereafter, proteinase K (10 µL of a 20 mg/mL solution) and SDS (10 µL of a 20% solution) and the solutions were incubated at 37° C for 1 h. DNA was isolated by the phenol/chloroform method and precipitated with ethanol.28 DNA was washed twice with 70% ethanol and dried at room temperature. The DNA was resuspended in 200 µL of sterile water and quantified with a NanoDrop™ 1000 Spectrophotometer (Thermo Fisher Scientific). DNA (5 µg) of each sample was spiked with isotopically labeled internal standards at a level of 1 adduct per 107 bases. DNA from the same concentrations of the individually treated 4-ABP and HAA hepatocyte samples were then pooled.
The enzymatic digestion of DNA was conducted in 5 mM Bis-Tris-HCl buffer (pH 7.1) with DNase I for 1.5 h, followed by incubation with nuclease P1 for 3 h, and then by digestion with alkaline phosphatase and phosphodiesterase for 18 h.53 These enzyme digestion conditions were shown to be highly efficient in the recovery of the dG-C8 adducts of PhIP, MeIQx, and 4-ABP from calf thymus DNA modified by these carcinogens.26,29 The samples were vacuum centrifuged to dryness and resuspended in 1:1 water:DMSO (30 µL). Following centrifugation (22,000 g for 5 min), the supernatant was transferred to capillary LC vials.
UPLC-ESI-MS/MS3 Measurement of DNA Adducts
The DNA adduct analyses were conducted with a Waters NanoAcquity UPLC system (Waters Corp., New Milford, MA) equipped with a Waters Symmetry trap column (180 µm × 20 mm, 5 µm particle size), a Michrom C18 AQ column (0.3 × 150 mm, 3 µm particle size, Michrom Bioresources Inc., Auburn, CA) and a Michrom CaptiveSpray™ source interfaced to a linear quadrupole ion-trap mass spectrometer (LTQ Velos, Thermo Fisher, San Jose, CA). Samples (10 µL) were injected on to the trap column and washed with 100% A (solvent composition: 0.01% HCO2H and 10% CH3CN) for 3 min, to remove non-modified deoxynucleosides. Thereafter, the adducts were back-flushed on to the analytical column and separated with a gradient. The solvent conditions were held at 100% A for 2 min, followed by a linear gradient to 100% B (solvent composition: 95% CH3CN containing 0.01% HCO2H) over 20 min at a flow rate of 5 µL/min.
Xcalibur version 2.07 software was used for data manipulations. Analyses were conducted in the positive ionization mode. Representative optimized instrument tuning parameters were as follows: capillary temperature, 270 °C; source spray voltage, 1.5 kV; source current, 0.3 µA; no sheath gas, sweep gas, or auxiliary gas was employed; capillary voltage, 32 V; tube lens voltage, 110 V. Helium was used as the collision damping gas in the ion trap and was set at a pressure of 1 mTorr. The LTQ MS was employed in the tandem MS/MS scan mode to monitor the loss of deoxyribose from the protonated molecules of the adducts ([M + H − 116]+), followed by the consecutive reaction monitoring scan mode at the MS3 scan stage, to characterize the product ions of the aglycone adducts [BH2]+. The ions monitored in MS → MS2 → MS3 scan modes were as follows: dG-C8-MeIQx (m/z 479.1 → 363.1 → 239.2, 318.4, 346.4); dG-C8-[2H3C]-C8-MeIQx (m/z 482.1 → 366.1 → 242.2, 321.5, 349.5); dG-AαC (m/z 449.1 → 333.1 → 209.2, 291.4, 316.4); [13C10]-dG-AαC (m/z 459.1 → 338.1 → 210.2, 295.5, 321.5); dG-C8-4-ABP (m/z 435.1 → 319.1 → 249.2, 277.3, 302.4); [13C10]-dG-C8-4-ABP (m/z 445.1 → 324.1 → 252.3, 281.4, 307.4). The reconstructed ion chromatograms containing these extracted ions produced at the MS3 scan stage were used for quantitative measurements of the adducts and their respective internal standards.
UPLC-ESI/MS2 Measurements of Supernatants to Assess Biotransformation of AαC, MeIQx, and 4-ABP
The cell culture media (50 µL) from the same time points of individually treated carcinogens were pooled and then the equivalent of 1000 pg of [13C6]-AαC, [2H9]-4-ABP, and 3-[2H3C]-MeIQx was added, followed by CH3OH (150 µL) and the mixtures were incubated on ice for 1 h, to promote the precipitation of salts and protein. The samples were then centrifuged at 15000 g for 3 min at 4 °C, and the supernatants were transferred to capLC recovery vials. The analyses were performed with a NanoAcquity UPLC system equipped with a Michrom C18 AQ column (0.3 × 150 mm, 3 µm particle size). The analytes were separated by a gradient: solvent A was 0.01% HCO2H in H2O, and solvent B contained 0.01% HCO2H and 5% H2O in CH3CN. The flow rate was set at 5 µL/min, starting at 95% A and increased by a linear gradient to 99% B over 20 min, and then holding for 1 min.
The mass-spectral data were acquired on a Finnigan Quantum Ultra Triple Stage Quadrupole MS (Thermo Fisher, San Jose, CA) and processed with Xcalibur version 2.07 software. Analyses were conducted in the positive ionization mode and employed an Advance CaptiveSpray (Michrom Bioresources Inc, Auburn, CA). The spray voltage was set at 1400 V; the in-source fragmentation was 5 V; and the capillary temperature was 200 °C. There was no sheath or auxiliary gas. The peak widths (Q1 and Q3) were set at 0.7 Da. The measurement of the chemicals was done by selected reaction monitoring (SRM). The following transitions and collision energies were used for the quantification of MeIQx, AαC and 4-ABP: MeIQx and [2H3C]-MeIQx: m/z 214.1 → 199.1 and 217.1 → 199.1, at 32 eV; the transitions of AαC and [13C6]-AαC were m/z 184.1 → 167.1 and 190.1 → 173.1 at 27 eV; and the transitions employed for 4-ABP and [2H9]-4-ABP were m/z 170.1 → 151.9 and 179.1 → 160.1, 159.1, 158.1 at 25 eV. The dwell time for each transition was 5 ms. Argon was used as the collision gas and was set at 1.5 mTorr
Calibration Curves
Calibration curves were constructed with [13C10]-dG-C8-AαC, [13C10]-dG-C8-4-ABP and dG-C8-[2H3C]-MeIQx set at 10 adducts per 108 deoxynucleosides with unlabeled DNA adducts added at a level of 0, 1 – 1000 adducts per 109 nucleotides (7 calibrant levels) in calf thymus DNA digest (5 µg). The calibration curves were done in triplicate at each level, and the data were fitted to a straight line (area of response of the adduct/internal standard versus the level of adduct per 108 nucleosides) using ordinary least-squares with equal weightings. The coefficient of determination (r2) values of the slopes exceeded 0.998. The estimates of unmetabolized AαC, MeIQx and 4-ABP in the media were determined by a single point estimate of the area ratio of the unmetabolized carcinogen/internal standard.
RESULTS
P450 1A Activity in Human Hepatocytes
Metabolism studies with human liver microsomes or recombinant P450s reveal that P450 1A2 is a major P450 isoform involved in the bioactivation of HAAs and 4-ABP.43–45 The basal activities of P450 1A were measured in human hepatocytes from eight donors (Table 1) using ethoxyresorufin and methoxyresorufin as substrates. These alkoxyresorufin homologues undergo dealkylation, by both P450s 1A and 1A2, at variable rates.50 As we previously reported,46 a wide interindividual variation in P450 1A enzyme activity was observed among the 8 donors. The variation of P450 activity was greater for MROD than for EROD activity: the EROD activity varied from 0.16 to 0.50 pmol/min/mg protein, whereas MROD activity ranged from 0.08 to 1.46 pmol/min/mg protein. These levels of enzyme activities overlap the ranges of activities measured in human hepatocytes reported in our previous studies.54,55 The levels of expression of P450 1A1 and 1A2 protein were determined in hepatocytes from two donors with a sufficient amount of cells: Western blot analysis revealed that only the P450 1A2 protein was expressed (Unpublished observations, S. Langouët). The findings are consistent with previous studies showing that P450 1A1 protein is rarely detected in human liver.56,57
Table 1.
P450 1A basal activities of EROD and MROD measured in human hepatocytes
| ERODa 24 h | ERODa + Furafylline | MRODa 24 h | MRODa + Furafylline | |
|---|---|---|---|---|
| Donor 1 | 0.16 ± 0.02 | - | 0.08 ± 0.07 | - |
| Donor 2 | 0.44 ± 0.04 | - | 0.31 ± 0.07 | - |
| Donor 3 | 0.50 ± 0.07 | - | 0.37 ± 0.03 | - |
| Donor 4 | 0.27 ± 0.07 | 0.16 ± 0.02** | 1.04 ± 0.03 | 0.21 ± 0.09** |
| Donor 5 | 0.22 ± 0.01 | 0.16 ± 0.01** | 1.46 ± 0.18 | 0.15 ± 0.01** |
| Donor 6 | 0.33 ± 0.04 | 0.28 ± 0.09 | 0.48 ± 0.01 | 0.30 ± 0.05* |
| Donor 7 | 0.21 ± 0.01 | - | 0.58 ± 0.11 | - |
| Donor 8 | 0.27 ± 0.02 | - | 0.77 ± 0.02 | - |
activities are expressed in pmol/min/mg protein, mean ± SD (N = 4 measurements), after 60 h of culture
Due to limited number of cells, the effect of furafylline was studied only with donors 4, 5 and 6.
(Unpaired Sudent t- test: *p < 0.004; **p < 0.001)
Furafylline, a selective mechanistic-based inhibitor of P450 1A2,58 was more effective in diminution of MROD than EROD activity (Table 1). On the basis of MROD activity, P450 1A2 was inhibited by 80% and 91%, respectively, in hepatocytes of donors 4 and 5, and inhibition of P450 1A2 occurred by 38% in the hepatocytes of donor 6. The inhibition of EROD activity was significant but less pronounced than for MROD. Based on the strong inhibition of HAA- and 4-ABP-DNA adduct formation (vide infra), we surmise that a portion of the remaining O-dealkylase activities in hepatocytes is attributed to P450s other than P450 1A2.59
Metabolism and Bioactivation of AαC, MeIQx, and 4-ABP in Human Hepatocytes
The extent of metabolism of AαC, MeIQx, and 4-ABP in hepatocytes in primary culture treated with the carcinogens (1 µM) was estimated in donor 3, by measuring the amount of parent amine remaining in the media over time. The metabolism of AαC and MeIQx was more extensive than that of 4-ABP in this hepatocyte sample. After the 8 h treatment, 35% of the initial concentration of 4-ABP remained in the medium, whereas MeIQx and AαC were undetected (limit of detection was <0.1% of initial concentration) (Supporting Information, Figure S-1). These results are consistent with our previous findings showing that the metabolism of 4-ABP was slower than the rate of metabolism of HAAs.28,46 The cell media were measured every 12 h after the 4-ABP and HAAs had been removed. The analyses showed that neither 4-ABP nor HAAs were stored within the cells and excreted into the media over time.
The Role of P4540 1A2 in DNA Adduct Formation of AαC, MeIQx, and 4-ABP in Human Hepatocytes
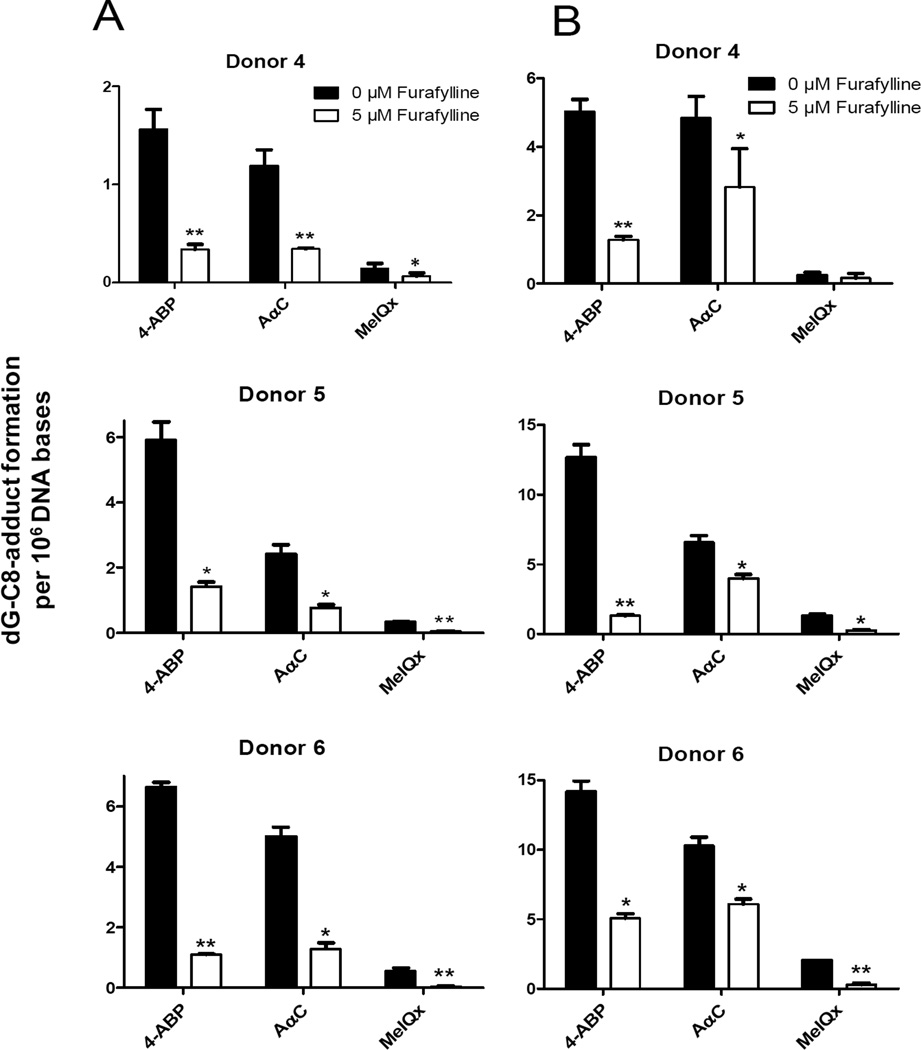
The contribution of P450 1A2 to activate AαC, MeIQx, and 4-ABP was assessed by measuring DNA adduct formation in cells pretreated with furafylline for 24 h in cultures of human hepatocytes from three different donors. Furafylline strongly inhibited the formation of DNA adducts in hepatocytes exposed to these procarcinogens (1 µM): DNA adduct formation was reduced by 70 to 90% (Figure 2). DNA adduct formation was also inhibited by furafylline, when the concentration of procarcinogens was increased to 10 µM, although the inhibition was less potent. The findings imply that P450 1A2 is a major P450 isoform involved in the bioactivation of AαC, MeIQx, and 4-ABP in human hepatocytes.
Figure 2.
DNA adduct levels formed in human hepatocytes treated with AαC, 4-ABP or MeIQx at concentrations (A) 1 µM or (B) 10 µM for 24 h without or with pretreatment of cells with furafylline (5 µM). DNA adducts were measured independently in duplicate or triplicate, and the data are plotted as the mean and standard deviation of the adduct levels donors 4, 5 and 6). Treatment with furafylline resulted in a significant decrease in adduct levels for all treatments (unpaired Student t test, *p < 0.02, **p < 0.002) except for MeIQx adduct formation at 10 µM for donor 4.
Concentration-Dependent Formation of AαC- and 4-ABP-DNA Adducts in Human Hepatocytes
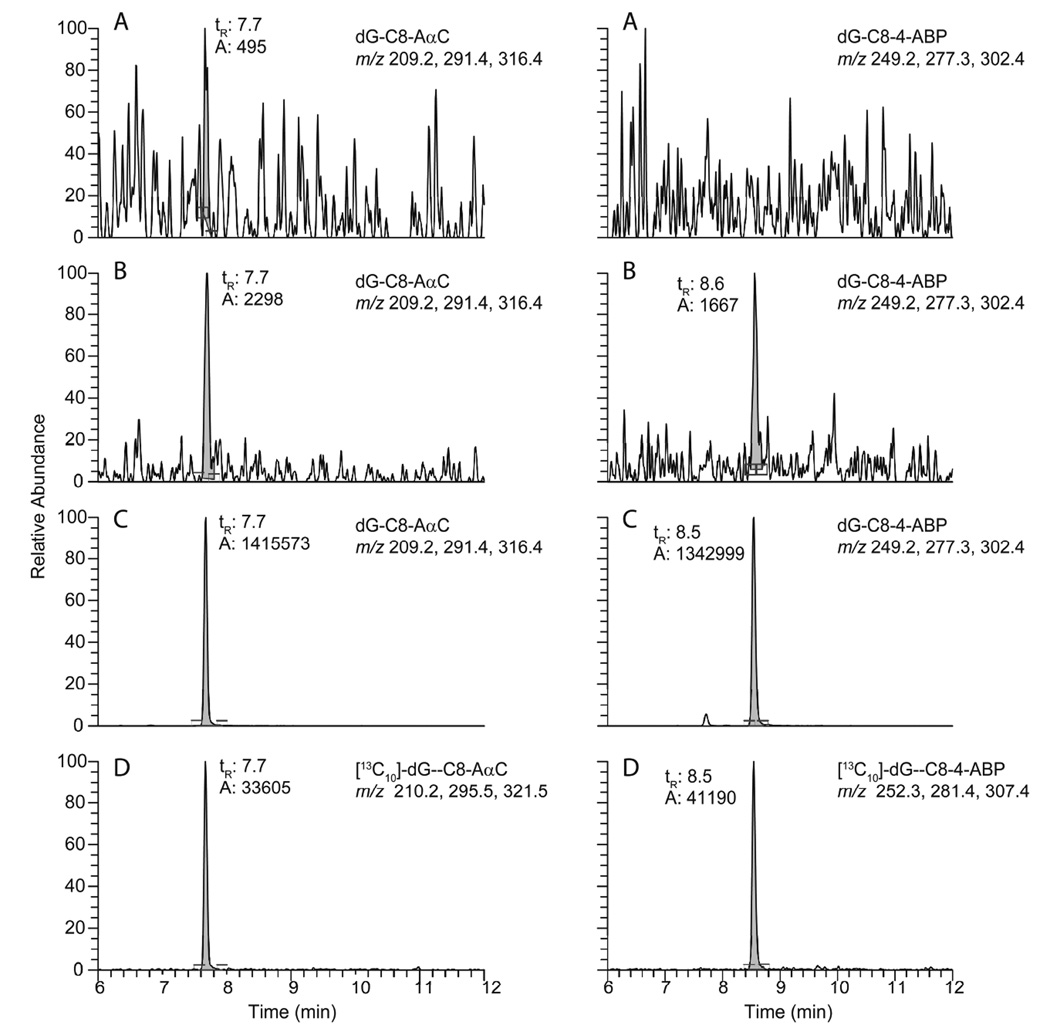
We examined the capacity of hepatocytes to metabolize AαC and 4-ABP to form DNA adducts as a function of procarcinogen concentration. The levels of AαC- and 4-ABP-DNA adducts were determined in hepatocyte primary culture from 3 different donors with concentrations of carcinogens ranging from 1 nM to 10 µM. An exposure period of 8 h was chosen to investigate the concentration-response relationship of DNA adduct formation, because the highest levels of adducts were formed at this time point in our earlier study with primary cultured human hepatocytes.28 Representative UPLC-ESI/MS3 chromatograms of DNA adducts obtained from untreated, and cells exposed to AαC- and 4-ABP at concentrations of 1 nM or 1 µM are presented in Figure 3. The major DNA adducts were identified as the dG-C8 adducts. The treatment of hepatocytes with MeIQx was only conducted at a concentration of 1 µM, and the level of adducts formed were ~5-fold lower than for AαC and 4-ABP. A UPLC-ESI/MS3 chromatogram for dG-C8-MeIQx formation is shown in Supporting Information, Figure S-2. The product ion spectra for all three dG-C8 adducts at the MS3 scan stage are shown in Supporting Information, Figure S-3.
Figure 3.
UPLC-ESI/MS3 chromatograms of dG-C8-AαC and dG-C8-4-ABP in (A) untreated hepatocytes, (B) hepatocytes treated with 0.001 µM carcinogen, (C) hepatocytes exposed to 1 µM carcinogen for 8 h, and (D) internal standards [13C10]-dG-C8-AαC and [13C10]-dG-C8-4-ABP were added at a level of 1 adduct per 107 DNA bases.
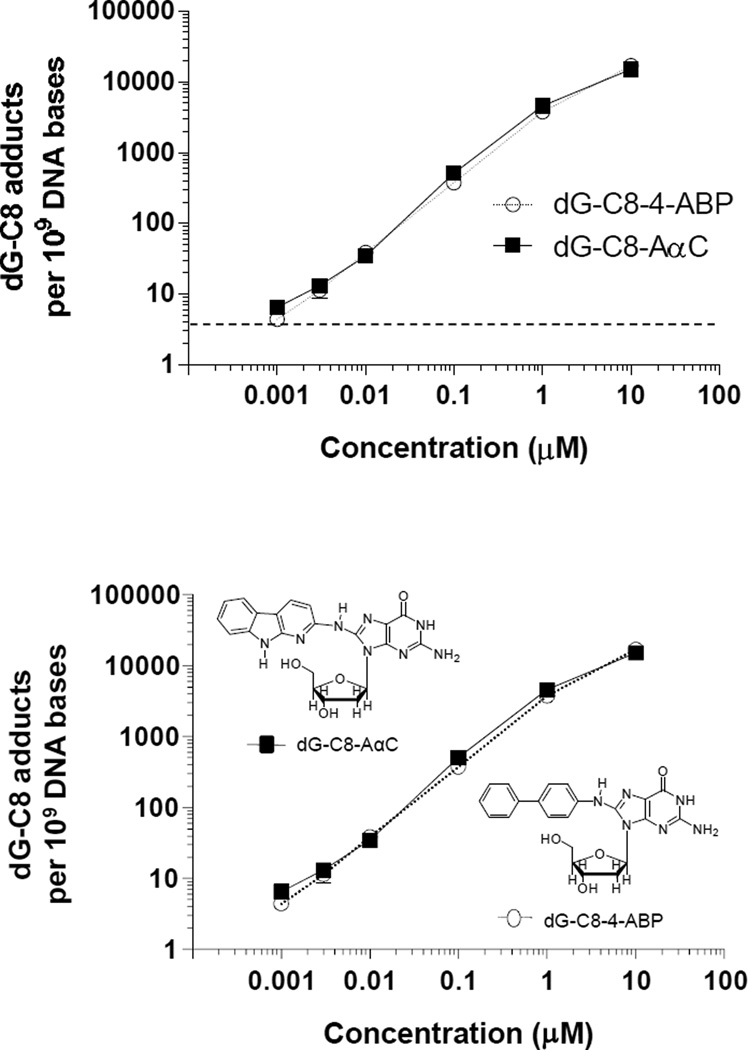
The formation of dG-C8-AαC and dG-C8-4-ABP adducts occurred in a concentration-dependent manner, and DNA adducts were detected at the lowest concentration of exposure (1 nM). The levels of adducts formed in hepatocytes of donor 3 as a function of concentration of AαC or 4-ABP are depicted Figure 4, and the levels of adduct formation as a function of concentration for two other donors are reported in Supporting Information (Table S-1). The level of dG-C8 adduct formation began to reach a plateau at the highest concentration of exposure to AαC and 4-ABP (10 µM), and a power function was a better fit of the data than a linear regression curve. However, the coefficient of determination values (r2) for the linear regression analysis for both dG-C8-AαC and dG-C8-4-ABP formation were >0.999, when the highest concentration of procarcinogen treatment was excluded from the analysis of the data (AαC: Y = 4.559(X) + 1 × 10−8 and 4-ABP: Y = 3.768(X) + 3 × 10−10, where X = concentration in molarity and Y = adduct level per DNA base; the 95% confidence intervals of the Y intercepts include the origin when X = 0).
Figure 4.
DNA adduct formation in human hepatocyte donor 3 treated with AαC or 4-ABP at concentrations of 0.001, 0.003, 0.010, 0.10, 0.50, 1.0, or 10.0 µM. DNA adducts were measured in duplicate or triplicate, and the adduct levels were plotted as the mean and standard deviation. The horizontal dashed line delineates the adduct level for the limit of quantification of DNA adducts.
Persistence of dG-C8 Adducts of AαC, MeIQx, and 4-ABP in Human Hepatocytes
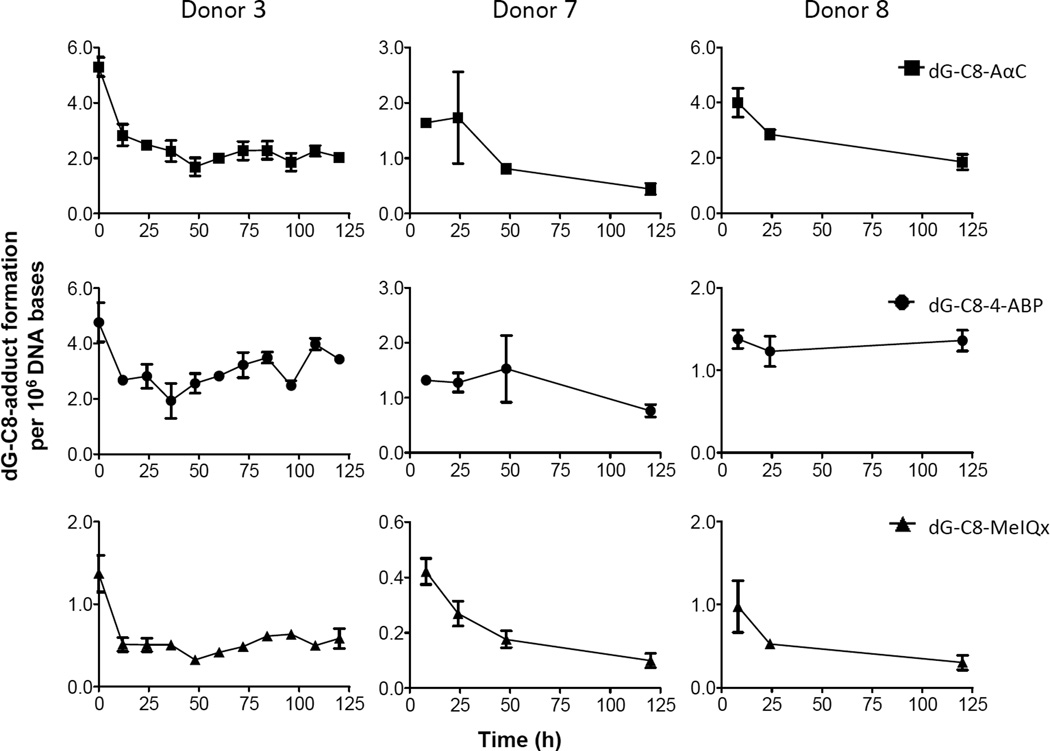
The persistence of DNA adducts was investigated in primary hepatocytes from three donors (Figure 5). For donor 3, there was a sufficient number of hepatocytes to measure the level of DNA adducts over multiple time points. The amount of hepatocytes for donor 7 and 8 were limiting, and the levels of adducts were measured at 2 or 3 time points up to 120 h after treatment with the carcinogens. For donor 3, the initial levels of adducts after 8h of treatment were: dG-C8-AαC 530 ± 34; dG-C8-4-ABP 476 ± 17; and dG-C8-MeIQx 137 ± 23 per 108 bases. The amounts of adducts remaining in the cells were then measured every 12 h over 5 days (Figure 5). The adduct levels for each carcinogen decreased to about half of the initial levels 12 – 24 h after the exposure to the compounds had ceased. Thereafter, the remaining adducts persisted during the next 4 – 5 days. The rates of adduct removal displayed appreciable interindividual variation for donors 7 and 8. The dG-C8 adduct of 4-ABP was the most persistent lesion, followed by dG-C8-AαC and then dG-C8-MeIQx in the hepatocytes of the three donors used in this study.
Figure 5.
Kinetics of DNA adduct removal. Time 0 h corresponds to the level of adducts present at the end of the 8 h treatment with AαC, MeIQx, or 4-ABP (1 µM), when the carcinogens were removed from the media. Adduct measurements were made with either 2 or 3 independent cell cultures (donors 3, 7 and 8). Data are expressed as the mean and the standard deviation.
DISCUSSION
Liver is by far the most metabolically active tissue in the biotransformation of HAAs.17 We showed previously that human hepatocytes in primary culture closely reflect the metabolism of HAAs in vivo; the metabolite profile of MeIQx and PhIP in primary human hepatocytes closely matched the urinary metabolite profile of both HAAs in healthy humans.46,60,61 In this study, we have examined the capacity of human hepatocytes to bioactivate HAAs and 4-ABP to DNA damaging agents and the ensuing repair of the resultant DNA lesions. In vitro studies conducted with human liver microsomes or recombinant human P450s have implicated P450 1A2 as the primary P450 isoform involved in N-oxidation and bioactivation of 4-ABP and HAAs.43–45 The treatment of hepatocytes with furafylline, a selective, mechanism-based inhibitor of P450 1A2,58 show that P450 1A2 is also the primary P450 isoform in human hepatocytes involved in N-oxidation of HAAs and 4-ABP, and the catalytic activity of P450 1A2 is critical for DNA adduct formation (Figure 2).
Human hepatocytes are highly efficient in the bioactivation of AαC, MeIQx, and 4-ABP into reactive intermediates that bind to DNA. In fact, the levels of adduct formation are comparable to or greater than the levels of dG-C8 adducts of all three genotoxicants formed in Chinese hamster ovary (CHO) cells stably transfected with P450 1A1 or P450 1A2 co-expressed with N-acetyltransferase (NAT2*4).62–64 The global DNA adduct formation and the genotoxicity of AαC, MeIQx, and 4-ABP in the hypoxanthine phosphoribosyl transferase (hprt) gene of these CHO cell lines revealed that the potential of the dG-C8 adducts of AαC and MeIQx to induce hprt mutations was greater than that of dG-C8-4-ABP, when the mutation frequency was normalized to the levels of the dG-C8 adducts.
DNA adduct formation of AαC and 4-ABP in human hepatocytes occurred in a linear, concentration-dependent manner over a thousand-fold exposure range (Figure 4). The lowest concentration of procarcinogen assayed (1 nM) may approach the exposure levels of AαC in tobacco smokers.11 DNA adducts are regarded as one important factor in the carcinogenic potential of a genotoxicant, and DNA adducts have been used for interspecies extrapolation of toxicity data for human risk assessment.37,38 The carcinogenic potential of genotoxic chemicals in rodents has been assessed by the carcinogen DNA binding index and bench mark dose values.39–41 The doses of chemicals and the levels of their DNA adducts required at the steady-state to induce liver tumors in rodents vary greatly.39–41 The steady-state levels of some types of DNA adducts in rats chronically exposed to a single carcinogen, including HAAs or arylamines, generally must be greater than 100 adducts per 108 DNA bases to increase the frequencies of tumors by 10% or greater than the spontaneous background levels.41 The levels of dG-C8-AαC and dG-C8-4-ABP formed in human hepatocytes are ~5 adducts per 109 DNA bases, following a single exposure to AαC or 4-ABP at the lowest concentration assayed (1 nM); however, because of their persistence, the levels of adduct are likely to increase during repeated exposures. The development of human cancer is complex and requires a number of steps, including multiple mutations, genetic alterations, and cell proliferation.65 AαC is one of many genotoxicants present in tobacco smoke. AαC may act as an initiating agent in the development of human cancer during chronic smoking, in which many other mutagens, carcinogens, tumor promoters, and factors stimulating tumor progression exist.7
There are two reports on the genotoxicity of AαC in human cells. One study reported an induction of DNA double strand breaks and micronucleus formation in human lymphoblastoid cell line MCL-5 cells treated with AαC at concentrations above 50 nM.66 Another study reported that AαC induced double strand breaks in peripheral blood lymphocytes and micronuclei in the human hepatoma HepG2 cell line, when employing even higher concentrations of AαC.67 Our data show that human hepatocytes in primary culture are far more susceptible to the genotoxicity of AαC than MCL-5 and HepG2 cell lines, certainly because of the superior capacity of hepatocytes to bioactivate AαC. A possible explanation for the high levels of AαC-DNA adducts formed in hepatocytes may be attributed to UDP-glucuronosyltransferase (UGTs) enzymes. Usually UGTs are important enzymes involved in the detoxication of procarcinogens; however, we discovered that several UGTs bioactivate HONH-AαC and catalyze the formation of an O-glucuronide conjugate of HONH-AαC, a reactive intermediate that binds covalently to DNA.68 In addition, NAT2 and SULT1A1, which are highly expressed in liver,69,70 catalyze the binding of HONH-AαC to DNA.71
Approximately half of the dG-C8-AαC, dG-C8-MeIQx, and dG-C8-4-ABP adducts underwent repair within the 12 –24 h following the removal of the carcinogens from the media for two of the hepatocyte donors. Thereafter, the remaining adducts persisted over the next 120 h. In the case of donor 8, dG-C8-4-ABP was stable over the entire study. NMR solution structural studies have shown that several dG-C8-HAA and dG-C8-arylamine adducts in some oligonucleotide sequence contexts adopt the glycosidic torsion angle in the syn conformation instead of the normally occurring anti conformation.72–74 These conformational changes in the DNA helix induced by HAA–purine or arylamine-purine adducts are important determinants of the adducts’ biological effects and miscoding properties during translesional syntheses with polymerases,75–77 and also influence the adducts’ persistence and rate of adduct removal in vivo,78–80 by the nucleotide excision repair (NER) complex.75 NER is the primary pathway responsible for the removal of bulky DNA lesions, including those formed by aromatic amines and HAAs.81,82 There are two subpathways of NER: global-genome repair (GG-NER) and transcription-coupled repair (TC-NER). These enzyme complexes only differ by the manner in which the substrate lesions are recognized, thereafter, the two enzyme complexes share a common set of steps to complete the repair process.81 Persistent adducts are viewed as most biologically relevant, because the unrepaired DNA adducts can be bypassed by error-prone DNA polymerases, leading to the accumulation of mutations.65,77 We surmise that the initial rapid removal of dG-C8 adducts of AαC, MeIQx, and 4-ABP adducts occurred either by GG-NER or TC-NER of actively transcribed genes in hepatocytes, and the persistent adducts resided in sequence contexts that minimally perturbed DNA conformation and escaped GG-NER,81,83 or the remaining adducts were situated in transcriptionally silent portions of the genome. Our findings show that a significant proportion of the dG-C8-AαC, dG-C8-MeIQx, and dG-4-ABP adducts in genomic DNA persist in human hepatocytes.
Tobacco smoking is a recognized risk factor for cancers of the digestive tract 33,34 and an independent risk factor for hepatocellular carcinoma.35,36 Several epidemiologic studies have reported that frequent consumption of well-done meat containing HAAs increase the risk of colorectal cancer, particularly in individuals who harbored rapid phenotype for both P450 1A2 and NAT2, which bioactivate AαC and other HAAs, and the risk was greatly increased in smokers.6,84 The high level of adduct formation and the relative persistence of the dG-C8-AαC in hepatocytes suggest that dG-C8-AαC can serve as a biomarker to assess the exposure and DNA damage induced by AαC in liver and possibly extrahepatic tissues of tobacco smokers. The considerably higher levels of AαC present in tobacco smoke in comparison to 4-ABP, other aromatic amines and HAAs, combined with the propensity of AαC to undergo bioactivation by phase I and II enzymes expressed in the liver and extrahepatic tissues provide a biochemical mechanism for a role for AαC in the etiology of tobacco-associated cancers.
Supplementary Material
ACKNOWLEDGEMENTS
Financial support: This work was funded by the Institut National de la Santé et de la Recherche Médicale (Inserm), la Ligue contre le cancer Grand Ouest, ANSES (PNR EST-11-152), l’université de Rennes 1, and supported by Grants R01CA122320 (RJT) and R01CA13470 (RJT) from the National Cancer Institute. GN and MB are recipients of a fellowship from ANSES and the région Bretagne.
List of abbreviations
- AαC
2-amino-9H-pyrido[2,3-b]indole
- HONH-AαC
2-hydroxyamino-9H-pyrido[2,3-b]indole
- PhIP
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine
- MeIQx
2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline
- HONH-MeIQx
2-hydroxyamino-3,8-dimethylimidazo[4,5-f]quinoxaline
- 4-ABP
4-aminobiphenyl
- HONH-4-ABP
4-hydroxyaminobiphenyl
- dG-C8-4-ABP
N-(deoxyguanosin-8-yl)-4-ABP
- dG-C8-AαC
N-(deoxyguanosin-8-yl)-AαC
- dG-C8-MeIQx
N-(deoxyguanosin-8-yl)-MeIQx
- CHO
Chinese hamster ovary
- HAA
heterocyclic aromatic amine
- hprt
hypoxanthine phosphoribosyl transferase
- EROD
ethoxyresorufin O-deethylase
- GG-NER
global-genome repair
- MROD
methoxyresorufin O-demethylase
- NAT
N-acetyltransferase
- NER
nucleotide excision repair
- ppb
part-per-billion
- SULT
Sulfotransferase
- TC-NER
transcription-coupled nucleotide excision repair
- UGT
UDP-glucuronosyltransferase
- UPLC-ESI/MSn
ultra performance liquid chromatography−electrospray ionization/multistage scan mass spectrometry
Footnotes
Conflicts of interests: no conflict of interest
SUPPORTING INFORMATION:
Level of adducts of AαC and 4-ABP formed in hepatocytes (Table S-1). Amount of unmetabolized AαC, 4-ABP, and MeIQx present in cell culture media (Figure S-1). UPLC-ESI/MS3 chromatogram for dG-C8-MeIQx adduct formation in human hepatocytes (Figure S-2). The product ion spectra for dG-C8-AαC, dG-C8-4-ABP, and dG-C8-MeIQx (Figure S-3). This material is available free of charge via the internet at http//pubs.acs.org.
Reference List
- 1.International Agency for Research on Cancer. Tobacco smoke. Vol 38. Lyon, France: 1986. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Google Scholar]
- 2.International Agency for Research on Cancer. Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. Vol. 56. Lyon, France: 1993. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Google Scholar]
- 3.Zheng W, Lee SA. Well-done meat intake, heterocyclic amine exposure, and cancer risk. Nutr. Cancer. 2009;61:437–446. doi: 10.1080/01635580802710741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joshi AD, Corral R, Catsburg C, Lewinger JP, Koo J, John EM, Ingles SA, Stern MC. Red meat and poultry, cooking practices, genetic susceptibility and risk of prostate cancer: results from a multiethnic case-control study. Carcinogenesis. 2012;33:2108–2118. doi: 10.1093/carcin/bgs242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cross AJ, Ferrucci LM, Risch A, Graubard BI, Ward MH, Park Y, Hollenbeck AR, Schatzkin A, Sinha R. A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Res. 2010;70:2406–2414. doi: 10.1158/0008-5472.CAN-09-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Marchand L, Hankin JH, Pierce LM, Sinha R, Nerurkar PV, Franke AA, Wilkens LR, Kolonel LN, Donlon T, Seifried A, Custer LJ, Lum-Jones A, Chang W. Well-done red meat, metabolic phenotypes and colorectal cancer in Hawaii. Mutat. Res. 2002;506–507:205–214. doi: 10.1016/s0027-5107(02)00167-7. [DOI] [PubMed] [Google Scholar]
- 7.Sugimura T, Wakabayashi K, Nakagama H, Nagao M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004;95:290–299. doi: 10.1111/j.1349-7006.2004.tb03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshida D, Matsumoto T, Yoshimura R, Matsuzaki T. Mutagenicity of amino-alpha-carbolines in pyrolysis products of soybean globulin. Biochem. Biophys. Res. Commun. 1978;83:915–920. doi: 10.1016/0006-291x(78)91482-1. [DOI] [PubMed] [Google Scholar]
- 9.Skog K, Augustsson K, Steineck G, Stenberg M, Jagerstad M. Polar and non-polar heterocyclic amines in cooked fish and meat products and their corresponding pan residues. Food Chem. Toxicol. 1997;35:555–565. doi: 10.1016/s0278-6915(97)00021-5. [DOI] [PubMed] [Google Scholar]
- 10.Manabe S, Izumikawa S, Asakuno K, Wada O, Kanai Y. Detection of carcinogenic amino-alpha-carbolines and amino-gamma-carbolines in diesel-exhaust particles. Environ. Pollut. 1991;70:255–265. doi: 10.1016/0269-7491(91)90013-m. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida D, Matsumoto T. Amino-alpha-carbolines as mutagenic agents in cigarette smoke condensate. Cancer Lett. 1980;10:141–149. doi: 10.1016/0304-3835(80)90037-3. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Ashley DL, Watson CH. Quantitative analysis of six heterocyclic aromatic amines in mainstream cigarette smoke condensate using isotope dilution liquid chromatography-electrospray ionization tandem mass spectrometry. Nicotine. Tob. Res. 2011;13:120–126. doi: 10.1093/ntr/ntq219. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann D. Letters to the editor: Tobacco smoke components. Beiträge zur Tabakforschung Int. 1998;18:49–52. [Google Scholar]
- 14.Turesky RJ, Yuan JM, Wang R, Peterson S, Yu MC. Tobacco smoking and urinary levels of 2-amino-9H-pyrido[2,3-b]indole in men of Shanghai, China. Cancer Epidemiol. Biomarkers Prev. 2007;16:1554–1560. doi: 10.1158/1055-9965.EPI-07-0132. [DOI] [PubMed] [Google Scholar]
- 15.Kriek E. Fifty years of research on N-acetyl-2-aminofluorene, one of the most versatile compounds in experimental cancer research. J. Cancer Res. Clin. Oncol. 1992;118:481–489. doi: 10.1007/BF01225261. [DOI] [PubMed] [Google Scholar]
- 16.Felton JS, Jagerstad M, Knize MG, Skog K, Wakabayashi K. Contents in foods, beverages and tobacco. In: Nagao M, Sugimura T, editors. Food Borne Carcinogens Heterocyclic Amines. Chichester, England: John Wiley & Sons Ltd; 2000. pp. 31–71. [Google Scholar]
- 17.Turesky RJ, Le Marchand L. Metabolism and biomarkers of heterocyclic aromatic amines in molecular epidemiology studies: lessons learned from aromatic amines. Chem. Res. Toxicol. 2011;24:1169–1214. doi: 10.1021/tx200135s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guengerich FP, Shimada T. Oxidation of toxic and carcinogenic chemicals by human cytochrome P-450 enzymes. Chem. Res. Toxicol. 1991;4:391–407. doi: 10.1021/tx00022a001. [DOI] [PubMed] [Google Scholar]
- 19.Beland FA, Beranek DT, Dooley KL, Heflich RH, Kadlubar FF. Arylamine-DNA adducts in vitro and in vivo: their role in bacterial mutagenesis and urinary bladder carcinogenesis. Environ. Health Perspect. 1983;49:125–134. doi: 10.1289/ehp.8349125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talaska G, al Juburi AZ, Kadlubar FF. Smoking related carcinogen-DNA adducts in biopsy samples of human urinary bladder: identification of N-(deoxyguanosin-8-yl)-4-aminobiphenyl as a major adduct. Proc. Natl. Acad. Sci. U. S. A. 1991;88:5350–5354. doi: 10.1073/pnas.88.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zayas B, Stillwell SW, Wishnok JS, Trudel LJ, Skipper P, Yu MC, Tannenbaum SR, Wogan GN. Detection and quantification of 4-ABP adducts in DNA from bladder cancer patients. Carcinogenesis. 2007;28:342–349. doi: 10.1093/carcin/bgl142. [DOI] [PubMed] [Google Scholar]
- 22.Kimura S, Kawabe M, Ward JM, Morishima H, Kadlubar FF, Hammons GJ, Fernandez-Salguero P, Gonzalez FJ. CYP1A2 is not the primary enzyme responsible for 4-aminobiphenyl-induced hepatocarcinogenesis in mice. Carcinogenesis. 1999;20:1825–1830. doi: 10.1093/carcin/20.9.1825. [DOI] [PubMed] [Google Scholar]
- 23.Wang LY, Chen CJ, Zhang YJ, Tsai WY, Lee PH, Feitelson MA, Lee CS, Santella RM. 4-Aminobiphenyl DNA damage in liver tissue of hepatocellular carcinoma patients and controls. Am. J. Epidemiol. 1998;147:315–323. doi: 10.1093/oxfordjournals.aje.a009452. [DOI] [PubMed] [Google Scholar]
- 24.International Agency for Research on Cancer. Personal habits and indoor combustions. Vol. 100E. Lyon, France: 2012. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: [PMC free article] [PubMed] [Google Scholar]
- 25.International Agency for Research on Cancer. Tobacco smoke and involuntary smoking. Vol. 83. Lyon, France: 2004. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [PMC free article] [PubMed] [Google Scholar]
- 26.Turesky RJ, Rossi SC, Welti DH, Lay JO, Jr, Kadlubar FF. Characterization of DNA adducts formed in vitro by reaction of N-hydroxy-2-amino-3-methylimidazo[4,5-f]quinoline and N-hydroxy-2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline at the C-8 and N2 atoms of guanine. Chem. Res. Toxicol. 1992;5:479–490. doi: 10.1021/tx00028a005. [DOI] [PubMed] [Google Scholar]
- 27.Pfau W, Schulze C, Shirai T, Hasegawa R, Brockstedt U. Identification of the major hepatic DNA adduct formed by the food mutagen 2-amino-9H-pyrido[2,3-b]indole (AaC) Chem. Res. Toxicol. 1997;10:1192–1197. doi: 10.1021/tx9701182. [DOI] [PubMed] [Google Scholar]
- 28.Nauwelaers G, Bessette EE, Gu D, Tang Y, Rageul J, Fessard V, Yuan JM, Yu MC, Langouet S, Turesky RJ. DNA adduct formation of 4-aminobiphenyl and heterocyclic aromatic amines in human hepatocytes. Chem Res. Toxicol. 2011;24:913–925. doi: 10.1021/tx200091y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bessette EE, Spivack SD, Goodenough AK, Wang T, Pinto S, Kadlubar FF, Turesky RJ. Identification of carcinogen DNA adducts in human saliva by linear quadrupole ion trap/multistage tandem mass spectrometry. Chem. Res. Toxicol. 2010;23:1234–1244. doi: 10.1021/tx100098f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baranczewski P, Moller L. Relationship between content and activity of cytochrome P450 and induction of heterocyclic amine DNA adducts in human liver samples in vivo and in vitro. Cancer Epidemiol. Biomarkers Prev. 2004;13:1071–1078. [PubMed] [Google Scholar]
- 31.Zhang XB, Felton JS, Tucker JD, Urlando C, Heddle JA. Intestinal mutagenicity of two carcinogenic food mutagens in transgenic mice: 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and amino(alpha)carboline. Carcinogenesis. 1996;17:2259–2265. doi: 10.1093/carcin/17.10.2259. [DOI] [PubMed] [Google Scholar]
- 32.Okonogi H, Ushijima T, Shimizu H, Sugimura T, Nagao M. Induction of aberrant crypt foci in C57BL/6N mice by 2-amino-9H-pyrido[2,3-b]indole (A alphaC) and 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) Cancer Lett. 1997;111:105–109. doi: 10.1016/s0304-3835(96)04505-3. [DOI] [PubMed] [Google Scholar]
- 33.Giovannucci E. An updated review of the epidemiological evidence that cigarette smoking increases risk of colorectal cancer. Cancer Epidemiol. Biomarkers Prev. 2001;10:725–731. [PubMed] [Google Scholar]
- 34.Luchtenborg M, White KK, Wilkens L, Kolonel LN, Le Marchand L. Smoking and colorectal cancer: different effects by type of cigarettes? Cancer Epidemiol. Biomarkers Prev. 2007;16:1341–1347. doi: 10.1158/1055-9965.EPI-06-0519. [DOI] [PubMed] [Google Scholar]
- 35.Lee YC, Cohet C, Yang YC, Stayner L, Hashibe M, Straif K. Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. Int. J. Epidemiol. 2009;38:1497–1511. doi: 10.1093/ije/dyp280. [DOI] [PubMed] [Google Scholar]
- 36.Vineis P, Alavanja M, Buffler P, Fontham E, Franceschi S, Gao YT, Gupta PC, Hackshaw A, Matos E, Samet J, Sitas F, Smith J, Stayner L, Straif K, Thun MJ, Wichmann HE, Wu AH, Zaridze D, Peto R, Doll R. Tobacco and cancer: recent epidemiological evidence. J. Natl. Cancer Inst. 2004;96:99–106. doi: 10.1093/jnci/djh014. [DOI] [PubMed] [Google Scholar]
- 37.Miller EC. Some current perspectives on chemical carcinogenesis in humans and experimental animals: Presidential address. Cancer Res. 1978;38:1479–1496. [PubMed] [Google Scholar]
- 38.Jarabek AM, Pottenger LH, Andrews LS, Casciano D, Embry MR, Kim JH, Preston RJ, Reddy MV, Schoeny R, Shuker D, Skare J, Swenberg J, Williams GM, Zeiger E. Creating context for the use of DNA adduct data in cancer risk assessment: I. Data organization. Crit. Rev. Toxicol. 2009;39:659–678. doi: 10.1080/10408440903164155. [DOI] [PubMed] [Google Scholar]
- 39.Otteneder M, Lutz WK. Correlation of DNA adduct levels with tumor incidence: carcinogenic potency of DNA adducts. Mutat. Res. 1999;424:237–247. doi: 10.1016/s0027-5107(99)00022-6. [DOI] [PubMed] [Google Scholar]
- 40.Poirier MC, Beland FA. DNA adduct measurements and tumor incidence during chronic carcinogen exposure in animal models: Implications for DNA adduct-based human cancer risk assessment. Chem. Res. Toxicol. 1992;5:749–755. doi: 10.1021/tx00030a003. [DOI] [PubMed] [Google Scholar]
- 41.Paini A, Scholz G, Marin-Kuan M, Schilter B, O'Brien J, van Bladeren PJ, Rietjens IM. Quantitative comparison between in vivo DNA adduct formation from exposure to selected DNA-reactive carcinogens, natural background levels of DNA adduct formation and tumour incidence in rodent bioassays. Mutagenesis. 2011;26:605–618. doi: 10.1093/mutage/ger022. [DOI] [PubMed] [Google Scholar]
- 42.Guillouzo A, Morel F, Langouet S, Maheo K, Rissel M. Use of hepatocyte cultures for the study of hepatotoxic compounds. J Hepatol. 1997;262(Suppl):73–80. doi: 10.1016/s0168-8278(97)80499-0. [DOI] [PubMed] [Google Scholar]
- 43.Butler MA, Iwasaki M, Guengerich FP, Kadlubar FF. Human cytochrome P-450PA (P450IA2), the phenacetin O-deethylase, is primarily responsible for the hepatic 3-demethylation of caffeine and N-oxidation of carcinogenic arylamines. Proc. Natl. Acad. Sci. U. S. A. 1989;86:7696–7700. doi: 10.1073/pnas.86.20.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raza H, King RS, Squires RB, Guengerich FP, Miller DW, Freeman JP, Lang NP, Kadlubar FF. Metabolism of 2-amino-alpha-carboline. A food-borne heterocyclic amine mutagen and carcinogen by human and rodent liver microsomes and by human cytochrome P4501A2. Drug Metab. Dispos. 1996;24:395–400. [PubMed] [Google Scholar]
- 45.Turesky RJ, Constable A, Richoz J, Varga N, Markovic J, Martin MV, Guengerich FP. Activation of heterocyclic aromatic amines by rat and human liver microsomes and by purified rat and human cytochrome P450 1A2. Chem. Res. Toxicol. 1998;11:925–936. doi: 10.1021/tx980022n. [DOI] [PubMed] [Google Scholar]
- 46.Turesky RJ, Guengerich FP, Guillouzo A, Langouet S. Metabolism of heterocyclic aromatic amines by human hepatocytes and cytochrome P4501A2. Mutat. Res. 2002;506–507:187–195. doi: 10.1016/s0027-5107(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 47.Sparfel L, Langouet S, Fautrel A, Salles B, Guillouzo A. Investigations on the effects of oltipraz on the nucleotide excision repair in the liver. Biochem. Pharmacol. 2002;63:745–749. doi: 10.1016/s0006-2952(01)00906-6. [DOI] [PubMed] [Google Scholar]
- 48.Turesky RJ, Freeman JP, Holland RD, Nestorick DM, Miller DW, Ratnasinghe DL, Kadlubar FF. Identification of aminobiphenyl derivatives in commercial hair dyes. Chem. Res. Toxicol. 2003;16:1162–1173. doi: 10.1021/tx030029r. [DOI] [PubMed] [Google Scholar]
- 49.Jones CR, Sabbioni G. Identification of DNA adducts using HPLC/MS/MS following in vitro and in vivo experiments with arylamines and nitroarenes. Chem. Res. Toxicol. 2003;16:1251–1263. doi: 10.1021/tx020064i. [DOI] [PubMed] [Google Scholar]
- 50.Eugster H-P, Probst M, Würgler FE, Sengstag C. Caffeine, estradiol, and progesterone interact with human CYP1A1 and CYP1A2. Drug Metab. and Disp. 1993;21:43–49. [PubMed] [Google Scholar]
- 51.Langouët S, Coles B, Morel F, Becquemont L, Beaune P, Guengerich FP, Ketterer B, Guillouzo A. Inhibition of CYP1A2 and CYP3A4 by oltipraz results in reduction of aflatoxin B1 metabolism in human hepatocytes in primary culture. Cancer Res. 1995;55:5574–5579. [PubMed] [Google Scholar]
- 52.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 53.Goodenough AK, Schut HA, Turesky RJ. Novel LC-ESI/MS/MSn method for the characterization and quantification of 2'-deoxyguanosine adducts of the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by 2-D linear quadrupole ion trap mass spectrometry. Chem. Res. Toxicol. 2007;20:263–276. doi: 10.1021/tx0601713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Langouët S, Paehler A, Welti DH, Kerriguy N, Guillouzo A, Turesky RJ. Differential metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5- b]pyridine in rat and human hepatocytes. Carcinogenesis. 2002;23:115–122. doi: 10.1093/carcin/23.1.115. [DOI] [PubMed] [Google Scholar]
- 55.Langouët S, Welti DH, Kerriguy N, Fay LB, Huynh-Ba T, Markovic J, Guengerich FP, Guillouzo A, Turesky RJ. Metabolism of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in human hepatocytes: 2-amino-3-methylimidazo[4,5-f]quinoxaline-8-carboxylic acid is a major detoxification pathway catalyzed by cytochrome P450 1A2. Chem. Res. Toxicol. 2001;14:211–221. doi: 10.1021/tx000176e. [DOI] [PubMed] [Google Scholar]
- 56.Guengerich FP, Turvy CG. Comparison of levels of several human microsomal cytochrome P-450 enzymes and epoxide hydrolase in normal and disease states using immunochemical analysis of surgical liver samples. J. Pharmacol. Exp. Ther. 1991;256:1189–1194. [PubMed] [Google Scholar]
- 57.Liu N, Zhang QY, Vakharia D, Dunbar D, Kaminsky LS. Induction of CYP1A by benzo[k]fluoranthene in human hepatocytes: CYP1A1 or CYP1A2? Arch. Biochem. Biophys. 2001;389:130–134. doi: 10.1006/abbi.2001.2323. [DOI] [PubMed] [Google Scholar]
- 58.Kunze KL, Trager WF. Isoform-selective mechanism-based inhibition of human cytochrome P450 1A2 by furafylline. Chem. Res. Toxicol. 1993;6:649–656. doi: 10.1021/tx00035a009. [DOI] [PubMed] [Google Scholar]
- 59.Burke MD, Thompson S, Elcombe CR, Halpert J, Haaparanta T, Mayer RT. Ethoxy-, pentoxy- and benzyloyphenoxazones and homologues: a series of substrates to distinguish between different induced cytochome P-450. Biochem. Pharmacol. 1985;34:3337–3345. doi: 10.1016/0006-2952(85)90355-7. [DOI] [PubMed] [Google Scholar]
- 60.Gu D, Raymundo MM, Kadlubar FF, Turesky RJ. Ultraperformance liquid chromatography-tandem mass spectrometry method for biomonitoring cooked meat carcinogens and their metabolites in human urine. Anal. Chem. 2011;83:1093–1101. doi: 10.1021/ac102918b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malfatti MA, Dingley KH, Nowell-Kadlubar S, Ubick EA, Mulakken N, Nelson D, Lang NP, Felton JS, Turteltaub KW. The urinary metabolite profile of the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine is predictive of colon DNA adducts after a low-dose exposure in humans. Cancer Res. 2006;66:10541–10547. doi: 10.1158/0008-5472.CAN-06-1573. [DOI] [PubMed] [Google Scholar]
- 62.Bendaly J, Zhao S, Neale JR, Metry KJ, Doll MA, States JC, Pierce WM, Jr, Hein DW. 2-Amino-3,8-dimethylimidazo-[4,5-f]quinoxaline-induced DNA adduct formation and mutagenesis in DNA repair-deficient Chinese hamster ovary cells expressing human cytochrome P4501A1 and rapid or slow acetylator N-acetyltransferase 2. Cancer Epidemiol. Biomarkers Prev. 2007;16:1503–1509. doi: 10.1158/1055-9965.EPI-07-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bendaly J, Doll MA, Millner LM, Metry KJ, Smith NB, Pierce WM, Jr, Hein DW. Differences between human slow N-acetyltransferase 2 alleles in levels of 4-aminobiphenyl-induced DNA adducts and mutations. Mutat. Res. 2009;671:13–19. doi: 10.1016/j.mrfmmm.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turesky RJ, Bendaly J, Yasa I, Doll MA, Hein DW. The impact of NAT2 acetylator genotype on mutagenesis and DNA adducts from 2-amino-9H-pyrido[2,3-b]indole. Chem. Res. Toxicol. 2009;22:726–733. doi: 10.1021/tx800473w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Loeb LA, Harris CC. Advances in chemical carcinogenesis: a historical review and prospective. Cancer Res. 2008;68:6863–6872. doi: 10.1158/0008-5472.CAN-08-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pfau W, Martin FL, Cole KJ, Venitt S, Phillips DH, Grover PL, Marquardt H. Heterocyclic aromatic amines induce DNA strand breaks and cell transformation. Carcinogenesis. 1999;20:545–551. doi: 10.1093/carcin/20.4.545. [DOI] [PubMed] [Google Scholar]
- 67.Majer BJ, Kassie F, Sasaki Y, Pfau W, Glatt H, Meinl W, Darroudi F, Knasmuller S. Investigation of the genotoxic effects of 2-amino-9H-pyrido[2,3-b]indole in different organs of rodents and in human derived cells. J Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004;802:167–173. doi: 10.1016/j.jchromb.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 68.Tang Y, LeMaster DM, Nauwelaers G, Gu D, Langouet S, Turesky RJ. UDP-Glucuronosyltransferase-mediated metabolic activation of the tobacco carcinogen 2-amino-9H-pyrido[2,3-b]indole. J. Biol. Chem. 2012;287:14960–14972. doi: 10.1074/jbc.M111.320093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hein DW, Doll MA, Fretland AJ, Leff MA, Webb SJ, Xiao GH, Devanaboyina US, Nangju NA, Feng Y. Molecular genetics and epidemiology of the NAT1 and NAT2 acetylation polymorphisms. Cancer Epidemiol. Biomarkers Prev. 2000;9:29–42. [PubMed] [Google Scholar]
- 70.Teubner W, Meinl W, Florian S, Kretzschmar M, Glatt H. Identification and localization of soluble sulfotransferases in the human gastrointestinal tract. Biochem. J. 2007;404:207–215. doi: 10.1042/BJ20061431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.King RS, Teitel CH, Kadlubar FF. In vitro bioactivation of N-hydroxy-2-amino-alpha-carboline. Carcinogenesis. 2000;21:1347–1354. [PubMed] [Google Scholar]
- 72.Patel DJ, Mao B, Gu Z, Hingerty BE, Gorin A, Basu AK, Broyde S. Nuclear magnetic resonance solution structures of covalent aromatic amine-DNA adducts and their mutagenic relevance. Chem. Res. Toxicol. 1998;11:391–407. doi: 10.1021/tx9702143. [DOI] [PubMed] [Google Scholar]
- 73.Brown K, Hingerty BE, Guenther EA, Krishnan VV, Broyde S, Turteltaub KW, Cosman M. Solution structure of the 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine C8-deoxyguanosine adduct in duplex DNA. Proc. Natl. Acad. Sci. U.S.A. 2001;98:8507–8512. doi: 10.1073/pnas.151251898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang F, Elmquist CE, Stover JS, Rizzo CJ, Stone MP. DNA sequence modulates the conformation of the food mutagen 2-amino-3-methylimidazo[4,5-f]quinoline in the recognition sequence of the NarI restriction enzyme. Biochemistry. 2007;46:8498–8516. doi: 10.1021/bi700361u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cho B. Structure-function characteristics of aromatic amine-DNA adducts. In: Geacintov NE, Broyde S, editors. The Chemical Biology of DNA Damage. Weinheim: Wiley-VCH; 2010. pp. 217–238. [Google Scholar]
- 76.Choi JY, Stover JS, Angel KC, Chowdhury G, Rizzo CJ, Guengerich FP. Biochemical basis of genotoxicity of heterocyclic arylamine food mutagens: Human DNA polymerase eta selectively produces a two-base deletion in copying the N2-guanyl adduct of 2-amino-3-methylimidazo[4,5-f]quinoline but not the C8 adduct at the NarI G3 site. J. Biol. Chem. 2006;281:25297–25306. doi: 10.1074/jbc.M605699200. [DOI] [PubMed] [Google Scholar]
- 77.Broyde S, Wang L, Zhang L, Rechkoblit O, Geacintov NE, Patel DJ. DNA adduct structure-function relationships: comparing solution with polymerase structures. Chem. Res. Toxicol. 2008;21:45–52. doi: 10.1021/tx700193x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Howard PC, Casciano DA, Beland FA, Shaddock JG., Jr The binding of N-hydroxy-2-acetylaminofluorene to DNA and repair of the adducts in primary rat hepatocyte cultures. Carcinogenesis. 1981;2:97–102. doi: 10.1093/carcin/2.2.97. [DOI] [PubMed] [Google Scholar]
- 79.Culp SJ, Poirier MC, Beland FA. Biphasic removal of DNA adducts in a repetitive DNA sequence after dietary administration of 2-acetylaminofluorene. Environ. Health Perspect. 1993;99:273–275. doi: 10.1289/ehp.9399273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turesky RJ, Box RM, Markovic J, Gremaud E, Snyderwine EG. Formation and persistence of DNA adducts of 2-amino-3-methylimidazo[4,5-f]quinoline in the rat and nonhuman primates. Mutat. Res. 1997;376:235–241. doi: 10.1016/s0027-5107(97)00048-1. [DOI] [PubMed] [Google Scholar]
- 81.Gillet LC, Scharer OD. Molecular mechanisms of mammalian global genome nucleotide excision repair. Chem. Rev. 2006;106:253–276. doi: 10.1021/cr040483f. [DOI] [PubMed] [Google Scholar]
- 82.Reeves DA, Mu H, Kropachev K, Cai Y, Ding S, Kolbanovskiy A, Kolbanovskiy M, Chen Y, Krzeminski J, Amin S, Patel DJ, Broyde S, Geacintov NE. Resistance of bulky DNA lesions to nucleotide excision repair can result from extensive aromatic lesion-base stacking interactions. Nucleic Acids Res. 2011;39:8752–8764. doi: 10.1093/nar/gkr537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mu H, Kropachev K, Wang L, Zhang L, Kolbanovskiy A, Kolbanovskiy M, Geacintov NE, Broyde S. Nucleotide excision repair of 2-acetylaminofluorene- and 2-aminofluorene-(C8)-guanine adducts: molecular dynamics simulations elucidate how lesion structure and base sequence context impact repair efficiencies. Nucleic Acids Res. 2012;40:9675–9690. doi: 10.1093/nar/gks788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nothlings U, Yamamoto JF, Wilkens LR, Murphy SP, Park SY, Henderson BE, Kolonel LN, Le Marchand L. Meat and heterocyclic amine intake, smoking, NAT1 and NAT2 polymorphisms, and colorectal cancer risk in the multiethnic cohort study. Cancer Epidemiol. Biomarkers Prev. 2009;18:2098–2106. doi: 10.1158/1055-9965.EPI-08-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.