The divalent cation calcium (Ca2+) is best known in cardiomyocytes for its role in excitation-contraction coupling, where it triggers myosin-actin cross-bridge formation. However, Ca2+ regulates myriad fundamental processes in all cells. Among these are the activities of enzymes, channels, and pumps, transcription, translation, motility, exocytosis, metabolism, growth, differentiation, and death1. Mitochondria are the primary venue for some of these processes, such as metabolism and cell death. This raises a question that appears seemingly straightforward, but has proven to be complex and contentious: How does Ca2+ get from its major storage depot, the sarcoplasmic/endoplasmic reticulum (SR/ER), to mitochondria? In this issue of Circulation Research, Chen et al2 address this issue in cardiomyocytes.
Uptake of Ca2+ by mitochondria is critical for coupling the energy demands of cardiac work with metabolism3. These demands can change on a beat-to-beat basis necessitating a messenger, such as Ca2+, with a rapid response time. The most intensive catabolism of substrates occurs in the mitochondrial matrix via the Krebs cycle, several rate-limiting enzymes of which are Ca2+-dependent. The phasic increases in cytosolic Ca2+ with each heart beat, which are more frequent or larger under conditions of increased energy demand (e.g. increased heart rate or contractility), are sensed and averaged by the complex buffering system in mitochondria to accelerate cardiac metabolism through uptake of Ca2+ into the matrix4.
The story of mitochondrial Ca2+ handling is long and convoluted, but a bolus of clarity was recently infused with the molecular identification of the mitochondrial Ca2+ uniporter (MCU) in the inner mitochondrial membrane (IMM)5, 6 (Figure 1). Ca2+ traverses the outer mitochondrial membrane (OMM) through non-specific high-conductance voltage dependent anion channels (VDAC) to reach the intermembrane space. The MCU, which is highly selective for Ca2+, then moves Ca2+ from the intermembrane space to the matrix.
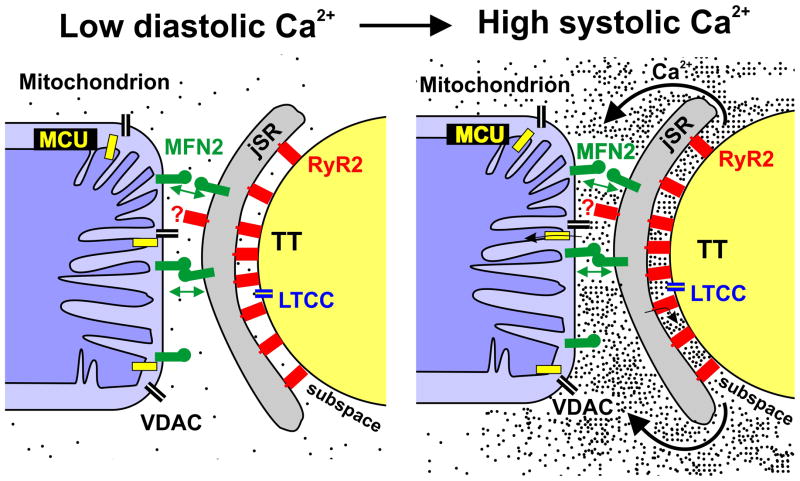
Figure 1. Ca2+ microdomains in heart.
Mitochondria span Z-disk to Z-disk and in close proximity to the junctional SR (jSR). The inner mitochondrial membrane contains the mitochondrial Ca2+ uniporter (MCU), and the outer mitochondrial membrane contains non-specific Ca2+-permeable voltage dependent anion channels (VDAC). The dynamin-related GTPase mitofusin-2 (MFN2) in the jSR membrane engages in homotypic interactions with MFN2 in the outer mitochondrial membrane (indicated by double arrows). During systole, extracellular Ca2+ enters the subspace between the transverse tubule (TT) and the jSR through L-type Ca2+ channels (LTCC) in the membrane of the TT and the sarcolemma (SL), and this Ca2+ triggers the release of large amounts of additional Ca2+ from the jSR into the subspace via ryanodine receptors (RyR2). The very high local concentrations of Ca2+ in the subspace subsequently diffuse to create microdomains of high Ca2+ around the ends of the mitochondria. Ca2+ can cross the outer mitochondrial membrane through VDACs and be transported into the matrix via the MCU. Rogue RyR216 (indicated by the question mark) may be found in the microdomain.
ATP production by aerobic metabolism requires a large negative voltage gradient across the IMM, which is generated by the electron transport chain. However, these conditions also favor Ca2+ entry into the matrix via the MCU. Perhaps to protect cardiomyocytes from the potentially lethal consequences of mitochondrial Ca2+ overload, the MCU is relatively insensitive to the average ambient cytosolic Ca2+ concentrations in cardiomyocytes (~100 nM diastole and 0.5 μM systole). Yet, there needs to be some conditions under which the MCU can accomplish its job of bringing Ca2+ into the matrix. This conundrum gave rise to the concept of Ca2+ microdomains, localized areas of high Ca2+ concentration7, 8. Microdomains with Ca2+ concentrations ~10 μM flank the ends of intermyofibrillar mitochondria in cardiomyocytes9 (Figure 1), and are adequate to drive efficient uptake of Ca2+ into the matrix via the MCU. These microdomains, which persist 10–20 ms per heart beat, originate in the “subspace” between the transverse tubules and the junctional sarcoplasmic reticulum (jSR). The influx of Ca2+ through L-type Ca2+ channels in the transverse tubules triggers the massive release of Ca2+ from the jSR to the subspace via ryanodine receptors, most of which face the transverse tubules10. The resulting very high concentrations of Ca2+ in the subspace (100–300 μM)11 then diffuses ~100 nanometers to produce the Ca2+ microdomains in juxtaposition to the mitochondria.
For Ca2+ microdomains to exist in proximity to mitochondria, the SR/ER and mitochondria must be held in close apposition. While contact points between SR/ER and mitochondria have been recognized and membrane fractions containing SR/ER and mitochondrial proteins (“mitochondrial associated membranes”) have been isolated, the molecular basis of SR/ER-mitochondrial tethering remained obscure until recently. A major breakthrough was provided by experiments in non-excitable cells demonstrating that the dynamin-related GTPase mitofusin 2 (MFN2) is critical in linking the two organelles12. MFN2 in the ER membrane interacts with MFN2 in the outer mitochondrial membrane as well as with MFN1, a homologous protein located solely on the outer mitochondrial membrane, to bring ER and mitochondria together. Reconstitution experiments in knockout cells have shown, however, that only MFN2 specifically at the ER is indispensable in this tethering process.
In the present study, Chen et al conduct experiments that move from Drosophila to mice to interrogate the consequences of mitofusin-mediated mitochondrial-SR tethering across cardiac evolution. The starting point of these investigations is that Ca2+ microdomains are important in overcoming the low efficiency of the MCU in moving Ca2+ into the mitochondrial matrix. The authors ask how microdomains are created in cardiomyocytes, and what are their downstream consequences? Since microdomains reflect the close apposition of SR and mitochondria, the investigators sought to differentiate between two hypotheses: Was the nearness of these organelles in cardiomyocytes mediated by contact points dependent on MFN2, as in non-excitable cells? Alternatively, was the physical sandwiching of the junctional SR (jSR) between T-tubules and mitochondria, by itself, sufficient to account for this close proximity? To sort out these possibilities, Chen et al first determined the effect of RNAi-mediated suppression of the sole Drosophila mitofusin, MARF, in fruit fly heart tubes; phasic SR Ca2+ release increased, possibly reflecting decreased SR-to-mitochondrial Ca2+ transfer. Then, they created mice in which each of the two vertebrate mitofusins, MFN1 or MFN2, was individually deleted specifically in cardiomyocytes beginning in postnatal life. Comparing ablation of MFN1 and MFN2 controlled for the potential confounding factor of MFN2 as a mediator (along with MFN1) of fusion between mitochondria. Cardiomyocyte-specific inactivation of MFN1 would, therefore, have the same effects on mitochondrial fusion as MFN2, but would not affect SR-mitochondrial coupling.
The most important finding of this study is that the contact area between SR and mitochondria in cardiomyocytes is reduced ~30% in MFN2 knockout mice as compared with wild type controls, an abnormality not observed in MFN1 knockout mice. This structural uncoupling correlated with decreased SR to mitochondrial Ca2+ transfer in isolated cardiomyocyte experiments. Moreover, the conclusion that MFN2 deletion decreases SR to mitochondrial Ca2+ transfer was strengthened by the observation that loss of MFN2 actually increases SR to cytosol release, although the precise mechanism of the latter is not known. The study went on to show that the depressed transfer of Ca2+ to mitochondria in cells lacking MFN2 is of physiological significance as it impairs the Krebs cycle. These abnormalities are most dramatic under conditions of increased workload, as modeled by isoproterenol administration combined with accelerated rates of cellular pacing. While wild type cardiomyocytes transiently augment the rate of SR to mitochondria Ca2+ transfer to drive the Krebs cycle, MFN2 null cardiomyocytes can do neither. Taken together, these data indicate that MFN2 is critical in coupling cardiac work with metabolism, most likely by maintaining the intimacy between SR and mitochondria required for optimal Ca2+ transfer.
The main lesson we learn from this study is that a commonality exists in the mechanisms that maintain SR/ER-mitochondrial apposition in non-excitable cells and cardiomyocytes. While the rigid architecture of the latter may contribute to bringing these organelles together, it does not suffice, and MFN2 is critical. Given the conservation of MFNs, why consider that cell type-specific differences might exist? One compelling reason is that cardiomyocytes have already proven to differ drastically with respect to their glacial rates of mitochondrial fusion13, another key function of these proteins.
The investigators excluded many of the alternative explanations for their data, the most obvious being MFN2-related alterations in the electrical potential difference across the IMM, a key driving force for Ca2+ import via the MCU. One possibility that was not investigated, however, is whether MFN2 plays a direct role in mitochondrial Ca2+ uptake, independent of in its tethering of SR/ER to mitochondria. This notion could be tested using isolated mitochondria. While there is currently no evidence for this hypothesis, it would not be surprising for MFN2 to have yet additional functions. In this vein, MFN2 has been implicated in the Ca2+ sensitivity of the mitochondrial permeability transition pore14, 15.
The results of the current study appear to differ from those of Papanicolaou et al, who concluded that MFN2 deletion does not alter the relationship of SR to mitochondria in cardiomyocytes14. This discrepancy, however, appears to be largely the result of variation in the parameters that were assessed. The conclusions of the present study are based on decreases in the contact area between SR and mitochondria, similar to the major parameter evaluated in the initial study in non-excitable cells12. A trend toward increased distance between jSR and mitochondria was observed in the present study, but did not reach statistical significance. In contrast, Papanicolaou et al found that MFN2 absence does not affect the distance between the transverse tubule and mitochondria. The magnitude of this distance, however, is much larger than the gap between jSR and mitochondria and may, therefore, have obscured small changes in the latter. Rates of Ca2+ uptake were not assessed in Papanicolaou et al.
Mice with combined deletion of MFN1 and MFN2 manifest a spontaneous lethal dilated cardiomyopathy13. In contrast, the present study shows that mice with inactivation of MFN2 (or MFN1) alone have normal cardiac dimensions and function. This may not be surprising given the low metabolic demands of the basal state. It will be interesting to test the effects of various energy-demanding stresses on cardiac function in these mice. If the pathway described in this report exists to match bioenergetics with increased work load, one would predict that increases in preload, afterload, chronotropy, or inotropy would be poorly tolerated in MFN2 knockout mice.
Acknowledgments
Sources of Funding
This work was supported by grants from National Institutes of Health (5R01HL060665-14 (RNK), 5P30CA013330-39 (RNK), 5P60DK020541-34 (RNK), 5R01HL106059-02 (WJL), 5R01HL105239-02 (WJL), and 5P01AG025532-05 (RR)); Leducq North American-European Atrial Fibrillation Research Alliance (WJL); European Union Seventh Framework Program (FP7) Georg August University, “Identification and therapeutic targeting of common arrhythmia trigger mechanisms” (WJL); Italian Ministries of Health (Ricerca Finalizzata) and of Education, University and Research (PRIN, FIRB) (RR); the European Union (ERC mitoCalcium, no. 294777 and FP7 “MyoAGE”, no. 223576) (RR); Cariparo Foundation (Padua) (RR); the Italian Association for Cancer Research (AIRC) (RR); Telethon-Italy (GPP1005A) (RR); and the A. G. Leventis Foundation (KK). R.N.K. is supported by the Dr. Gerald and Myra Dorros Chair in Cardiovascular Disease. We are most grateful to the Wilf Family for their ongoing generosity and support.
Footnotes
Disclosures
None.
References
- 1.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Csordás G, Jowdy C, Schneider TG, Csordás N, Wang W, Liu Y, Kohlhaas M, Meiser M, Bergem S, Nerbonne JM, Dorn GW, Maack C. Mitofusin 2–containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via inter-organelle ca2+ crosstalk. Circulation research. 2012;111:xxx–xxx. doi: 10.1161/CIRCRESAHA.112.266585. in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiological reviews. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 4.Rizzuto RDSD, Rafaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signaling. Nat Rev Mol Cell Biol. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 5.De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies mcu as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high ca2+ close to ip3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 8.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 9.Chikando AC, Kettlewell S, Williams GS, Smith G, Lederer WJ. Ca2+ dynamics in the mitochondria - state of the art. Journal of molecular and cellular cardiology. 2011;51:627–631. doi: 10.1016/j.yjmcc.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franzini-Armstrong C, Protasi F, Ramesh V. Shape, size, and distribution of ca 2+ release units and couplons in skeletal and cardiac muscles. Biophys J. 1999;77:1528–1539. doi: 10.1016/S0006-3495(99)77000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams GS, Chikando AC, Tuan HT, Sobie EA, Lederer WJ, Jafri MS. Dynamics of calcium sparks and calcium leak in the heart. Biophys J. 2011;101:1287–1296. doi: 10.1016/j.bpj.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Liu Y, Dorn GW., 2nd Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circulation research. 2011;109:1327–1331. doi: 10.1161/CIRCRESAHA.111.258723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papanicolaou KN, Khairallah RJ, Ngoh GA, Chikando A, Luptak I, O’Shea KM, Riley DD, Lugus JJ, Colucci WS, Lederer WJ, Stanley WC, Walsh K. Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Molecular and cellular biology. 2011;31:1309–1328. doi: 10.1128/MCB.00911-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whelan RS, Konstantinidis K, Wei AC, Chen Y, Reyna DE, Jha S, Yang Y, Calvert JW, Lindsten T, Thompson CB, Crow MT, Gavathiotis E, Dorn GW, 2nd, O’Rourke B, Kitsis RN. Bax regulates primary necrosis through mitochondrial dynamics. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6566–6571. doi: 10.1073/pnas.1201608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sobie EA, Guatimosim S, Gomez-Viquez L, Song LS, Hartmann H, Saleet JM, Lederer WJ. The ca(2+) leak paradox and “rogue ryanodine receptors”: Sr ca(2+) efflux theory and practice. Prog Biophys Mol Biol. 2006;90:172–185. doi: 10.1016/j.pbiomolbio.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]