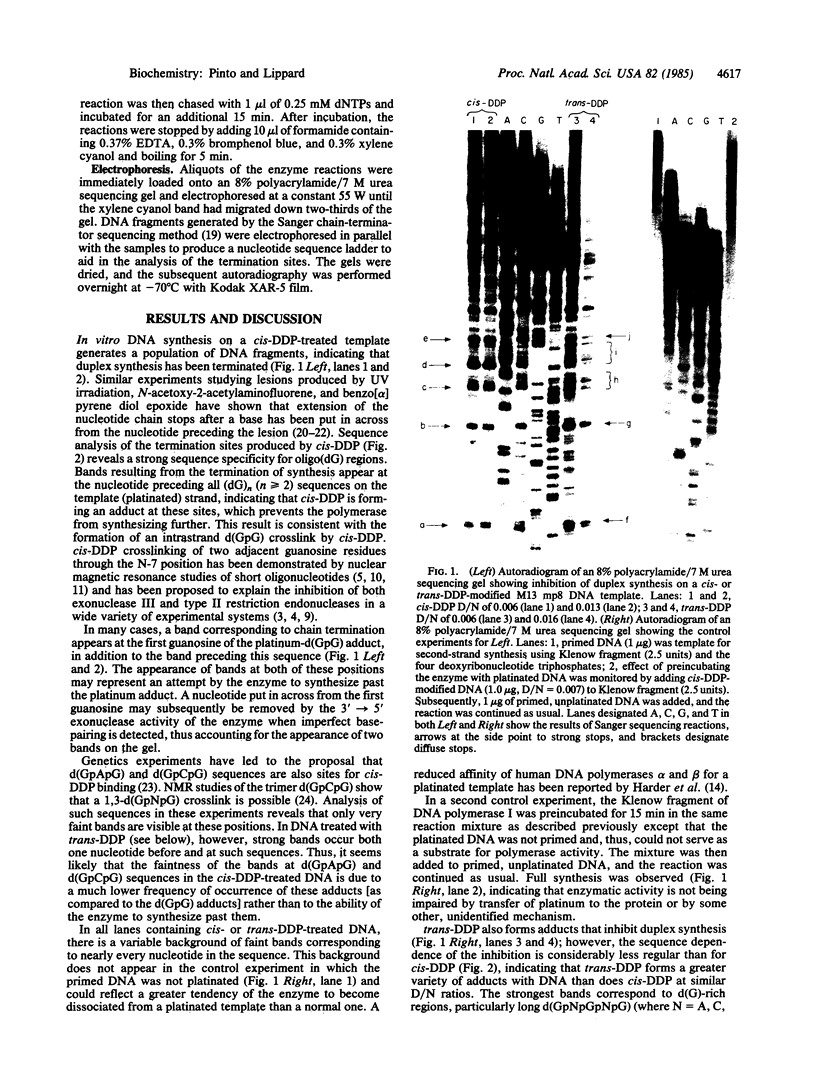
Abstract
Inhibition of DNA replication by the antitumor drug cis-diamminedichloroplatinum (II) (cis-DDP) has been proposed to be responsible for its cytotoxicity. Treatment of primed phage M13 mp8 viral DNA templates with the drug followed by second-strand synthesis using large fragment DNA polymerase I reveals that cis-DDP forms an adduct with DNA that inhibits DNA synthesis in vitro. This inhibition occurs at all (dG)n (n greater than or equal to 2) sequences in the template strand, confirming that these regions are the major cis-DDP binding sites on DNA. trans-Diamminedichloroplatinum (II), which is inactive as a drug, also forms adducts that inhibit DNA synthesis. Although considerably lower specificity is observed with the trans isomer, there appears to be a preference for d(GpNpG) sequences, where N is any intervening nucleotide. The monofunctional adduct formed between chlorodiethylenetriamineplatinum(II) chloride and DNA does not inhibit DNA synthesis in this system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brouwer J., van de Putte P., Fichtinger-Schepman A. M., Reedijk J. Base-pair substitution hotspots in GAG and GCG nucleotide sequences in Escherichia coli K-12 induced by cis-diamminedichloroplatinum (II). Proc Natl Acad Sci U S A. 1981 Nov;78(11):7010–7014. doi: 10.1073/pnas.78.11.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtinger-Schepman A. M., van der Veer J. L., den Hartog J. H., Lohman P. H., Reedijk J. Adducts of the antitumor drug cis-diamminedichloroplatinum(II) with DNA: formation, identification, and quantitation. Biochemistry. 1985 Jan 29;24(3):707–713. doi: 10.1021/bi00324a025. [DOI] [PubMed] [Google Scholar]
- Harder H. C., Rosenberg B. Inhibitory effects of anti-tumor platinum compounds on DNA, RNA and protein syntheses in mammalian cells in virtro. Int J Cancer. 1970 Sep 15;6(2):207–216. doi: 10.1002/ijc.2910060207. [DOI] [PubMed] [Google Scholar]
- Harder H. C., Smith R. G., Leroy A. F. Template primer inactivation by cis- and trans-dichlorodiammine platinum for human DNA polymerase alpha, beta, and Rauscher murine leukemia virus reverse transcriptase, as a mechanism of cytotoxicity. Cancer Res. 1976 Oct;36(10):3821–3829. [PubMed] [Google Scholar]
- Howle J. A., Gale G. R. Cis-dichlorodiammineplatinum (II). Persistent and selective inhibition of deoxyribonucleic acid synthesis in vivo. Biochem Pharmacol. 1970 Oct;19(10):2757–2762. doi: 10.1016/0006-2952(70)90102-4. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Moore P. D., Bose K. K., Rabkin S. D., Strauss B. S. Sites of termination of in vitro DNA synthesis on ultraviolet- and N-acetylaminofluorene-treated phi X174 templates by prokaryotic and eukaryotic DNA polymerases. Proc Natl Acad Sci U S A. 1981 Jan;78(1):110–114. doi: 10.1073/pnas.78.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. D., Rabkin S. D., Osborn A. L., King C. M., Strauss B. S. Effect of acetylated and deacetylated 2-aminofluorene adducts on in vitro DNA synthesis. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7166–7170. doi: 10.1073/pnas.79.23.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P., Strauss B. S. Sites of inhibition of in vitro DNA synthesis in carcinogen- and UV-treated phi X174 DNA. Nature. 1979 Apr 12;278(5705):664–666. doi: 10.1038/278664a0. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B., Gordon L. K., Haseltine W. A. Use of exonuclease III to determine the site of stable lesions in defined sequences of DNA: the cyclobutane pyrimidine dimer and cis and trans dichlorodiammine platinum II examples. Nucleic Acids Res. 1981 Sep 25;9(18):4595–4609. doi: 10.1093/nar/9.18.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone P. J., Kelman A. D., Sinex F. M. Resolution of alpha, beta and gamma DNA of Saccharomyces cerevisiae with the antitumor drug cis-Pt (NH3)2CL2. Evidence for preferential drug binding by GpG sequences of DNA. J Mol Biol. 1976 Jul 15;104(4):793–801. doi: 10.1016/0022-2836(76)90182-0. [DOI] [PubMed] [Google Scholar]
- Stone P. J., Kelman A. D., Sinex F. M. Specific binding of antitumour drug cis-Pt(NH3)2C12 to DNA rich in guanine and cytosine. Nature. 1974 Oct 25;251(5477):736–737. doi: 10.1038/251736a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S., Farquhar M. N. Specific measurement of DNA in nuclei and nucleic acids using diaminobenzoic acid. Anal Biochem. 1978 Aug 15;89(1):35–44. doi: 10.1016/0003-2697(78)90724-8. [DOI] [PubMed] [Google Scholar]
- Zwelling L. A., Kohn K. W., Ross W. E., Ewig R. A., Anderson T. Kinetics of formation and disappearance of a DNA cross-linking effect in mouse leukemia L1210 cells treated with cis- and trans-diamminedichloroplatinum(II). Cancer Res. 1978 Jun;38(6):1762–1768. [PubMed] [Google Scholar]





