Abstract
Mustached bats emit echolocation and communication calls containing both constant frequency (CF) and frequency-modulated (FM) components. Previously we found that 86% of neurons in the ventral division of the external nucleus of the inferior colliculus (ICXv) were directionally selective for linear FM sweeps and that selectivity was dependent on sweep rate. The ICXv projects to the suprageniculate nucleus (Sg) of the medial geniculate body. In this study, we isolated 37 single units in the Sg and measured their responses to best excitatory frequency (BEF) tones and linear 12-kHz upward and downward FM sweeps centered on the BEF. Sweeps were presented at durations of 30, 12, and 4 ms, yielding modulation rates of 400, 1,000, and 3,000 kHz/s. Spike count versus level functions were obtained at each modulation rate and compared with BEF controls. Sg units responded well to both tones and FM sweeps. BEFs clustered at 58 kHz, corresponding to the dominant CF component of the sonar signal. Spike count functions for both tones and sweeps were predominantly non-monotonic. FM directional selectivity was significant in 53–78% of the units, depending on modulation rate and level. Units were classified as up-selective (52%), down-selective (24%), or bi-directional (non-selective, 16%); a few units (8%) showed preferences that were either rate- or level-dependent. Most units showed consistent directional preferences at all SPLs and modulation rates tested, but typically showed stronger selectivity at lower sweep rates. Directional preferences were attributable to suppression of activity by sweeps in the non-preferred direction (~80% of units) and/or facilitation by sweeps in the preferred direction (~20–30%). Latencies for BEF tones ranged from 4.9 to 25.7 ms. Latencies for FM sweeps typically varied linearly with sweep duration. Most FM latency-duration functions had slopes ranging from 0.4 to 0.6, suggesting that the responses were triggered by the BEF. Latencies for BEF tones and FM sweeps were significantly correlated in most Sg units, i.e., the response to FM was temporally related to the occurrence of the BEF in the FM sweep. FM latency declined relative to BEF latency as modulation rate increased, suggesting that at higher rates response is triggered by frequencies in the sweep preceding the BEF. We conclude that Sg and ICXv units have similar, though not identical, response properties. Sg units are predominantly upsweep selective and could respond to either or both the CF and FM components in biosonar signals in a number of echolocation scenarios, as well as to a variety of communication sounds.
INTRODUCTION
Frequency modulations (FMs) are ubiquitous acoustic features in animal communication sounds and formant transitions of speech. Bats have proven to be excellent models for studying the processing of such complex sounds in the mammalian auditory system, as FM is the most common signal used in echolocation (Simmons and Stein 1980; Simmons et al. 1979), and is incorporated into many communication sounds as well.
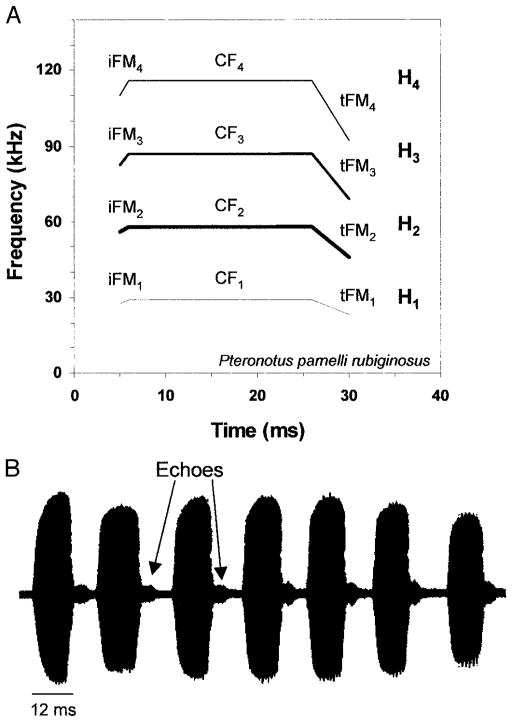
Parnell’s mustached bat (Pteronotus parnelli) is a neotropical aerial insectivore. FM elements occur in both the echolocation call (Novick and Vaisnys 1964; Schnitzler 1970) and the communication sound repertoire (Kanwal et al. 1994). The echolocation call has a total duration that varies from 5 to 30 ms depending on bat-target distance. Each pulse consists of a long constant-frequency (CF) component preceded and terminated by brief upward and downward FM components (Fig. 1). The acoustic characteristics of the CF and FM components adapt them to different echolocation tasks. The initial upward FM component is variable in bandwidth (ranging from 2 to 6 kHz in the dominant second harmonic) and low in amplitude; its role in echolocation remains uncertain. The role of the long CF and terminal FM components is much better understood. When reflected from an object, the downward sweeping terminal FM component conveys spatial information, including target azimuth, elevation, and range, as well as fine textural and shape information (Simmons and Stein 1980; Simmons et al. 1979). Its utility is such that it is used by nearly all insectivorous bats pursuing flying prey. In contrast, echoes of the long CF component are best suited for detecting local prey motion, as the fluttering wings of insect prey induce rapid FMs in this signal via the Doppler effect. Behavioral experiments have shown that mustached bats will not attack an insect unless its wings are fluttering (Goldman and Henson 1977). Thus FM primarily occurs in two forms, quasi-linear aperiodic modulations conveying target range and fine feature information via the terminal FM component and periodic modulations conveying flutter information via the CF component. Both types of FM are important for identifying, localizing, and capturing insect prey.
FIG. 1.
A: sonogram of the mustached bat biosonar signal. Intensities of the constant frequency (CF), initial FM (iFM), and terminal FM (tFM) components of the 4 prominent harmonics (H1–H4) are indicated with different line widths. The duration of this signal corresponds to signals emitted during the “search phase” of echolocation. The downward sweeping tFM2 component in search phase signals has a bandwidth of 10–12 kHz and a duration of ~4 ms, corresponding to the fastest FM sweeps used in our experiments. The upward sweeping iFM2 component has a bandwidth of ~2 kHz and a duration of ~1–2 ms. B: temporal waveforms of a train of sonar signals emitted by an echolocating mustached bat during the approach to a target. Note how the amplitude of the signals rises rather gradually to a maximum. The duration of each pulse is ~12 ms, matching one of the stimulus durations in the experiments reported here.
Recent experiments in our lab identified a ventral subdivision of the external (or lateral) nucleus of the inferior colliculus (ICXv) containing auditory units directionally selective for linear FM sweeps (Gordon and O’Neill 1998, 2000). The proportion of units found to be directionally selective was much higher in the ICXv compared with units recorded in the central nucleus of the inferior colliculus (ICC) in the same experiments (86 vs. 65%). The vast majority of ICXv units not only expressed a greater degree of directionality, but they also expressed significant directional preferences over a wider range of modulation rates than ICC units. Contrary to initial expectations, for units expressing significant directionality, 92% of ICXv and 77% of ICC units preferred upward over downward FM sweeps. Aside from the weak and variable initial FM in the sonar pulse mentioned in the preceding text, upward FM sweeps are prominent in a variety of communication sounds and in the periodic FM signals reflected by fluttering targets.
Tract tracing studies have revealed that the ICXv receives afferents from only one nucleus in the brain stem, the nucleus of the central acoustic tract (NCAT) (Casseday et al. 1989; Gordon and O’Neill 2000), which is otherwise known as the anterolateral periolivary nucleus. The central acoustic tract (CAT) was first described in mouse by Ramon y Cajal (1911) and later identified in cat by Papez (1929). More recently, Casseday et al. (1989) corroborated these findings with modern techniques, showing not only that NCAT was the source of the pathway but that it also projects to the deep layers of the superior colliculus (SCd), the pretectal area, and the suprageniculate nucleus (Sg) of the medial geniculate body (MGB). Sg receives projections from NCAT, the ICC, and in particular, the ICX (including ICXv), although the precise origin of the projections was somewhat compromised by large injection sites that invaded the nearby regions of the MGB. Our experiments verified the projection of ICXv to Sg and also showed that it projects to the SCd and pretectal area in parallel with projections from NCAT (Gordon and O’Neill 2000). ICXv may also send input to the region of the MGB surrounding the Sg, as the extent of terminal labeling exceeded the boundaries of the Sg somewhat. CAT thereby makes both direct connections to the Sg, bypassing the ICC, and indirect connections to the Sg via the ICXv (Gordon and O’Neill 2000) and perhaps the SCd (Casseday et al. 1989; Covey et al. 1987).
The primary motivation for the present study was to determine whether Sg neurons also show strong preferences for FM sweeps like units in the ICXv. Ultimately, we wish to understand the underlying mechanisms for directional selectivity and whether CAT plays an important role in processing FM signals in the mustached bat. Little has been published in any species about how Sg neurons respond to complex sounds. In the mustached bat, FM responses, in the form of FM-FM combination-sensitive neurons, have been found in the dorsal division of the MGB, within which the Sg nucleus resides (Olsen and Suga 1991; Wenstrup 1999). In the most comprehensive mapping of response properties in the mustached bat MGB published to date, only four neurons were characterized in the Sg (Wenstrup 1999). In this paper, we describe the response of a population of Sg neurons to upward- and downward-sweeping FM stimuli over a variety of modulation rates and sound pressure levels. By revealing how Sg processes FM signals, we provide new information regarding the role of Sg in processing complex sounds behaviorally relevant to this species.
METHODS
Preparation
We recorded from male and female Parnell’s mustached bats (Pteronotus parnelli rubiginosus) wild-caught in Trinidad. The bats were housed in a large temperature- and humidity-controlled flight room and fed fortified mealworms. All husbandry and experimental procedures were approved by the University Committee on Animal Resources.
Single-unit recording and data collection
Surgery to expose the skull and cement a head-restraining post to the surface was performed under methoxyflurane anesthesia (Meto-fane, Pittman-Moore) using techniques described previously (O’Neill 1985). Recordings were performed on unanesthetized animals restrained comfortably in a custom stereotaxic device (Schuller et al. 1986). Penetrations were guided using an atlas of the mustached bat brain calibrated to the stereotaxic frame. Single-unit action potentials were recorded extracellularly using glass micropipette electrodes with tip diameters of 2–5 μm, filled with 1 M sodium acetate. A 125-μm-diam bare tungsten wire was implanted in contact with the cerebral cortex to serve as an indifferent electrode. Spikes were amplified (Dagan 2400, Minneapolis, MN) and band-pass filtered (0.5–4.0 kHz, simple R-C; Krohn-Hite 3202). Single units were isolated based on spike amplitude and waveform using a window discriminator (BAK Electronics, Germantown, MD). Discrimination of single units from fibers was based on spike shape and duration.
Stimuli were presented within a data acquisition period of 100 ms, repeated at 4 Hz (250-ms interstimulus interval), for a total of 50 repetitions. Stimuli were delayed by 20 ms from the onset of the acquisition period. Spike times relative to the onset of the acquisition period were collected with a resolution of 10 μs by a KWV-11 real-time programmable clock running on a Micro PDP-11/23+ computer (Digital Equipment, Maynard, MA). Peristimulus-time histograms (PSTHs) with binwidths of 500 μs were displayed in real time. Spike data were analyzed within two time windows. Background activity was quantified by counting spikes in the period 20 ms before stimulus onset. Stimulus-driven activity was quantified by counting spikes in a second 40-ms window after stimulus onset. In some cases, the onset and/or duration of the second window was adjusted to compensate for longer response latencies or high levels of background activity.
Stimulus generation and presentation
Acoustical stimuli were presented through a 3.75-cm-diam electrostatic transducer (model T2004C, Polaroid, Cambridge, MA) placed 17 cm from the bat’s head along the acoustic axis of the pinna at 60 kHz (25° from the midline contralateral to the recording site in the horizontal plane). Sine-wave tone bursts were generated by a function generator (Wavetek 111, San Diego CA) and shaped and gated with linear rise-fall times of 0.5 ms using a programmable electronic switch (Wilsonics BSIT, San Diego, CA). Three stimulus durations (30, 12, and 4 ms) were used. Linear FM sweeps with a fixed 12-kHz bandwidth were generated at modulation rates of 400, 1,000, and 3,000 kHz/s (corresponding to durations of 30, 12, and 4 ms, respectively) using a custom-built ramped voltage generator. Stimulus timing, triggering, and repetition rate were controlled by a Master 8 stimulator (AMPI, Jerusalem, Israel). The 12-kHz bandwidth and 3,000-kHz/s modulation rate stimulus mimics the second harmonic of the terminal FM sweep emitted during the “search” phase of echolocation prior to detection of a target. The two slower modulation rates are outside the range of terminal FM sweeps emitted by mustached bats but correspond to FM rates found in some of the species communication sounds. Mustached bats are not known to vary the bandwidth of the terminal FM component, although microstimulation experiments have shown that the relative amplitudes of the harmonics may be actively varied (Gooler and O’Neill 1987).
The maximum sound pressure level (SPL, dB re 20 μPa) of the system was calibrated daily using a ¼-in condenser microphone (Type 4135, Brüel and Kjær) and a true-RMS measuring amplifier (Type 2610, Brüel and Kjær). Speaker output was essentially flat (deviated less than ±0.7 dB) over the 50- to 75-kHz frequency band where most units were responsive. Maximum sound level delivered was 85 dB SPL over this frequency range.
Experimental procedure
Search stimuli consisted of tone bursts varied in frequency and amplitude under manual control. After isolating a unit, the best excitatory frequency (BEF) was determined at the minimum threshold (MT) for each unit. MT was defined as the pure tone level eliciting a barely noticeable response as determined audiovisually. The MT was validated after obtaining spike-count versus SPL functions at BEF. Spike-count functions were gathered under computer control for each stimulus duration, starting at 10 dB below MT (MT – 10 dB) and incrementing in 10-dB steps to maximum speaker output using a programmable attenuator (Wilsonics PATT, San Diego, CA). Next, spike-count functions were obtained with “BEF-centered” FM sweeps. For example, downward FM sweeps began above BEF by 6 kHz and ended 6 kHz below. Up-sweeps spanned the same frequency band but in reverse time order. Consequently, the BEF in such sweeps occurred at a time delay equal to one-half the duration of the sweep. Spike-count functions were obtained for both upward and downward FM directions at each of the three FM sweep rates.
Data analysis
DIRECTIONAL SELECTIVITY ANALYSIS
As in previous studies (Britt and Starr 1976; Gordon and O’Neill 1998; Heil and Irvine 1998; Heil et al. 1992b; Mendelson and Cynader 1985; Phillips et al. 1985), a conventional directional selectivity index (DS) was calculated to describe FM directional preference using the equation: DS = (U − D)/(U + D), where U and D are the spike counts to upward and downward FM sweeps, respectively. DS can vary from +1 to −1, where the sign indicates preference for upward (+) or downward (−) sweeps, and 0 indicates no preference (i.e., equal response). FM directional selectivity was considered significant if the number of spikes (for 50 stimulus repetitions) was at least twice as large in one direction as the other ( DS ≥ 0.33). It should be noted that, as with any such ratio, the usefulness of this statistic is severely compromised by low spike counts. Therefore we did not consider DS significant for conditions where the total spike count to the preferred FM direction was <10 spikes over 50 stimulus repetitions (response probability ≤20%).
FIRST-SPIKE LATENCY ANALYSIS
Latency was estimated from the median of first-spike latencies collected over 50 presentations. We used data obtained at MT +20 dB where median latencies reached an asymptotic minimum for nearly all units. To capture driven activity only, the window for obtaining the first-spike latency started 3.5 ms after the onset of the stimulus and ended 80 ms later. For analysis, we excluded units with responses within this window with response probabilities <0.2 (i.e., < 10 spikes per 50 stimulus repetitions). Units with first-spike latencies >45 ms were also excluded because the PST histograms showed that the discharges at that time were likely offset responses.
Histology
Focal iontophoretic injections of biotinylated dextran amine (BDA, 10,000 MW, Molecular Probes, Eugene, OR) were made 1–1.5 wk prior to death to verify recording sites histologically. One or more injections (10% by weight solution of BDA in pH 7.4 phosphate-buffered saline) were made at selected stereotaxic coordinates with a micropipette with a tip diameter of 8–10 μm guided by recordings of multiunit activity. Pulsed 3.0-μA constant DC current was delivered for 5–8 min at a 50% duty cycle (7-s on, 7-s off; Transkinetics/Midgard, St. Louis, MO). At the conclusion of experiments, the bats were given an overdose of pentobarbital sodium (100 mg/kg) and perfused intracardially with 4% buffered paraformaldehyde fixative. The brains were carefully dissected and cryoprotected by submerging them in successive 10 and 30% solutions of sucrose in fixative. Thirty-micrometer sections were cut in a cryostat at −18°C (Frigocut 2800e, Leica-Jung, Germany) and divided into four interleaved sets. Two sets of sections were processed with a Neurotrace BDA-10,000 kit (Molecular Probes). One BDA-immunostained set was then counterstained with cresyl violet acetate (Sigma-Aldrich, St. Louis, MO). The sections were mounted, cleared, and coverslipped in Permount (VWR International, West Chester, PA). Digital photomicrographs were made with a SPOT Insight color camera and imaging software (Diagnostic Instruments, Sterling Heights, MI). Images were subsequently processed with Photoshop 6.0 (Adobe Systems, San Jose, CA).
Reconstruction of unit locations
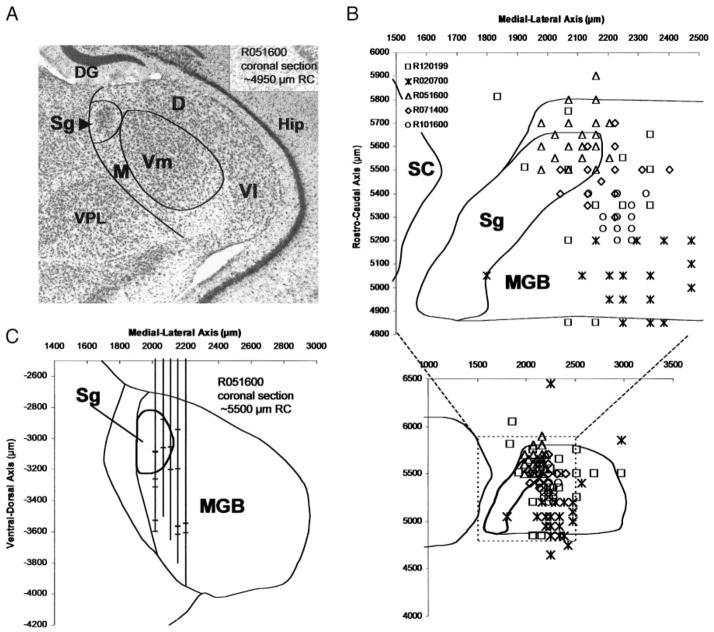
Unit stereotaxic coordinates were corrected by first matching the section containing the injection site center with the corresponding section in the mustached bat stereotaxic atlas. The injection site was then projected onto the atlas section to determine its “atlas coordinates.” The difference between the atlas coordinates of the injection site and the electrode coordinates in the stereotaxic frame was then used to correct the coordinates of all units, after taking into account tissue shrinkage during histological processing (typically 10% for BDA). Unit locations were then reconstructed by plotting their position onto the nearest corresponding atlas sections (e.g., Fig. 2C). All units reported in this paper were located within the caudolateral Sg as defined by Winer and Wenstrup (1994a, b).
FIG. 2.
A: Nissl-stained transverse section of the suprageniculate nucleus (Sg), medial geniculate body (MGB), and surrounding thalamus. Boundary of MGB is indicated by leftmost line; other lines indicate cytoarchitectonic divisions of the MGB. Sg neurons are among the largest in the MGB, and the nucleus is considered part of the dorsal division (D). B: stereotaxic reconstructions of electrode penetrations from 5 bats verified with biotinylated dextran amine (BDA) injections. The upper plot is a reconstructed 1-mm2 horizontal section at a depth of 2,800 μm from the cortical surface overlying the Sg. The area of this section relative to the entire MGB at this depth is represented by the dotted outline in bottom inset. Penetrations (n = 111, 17 in Sg) are plotted individually (with some overlap), and each of the 5 bats is represented by a different symbol. Penetrations primarily sampled units in the caudolateral Sg. C: representative section from 1 bat demonstrating reconstruction of unit locations (crossbars) within penetrations (vertical lines). Vl, Vm, lateral and medial subdivisions of the ventral division of MGB; D, M, dorsal and medial division of MGB; VPL, ventral posterior lateral nucleus; Hip, hippocampus; DG, dentate gyrus of Hip.
RESULTS
The photomicrograph in Fig. 2A shows the location of the Sg in a Nissl-stained cross-section of the MGB from one experimental animal. We made 17 penetrations passing through the Sg of a total of 111 penetrations made in and around the MGB in five bats (Fig. 2B, bottom inset). Because the nucleus is only about 200–300 μm thick, only two or three Sg units could typically be isolated in each penetration passing through it, as demonstrated in a single reconstructed section (Fig. 2C) from the same bat shown in Fig. 2A. Based on stereotaxic reconstruction of recording sites, all units isolated in the Sg (n = 37) were from the caudolateral third of the nucleus (Fig. 2B, top graph). The remaining units (n = 91) were sampled in a regular grid of penetrations covering nearly the entire rostro-caudal extent of the MGB, and were localized to the dorsal division, medial division, and the medial subdivision of the ventral division (D, M, and Vm, respectively, Fig. 2A).
BEFs
Wenstrup et al. (1994) found that the Sg received afferent input from the ICC representing most of the spectrum audible to the species. Nearly all afferents from the ICXv would be tuned to the CF2 frequencies of the sonar pulse, which lies between 58 and 59 kHz in P. parnelli rubiginosus (Gordon and O’Neill 2000). The sample of units we recorded in the caudal Sg had a mean BEF of 59.94 ± 7.42 (SD) kHz (n = 36). The distribution of BEFs is shown in Fig. 3. Seventy-two percent of the units (n = 26) had BEFs in the CF2 range from 58 to 59 kHz, 13.5% were tuned to the FM2 band from 48 to 58 kHz (5 units), and the remaining units (16.2%, 6 units) had BEFs >59 kHz. One unit responded to FM only and therefore had no specific BEF.
FIG. 3.
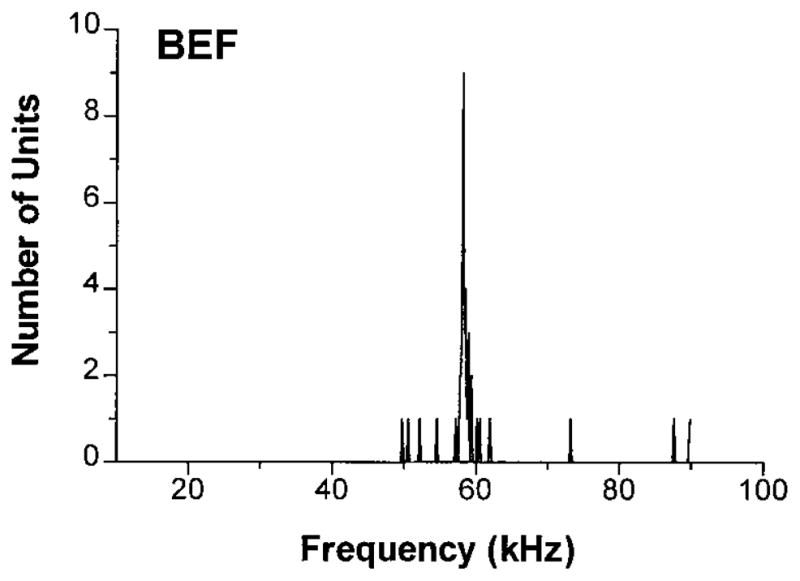
Distribution of best excitatory frequencies (BEF) in caudolateral Sg. BEFs are tightly clustered around 58 kHz.
Discharge patterns and BEF spike-count functions
Based on PSTHs obtained with 30-ms BEF tones at 10–20 dB above MT, we classified temporal discharge patterns of the units as phasic or tonic. Phasic units were defined as those that showed an ON response that declined to background levels within 4–5 ms after stimulus onset. Tonic units were defined as those showing an ON response that was sustained throughout the duration of the stimulus, with or without a decline in firing rate. For BEF tones, the vast majority of the units responded phasically (25 of 29, 86.2%). By contrast, for FM sweeps of the same duration (30 ms, 400 kHz/s sweep rate), the number of phasic units declined (20 of 31, 64.5%) while tonic responses increased.
Out of the 37 Sg units recorded, complete sets of spike-count versus level functions were obtained in 25 units with both BEF tones and FM sweeps at all three durations (4, 12, and 30 ms). Partial sets with only one or two durations were obtained in the remaining 12 units. In response to 30-ms BEF tones, 62% of the 30 units tested were nonmonotonic. When presented with 30-ms long FM sweeps, nearly the same proportion of non-monotonic units was observed (61%, 20 of 33). However, in nine units (27%), the shape of the spike count function was stimulus dependent, changing from monotonic to tones to nonmonotonic for sweeps (4 units), or vice versa (5 units). Except for a few units showing poor responses to 4-ms stimuli, the shape of the spike-count function remained the same with the two shorter stimuli.
Directional responses to FM sweeps
Five Sg units responded to tones but poorly or not at all to sweeps, and one unit responded to FM but not tones. The remaining units all showed responses to FM sweeps and were classified into six categories. Table 1 shows the number of Sg units in each category. A unit was defined as up or down selective if its spike count in one sweep direction was at least two times that in the other ( DS ≥ 0.3), and it was consistently selective for one direction at a minimum of three successive SPLs above minimum threshold, over all three modulation rates tested. Rate-selective units had significant DS values at only one or two sweep rates. Level-dependent units were significantly directional only over a restricted range of SPLs at all three sweep rates as evidenced by a peak in the nonmonotonic spike-count versus SPL functions for FM sweeps. Variably selective units exhibited directional selectivity but without a consistent selectivity for up or down sweeps over modulation rates or SPLs. Bi-directional or nonselective units responded well to FM sweeps in either direction ( DS ≤ 0.3). The responses of FM-suppressed units were significantly lower for FM sweeps than for BEF tones.
TABLE 1.
FM directional preference at different FM sweep rates
| Sg Unit Directionality*
|
|||
|---|---|---|---|
| Sweep rate, kHz/s | 400 | 1000 | 3000 |
| Sweep duration, ms | 30 | 12 | 4 |
| Up | 12 (40) | 13 (52) | 16 (46) |
| Down | 7 (23) | 6 (24) | 5 (14) |
| Rate dependent | 2 (7) | 1 (4) | 4 (11) |
| Level dependent | 2 (7) | 1 (4) | 0 (0) |
| Bi-directional | 7 (23) | 4 (16) | 10 (29) |
| Total† | 30 (100) | 25 (100) | 35 (100) |
Values in parentheses are percentages. Sg, suprageniculate nucleus.
Responses at 50–60 dB SPL.
Total number of units tested at each sweep rate/duration.
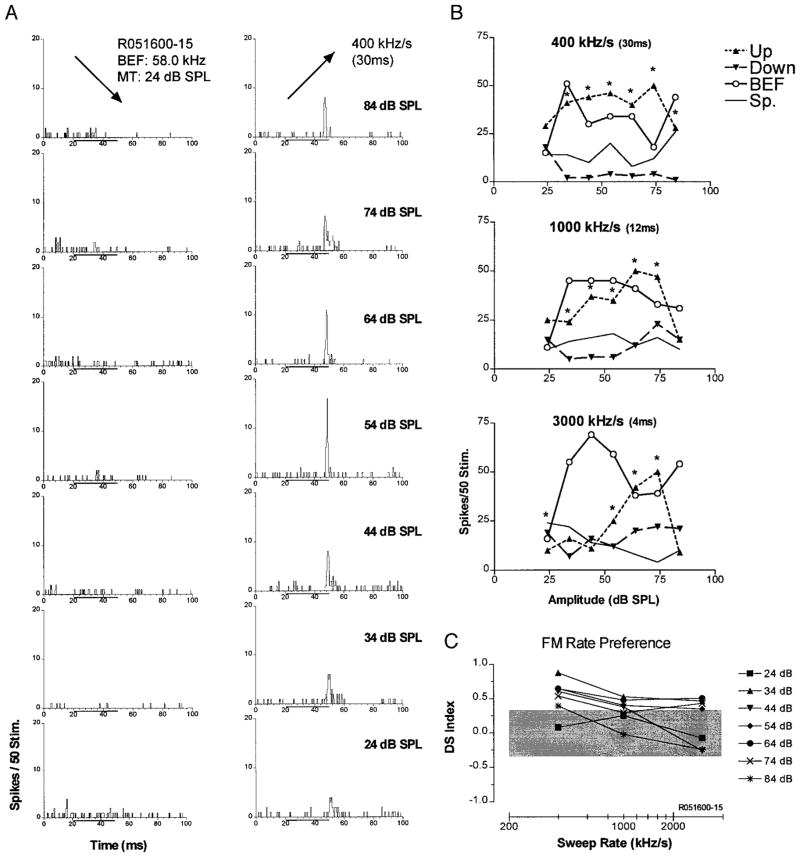
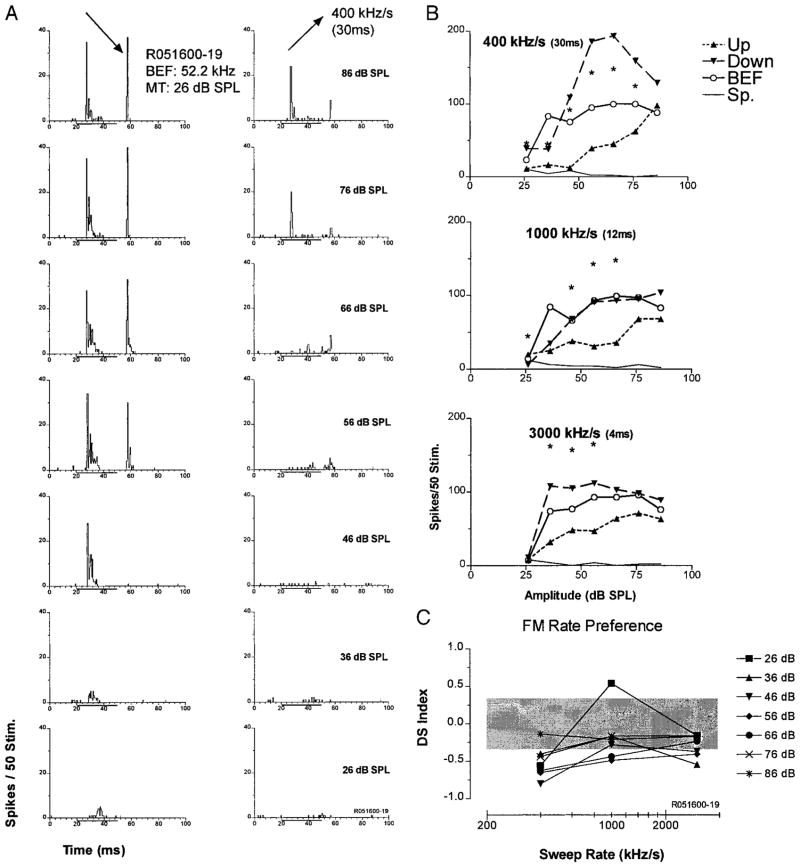
Figures 4–7 are similarly formatted to show examples of up-selective, down-selective, bi-directional, and rate-selective units recorded in the Sg. The majority of the Sg units were up-selective at each of the three sweep rates tested (Table 1). Figure 4 shows an example of an up-selective Sg unit with a BEF in the CF2 range. In Fig. 4A, PSTHs show responses to downward (left) and upward (right) 30-ms FM sweeps (400 kHz/s sweep rate) from BEF-tone threshold (21 dB SPL) to maximum SPL tested (81 dB SPL) in 10-dB steps. The unit responded almost exclusively to upward sweeps with a phasic long latency discharge pattern followed by a sustained period of suppression of spontaneous activity, particularly at higher SPLs.
FIG. 4.
Up-selective unit in the Sg. A: peristimulus time histograms (PSTHs) constructed with data from 50 presentations of the same stimulus at the indicated SPL and FM sweep direction. Left: responses to downward FM; right: responses to upward FM. B: spike counts are plotted as a function of SPL. Curves show responses to downward (down), upward FM (up), BEF tones (BEF), and spontaneous activity (Sp). The top-most plot is taken directly from the PSTHs in A. At the 400 kHz/s sweep rate, this unit had a significant directional preference [directional selectivity index (DS) > 0.3, indicated by *] for up-sweeps at all the SPLs tested except minimum threshold. A similar pattern was observed at 1,000 and 3,000 kHz/s (middle and bottom). C: DS as a function of sweep rate at all SPLs tested.
 , nonsignificant (DS < 0.3) directional selectivity. In this unit, selectivity declined as sweep rate increased.
, nonsignificant (DS < 0.3) directional selectivity. In this unit, selectivity declined as sweep rate increased.
FIG. 7.
Complex FM selectivity in Sg unit. Plots are organized identically to those in Fig. 4. The unit’s response to the slowest rate of modulation shown in B, top, indicates that over a wide range of SPLs the unit was selective for down sweeps. When tested at the two faster sweep rates (B, middle and bottom), the unit became selective for up sweeps. C: a summary of the unit’s directional preferences shows a clear and consistent rate-dependent shift in directional preference for all the SPLs tested.
Spike-count functions to up-sweeps, down-sweeps, BEF pure tones and spontaneous activity are plotted for comparison in the top graph of Fig. 4B. Spikes were counted in a 40-ms window after stimulus onset. For 30 ms upward FM sweeps, this unit had a significant directional preference (denoted by *) at each SPL tested except BEF threshold. Although the response declined at the highest level tested (84 dB SPL), the spike-count function for up sweeps grew slowly to a peak over most of the dynamic range above MT + 10 dB. The spike-count function for BEF tones was nonmonotonic, with a best amplitude at 10 dB above threshold. The response to upward FM sweeps had a lower threshold and exceeded the response to tones at all levels except for the BEF best amplitude. By contrast, the response to downward FM sweeps was suppressed well below that of the spontaneous activity.
A similar pattern and magnitude of upward FM preference was observed for 12-ms (1,000 kHz/s) sweeps (Fig. 4B, middle); however, the directional preference was not significant at the lowest and highest SPLs tested. Similarly, the response to 4-ms (3,000 kHz/s) sweeps (Fig. 4B, bottom) showed an overall preference for up-sweeping FM, but that preference was significant for only four of the seven stimulus SPLs tested. In addition, the response to low-level up-sweeps was much lower than the pure tone response at those levels.
The spike-count functions for duration-matched pure tone stimuli were examined to ascertain whether a rate-selective response to FM reflected underlying duration selectivity. For this unit, all tone durations elicited a nonmonotonic response to increasing SPL. The unit clearly showed a preference for short-duration stimuli, responding to 4-ms tones of 45–55 dB SPL almost threefold more than it did to 30-ms tones of the same amplitude (Fig. 4B compared across the 3 durations). Interestingly, this preference for brief tonal stimuli did not correlate with a preference for brief FM sweeps with high modulation rates. In fact, this unit responded best to FM sweeps with long durations and slow modulation rates (400 kHz/s) and showed a progressive decline in response to up sweeps with increases in modulation rate. Sweep rate selectivity for FM was clearly not related to duration tuning in this unit.
Figure 4C summarizes the unit’s directional selectivity at all SPLs and modulation rates tested. DS indices were found to be significant for 14 of the 21 rate/SPL combinations tested. Overall, the unit became less directionally selective at faster sweep rates. In addition, at all sweep rates tested, the unit tended to exhibit the greatest directionality in the midrange of SPLs. This progressive decline in directionality with increased modulation rate appears to be related to decreases in both the excitation evoked by up sweeps and the degree of suppression evoked by down-sweeps (Fig. 4B).
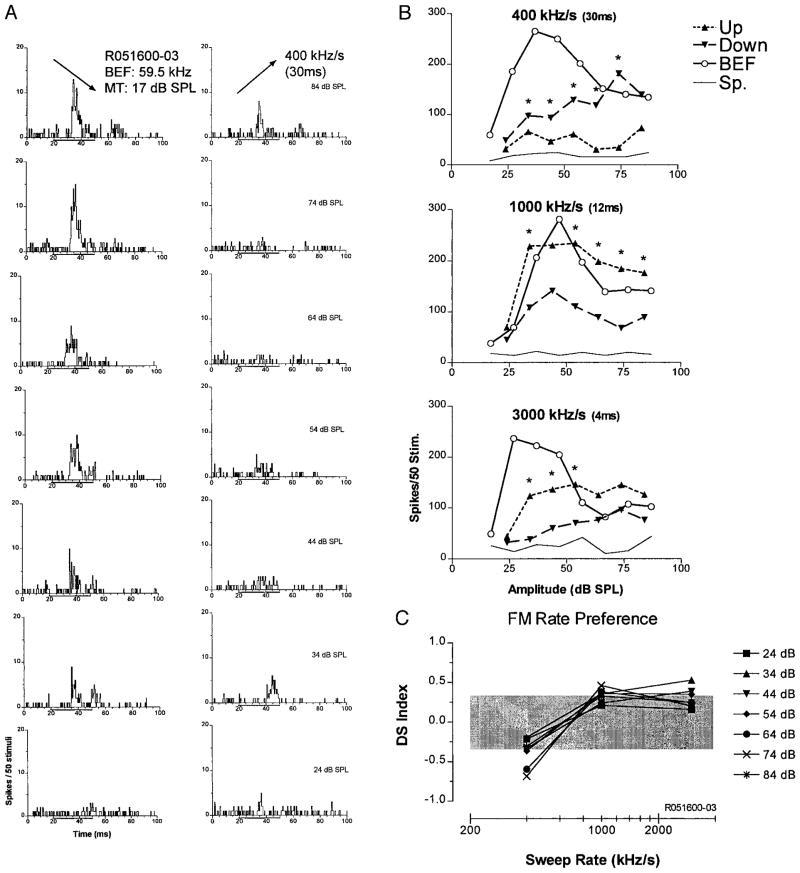
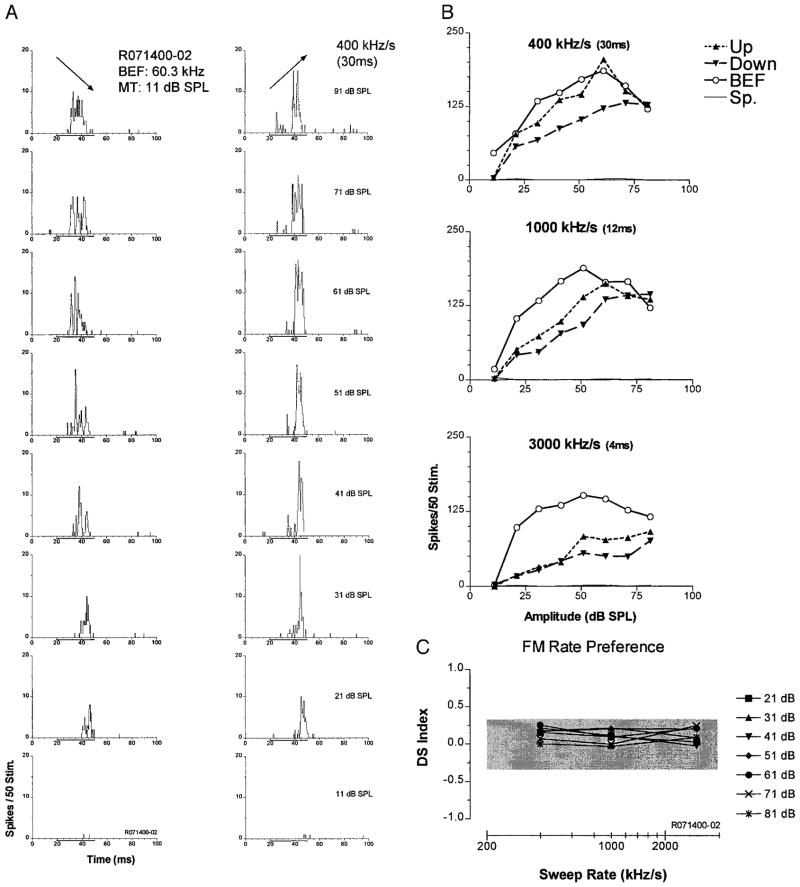
Figure 5 shows an example of a down-selective unit, the second largest class of FM selectivity. This unit’s BEF was in the FM2 frequency range. In response to downward FM sweeps, the unit exhibited a short-latency ON response at threshold that progressed to an ON-OFF response at higher levels (Fig. 5A). By contrast, for upward sweeps, there was virtually no response at low levels, a weak OFF response at moderate levels, and an ON-OFF response at high levels. Directional selectivity for downward sweeps was maintained not only across SPL, but also across all sweep rates (Fig. 5B). As seen in Fig. 5B, top, the unit was significantly selective for downward FM at slow sweep rates (400 kHz/s) at all SPLs save the highest and was facilitated relative to the BEF pure tone response over much of this range. Unlike the up-selective unit depicted in Fig. 4, which was suppressed by sweeps in the nonpreferred direction, this unit did respond to sweeps in the opposite direction. However, the threshold for up sweeps was ≥20 dB higher than that for BEF tones and down sweeps, and responses above that level were clearly less than that to BEF tones or down sweeps of the same amplitude.
FIG. 5.
Down-selective unit in the Sg. Plots are organized identically to those in Fig. 4. A: PSTHs from 14 rate/intensity combinations with responses to down sweeps (left) and to up sweeps (right). B: the unit was selective for downward modulations over a wide range of intensities. Selectivity was reduced slightly at the two faster sweep rates tested (bottom two plots); however, response to pure tones did not change with different durations. At the slowest rate, the response to down FM was greater than the pure tone response at several intensities. C: directional selectivity over most of the SPLs tested declined as a function of sweep rate.
At the higher modulation rates, responses to downward FM declined, whereas that to BEF tones and upward sweeps remained about the same. Directionality was also more dependent on level (Fig. 5B, middle and bottom). As in the up-selective unit described previously, this unit became less directionally selective for FM at higher sweep rates (Fig. 5C). Unlike the up-selective unit, this unit responded very similarly for BEF tones at all three durations.
Bi-directional or nonselective units responded to FM in both sweep directions ( DS ≤ 0.3), as shown by the example in Fig. 6. This unit had little or no spontaneous activity. Responses to upward or downward FM were similar in magnitude with a small but consistent preference for up sweeps (Fig. 6B). The unit was not rate selective (Fig. 6, B and C), but responses to both sweep directions declined relative to pure tone response as modulation rates increased. The discharge patterns clearly differed with sweep direction (Fig. 6A), suggesting that spike discharge probability was sensitive to the order of frequencies in the stimulus. This unit also demonstrates how sweep direction could be discriminated from differences in temporal discharge pattern and/or latency rather than response magnitude. Unfortunately, current schemes for classifying directionality do not consider temporal discharge patterns.
FIG. 6.
Bi-directional (nonselective) unit in the Sg. Plots are organized identically to those in Fig. 4. A: PSTHs from 16 rate/intensity combinations with responses to down sweeps (left) and to up sweeps (right). B: the unit showed robust responses to both down and up sweeps at all intensities and rates tested. C: summary of FM preferences. Unit was bi-directional at all combinations of modulation rate and SPL tested.
A very small number of units exhibited less consistent patterns of response to FM. Figure 7 shows a unit whose directional preference changed with sweep rate. This “rate-dependent” unit responded well to downward FM sweeps at modulation rates of 400 kHz/s, but very poorly for up sweeps. The response in both sweep directions was much less than that for tones (Fig. 7, A and B, top). However, at the two more rapid sweep rates, the unit shifted its selectivity in favor of up sweeps. The strongest response to FM was to up sweeps at 1,000 kHz/s modulation rate, where the response matched or exceeded that for tones (Fig. 7B, middle). As in the previously illustrated cases, such rate selectivity cannot be attributed to duration selectivity, as there was no consistent preference for short-duration stimuli evident in the BEF spike-count functions. The rate preference summary in Fig. 7C shows clearly that the unit was selective for down sweeps at slower sweep rates and up sweeps at faster rates, independent of SPL.
Directional selectivity in Sg units
The graphs in Fig. 8 and Table 1 summarize the directional preferences of Sg units, expressed as the percentage of units that were up selective, down selective or bi-directional/nonselective. This comparison is further subdivided into low, middle, and high SPL ranges for each of the three sweep rates. Two patterns emerged from this analysis. First, directional selectivity was significant for 53–78% of the units, depending on modulation rate and SPL. Second, save for one condition (400 kHz/s, 70–80 dB SPL), up-selective units outnumbered down-selective units by factors ranging from 2:1 to 3:1, depending on modulation rate (Table 1). The preference for up sweeps in the Sg is similar to, but less than, that found in the ICXv (Gordon and O’Neill 2000).
FIG. 8.
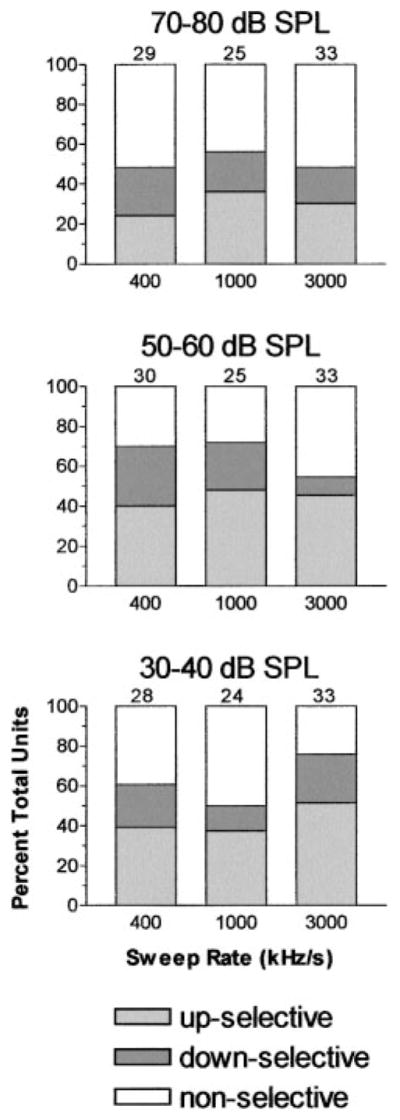
Summaries of the directional preferences found at the 3 sweep rates (x axis) and three ranges of SPL. In the range of 70–80 dB SPL, ~51% of units in the Sg had a directional preference. Furthermore, directional units were more likely to be up selective (light gray bars) than down selective (dark gray bars). This pattern of selectivity preference was evident at both the 50–60 dB SPL and the 30–40 dB SPL ranges, where more units were directionally selective.
Directionally dependent facilitation and suppression
Directional preferences could theoretically arise from suppression of responses to sweeps in the nonpreferred direction, but no change in the preferred direction relative to the BEF response; facilitation in the preferred direction, with no change in the non-preferred direction relative to control; or a combination of suppression and facilitation. In our previous studies of the ICXv, FM directional preferences always resulted from suppression by the nonpreferred sweep direction; sweeps in the preferred direction were never more potent than BEF pure tones at eliciting response. However, as can be seen for the up- and down-selective units in Figs. 4 and 5, FM sweeps could elicit both facilitation and suppression either alone or in combination.
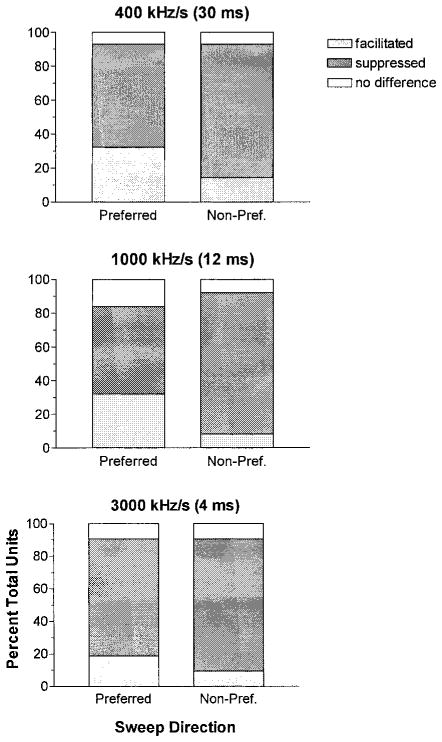
We categorized directionally selective Sg units (between 25 and 32 units, depending on sweep rate) as “facilitated” or “suppressed” if their response to FM sweeps was higher or lower than SPL-matched BEF tones by ≥20%. For simplicity, we limited our analysis to the spike count obtained at the optimal SPL for FM response. For preferred sweep directions, the majority of units responded less well to FM than to BEF, with fully 61, 52, and 72% showing suppression at 400-, 1,000-, and 3,000 kHz/s, respectively (Fig. 9). Directional preference in 79–84% of the population was mainly attributable to even greater levels of suppression for sweeps in the nonpreferred direction. However, facilitation was evident in 20–30% of the units. Directional preferences were enhanced by facilitation in the preferred sweep direction combined with suppression in the nonpreferred direction in 4 units (e.g., the unit in Fig. 4). Interestingly, in a few units (8–14% depending on sweep rate) both sweep directions elicited facilitation relative to BEF tones.
FIG. 9.
Prevalence of facilitation and suppression by FM sweeps in the preferred (left) and nonpreferred (right) directions as a function of modulation rate/stimulus duration. Facilitation and suppression were defined as an increase or decrease in response relative to BEF tone burst control by ≥20%. Suppression dominated for both preferred, and especially, nonpreferred sweeps. Facilitation for preferred sweeps was seen in 1/5 to 1/3 of the units, depending on modulation rate.
Threshold differences for tones and FM sweeps
Specialization for FM sweeps is often indicated by lower thresholds for sweeps than for BEF tones. Averaging across all modulation rates for FM sweeps in the preferred direction, we found that 19% of Sg units had minimum thresholds for preferred FM sweeps that were lower (by ≥3 dB) than their BEF thresholds. However, the majority of units (55%) had higher FM thresholds, and 26% had thresholds within ±3 dB of BEF threshold. As one might expect, for nonpreferred sweeps the number of units with higher FM thresholds grew (66%), but FM thresholds still remained lower in 12%, and equal to BEF thresholds in 22%, of the units. Only one unit in our sample responded strongly to FM sweeps but showed no response to BEF tones. This unit was strongly up-sweep selective at all three modulation rates (DS = 0.67–0.89).
Latency for BEF tones vs. FM sweeps
Casseday et al. (1989) suggested that the central acoustic tract, which directly projects from the NCAT to the Sg, could subserve rapid processing of acoustic information by the fore-brain. Our data partially support this hypothesis. Table 2 compares the first-spike latencies for BEF tones in Sg units with samples from the ICXv and the anterolateral division of ICC reported in a previous study (Gordon and O’Neill 2000). Figure 10A shows the distribution of BEF latencies in the Sg. For BEF tones, the median latencies of 25% of the Sg units ranged from 5 to 7 ms. This latency range completely overlaps units in the ICXv, which receives input from NCAT, as well as short-latency units in the deep ICC. However, the latencies of the remaining 75% of the Sg population are longer than those in the deep ICC and ICXv, suggesting that most units receive their dominant excitatory input from the IC rather than directly from the NCAT.
TABLE 2.
Comparison of latencies in Sg and IC
| Sg | ICXv | ICC* | |
|---|---|---|---|
| Median, ms | 11.3 | 6.0 | 9.5 |
| Interquartile range | 10.2 | 1.0 | 3.9 |
| Mean, ms | 12.8 | 6.1 | 9.5 |
| 95% Confidence interval | 4.6 | 0.4 | 2.4 |
| Range | 4.9–25.7 | 4.8–10.0 | 6.0–18.0 |
| n | 30 | 60 | 22 |
Median first-spike latency for 30-ms best excitatory frequency (BEF) tones, 20–30 dB above minimum threshold. ICXv, ventral division of the external nucleus of the inferior colliculus; IC, inferior colliculus; ICC, central nucleus of the IC.
Units recorded primarily in the anterolateral division of ICC, which represents frequencies below CF2.
FIG. 10.
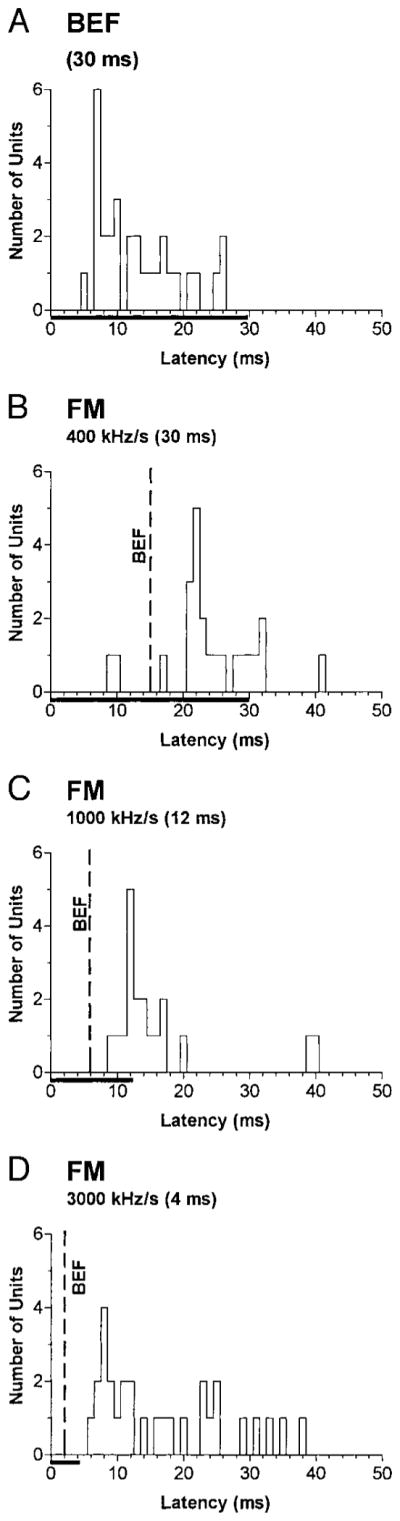
Distributions of median 1st-spike latencies to BEF tones (A) vs. 30-ms (B), 12-ms (C), and 4-ms (D) FM sweeps. The horizontal bars on the x axes indicate the time course of the stimuli. The vertical lines labeled “BEF” in B–D indicate the point in the FM stimulus where the frequency crossed the BEF (i.e., 15, 6, and 2 ms after stimulus onset, respectively). Latencies for sweeps would overlap those for BEF tones after subtracting the shift in BEF relative to sweep onset, suggesting that the effective instantaneous frequency (EFi) is near the BEF in most units.
Figure 10, B–D, shows the distribution of latencies for preferred FM sweeps with durations of 30, 12, and 4 ms. If FM latencies were locked to the sweep onset transient, then the distributions of BEF and FM latencies would largely overlap. However, FM latencies were shifted relative to sweep onset by ~15-ms for the 30-ms sweeps (Fig. 10B), and 6 ms for the 12-ms sweeps (Fig. 10C). Latencies for 4-ms sweeps mostly overlapped those for BEF tones (Fig. 10D), but these stimuli are too brief to reveal whether a shift occurred. The fact that the shifts with 30- and 12-ms sweeps equal about one-half the sweep duration suggests that the responses were temporally related to the midpoint of the sweep where the instantaneous frequency equals each unit’s BEF (vertical dashed lines in Fig. 10, B–D). This possibility is more carefully considered in the following section.
Are responses to FM sweeps triggered by an “effective frequency” within the sweep?
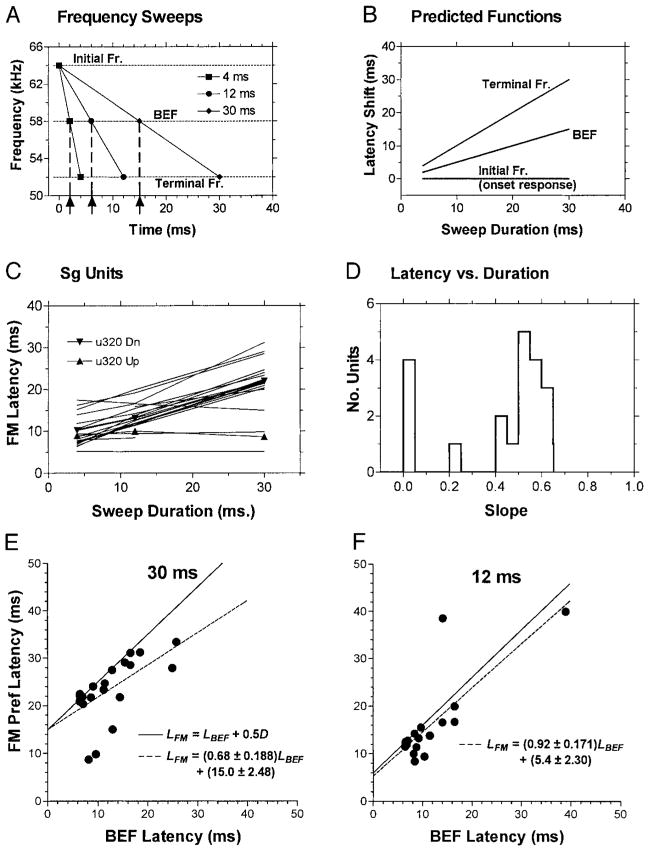
Most auditory neurons have latencies that are time-locked to the amplitude transient at tone burst onset. Consequently, latencies for BEF tones do not vary appreciably with stimulus duration. Latencies for FM sweeps might also be time-locked to the onset transient and show no duration dependence for either sweep direction. However, many studies have attributed responses to FM sweeps to stimulation of an effective instantaneous frequency (EFi) as the sweep traverses the response area (Bodenhamer et al. 1979; Britt and Starr 1976; Heil and Irvine 1998; Heil et al. 1992b; Sinex and Geisler 1981). In this case, FM sweep latency would vary with sweep duration. The growth (slope) of the latency versus duration function would depend on the time of occurrence of the EFi in the sweep, progressively increasing from 0 if EFi equals the initial frequency in the sweep, to a maximum of 1 if EFi equals the terminal frequency in the sweep. Figure 11, A and B, illustrates how latency would shift for the three durations of constant-bandwidth BEF-centered FM sweeps used in our study. Figure 11A shows the time course of frequency change for 12 kHz downward sweeps of 4, 12, and 30 ms. Figure 11B shows how the slope of the latency –duration function would grow for the initial, middle (i.e., BEF) and terminal frequencies in the sweep. Units in which the EFi equals the BEF would be expected to have latency –duration functions with slopes near 0.5 (“BEF” in Fig. 11B).
FIG. 11.
A: variation in frequency as a function of time for 4-, 12-, and 30-ms downward FM sweeps, illustrating how particular frequencies in the sweep shift in time with increases in sweep duration. The initial, middle (i.e., the BEF), and terminal frequencies shift by factors of 0, 0.5, and 1.0 times the sweep duration, respectively. ↑, the predicted latency shifts for units with an EFi equal to the BEF. B: predicted growth of latency with duration for the initial frequency (slope = 0), BEF (slope = 0.5), and terminal frequency (slope = 1.0) in fixed-bandwidth FM sweeps. C: latency vs. FM sweep duration functions in Sg neurons. Median 1st-spike latencies at 20 dB above MT are plotted for each duration tested (4, 12, and 30 ms). The majority of units show a monotonic, linear growth in latency with increasing sweep duration. This implies that the response to sweeps was triggered by an internal feature of the sweep, namely an EFi, which varied in time with modulation rate. D: distribution of slopes of the latency-duration functions in C. One group of units responds to sweep onset (slopes near 0), while the majority responds to the BEF in the sweep (slopes near 0.5). E and F: scatter plots with linear regression analysis of latency for FM sweeps vs. BEF tones for stimuli of 30- and 12-ms duration, respectively. - - -, the linear regression of FM against BEF latency; —, the relationship LFM = LBEF + 0.5 D. Most Sg units had nearly equal BEF and FM latencies (after subtracting 0.5 D, the temporal offset of the BEF in the sweep). The correlation was significant for Sg units at both sweep rates and for both directions of FM (not shown).
Figure 11C plots the latency-duration functions for preferred FM sweeps in Sg neurons, and Fig. 11D shows the distribution of the slopes of these functions. All except five units showed a growth of latency with duration. Of the five units with flat functions (slopes near 0; Fig. 11D), three were bi-directional/nonselective for FM and one was rate-dependent. These units responded to the onset transient with similar latencies for both sweep directions. The remaining unit (u320) had a relatively long duration-dependent latency for down sweeps, but a short-duration-independent latency for upsweeps (both curves are plotted in Fig. 11C for comparison).
In all other units, latency varied linearly with sweep duration, implying that the response was triggered by an internal feature of the sweep, namely an EFi, which varied in time with modulation rate. The slopes of these functions ranged between 0.4 and 0.6 (Fig. 11D); this implicates frequencies near the center of the sweep, i.e., near the BEF, as the EFi in these units.
If the BEF were the EFi, then the FM latency would be correlated with the BEF latency plus one-half the duration of the FM sweep. More formally, FM and BEF latencies for stimuli of equal duration would be related by the linear equation LFM = LBEF + ½D, where LFM is the latency for the FM sweep (centered on BEF), LBEF is the latency for the BEF tone, and D is the duration of the stimulus. Deviations below or above the line described by this equation would suggest that the response to FM is triggered by a frequency occurring earlier or later than the BEF in the sweep, respectively.
Figure 11, E and F, plots LFM for preferred sweep directions against LBEF for 30- and 12-ms stimuli, respectively. Table 3 provides a statistical summary of these data. The data for 4-ms stimuli were not analyzed because the variability made it impossible to resolve a 2-ms difference in the BEF and FM latencies for such short stimuli. Each graph predicts (—) equal latencies for BEF and FM, which intercepts the FM latency axis at 15 ms for the 30-ms duration stimuli and 6 ms for the 12-ms stimuli (i.e., ½D). Linear regression lines fit to the data are also shown (- - -). From these plots and the statistics in Table 3, it is clear that there was a significant correlation between LFM and LBEF for both 30- and 12-ms durations. In every unit except one outlier in the 12-ms data set, FM latencies were equal to or shorter than predicted by the hypothesis that the BEF was indeed the EFi. Moreover, as predicted from the preceding equation, the intercepts of the regression lines are nearly equal to ½D (15 and 6 ms, respectively).
TABLE 3.
Correlation of median first-spike latencies for FM sweeps versus BEF tones
| 30 ms | 12 ms | |
|---|---|---|
| Preferred FM vs. BEF | ||
| Pearson r | 0.63 | 0.79 |
| 95% confidence limits | 0.28–0.83 | 0.53–0.92 |
| P value | 0.002 | <0.0001 |
| n | 22 | 19 |
| Non-preferred FM vs. BEF | ||
| Pearson r | 0.45 | 0.79 |
| 95% confidence limits | 0.01–0.75 | 0.39–0.94 |
| P value | 0.04 | 0.002 |
| n | 20 | 12 |
These results strongly suggest that the BEF was the EFi for most, but not all, units. For 30-ms stimuli (Fig. 11E), fully 56.5% of Sg units had FM and BEF latencies differing by only ±2 ms (±6.67%) from equality. However, for 12-ms stimuli (Fig. 11F) using the same criterion (±6.67%, ±0.8 ms), only 31.6% of Sg units had FM and BEF latencies that were similar. Except for the single outlier in the 12-ms data mentioned in the preceding text, all units outside of these arbitrary boundaries had shorter latencies for FM than BEF. Three of these units were the same bi-directional onset responders mentioned previously.
In conclusion, for long FM sweeps with relatively slow modulation rates, the response of somewhat more than half the Sg units appears to be triggered by the BEF. However, for more rapid modulation rates, a higher percentage of units responded to frequencies preceding the BEF.
DISCUSSION
General summary
Our experiments showed that the majority of units in the caudolateral Sg responded strongly, but not exclusively, to FM sweeps. Between 50 and 75% of Sg units (depending on modulation rate) showed a significant sweep direction preference. In nearly all conditions up-selective units clearly outnumbered down-selective units. Only a small proportion of units showed a shift in selectivity with changes in modulation rate and/or SPL. Except for five units responsive only to tones, and one unit responsive only to FM, Sg units in the sample responded to both BEF tones and FM sweeps in one or both directions. Suppression in the nonpreferred sweep direction accounted for FM directional preferences in the majority of neurons, but facilitation in the preferred direction was also evident in ~20–30% of the population (depending on modulation rate). In the majority of units, the response to FM sweeps appeared to be elicited when the sweep crossed the unit’s BEF.
It is worth repeating at the outset of this discussion that the sample of Sg units recorded in these experiments was confined to the caudal 1/3 of the nucleus. Therefore our understanding of FM processing in the Sg will remain incomplete until further experiments are carried out in the more rostral portion of the nucleus.
Directional selectivity
The majority of units in the Sg were directionally selective and maintained selectivity for the same sweep direction at more than one SPL. The proportion of directionally selective cells equaled or exceeded that found in cat auditory cortex (ranging between 58 and 66%) (Heil and Irvine 1998; Mendelson and Cynader 1985; Tian and Rauschecker 1994). Despite the fact that the degree of directional preference showed some sweep rate dependence, most FM selective units in the Sg maintained consistent preferences for up or down sweeps over all three sweep rates tested and over a wide range of SPLs. Only one Sg unit responded to FM but not to tonal stimuli, whereas five units responded to tones but poorly or not at all to FM sweeps. Because we did not test for response to BEF-centered noise bursts with a bandwidth similar to the FM sweep, we are unable to state unequivocally that the former unit was “FM-specialized” (i.e., noise and tone “deaf”), or that the latter units were “tone-specialized” (FM and noise deaf), per the classification of Suga (1969).
Directional selectivity was not equally represented in the population responding to FM, as units preferred up sweeps to down sweeps by a factor of 2–3:1. The predominance of up selectivity in the Sg is noteworthy because nearly all studies examining directional selectivity at various levels of the bat auditory pathway have shown either an equal representation of upward and downward preferences or a predominance of downward preferences (Fuzessery 1994; Gordon and O’Neill, 2000; Huffman et al. 1998; Suga 1968, 1973; Suga et al. 1983; Vater 1981). The dominance of up-sweep preference in the Sg may reflect the nature of its afferent input from the ICXv, where units also overwhelmingly prefer up sweeps (83%) (Gordon and O’Neill 2000). However, it could just as well reflect input from other sources such as the ICC, where the proportion of up-selective neurons is lower but still approaches 50% of the units responding to FM (Gordon and O’Neill 2000), or from the NCAT and perhaps even the SCd, where unfortunately nothing is known about FM responsiveness. The distribution of latencies is of no particular help in resolving the source of this property, in that the shortest latencies in the Sg match, and the longest latencies exceed both the ICXv and the ICC (Table 2).
Regardless of how Sg units acquire directional preference, the proportion of directionally selective units in general, and upward selective units in particular, is clearly lower in the Sg than in the ICXv. This reduction in selectivity might result from any number of different factors, e.g., from a higher contribution of nonselective afferents projecting to the Sg from sources other than ICXv. Another possibility is that downward or bi-directional FM selectivity arises de novo in the Sg, shifting the representation away from that in the afferent population. Further experiments are needed to determine whether FM selectivity is created at the level of the Sg itself or whether it reflects interactions of afferents from lower levels that are already FM selective.
Responses to FM are attributable to an effective frequency within the sweep
Two lines of evidence support the conclusion that response to FM sweeps in most units is triggered when the sweep crosses frequencies at or near the BEF. First, in all but five units, the latency-duration functions for FM sweeps had slopes between 0.4 and 0.6. Second, BEF and FM latencies were highly correlated (r values ranging from 0.63 to 0.79, Table 3) after correcting for the temporal offset of BEF in the sweep, at least for relatively long durations and slow modulation rates. These observations suggest that BEF is the EFi for response to FM sweeps in the majority of units at low modulation rates and in a large minority at higher rates. In the remainder of the population, the FM latency relative to the BEF in the sweep was shorter than BEF latency, in some cases substantially so. A few of these units were simply responding to the onset transient in the sweep. However, many other units responded to an EFi that preceded the BEF particularly at the more rapid modulation rate of 1,000 kHz/s (Fig. 11F). This observation implies that units may respond to some other feature of the excitatory response area, e.g., the boundary of the response area, as modulation rate increases. Responses to FM sweeps have been attributed to the receptive field boundary in ICC neurons of Mexican free-tailed bats (Bodenhamer and Pollak 1981) and rats (Felsheim and Ostwald 1996), as well as in cortical neurons of cats (Heil et al. 1992a).
Facilitation by preferred sweep directions
Facilitation by preferred sweeps exceeding the response to BEF tones differentiates this sample of Sg neurons from those in the ICXv, which manifested directionality through suppression by nonpreferred FM sweeps (Gordon and O’Neill 1998, 2000). Until recordings are made in the NCAT to characterize responses to FM sweeps, it will be impossible to determine whether this amplification in the response to FM sweeps arises at the level of the Sg, as opposed to the NCAT-ICXv-Sg pathway, or the ICC. Regardless of its origin, as a rule facilitation appears to strengthen as one ascends the mustached bat auditory pathway. For example, neurons facilitated by combinations of first harmonic tones or FM sweeps and second, third, or even fourth harmonic tones or sweeps (“combination-sensitive FM-FM” neurons) are already common at the level of the ICC (Mittman and Wenstrup 1995; Portfors and Wenstrup 1999) but are not found at lower levels (Marsh and Wenstrup 2002; Portfors and Wenstrup 2001). Facilitation by FM-FM combinations is much less strong at the ICC level than at the level of either the MGB (Olsen and Suga 1991) or the auditory cortex (O’Neill and Suga 1982; Suga et al. 1983). At the cortical level, facilitation reaches a level where units respond only to combinations, and poorly or not at all to FM sweeps alone. Moreover, unlike ICXv, ICC, and Sg neurons, cortical FM-FM neurons overwhelmingly prefer downward FM sweeps, often showing little or no response to upsweeps or BEF tones (Fitzpatrick et al. 1991; Suga et al. 1983). FM-FM neurons respond best to sweeps mimicking the terminal FM components of the sonar signal, whereas ICXv and Sg neurons have BEFs matching only the CF2 component. No systematic study of directional preference for FM sweeps has yet been carried out in the cortical areas representing the CF2 frequencies. Consequently, at this time it is unclear whether the proportion of up-selective cells is maintained or altered at the cortical level.
Behavioral relevance of up-sweep selectivity
Sg neurons might be responsive to both the CF and FM components of biosonar signals during echolocation. First, most Sg neurons respond well to tones and have BEFs around 58 kHz. Consequently, they would probably respond well to the CF2 frequency in the sonar pulses emitted by a bat at rest but poorly to either the initial or terminal FM component. Second, mustached bats actively lower the frequency of the sonar signal to compensate precisely for Doppler shifts in the echoes reflected from objects moving toward the resting bat or when the bat is flying toward an object. This behavior drops the second harmonic in the emitted signal below the optimal range for Sg neurons; but in doing so, it stabilizes the echo at the best frequency of the bat’s ear 100–200 Hz above the CF2 frequency in the bat’s resting sonar signal. Consequently, Sg neurons might also respond to the CF2 in compensated echoes. Third, Sg neurons might respond to one or both FM2 components in the echo during Doppler-shift compensation but prior to stabilization. The FM components in uncompensated echoes would sweep through the response areas of most Sg neurons during this transition period. For 4-ms sweeps with amplitudes of 50–60 dB SPL (comparable to natural echoes), nearly half the population (46%) of Sg units were up selective, just under one-third (29%) were bi-directional/nonselective, and only 14% were down-selective (Table 1; Fig. 8). These statistics imply that about four of five units in Sg (i.e., up-selective and bi-directional units) would be responsive to the iFM component, but only about two of five (i.e., bi-directional and down-selective units) would be responsive to the tFM component. The combination of up-selective and bi-directional units could in theory mark the onset of the echo with phasic discharges triggered by the iFM component.
However, the overwhelming bias of Sg units toward the iFM component in such echoes is difficult to reconcile. Laboratory recordings of mustached bat sonar signals in past studies (Fitzpatrick et al. 1991; Gooler and O’Neill 1987; Henze and O’Neill 1991) showed that the bandwidth and duration of the iFM component varies from about one-fifth to one-half that of the tFM sweep, and its amplitude is always lower (cf. Fig. 1B). No systematic studies have assessed the possible function of this component in echolocation, and it has often been dismissed (in retrospect, perhaps prematurely) as an unintended byproduct of vocalization. Whatever its significance, the iFM would be strongly attenuated in the echoes from small targets, and up-selective or bi-directional Sg neurons would at best be poorly activated by such a weak and brief stimulus. By contrast, down-selective and bi-directional Sg units might respond well to the much stronger terminal FM2 component in uncompensated echoes.
There are two other possible sources of FM in the mustached bat’s acoustic biotope that would perhaps be more appropriate stimuli for Sg units. One source would be communication sounds. The mustached bat has one of the largest communication sound repertoires known in mammals, with >20 discrete utterances documented in captive colonies (Kanwal et al. 1994). Eight of these utterances contain prominent FM components, six of which contain up sweeps, some of which are longer in duration than the FM components of the sonar signal. Unfortunately, there is at present no information regarding how frequently these different vocalizations are produced either in captivity or in the wild. Nor do we know anything about their significance in a social context. Further study is necessary to determine whether the ratio of up and down FM sweeps in communication calls relates in any way to the ratio of up- to down-selective units in the Sg.
FM stimuli are also present in the echoes reflected from flying insects. Periodic FM and AM due to the Doppler effect (Schnitzler et al. 1983) are carried by such signals. In fact, modulated echoes are the only signals that elicit pursuit and prey capture in Doppler-compensating bats like Pteronotus (Goldman and Henson 1977), and Doppler-shift compensation probably evolved to permit mustached bats to utilize flutter information to hunt flying prey in cluttered environments. These periodic modulations are mainly carried by echoes of the CF2 component of the biosonar signal and are ideally situated to traverse the response areas and BEFs of Sg units. Such echoes contain alternating upward- and downward-sweeping FM components that vary in modulation rate, amplitude, bandwidth, and modulation frequency, depending on the size and wing beat rate of the fluttering target (Schuller 1984). These complex modulations contain potentially valuable information about an insect’s identity and orientation in space relative to the approaching bat (Schnitzler and Ostwald 1983). The time it takes a bat to identify, pursue, and capture a flying insect is often ≤1 s. Therefore the cognitive processes leading to a decision to capture a target must be supported by neuronal pathways optimized for processing modulations with extreme speed and accuracy. The Sg, located at the terminus of the CAT and strategically positioned to distribute information in parallel to the auditory and frontal cortices, superior colliculus, and lateral amygdala, may play an important role in this demanding process.
In conclusion, resolving the role of directionally selective units both in the Sg and in the ICXv will require further investigation of other aspects of FM processing relevant to communication sounds and fluttering target echoes.
Acknowledgments
The authors thank J. Housel for participation in the experiments, care of the animals, and technical support. We are grateful to Dr. Jagmeet Kanwal, Georgetown University, who provided the bats for this study under permit No. 1845 from the Trinidad/Tobago Ministry of Agriculture, Land, and Marine Resources. We are also indebted to the two anonymous reviewers whose insightful comments were extremely helpful in crafting the final manuscript.
This work was supported by National Institute on Deafness and Other Communication Disorders Grant 5-R01-DC-3717.
References
- Bodenhamer RD, Pollak GD. Time and frequency domain processing in the inferior colliculus of echolocating bats. Hear Res. 1981;5:317–335. doi: 10.1016/0378-5955(81)90055-1. [DOI] [PubMed] [Google Scholar]
- Bodenhamer R, Pollak GD, Marsh DS. Coding of fine frequency information by echoranging neurons in the inferior colliculus of the Mexican free-tailed bat. Brain Res. 1979;171:530–535. doi: 10.1016/0006-8993(79)91057-6. [DOI] [PubMed] [Google Scholar]
- Britt R, Starr A. Synaptic events and discharge patterns of cochlear nucleus cells. II. Frequency modulated tones. J Neurophysiol. 1976;39:179–194. doi: 10.1152/jn.1976.39.1.179. [DOI] [PubMed] [Google Scholar]
- Casseday JH, Kobler JB, Isbey SF, Covey E. Central acoustic tract in an echolocating bat: an extralemniscal auditory pathway to the thalamus. J Comp Neurol. 1989;287:247–259. doi: 10.1002/cne.902870208. [DOI] [PubMed] [Google Scholar]
- Covey E, Hall WC, Kobler JB. Subcortical connections of the superior colliculus in the mustache bat Pteronotus parnellii. J Comp Neurol. 1987;263:179–197. doi: 10.1002/cne.902630203. [DOI] [PubMed] [Google Scholar]
- Felsheim C, Ostwald J. Responses to exponential frequency modulations in the rat inferior colliculus. Hear Res. 1996;98:137–151. doi: 10.1016/0378-5955(96)00078-0. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DC, Suga N, Misawa H. Are the initial frequency-modulated components of the mustached bat’s biosonar pulses important for ranging? J Neurophysiol. 1991;66:1951–1964. doi: 10.1152/jn.1991.66.6.1951. [DOI] [PubMed] [Google Scholar]
- Fuzessery ZM. Response selectivity for multiple dimensions of frequency sweeps in the pallid bat inferior colliculus. J Neurophysiol. 1994;72:1061–1079. doi: 10.1152/jn.1994.72.3.1061. [DOI] [PubMed] [Google Scholar]
- Goldman LJ, Henson OW. Prey recognition and selection by the constant frequency bat Pteronotus p. parnellii. Behav Ecol Sociobiol. 1977;2:411–419. [Google Scholar]
- Gooler DM, O’Neill WE. Topographic representation of vocal frequency demonstrated by microstimulation of anterior cingulate cortex in the echo-locating bat Pteronotus parnelli parnelli. J Comp Physiol [A] 1987;161:283–294. doi: 10.1007/BF00615248. [DOI] [PubMed] [Google Scholar]
- Gordon M, O’Neill WE. Temporal processing across frequency channels by FM selective auditory neurons can account for FM rate selectivity. Hear Res. 1998;122:97–108. doi: 10.1016/s0378-5955(98)00087-2. [DOI] [PubMed] [Google Scholar]
- Gordon M, O’Neill WE. An extralemniscal component of the mustached bat inferior colliculus selective for direction and rate of linear frequency modulations. J Comp Neurol. 2000;426:165–181. doi: 10.1002/1096-9861(20001016)426:2<165::aid-cne1>3.0.co;2-i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil P, Irvine DRF. Functional specialization in auditory cortex: responses to frequency-modulated stimuli in the cat’s posterior auditory field. J Neurophysiol. 1998;79:3041–3059. doi: 10.1152/jn.1998.79.6.3041. [DOI] [PubMed] [Google Scholar]
- Heil P, Langner G, Scheich H. Processing of frequency-modulated stimuli in the chick auditory cortex analogue: evidence for topographic representations and possible mechanisms of rate and directional sensitivity. J Comp Physiol [A] 1992a;171:583–600. doi: 10.1007/BF00194107. [DOI] [PubMed] [Google Scholar]
- Heil P, Rajan R, Irvine DRF. Sensitivity of neurons in cat primary auditory cortex to tones and frequency-modulated stimuli. I. Effects of variation of stimulus parameters. Hear Res. 1992b;63:108–134. doi: 10.1016/0378-5955(92)90080-7. [DOI] [PubMed] [Google Scholar]
- Henze D, O’Neill WE. The emission pattern of vocalizations and directionality of the sonar system in the echolocating bat Pteronotus parnelli. J Acoust Soc Am. 1991;89:2430–2434. doi: 10.1121/1.400975. [DOI] [PubMed] [Google Scholar]
- Huffman RF, Argeles PC, Covey E. Processing of sinusoidally frequency modulated signals in the nuclei of the lateral lemniscus of the big brown bat Eptesicus fuscus. Hear Res. 1998;126:161–180. doi: 10.1016/s0378-5955(98)00165-8. [DOI] [PubMed] [Google Scholar]
- Kanwal JS, Matsumura S, Ohlemiller K, Suga N. Analysis of acoustic elements and syntax in communication sounds emitted by mustached bats. J Acoust Soc Am. 1994;96:1229–1254. doi: 10.1121/1.410273. [DOI] [PubMed] [Google Scholar]
- Marsh RA, Wenstrup JJ. Responses to combinations of tones in the cochlear nucleus of awake mustached bats. Abstr Assoc Res Otolaryngol. 2002;25:37. [Google Scholar]
- Mendelson JR, Cynader MS. Sensitivity of cat primary auditory cortex (A1) neurons to the direction and rate of frequency modulation. Brain Res. 1985;327:331–335. doi: 10.1016/0006-8993(85)91530-6. [DOI] [PubMed] [Google Scholar]
- Mittmann DH, Wenstrup JJ. Combination-sensitive neurons in the inferior colliculus. Hear Res. 1995;90:185–191. doi: 10.1016/0378-5955(95)00164-x. [DOI] [PubMed] [Google Scholar]
- Novick A, Vaisnys JR. Echolocation of flying insects by the bat Chilonycteris parnellii. Biol Bull. 1964;127:478–488. [Google Scholar]
- Olsen JF, Suga N. Combination-sensitive neurons in the medial geniculate body of the mustached bat: encoding of target range information. J Neurophysiol. 1991;65:1275–1296. doi: 10.1152/jn.1991.65.6.1275. [DOI] [PubMed] [Google Scholar]
- O’Neill WE. Responses to pure tones and linear FM components of the CF-FM biosonar signal by single units in the inferior colliculus of the mustached bat. J Comp Physiol [A] 1985;157:797–815. doi: 10.1007/BF01350077. [DOI] [PubMed] [Google Scholar]
- O’Neill WE, Suga N. Encoding of target range and its representation in the auditory cortex of the mustached bat. J Neurosci. 1982;2:17–31. doi: 10.1523/JNEUROSCI.02-01-00017.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papez JW. Central acoustic tract in cat and man. Anat Rec. 1929;42:60. [Google Scholar]
- Phillips DP, Mendelson JR, Cynader MS, Douglas RM. Responses of single neurones in cat auditory cortex to time-varying stimuli: frequency-modulated tones of narrow excursion. Exp Brain Res. 1985;58:443–454. doi: 10.1007/BF00235862. [DOI] [PubMed] [Google Scholar]
- Portfors CV, Wenstrup JJ. Delay-tuned neurons in the inferior colliculus of the mustached bat: implications for analyses of target distance. J Neurophysiol. 1999;82:1326–1338. doi: 10.1152/jn.1999.82.3.1326. [DOI] [PubMed] [Google Scholar]
- Portfors CV, Wenstrup JJ. Responses to combinations of tones in the nuclei of the lateral lemniscus. J Assoc Res Otolaryngol. 2001;2:104–117. doi: 10.1007/s101620010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon y Cajal S. Histologie du Systeme Nerveux de l’Homme et des Vertebres. Vol. 2 Paris: Maloine; 1911. [Google Scholar]
- Schnitzler HU. Echoortung bei der Fledermaus Chilonycteris rubiginosa. Z Vgl Physiol. 1970;68:25–38. [Google Scholar]
- Schnitzler HU, Menne D, Kober R, Heblich K. The acoustical image of fluttering insects in echolocating bats. In: Huber F, Markl H, editors. Neuroethology and Behavioral Physiology. Roots and Growing Points. Berlin: Springer-Verlag; 1983. pp. 235–250. [Google Scholar]
- Schnitzler HU, Ostwald J. Adaptations for the detection of fluttering insects by echolocation in horseshoe bats. In: Ewart JP, Capranica RR, Ingle DJ, editors. Advances in Vertebrate Neuroethology. New York: Plenum; 1983. pp. 801–827. [Google Scholar]
- Schuller G. Natural ultrasonic echoes from wing beating insects are encoded by collicular neurons in the CF-FM bat Rhinolophus ferrumequinum. J Comp Physiol [A] 1984;155:121–128. [Google Scholar]
- Schuller G, Radtke-Schuller S, Betz M. A stereotaxic method for small animals using experimentally determined reference profiles. J Neurosci Methods. 1986;18:339–350. doi: 10.1016/0165-0270(86)90022-1. [DOI] [PubMed] [Google Scholar]
- Simmons JA, Fenton MB, O’Farrell MJ. Echolocation and pursuit of prey by bats. Science. 1979;203:16–21. doi: 10.1126/science.758674. [DOI] [PubMed] [Google Scholar]
- Simmons JA, Stein RA. Acoustic imaging in bat sonar: echolocation signals and the evolution of echolocation. J Comp Physiol [A] 1980;135:61–84. [Google Scholar]
- Sinex DG, Geisler CD. Auditory-nerve fiber responses to frequency modulated tones. Hear Res. 1981;4:127–148. doi: 10.1016/0378-5955(81)90001-0. [DOI] [PubMed] [Google Scholar]
- Suga N. Analysis of frequency-modulated and complex sounds by single auditory neurons of the bat. J Physiol (Lond) 1968;198:51–80. doi: 10.1113/jphysiol.1968.sp008593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga N. Classification of inferior collicular neurones of bats in terms of responses to pure tones. FM sounds, and noise bursts. J Physiol (Lond) 1969;200:555–574. doi: 10.1113/jphysiol.1969.sp008708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga N. Feature extraction in the auditory system of bats. In: Møller ARR, editor. Basic Mechanisms in Hearing. New York: Academic; 1973. pp. 675–744. [Google Scholar]
- Suga N, O’Neill WE, Kujirai K, Manabe T. Specificity of “combination sensitive” neurons for processing complex biosonar signals in the auditory cortex of the mustached bat. J Neurophysiol. 1983;49:1573–1626. doi: 10.1152/jn.1983.49.6.1573. [DOI] [PubMed] [Google Scholar]
- Tian B, Rauschecker JP. Processing of frequency-modulated sounds in the cat’s anterior auditory field. J Neurophysiol. 1994;71:1959–1975. doi: 10.1152/jn.1994.71.5.1959. [DOI] [PubMed] [Google Scholar]
- Vater M. Single-unit responses to linear frequency modulations in the inferior colliculus of the greater horseshoe bat Rhinolophus ferrumequinum. J Comp Physiol [A] 1981;141:249–264. [Google Scholar]
- Wenstrup JJ. Frequency organization and responses to complex sounds in the medial geniculate body of the mustached bat. J Neurophysiol. 1999;82:2528–2544. doi: 10.1152/jn.1999.82.5.2528. [DOI] [PubMed] [Google Scholar]
- Wenstrup JJ, Larue DT, Winer JA. Projections of physiologically defined subdivisions of the inferior colliculus in the mustached bat: targets in the medial geniculate body and extrathalamic nuclei. J Comp Neurol. 1994;346:207–236. doi: 10.1002/cne.903460204. [DOI] [PubMed] [Google Scholar]
- Winer JA, Wenstrup JJ. The neurons of the medial geniculate body in the mustached bat (Pteronotus parnellii) J Comp Neurol. 1994a;346:183–206. doi: 10.1002/cne.903460203. [DOI] [PubMed] [Google Scholar]
- Winer JA, Wenstrup JJ. Cytoarchitecture of the medial geniculate body in the mustached bat (Pteronotus parnellii) J Comp Neurol. 1994b;346:161–182. doi: 10.1002/cne.903460202. [DOI] [PubMed] [Google Scholar]