Abstract
Reported results of high-dose therapy (HDT) reflect the combined effect of initial therapy and HDT. The incremental contribution of HDT is often difficult to analyze with varying degrees of response pre-HDT. Here we analyze results of HDT in patients with measurable disease at transplant, defined as a serum or 24 h urine M protein of >1.0 g per 100 ml and >200 mg per day, respectively. Paraprotein responses were calculated using measurements prior to HDT and the lowest subsequent measurement. A total of 431 patients were studied; 264 (61.3%) transplanted within 1-year of diagnosis. An additional reduction in paraprotein by 50% following HDT was seen in 86% patients; with 129 patients (30%) obtaining a 90% reduction. Patients with at least a 90% reduction had longer time to progression with no overall survival advantage and this was independent of other prognostic factors for decreased risk of progression. This study provides an estimate of the degree of tumor reduction provided by HDT, in addition to that provided by the initial therapy. In this group of patients with measurable disease after initial therapy, HDT therapy leads to complete responses in nearly a quarter of the patients and a 90% reduction in another 7%, an outcome associated with better progression-free survival.
Keywords: multiple myeloma, high-dose therapy, survival, response, SCT
Introduction
High-dose therapy (HDT) and autologous SCT (ASCT) have been shown to improve survival in patients with myeloma compared to conventional chemotherapy in randomized trials and remain the standard of care for patients eligible to undergo the procedure.1–3 Patients with newly diagnosed myeloma either proceed to HDT after 4–6 cycles of initial therapy with different regimens or consider the procedure at the time of relapse from their initial therapy. Randomized trials have not demonstrated any survival advantage for the early HDT approach, although it appears to provide a better quality of life in terms of the time with out treatment and symptoms.4 Since the initial trials of HDT in myeloma, several approaches have been tried to enhance the efficacy of transplantation as a therapeutic modality in myeloma. These have included the use of higher doses of melphalan,5,6 incorporation of newer agents into the conditioning regimen7 as well as the use of tandem transplant.8 Although the use of tandem ASCT results in improved survival in a subgroup of patients failing to achieve at least a very good partial response (VGPR) from initial transplant, other approaches have not been successful.
In patients undergoing a planned induction therapy in preparation for HDT, the reported response rates usually represent the cumulative effect of the induction therapy as well as the conditioning therapy used for the SCT. Although results from some of the clinical trials report the proportion of patients in each response category at each stage of the therapy, these responses are calculated based on the disease measurements at the time of diagnosis and the individual contribution of the HDT itself is often difficult to discern.9 Assessment of the paraprotein reduction following HDT will give us a better idea of the individual contribution of HDT in terms of disease control and will allow comparisons of approaches trying to intensify the HDT process. We examined a cohort of patients with measurable disease at the time of SCT and assessed their response to HDT by comparing the best response to HDT (lowest disease measurements post-HDT) with the pre-HDT measurements to evaluate the impact of high-dose melphalan or melphalan plus TBI.
Patients and methods
A total of 431 patients with multiple myeloma, who underwent stem cell collection and ASCT between June 1989 and October 2005 at Mayo Clinic, Rochester, were included in this study. We identified patients from our transplant database with measurable disease at the time of initiation of transplant as defined by a serum M protein of ≥1.0 g per 100 ml, or 24 h urine M protein ≥200 mg. Data from patients undergoing SCT are captured prospectively into a database that is continuously updated. Complete follow-up was available for all the patients. All patients had provided written informed consent for use of their medical records. Approval of the Mayo Foundation Institutional Review Board was obtained in accordance with Federal regulations and the Declaration of Helsinki.
Stem cells were collected using G-CSF alone in 121 patients (28%). G-CSF was administered s.c. (10 μg/kg) daily until the completion of PBSC collection with aphereses beginning on the fifth day after starting G-CSF. The remaining 310 (72%) patients had stem cells collected after administration of CY 1.5 g/m2 per day for 2 consecutive days, followed by GM-CSF or G-CSF at 5 μg/kg starting on day 3 and continuing through the period of granulocytopenia. Apheresis was performed once the total WBC count exceeded 500 per μl during the initial period and a CD34 count of 10 per μl was used as threshold more recently. Prior to 1997 the target for the apheresis procedure was 5 × 108 mononuclear cells per kg, which was subsequently replaced by quantitation of CD34+ cells and the minimum target became 4 × 106 CD34+ cells per kg.
Total 304 patients (71%) underwent conditioning with melphalan alone, at 200 mg/m2 given either as a single dose on day −1 or split over 2 days (100 mg/m2, days −2 and −1). In 23 patients (5%) melphalan dose was reduced to 100–140 mg/m2 due to advanced age, renal insufficiency or poor performance status. All transplants since June 2000 were carried out with melphalan 200 mg/m2 conditioning, reflecting a change in clinical practice based on results of randomized trials. Prior to this date 104 patients (24%) received melphalan at 140 mg/m2 on day −4, followed by total body radiation of 2 Gy twice daily on days −3 to −1.
BM plasma cell labeling index (PCLI), cytogenetics, β2 microglobulin and other laboratory variables were assessed pretransplantation. PCLI, a measure of DNA synthesis, is determined using a slide-based immunoflourescence method on BM samples, as previously described and is considered high when ≥1%.10 Responses were defined according to the European Blood and Marrow Transplant criteria, using the measurements from just before HDT and the lowest measurement obtained post transplantation.11 VGPR required a 90% reduction in the serum M component with urine protein <100 mg for 24 h. Overall survival (OS) was defined as the time from transplantation or from the date of initial diagnosis of myeloma to the date of death or last follow-up, as the case may be. The χ2- and Fisher’s exact tests were used to compare differences among the patient groups for nominal variables and the nonparametric tests Mann–Whitney U-test or Kruskal–Wallis test were used for continuous variables. Kaplan–Meier analysis was used for analyzing OS and progression-free survival (PFS) and differences between survival curves were tested for statistical significance using the two-tailed log-rank test.12 Multivariate analysis was performed using Cox proportional hazards model13 or logistic regression.
Results
A total of 431 patients, 276 (64%) men, with a median age of 58 years (range, 32.6–74.9 years) at transplant, were included in this study. These patients represent 64% of the 677 patients who underwent HDT for a diagnosis of myeloma during the same time period, who had measurable disease at the time of HDT. The majority of patients (362, 84%) had defined measurable disease by virtue of a serum monoclonal protein ≥1.0 gm per 100 ml, with the rest having measurable disease based on urine M protein ≥200 mg for 24 h (69 patients, 16%). The median time from diagnosis to HDT was 7.7 months (range, 3 months to 16 years) with 264 (61%) patients undergoing SCT within 12 months of diagnosis (early transplants). A total of 341 (79%) of the patients have relapsed since HDT. At the time of this analysis, 200 (46%) of the patients were alive with a median follow up of 42.5 months (range, 11.9 months to 13.2 years) from HDT and 53.2 months (range, 17.6 months to 18.2 years) from diagnosis. The median number of treatment regimens before HDT was 1 (range, 1–5) with those undergoing HDT within 12 months of diagnosis receiving fewer therapies as expected (P =0.02). Nearly a third of the patients (144; 33%) were responding to the previous therapy or in a disease plateau at the time of transplant, 102 (24%) were primary refractory to initial therapy, 101 (23.5%) had relapsed off therapy and 84 (19.5%) were relapsing on the previous therapy. Baseline characteristics for the entire group as well as the groups going to transplant early and late are detailed separately in Table 1.
Table 1.
Baseline features at transplant
|
Characteristics Variable |
All patients (n = 431) Median (range) |
Early transplant (n = 264) Median (range) |
Late transplant (n = 167) Median (range) |
|---|---|---|---|
| Age at diagnosis | 56.6 (24–73) | 57.7 (32–73) | 54.4 (24–73) |
| Age at transplant | 57.8 (33–75) | 58.2 (33–73) | 57.1 (33–75) |
| Time to transplant (months) | 7.7 (3–196) | 6.2 (3–11.8) | 31.1 (12.1–196) |
| Serum M protein level | 2 (0–10.4) | 1.9 (0–6.9) | 2.3 (0–10.4) |
| BM plasma cell (%) | 21 (0–95) | 17 (0–95) | 29 (0–95) |
| Creatinine | 1.1 (0.6–10) | 1.1 (0.6–10) | 1.1 (0.6–2.8) |
| Gender: male (%) | 64 | 61.9 | 67.5 |
| Durie salmon stage (%) | |||
| 2 | 29.2 | 31.7 | 25.1 |
| 3 | 70.3 | 68.3 | 74.9 |
| Relapse/refractory (%) | 66.5 | 48.2 | 95.9 |
| PCLI >=1% (%) | 36.7 | 29.1 | 48.8 |
| β2 Microglobulin > 3.5 (%) | 12.9 | 12.3 | 13.9 |
| Bone disease (%) | 84.7 | 83.3 | 87 |
| Abnormal cytogenetics (%) | 30.3 | 23.8 | 40.9 |
Abbreviation: PCLI =plasma cell labeling index.
The overall partial response to HDT alone was 86%, 129 patients (30%) satisfied criteria for VGPR or better, of whom 98 patients (23%) satisfied criteria for CR. The partial response rate was higher among the early HDT group compared to the late HDT group (92 vs 82%, P<0.01); no difference was seen in the VGPR/CR rates (Table 2). The PR or better rates for patients in a disease plateau at the time of transplant, primary refractory to initial therapy, relapsed off therapy and relapsing on the previous therapy were 80, 82, 88 and 86%, respectively (P =NS) and there were no differences in the VGPR or CR rates. There were no differences in terms of response rates whether CY was used for stem cell mobilization.14 However, patients receiving Mel–TBI were more likely to attain a VGPR/CR compared to those receiving melphalan alone at 200 or 140 mg/m2 (44 vs 32%; P =0.02). However, this difference was mostly seen among those patients with relapsed or refractory disease at the time of HDT. In a logistic regression model examining pretransplant factors predicting for a VGPR post-HDT, a lower M-protein concentration in the serum or urine and the use of TBI-based conditioning regimen were significantly associated with attaining a VGPR.
Table 2.
Transplant characteristics
| Characteristics | All patients, % (n = 431) | Early transplant, % (n = 264) | Late transplant, % (n = 167) |
|---|---|---|---|
| Cytoxan for stem cell harvest | 72 | 70.4 | 74.6 |
| Conditioning regimen | |||
| Mel-200 | 70.8 | 79.6 | 56.8 |
| Mel-140 | 5.2 | 6.3 | 3.6 |
| Mel–TBI | 23.9 | 14.1 | 39.7 |
| ≥PR | 86 | 82.2 | 92.2 |
| ≥VGPR | 30 | 30 | 30 |
| CR | 22.8 | 23.1 | 22.2 |
Abbreviation: VGPR =very good partial response.
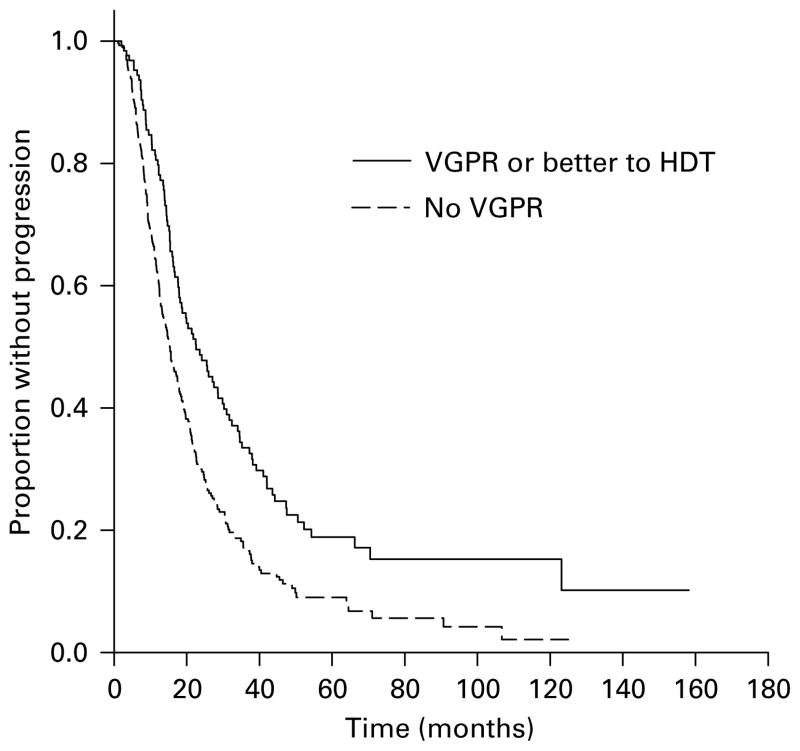
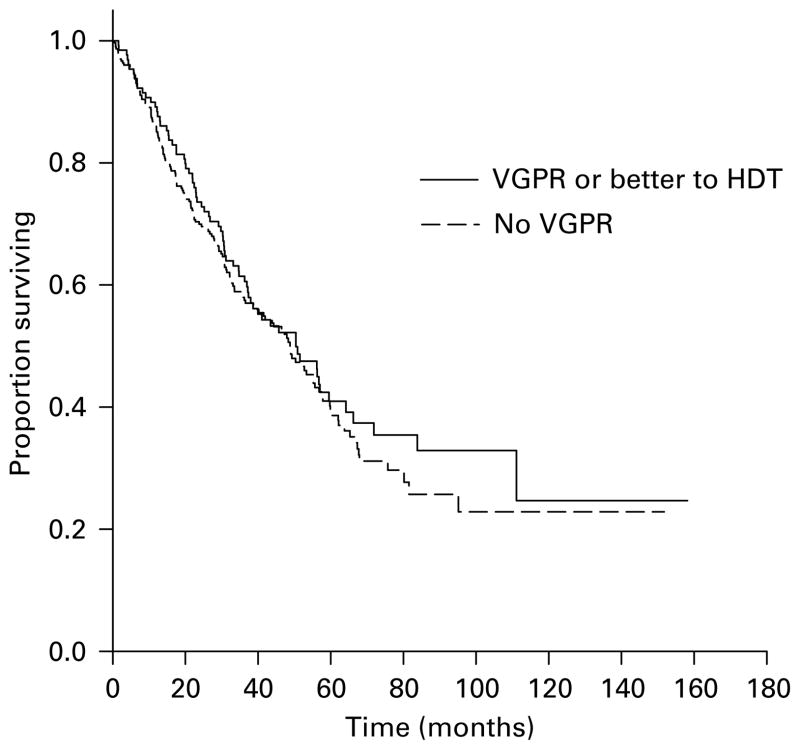
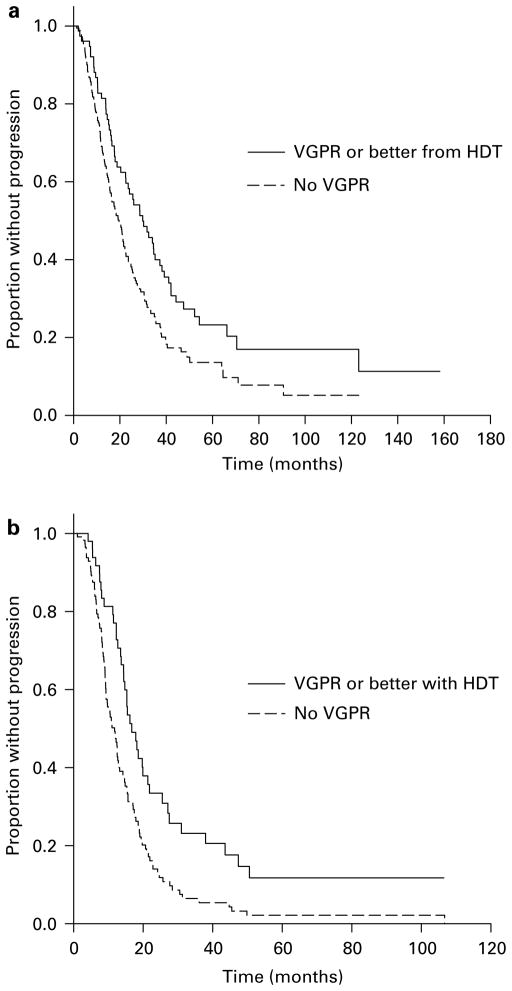
The median time to progression (TTP) for the entire group was 17.4 months (95% CI 15.6, 19.2 months) with the TTP being greater for those achieving a VGPR or better (22.6 vs 15.3 months, P<0.001; Figure 1) and for those achieving a CR (25.6 vs 15.3 months, P<0.001). However, there was no difference in terms of OS from HDT or from diagnosis based on the ability to achieve a VGPR (Figure 2). Also, the median TTP as well as OS from HDT was similar for the groups achieving at least a PR compared to those failing to do so. As expected, patients undergoing an early transplant had significantly better median TTP (21.3 vs 12.7 months, P<0.001) and OS from transplant (62 vs 30.6 months, P<0.001) and no difference in the OS from diagnosis. We examined the effect of achieving a VGPR on the TTP and OS post transplant in these groups separately. Among the patients undergoing early HDT, the median TTP from transplant for those achieving a VGPR was 29.9 vs 19.7 months (P<0.01) for those with no VGPR (Figure 3a) and there was no difference in the OS from transplant. Among the late transplants, the median TTP for those with a VGPR was 16.7 vs 11.8 months for those with no VGPR (P<0.001; Figure 3b). We also separately examined the effect of VGPR on TTP and OS among those in a plateau at HDT and those with active disease at HDT (primary refractory, relapsing off therapy or relapsing on therapy). Among patients with primary refractory or relapsed disease at HDT, the TTP was longer for those with a VGPR or better (19.8 vs 12.9 months; P<0.001) with no difference in the OS. Similarly, among those in a plateau at HDT, the median TTP was longer with a VGPR (34.5 vs 20.8 months; P<0.01) with no difference on OS. However, among patients receiving Mel/TBI conditioning, the higher VGPR rates did not translate into any improvement in the TTP compared to those receiving melphalan-only conditioning.
Figure 1.
Kaplan–Meier curves demonstrating the time to progression after high-dose therapy (HDT) based on response, very good partial response (VGPR) or more vs <VGPR. The survival curves were compared using log-rank test.
Figure 2.
Kaplan–Meier curves demonstrating the overall survival from high-dose therapy (HDT) based on response, very good partial response (VGPR) or more vs <VGPR. The survival curves were compared using log-rank test.
Figure 3.
Kaplan–Meier curves demonstrating the time to progression after high-dose therapy (HDT) based on response, very good partial response (VGPR) or more vs <VGPR among patients undergoing early HDT (a) and late HDT (b). The survival curves were compared using log-rank test.
We examined various pretransplant characteristics for their effect on the risk of progression. In a univariate analysis, late transplant (beyond 12 months from diagnosis), relapsed or primary refractory disease at time of transplant, PCLI ≥1%, presence of abnormal cytogenetics and lack of a VGPR were all predictive of early progression after HDT. In a multivariate analysis incorporating these factors, late transplant, PCLI ≥1%, presence of abnormal cytogenetics and lack of a VGPR were all independently predictive for a shorter median time to progression.
Finally, we also compared the outcome of the group of patients with measurable disease at HDT (431 patients) with the 246 patients without measurable disease at HDT. The median TTP for the patients without measurable disease at HDT was 22 months (95% CI, 17.5–25.5) compared to 17.4 months (95% CI, 15.6–19.2) for those with measurable disease at HDT (P<0.01). However, there was no significant difference in the OS from HDT.
Discussion
High-dose chemotherapy was initially conceived as a mechanism to overcome drug resistance in patients with myeloma by McElwain and Powles15 nearly three decades ago. This was further refined by addition of autologous stem cell rescue that enables rapid recovery of the blood counts and decreases the morbidity and mortality associated with prolonged cytopenias.16,17 Over the years, HDT has been increasingly used for the treatment of newly diagnosed myeloma patients where initial reduction of tumor burden is achieved through 4–6 months of induction with different regimens before proceeding to HDT.1–3 A substantial proportion of patients refractory to induction will obtain benefit from HDT.4
It is often difficult to discern the individual contribution of HDT from the pretransplant induction. We have examined a group of patients with measurable disease at the time of initiating stem cell harvest and HDT to provide a better idea of the degree of tumor reduction achieved by HDT. This group represents nearly two-thirds of patients going to HDT and as expected, and is enriched with patients who obtained a poor response to initial therapy or relapsed from their previous treatment by virtue of the requirement for a measurable M-protein pretransplant. It is known that patients with newly diagnosed myeloma refractory to initial treatment obtain the same degree of benefit from HDT as those patients responding to initial therapy.18–20 In the relapsed setting, although demonstration of chemosensitivity is not a prerequisite for HDT, patients who are progressing in the face of ongoing therapy have inferior outcomes compared to those with disease relapsing after discontinuation of therapy.21 In addition, given the known differences in the time to progression after HDT in patients going for early HDT compared those receiving later HDT, we chose to perform additional analyses comparing the effects of HDT in the two populations.
The majority of patients in this study achieved a 50% or greater reduction in their pre-HDT M protein and this response rate was higher in patients undergoing an early compared to late transplant and reflects the reduced sensitivity of tumor cells to melphalan in this heavily pretreated population. Nearly 80% of patients undergoing late transplant had previously been exposed to melphalan and had more prior regimens compared to 8% of patients in the early transplant group. Among the study patients, nearly a third achieved an over 90% reduction in the tumor burden or a VGPR including a CR in a quarter of the patients. There was no difference in terms of the VGPR or CR rate among the early transplants compared to the late transplants. However, one needs to take into consideration that some of the patients classified as PR after ASCT would have been classified as VGPR, if the response had been calculated based on the tumor measurements from diagnosis. This could partially explain the rate of VGPR seen with the early transplant group, which is lower than reported in other trials. The CR rate of 25% is also slightly lower than reported in some clinical trials and is lower than the CR rate for the entire transplant cohort at our institution (33%, data not shown). This is likely explained on the basis of exclusion of patients with more sensitive disease. The response rates seen here were independent of the disease status at HDT, whether in a disease plateau, primary refractory to initial therapy, relapsed off therapy or relapsing on the previous therapy, reflecting the ability of HDT to at least temporarily overcome drug resistance. This is consistent with previously published data demonstrating benefit for HDT in patients with refractory disease.
The degree of additional cytoreduction achieved by high-dose melphalan has an impact on the time to progression. In the current study, patients achieving a VGPR or better between completion of induction and transplantation had a longer time to progression compared to those who failed to achieve this degree of response to high-dose melphalan. This beneficial effect of tumor reduction was evident for both patients with early as well as the late HDT. The benefit of achieving VGPR or CR is consistent with other reports.22,23 However, it is of interest that similar rates of VGPR and CR were seen in the late transplant group, however the median time to progression remained inferior in this group of patients compared to those undergoing early transplant, likely a reflection of the altered disease biology following progression. In this study, patients achieving a CR prior to HDT have been excluded as we had focused on those patients who still had measurable levels of residual disease left behind after the initial therapy. However, previous analysis had shown that there was no difference in the outcome between those achieving a CR before or after HDT, highlighting the prognostic value of this degree of tumor reduction, whether a reflection of the HDT or of disease biology.24 However, as in some of the other studies where depth of response have clearly correlated with the time to progression and not OS, we did not see any impact of the depth of response on the OS.25 Other trials have failed to demonstrate any benefit on the PFS as well as in the case of the recent Intergroupe Francophone du Myélome trial comparing MPT regimen to two cycles of Mel-100.26
One of the interesting findings is the association between a TBI-based conditioning regimen and the higher rates of VGPR. This higher VGPR rate did not translate into a better TTP in this study. In the French trial that compared Mel-200 with Mel-140 and TBI, the CR rates were comparable for the two groups.27 In the current study, the differences were more significant for those patients who had relapsed or refractory disease at the time of HDT and could reflect the heightened activity of radiation in patients previously exposed to and not responding to traditional chemotherapy agents. It raises the question whether a melphalan TBI regimen may provide additional benefit for this group of patients, albeit likely with higher toxicity. This clearly will have to be answered in the setting of prospective clinical trials.
In conclusion, we provide some estimate of the degree of tumor reduction provided by the HDT in addition to that provided by the initial therapy. The data presented here are more applicable for patients with lower degrees of response to the initial therapy, given the study design. However, the numbers may allow comparison with novel conditioning regimens that are being evaluated to improve the efficacy rates of HDT.
Acknowledgments
This study was supported in part by Hematologic Malignancies Program, Mayo Clinic CR20 program, ASCO Young Investigator Award, Amgen Oncology Institute Junior Faculty Award (SK) and grants CA93842 and CA10080 from the National Cancer Institute; National Institutes of Health and the Department of Health and Human Services.
References
- 1.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 2.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 3.Fermand JP, Katsahian S, Divine M, Leblond V, Dreyfus F, Macro M, et al. High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55–65 years: long-term results of a randomized control trial from the Group Myelome-Auto-greffe. J Clin Oncol. 2005;23:9227–9233. doi: 10.1200/JCO.2005.03.0551. [DOI] [PubMed] [Google Scholar]
- 4.Fermand JP, Ravaud P, Chevret S, Divine M, Leblond V, Belanger C, et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998;92:3131–3136. [PubMed] [Google Scholar]
- 5.Moreau P, Milpied N, Mahe B, Juge-Morineau N, Rapp MJ, Bataille R, et al. Melphalan 220 mg/m2 followed by peripheral blood stem cell transplantation in 27 patients with advanced multiple myeloma. Bone Marrow Transplant. 1999;23:1003–1006. doi: 10.1038/sj.bmt.1701763. [DOI] [PubMed] [Google Scholar]
- 6.Moreau P, Kergueris MF, Milpied N, Le Tortorec S, Mahe B, Bulabois CE, et al. A pilot study of 220 mg/m2 melphalan followed by autologous stem cell transplantation in patients with advanced haematological malignancies: pharmacokinetics and toxicity. Br J Haematol. 1996;95:527–530. doi: 10.1046/j.1365-2141.1996.d01-1932.x. [DOI] [PubMed] [Google Scholar]
- 7.Giralt S, Bensinger W, Goodman M, Podoloff D, Eary J, Wendt R, et al. 166Ho-DOTMP plus melphalan followed by peripheral blood stem cell transplantation in patients with multiple myeloma: results of two phase 1/2 trials. Blood. 2003;102:2684–2691. doi: 10.1182/blood-2002-10-3250. [DOI] [PubMed] [Google Scholar]
- 8.Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet JG, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–2502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- 9.Barlogie B, Kyle RA, Anderson KC, Greipp PR, Lazarus HM, Hurd DD, et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. J Clin Oncol. 2006;24:929–936. doi: 10.1200/JCO.2005.04.5807. [DOI] [PubMed] [Google Scholar]
- 10.Greipp PR, Lust JA, O’Fallon WM, Katzmann JA, Witzig TE, Kyle RA. Plasma cell labeling index and beta 2-microglobulin predict survival independent of thymidine kinase and C-reactive protein in multiple myeloma. Blood. 1993;81:3382–3387. [PubMed] [Google Scholar]
- 11.Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan E, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 13.Cox D. Regression models and life tables. J R Stat Soc. 1972;34 :187–202. [Google Scholar]
- 14.Dingli D, Nowakowski GS, Dispenzieri A, Lacy MQ, Hayman S, Litzow MR, et al. Cyclophosphamide mobilization does not improve outcome in patients receiving stem cell transplantation for multiple myeloma. Clin Lymphoma Myeloma. 2006;6:384–388. doi: 10.3816/CLM.2006.n.014. [DOI] [PubMed] [Google Scholar]
- 15.McElwain TJ, Powles RL. High-dose intravenous melphalan for plasma-cell leukaemia and myeloma. Lancet. 1983;2:822–824. doi: 10.1016/s0140-6736(83)90739-0. [DOI] [PubMed] [Google Scholar]
- 16.Barlogie B, Hall R, Zander A, Dicke K, Alexanian R. High-dose melphalan with autologous bone marrow transplantation for multiple myeloma. Blood. 1986;67:1298–1301. [PubMed] [Google Scholar]
- 17.McElwain TJ, Selby PJ, Gore ME, Viner C, Meldrum M, Millar BC, et al. High-dose chemotherapy and autologous bone marrow transplantation for myeloma. Eur J Haematol Suppl. 1989;51 (Suppl 1989):152–156. doi: 10.1111/j.1600-0609.1989.tb01509.x. [DOI] [PubMed] [Google Scholar]
- 18.Singhal S, Powles R, Sirohi B, Treleaven J, Kulkarni S, Mehta J. Response to induction chemotherapy is not essential to obtain survival benefit from high-dose melphalan and auto-transplantation in myeloma. Bone Marrow Transplant. 2002;30 :673–679. doi: 10.1038/sj.bmt.1703717. [DOI] [PubMed] [Google Scholar]
- 19.Alexanian R, Dimopoulos MA, Delasalle KB, Hester J, Champlin R. Myeloablative therapy for primary resistant multiple myeloma. Stem Cells. 1995;13 (Suppl 2):118–121. doi: 10.1002/stem.5530130718. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Lacy MQ, Dispenzieri A, Rajkumar SV, Fonseca R, Geyer S, et al. High-dose therapy and autologous stem cell transplantation for multiple myeloma poorly responsive to initial therapy. Bone Marrow Transplant. 2004;34:161–167. doi: 10.1038/sj.bmt.1704545. [DOI] [PubMed] [Google Scholar]
- 21.Blade J, Rosinol L, Garcia-Sanz R, Lahuerta J, Hernandez M, Sureda A, et al. A PETHEMA study of high-dose therapy/stem cell support (HDT), including tandem transplant, in primary refractory multiple myeloma (MM): identification of two populations with different outcomes. J Clin Oncol (Meeting Abstracts) 2007;25 (18 Suppl):8021. [Google Scholar]
- 22.Rajkumar SV, Fonseca R, Dispenzieri A, Lacy MQ, Witzig TE, Lust JA, et al. Effect of complete response on outcome following autologous stem cell transplantation for myeloma. Bone Marrow Transplant. 2000;26:979–983. doi: 10.1038/sj.bmt.1702640. [DOI] [PubMed] [Google Scholar]
- 23.Harousseau J-L, Attal M, Moreau P, Garban F, Facon T, Avet-Loiseau H. The prognostic impact of complete remission (CR) plus very good partial remission (VGPR) in a double-transplantation program for newly diagnosed multiple myeloma (MM). Combined results of the IFM 99 trials. ASH Annual Meeting Abstracts. 2006;108:3077. [Google Scholar]
- 24.Dingli D, Pacheco JM, Nowakowski GS, Kumar SK, Dispenzieri A, Hayman SR, et al. Relationship between depth of response and outcome in multiple myeloma. J Clin Oncol. 2007;25:4933–4937. doi: 10.1200/JCO.2007.11.7879. [DOI] [PubMed] [Google Scholar]
- 25.Lenhoff S, Hjorth M, Turesson I, Westin J, Gimsing P, Wisloff F, et al. Intensive therapy for multiple myeloma in patients younger than 60 years. Long-term results focusing on the effect of the degree of response on survival and relapse pattern after transplantation. Haematologica. 2006;91:1228–1233. [PubMed] [Google Scholar]
- 26.Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity auto-logous stem cell transplantation in elderly patients with multiple myeloma (IFM 99–06): a randomised trial. Lancet. 2007;370:1209–1218. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 27.Moreau P, Facon T, Attal M, Hulin C, Michallet M, Maloisel F, et al. Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood. 2002;99:731–735. doi: 10.1182/blood.v99.3.731. [DOI] [PubMed] [Google Scholar]