Abstract
Purpose
The long-term impact of thalidomide plus dexamethasone (thal/dex) as primary therapy for newly diagnosed multiple myeloma (MM) is unknown. The goal of this study was to compare thalidomide plus dexamethasone versus placebo plus dexamethasone (placebo/dex)as primary therapy for newly diagnosed MM.
Patients and Methods
In this double-blind, placebo-controlled trial, patients with untreated symptomatic MM were randomized to thal/dex (arm A) or to placebo plus dexamethasone (dex) (arm B). Patients in arm A received oral thalidomide 50 mg daily, escalated to 100 mg on day 15, and to 200 mg from day 1 of cycle 2 (28-day cycles). Oral dex 40 mg was administered on days 1 through 4, 9 through 12, and 17 through 20 during cycles 1 through 4 and on days 1 through 4 only from cycle 5 onwards. Patients in arm B received placebo and dex, administered as in arm A. The primary end point of the study was time to progression. This study is registered at http://ClinicalTrials.gov (NCT00057564).
Results
A total of 470 patients were enrolled (235 randomly assigned to thal/dex and 235 to placebo/dex). The overall response rate was significantly higher with thal/dex compared with placebo/dex (63% v 46%), P < .001. Time to progression (TTP) was significantly longer with thal/dex compared with placebo/dex (median, 22.6 v 6.5 months, P < .001). Grade 4 adverse events were more frequent with thal/dex than with placebo/dex (30.3% v 22.8%).
Conclusion
Thal/dex results in significantly higher response rates and significantly prolongs TTP compared with dexamethasone alone in patients with newly diagnosed MM.
INTRODUCTION
Multiple myeloma (MM) accounts for approximately 10% of hematologic malignancies.1 Treatment consists of chemotherapy; the most common regimens are melphalan plus prednisone (MP), high-dose dexamethasone, and vincristine, doxorubicin, and dexamethasone (VAD).2 In some studies, high-dose therapy with autologous stem-cell transplantation (ASCT) has resulted in significantly prolonged survival compared with conventional-dose chemotherapy.3,4
Thalidomide plus dexamethasone (thal/dex) has shown encouraging results in newly diagnosed myeloma in small, uncontrolled trials.5-7 Some studies have reported the superiority of thal/dex compared with intravenous VAD based on short-term response rates.8-10 In a recent randomized trial, the Eastern Cooperative Oncology Group (ECOG) found significantly higher response rates with thal/dex compared with dexamethasone alone following 4 months of therapy (63% versus 41%).11 However, there was no data on long-term outcome measures, such as time to progression (TTP) and progression-free survival (PFS).
The goal of this clinical trial was to compare the response rate, TTP, and PFS of thal/dex versus dexamethasone alone in a randomized, placebo-controlled trial in patients with newly diagnosed MM.
PATIENTS AND METHODS
Eligibility
Patients were eligible if they had previously untreated symptomatic multiple myeloma, bone marrow plasmacytosis (≥10% plasma cells or sheets of plasma cells) or a biopsy-proven plasmacytoma, and measurable disease defined as serum monoclonal protein more than 1.0 g/dL and/or urine monoclonal protein of at least 200 mg/24 hours. Patients also needed to have a platelet count of at least 50,000 cells/mm3, absolute neutrophil count more than 1,000 cells/mm3, serum creatinine no higher than3 mg/dL, bilirubin 2 mg/dL or lower, and ALT and AST less than or equal to 3× the upper limit of normal. No prior systemic therapy, with the exception of bisphosphonates, was permitted. Patients were excluded if they had grade 2 or higher peripheral neuropathy, active infection, current or prior deep vein thrombosis, or Eastern Cooperative Oncology Group (ECOG) performance score of 3 or 4. Pregnant or nursing women were not eligible. Women of child-bearing potential who were unwilling to use a dual method of contraception and men who were unwilling to use a condom were not eligible. The study was approved by the institutional review boards in the participating institutions. Patients were enrolled between March 14, 2003, and April 11, 2005, in 77 participating institutions.
Randomization and Treatment Schedule
Patients were randomized in this double-blind placebo controlled trial to thal/dex (arm A) or placebo plus dexamethasone (placebo/dex; arm B). Random assignment was performed by a centralized telephone interactive voice response service with sequence of allocation concealed. Subjects were to be randomly assigned with a 1:1 randomization between the two treatment groups, stratified at random assignment by the following prognostic risk factors: age (< 65 years, ≥ 65 years), ECOG performance status score (0 or 1, 2), and beta-2M level (< 2.5 mg/L, ≥ 2.5 mg/L). Patients in arm A received thalidomide 50 mg orally daily, escalated to 100 mg on day 15, and to 200 mg from day 1 of cycle 2; dexamethasone 40 mg was administered orally on days 1 through 4, 9 through 12, and 17 through 20 during cycles 1 through 4 and on day 1 through 4 only beginning with cycle 5. Patients in arm B received placebo (identical to thalidomide administration) and dexamethasone was administered as in arm A. Cycles were 28 days long, and were repeated until progression or undue toxicity; there was no gap between cycles. No specific treatment recommendations were made for treatment after progression. Patients were allowed to interrupt therapy briefly for growth factor–supported stem-cell mobilization; however, patients who received nonprotocol therapy or transplantation had to go off study. Dose adjustments were permitted for toxicity. Patients who developed deep vein thrombosis (DVT) or pulmonary embolism (PE) were required to stop thalidomide therapy temporarily; patients were allowed to resume treatment after therapeutic anticoagulation (warfarin or low molecular weight heparin) was achieved with a 50% dose reduction based on experience in prior studies. Thromboprophylaxis was not mandated during this study; all patients were recommended to receive a bisphosphonate.
Response and Toxicity Criteria
The response criteria used were standard European Group for Blood and Bone Marrow Transplant (ie, EBMT or Bladé) criteria.12 Partial response (PR) was defined as at least a 50% reduction in the level of the serum monoclonal (M) protein and a reduction in 24-hour urinary M protein of at least 90% or to less than 200 mg, plus no increase in the number or size of lytic bone lesions or any other evidence of progressive disease by other parameters. Complete response (CR) required confirmed disappearance of the monoclonal protein in the serum and urine by immunofixation studies and less than 5% plasma cells on bone marrow examination. All response categories required confirmation by two consecutive measurements at least 6 weeks apart. In addition, patients were classified as having a very good partial response (VGPR) based on the International Myeloma Working Group (IMWG) response criteria.13 VGPR required either serum and urine M protein levels detectable only on immunofixation but not on electrophoresis or at least 90% reductions in serum M protein and 24-hour urine M protein to less than 100 mg/24 hours.
Disease progression required any one of the following criteria: (1) increase in serum monoclonal protein more than 25% above the lowest response level and an absolute increase of at least 5 g/L; (2) increase in urine monoclonal protein by 25% above the lowest remission value and an absolute increase in excretion by 200 mg/24 hours or greater; (3) increase in size of soft tissue plasmacytoma or appearance of a new plasmacytoma; (4) definite appearance of bone lesions or increase in the size of existing bone lesions; and (5) unexplained hypercalcemia more than 2.8 mmol/L (>11.5 mg/dL). For patients in CR, relapse included reappearance of monoclonal protein by immunofixation or protein electrophoresis of the serum or urine, or any other sign of progression. The National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 2, was used to classify and grade adverse events.
Statistical Design and Analysis
All analyses were performed on an intention-to-treat principle. The two principal investigators of the study (S.V.R. and J.B.) had complete access to the data and were responsible for the interpretation of the data and writing of the manuscript. The primary end point was TTP defined using EBMT criteria. TTP was defined as the time from random assignment to disease progression. TTP and all other measures of efficacy were calculated before initiation of any nonprotocol therapy including transplantation; any patient who received such therapy was censored. Thus the reported efficacy measures represent the sole effect of primary therapy with thal/dex or placebo/dex. PFS was defined as time from random assignment to disease progression or death resulting from any cause. Planned sample size was 218 eligible patients in each arm. The study was designed with a one-sided log-rank test (α = 0.025, allowing for one interim analysis) and 80% power to detect a 40% improvement in TTP (16.8 months in arm A v 12 months in arm B) when 282 patients progressed. A preplanned interim analysis of the primary end point and safety was performed by an independent Data Monitoring Committee (DMC). A P value less than .0015 at this interim analysis would indicate that arm A is superior to arm B based on an alpha-spending function of the O’Brien-Fleming type. At this interim analysis, the DMC recommend release of study results and the study was unblinded. The final data set was analyzed for response, progression, and survival by a response review committee blinded to treatment assignment.
Two-sided Fisher’s exact tests were used to test for difference in response rate and to assess homogeneity of baseline characteristics for categoric variables between the two arms. Two-sided F-tests from analysis of variance models with treatment as the independent variable were used to assess homogeneity of baseline characteristics between the arms for continuous variables. Survival analysis was done using the method described by Kaplan and Meier.14 Differences between survival curves were tested for statistical significance using a one-sided log-rank test. The study is registered at http://clinicaltrials.gov (NCT00057564.)
RESULTS
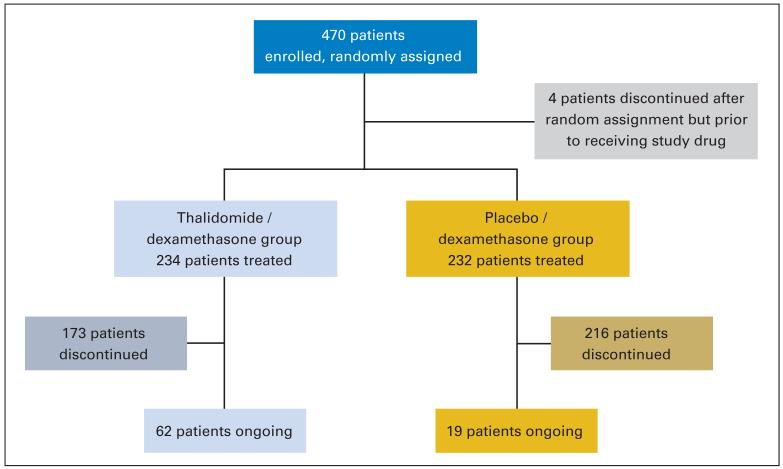
Patients were well matched between the two treatment arms; patient characteristics are listed on Table 1. Four hundred seventy patients were registered to the study. Four patients discontinued after random assignment but before receiving study drug (Fig 1). The median follow-up of the whole cohort is 18 months (95% CI, 17 to 19 months), with a median of 17 months for arm A and 18 months for arm B.
Table 1.
Characteristics of Eligible Patients
| Thalidomide Plus Dexamethasone (n = 235) |
Dexamethasone (n = 235) |
||||
|---|---|---|---|---|---|
| Characteristic | No. | % | No. | % | P |
| Age, years | .624 | ||||
| Mean | 64.0 | 64.4 | |||
| Standard deviation | 10.17 | 9.57 | |||
| Range | 39-86 | 31-84 | |||
| Sex | .927 | ||||
| Male | 118 | 50.2 | 120 | 51.1 | |
| Female | 117 | 49.8 | 115 | 48.9 | |
| Durie Salmon staging | .290 | ||||
| I/II | 78 | 33.2 | 90 | 38.3 | |
| III | 157 | 66.8 | 145 | 61.7 | |
| ECOG performance status |
.258 | ||||
| 0 | 40 | 17.0 | 54 | 23.0 | |
| 1 | 124 | 52.8 | 112 | 47.7 | |
| 2 | 70 | 68 | 28.9 | ||
| 3 | 0 | 0 | 1 | 0.4 | |
| Missing | 1 | 0.4 | 0 | 0 | |
| Type of M protein | .775 | ||||
| IgG | 179 | 76.2 | 169 | 71.9 | |
| IgA | 48 | 20.4 | 55 | 23.4 | |
| IgM | 1 | 0.4 | 1 | 0.4 | |
| Biclonal | 0 | 0 | 0 | 0 | |
| Light-chain only | 0 | 0 | 0 | 0 | |
| Missing | 7 | 3.0 | 10 | 4.3 | |
| Prior radiotherapy | .99 | ||||
| Yes | 28 | 11.9 | 29 | 12.3 | |
| No | 207 | 88.1 | 206 | 87.7 | |
| β-2 microglobulin | 200 | 85.1 | 199 | 84.7 | .922 |
| > 2.5 mg/L | |||||
| Lytic bone lesions | 185 | 78.7 | 188 | 8.0 | .909 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; Ig, immunoglobulin.
Fig 1.
Patient disposition.
The median duration of therapy was 6.9 months (range, 0.2 to 35.2 months) in the thal/dex arm and 6.4 months (range, 0 to 26.9 months) in the placebo/dex arm. The proportion of patients remaining receiving therapy longer than 1 year was 34% in the thal/dex arm and 20% in the placebo/dex arm. At 1 year, 32% of patients in the thal/dex arm continued treatment with thalidomide, and 29% with dexamethasone.
The median dose of thalidomide was 200 mg/d through cycle 27. The median dose of thalidomide decreased in subsequent cycles in patients who continued therapy as a result of dose reductions for adverse events; 75 to 100 mg cycle 28 to 31, and 50 mg in patients on therapy beyond cycle 32. Fifty-nine percent of patients in arm A had at least one dose reduction of thalidomide because of adverse effects during the course of the study compared with 40% of patients in arm B who needed at least one dose reduction in the placebo dose because of adverse effects. Primary reasons for dose reductions with thal/dex were infections (17%), muscle weakness (7%), neuropathy and related disorders (8%), and thrombosis (6%). Overall, only seven patients (1.5%) interrupted thalidomide/placebo therapy to pursue stem-cell collection.
Response to Therapy
On the basis of EBMT criteria, overall (CR + PR) response to therapy was significantly higher with thal/dex compared with placebo/dex (63% v 46%, respectively; P < .001; Table 2). A higher proportion of patients also achieved CR with thal/dex than with placebo/dex (7.7% v 2.6%; P = .02). On the basis of the IMWG criteria, the proportion of patients achieving CR or VGPR was 43.8% with thal/dex versus 15.8% with placebo/dex (P < .001).
Table 2.
Response to Therapy
| % |
|||
|---|---|---|---|
| Criteria | Thalidomide Plus Dexamethasone (n = 235) |
Dexamethasone (n = 235) |
P |
| Overall response (CR + PR) | 63 | 46 | < .001 |
| CR | 7.7 | 2.6 | |
| PR | 55.3 | 43.4 | |
| CR + VGPR (IMWG criteria) | 43.8 | 15.8 | < .001 |
Abbreviations: CR, complete response; PR, partial response; VGPR, very good PR; IMWG, International Myeloma Working Group.
Response to therapy with thal/dex was rapid, with a median time to response of 1.9 months compared with 4.6 months for placebo/dex (P < .001). Disease progression within the first 4 cycles of therapy was noted in 9.8% of patients receiving thal/dex and 14.0% of patients receiving dex alone.
Progression and Survival
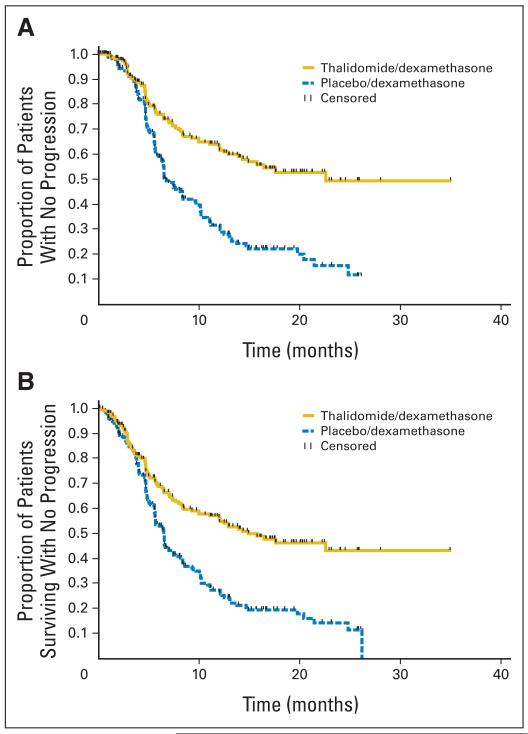
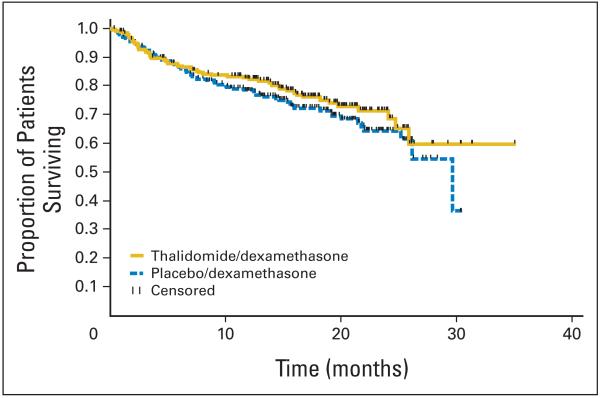
TTP was significantly longer with thal/dex compared with placebo/dex (median, 22.6 v 6.5 months, respectively; P < .001; Fig 2A). PFS was similarly better, with median times of 14.9 versus 6.5 months respectively (P < .001; Fig 2B). Overall survival curves for the two arms are provided in Figure 3; however, survival was not an end point for the study, and the study was not powered to compare differences in survival between arms. Moreover, only 26.6% of patients died on study so far (24.3% in the thal/dex arm and 28.9% in the placebo/dex arm).
Fig 2.
(A) Time to progression of patients receiving thalidomide plus dexamethasone (yellow) versus placebo plus dexamethasone (blue). Time to progression was 22.6 months for thalidomide/dexamethasone median and 6.5 months for placebo/dexamethasone (P < .001; hazard ratio [HR] = 0.43; 95% CI, 0.32 to 0.58). (B) Progression-free survival (PFS) of patients receiving thalidomide/dexamethasone (yellow) versus placebo plus dexamethasone (blue). Median PFS was 14.9 months for thalidomide/dexamethasone and 6.5 months for placebo/dexamethasone (P < .001; HR = 0.50; 95% CI, 0.38 to 0.64).
Fig 3.
Overall survival of patients receiving thalidomide and dexamethasone (yellow) versus placebo and dexamethasone (blue).
Response rates and TTP with placebo/dex were significantly inferior in patients with advanced-stage disease, as well as in patients with low beta-2 microglobulin (<5.5 mg/dL), or Durie-Salmon stage I/II disease.
Toxicity and Deaths
The most common toxicities are listed in Table 3. As expected, constipation, edema, tremor, dizziness were more commonly seen with thal/dex. In addition, grade 1 peripheral neuropathy (assessed as worst grade experienced by each patient) was noted in 32% of patients receiving thal/dex compared with 30% of patients receiving placebo/dex; grade 2 neuropathy in 19% versus 4% of patients, respectively. Table 4 lists the most common grade 3 or higher adverse events seen in the trial. Grade 3 or higher adverse events were more commonly seen with thal/dex than with dex alone (79.5% v 64.2%; P < .001). Grade 4 adverse events were similarly higher with thal/dex (30.3% v 22.8%, respectively).
Table 3.
Most Frequently Observed (> 15%) Adverse Events by Preferred Term (safety population)
| Thal/Dex (n = 234) |
Placebo/Dex (n = 232) |
|||
|---|---|---|---|---|
| Preferred Term* | No. | % | No. | % |
| Constipation | 116 | 49.6 | 49 | 21.1 |
| Peripheral edema | 80 | 34.2 | 57 | 24.6 |
| Tremor | 62 | 26.5 | 29 | 12.5 |
| Asthenia | 56 | 23.9 | 47 | 20.3 |
| Dizziness | 51 | 21.8 | 32 | 13.8 |
| Fatigue | 50 | 21.4 | 36 | 15.5 |
| Headache | 42 | 17.9 | 46 | 19.8 |
| Insomnia | 41 | 17.5 | 63 | 27.2 |
| Anemia NOS | 38 | 16.2 | 30 | 12.9 |
| Pyrexia | 37 | 15.8 | 42 | 18.1 |
| Hyperglycemia NOS | 36 | 15.4 | 32 | 13.8 |
| Weight increased | 35 | 15.0 | 42 | 18.1 |
| Pneumonia NOS | 35 | 15.0 | 28 | 12.1 |
Abbreviations: thal, thalidomide; dex, dexamethasone; NOS, not other wise specified.
Preferred terms are listed in descending order of frequency for the thal/dex column. Multiple occurrences of the same preferred term are counted only once per subject. Preferred terms were coded using MedDRA version 5.1.
Table 4.
Most Frequently Observed (> 3%) Grade 3 and 4 Adverse Events by Preferred Term (safety population)
| Thal/Dex (n = 234) |
Placebo/Dex (n = 232) |
|||
|---|---|---|---|---|
| Preferred Term* | No. | % | No. | % |
| Deep vein thrombosis | 27 | 11.5 | 4 | 1.7 |
| Pneumonia NOS | 17 | 7.3 | 14 | 6.0 |
| Pulmonary embolism | 16 | 6.8 | 4 | 1.7 |
| Hyperglycemia NOS | 14 | 6.0 | 12 | 5.2 |
| Anemia NOS | 14 | 6.0 | 7 | 3.0 |
| Fatigue | 12 | 5.1 | 10 | 4.3 |
| Asthenia | 11 | 4.7 | 4 | 1.7 |
| Atrial fibrillation | 11 | 4.7 | 8 | 3.4 |
| Muscle weakness NOS | 10 | 4.3 | 10 | 4.3 |
| Syncope | 8 | 3.4 | 1 | 0.4 |
| Peripheral neuropathy NOS | 8 | 3.4 | 0 | 0 |
| Weight increased | 8 | 3.4 | 4 | 1.7 |
| Neutropenia | 8 | 3.4 | 6 | 2.6 |
NOTE. Includes adverse events graded according to NCI-CTC and adverse events not defined in the NCI-CTC that were assigned grades 1 (mild), 2 (moderate), or 3 (severe).
Abbreviations: thal, thalidomide; dex, dexamethasone; NOS, not otherwise specified; NCI-CTC, National Cancer Institute Common Toxicity Criteria version 2.
Multiple occurrences of the same preferred term are counted only once per patient for the event with the highest toxicity grade. Preferred terms are coded using MedDRA version 5.1.
More patients discontinued therapy because of toxicity with thal/dex including pulmonary embolism (3%), neuropathy (3%), and infections (3%). Conversely, progressive disease was the most frequent cause for discontinuation of therapy with dexamethasone (Table A1, online only). There was no increased mortality with thal/dex and no evidence of increased risk of induction-related mortality: 19 and 18 deaths occurred in the thal/dex arm and dex arms, respectively, within the first four treatment cycles. The majority of deaths occurred because of disease progression (four with thal/dex, seven with dexamethasone), infection (pneumonia [n = 2], meningitis, and encephalitis; zero with thal/dex and four with dex), and DVT and/or PE (four with thal/dex and 0 with dexamethasone).
DVT was significantly more frequent with thal/dex compared with placebo/dex: 18.8% versus 5.6%, respectively. In the thal/dex arm, most DVTs (98%) occurred within the first 6 months of therapy. Incidence of DVT during the first four treatment cycles was 15.0% and 3.9% in the thal/dex and placebo/dex arms, respectively. The overall response rate to thal/dex was the same regardless of DVT occurrence; 68.2% in patients who had a DVT compared 61.8% in those without DVT. Most patients did not receive DVT prophylaxis; only 34% (thal/dex) and 29% (placebo/dex) of patients had some recorded use of aspirin during the defined time period. Eight of 44 thal/dex patients with a history of DVT had a second event, and two of 13 placebo/dex patients had a second thrombotic event. Forty-one of 234 patients in the thal/dex arm, and 38 of 232 patients in the placebo/dex arm received erythropoietic agents. The DVT rate in the thal/dex arm was significantly higher with use of erythropoietic agents (24.4% v 17.6%; P < .001); no difference was seen in the placebo/dex arm (5.3% v 5.7%, respectively).
DISCUSSION
Thalidomide first showed antimyeloma activity in a trial conducted in patients with advanced relapsed refractory myeloma.15,16 Several trials have since confirmed that thalidomide produces a response rate ranging from 25% to 35% when used as a single-agent in patients with relapsed refractory myeloma.17,18 Weber et al19 found that patients who had previously experienced treatment failure with thalidomide and dexamethasone individually respond again when the two drugs are combined. This observation led to several phase II clinical trials with thal/dex in relapsed multiple myeloma,20,21 as well as newly diagnosed disease.5-7 A subsequent ECOG randomized trial showed better responses with thal/dex compared with dexamethasone. However, there are no outcome data with long-term thal/dex therapy.
The present trial was designed in parallel with the ECOG trial to answer two separate questions concerning the efficacy of thal/dex: short-term (4 month) response rate in the ECOG trial, and long-term end point of TTP as primary end point in the present study primarily targeting patients unable or unwilling to undergo early ASCT.
Responses were observed in excess of 60% of patients with thal/dex. More importantly, the present study demonstrates that the depth of response is significantly higher with thal/dex; 43.8% of patients achieved CR or VGPR with thal/dex compared with only 15.8% with placebo/dex. Achievement of CR and VGPR are the best predictors of long-term outcome in myeloma.22,23
This trial provides the first comparative data on TTP and PFS with thal/dex in myeloma. As clearly shown, the significant improvement in response rates achieved with thal/dex does translate into longer TTP and PFS. The TTP seen with thal/dex of 22.6 months in this trial is somewhat lower than what is seen with ASCT (29 months).22 The PFS seen with dexamethasone is short (6.5 months), illustrating the major limitations of high-dose pulse dexamethasone as a single agent; previous studies have estimated a longer PFS of 13 months.24 The study excluded patients with serum creatinine more than 3 mg/dL, and hence the results cannot be extrapolated to such patients.
As expected, there is an increased risk of serious adverse events with thal/dex compared with placebo/dex. Early deaths caused by infections were low in both arms. The trial was initiated at a time when the high thrombosis risk with thal/dex,5,25 and the efficacy of thromboprophylaxis were not yet well established. Thus, patients in this trial did not receive routine thromboprophylaxis. A recent report by Palumbo et al26 provides strong evidence that routine thromboprophylaxis reduces the risk of DVT/PE when thalidomide is used in combination therapy for newly diagnosed multiple myeloma. This study, and recent reports with the thalidomide analog lenalidomide suggest that avoiding erythropoietic agents may decrease the incidence of DVT.27
The results of this study must be considered in the context of other advances occurring in the treatment of myeloma. The combination of melphalan, prednisone, and thalidomide (MPT) is emerging as the standard of care for patients who are not candidates for ASCT.26,28 MPT is preferable to thal/dex in patients who are not candidates for ASCT. On the other hand, patients who are candidates for ASCT need to avoid melphalan exposure, and thal/dex is an excellent oral regimen for such patients. Studies show that therapy with thal/dex does not significantly affect stem-cell yield.5,11 Some of the significant toxicity issues associated with thalidomide are also reduced when the drug is used for shorter periods (ie, for four cycles). As expected, in the absence of routine thromboprophylaxis the incidence of DVT/PE within four cycles was higher with thal/dex than with placebo/dex, and the rate of grade 3 peripheral neuropathy was 5.1%.
Other newly diagnosed regimens being tested need to be compared prospectively with thal/dex.29-32 In a phase II trial, lenalidomide in combination with dexamethasone has shown higher response rates than reported in the present study.33,34 However, lenalidomide seems to confer a similar risk for DVT.27 Lenalidomide is also associated with a greater incidence of hematologic adverse events.35 No comparative study has yet been conducted to our knowledge to evaluate the relative efficacy of the two drugs.
One limitation of the present trial was that overall survival comparisons were not possible because the trial was not powered to address the question, mainly because it was believed that a significant number of patients would go on to ASCT in disproportionate levels between the two arms, and at differing time points. Moreover, at the time of the final analysis, fewer than 27% of patients had died, making survival comparisons not possible.
This large phase III study provides precise estimates of the various categories of response, TTP, PFS, and rates of specific adverse events that are seen with thal/dex and dexamethasone in patients with newly diagnosed disease. Thal/dex should be considered a standard front-line regimen for the treatment of patients with myeloma who are candidates for ASCT. Future studies in MM should test new combinations, and explore the role of ASCT in the era of new drugs.36
Supplementary Material
Acknowledgments
Supported by Celgene Corporation, Summit, NJ; Pharmion Ltd, Windsor, United Kingdom; Public Health Service Grants No. CA93842, CA 107476, CA 62242, and CA 100080 (S.V.R.) from the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services; and Spanish Grant No. RD 06/0020/(L.R., J.B.).
Footnotes
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO’s conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Lela Lucy, Pharmion (C); Marta Olesnyckyj, Celgene (C); Zhinuan Yu, Celgene (C); Robert Knight, Celgene (C); Jerome B. Zeldis, Celgene (C) Consultant or Advisory Role: None Stock Ownership: Lela Lucy, Pharmion; Marta Olesnyckyj, Celgene; Zhinuan Yu, Celgene; Robert Knight, Celgene; Jerome B. Zeldis, Celgene Honoraria: Mohamad Hussein, Celgene Research Funding: S. Vincent Rajkumar, Celgene; Laura Rosinol, Celgene; Mohamad Hussein, Celgene; John Catalano, Celgene; Wieslaw Jedrzejczak, Celgene; Joan Bladé, Celgene Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: S. Vincent Rajkumar, Robert Knight, Joan Bladé
Financial support: Marta Olesnyckyj, Robert Knight, Jerome B. Zeldis
Administrative support: Marta Olesnyckyj, Robert Knight, Jerome B. Zeldis
Provision of study materials or patients: S. Vincent Rajkumar, Laura Rosinol, Mohamad Hussein, John Catalano, Wieslaw Jedrzejczak, Joan Bladé
Data analysis and interpretation: S. Vincent Rajkumar, Laura Rosinol, Lela Lucy, Zhinuan Yu, Joan Bladé
Manuscript writing: S. Vincent Rajkumar, Joan Bladé
Final approval of manuscript: S. Vincent Rajkumar, Laura Rosinol, Mohamad Hussein, John Catalano, Wieslaw Jedrzejczak, Lela Lucy, Marta Olesnyckyj, Zhinuan Yu, Robert Knight, Jerome B. Zeldis, Joan Bladé
The Appendix is included in the full-text version of this article, available online at www.jco.org. It is not included in the PDF version (via Adobe® Reader®).
REFERENCES
- 1.Sirohi B, Powles R. Multiple myeloma. Lancet. 2004;363:875–887. doi: 10.1016/S0140-6736(04)15736-X. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–1873. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 3.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 4.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 5.Rajkumar SV, Hayman S, Gertz MA, et al. Combination therapy with thalidomide plus dexamethasone for newly diagnosed myeloma. J Clin Oncol. 2002;20:4319–4323. doi: 10.1200/JCO.2002.02.116. [DOI] [PubMed] [Google Scholar]
- 6.Weber D, Rankin K, Gavino M, et al. Thalidomide alone or with dexamethasone for previously untreated multiple myeloma. J Clin Oncol. 2003;21:16–19. doi: 10.1200/JCO.2003.03.139. [DOI] [PubMed] [Google Scholar]
- 7.Cavo M, Zamagni E, Tosi P, et al. First-line therapy with thalidomide and dexamethasone in preparation for autologous stem cell transplantation for multiple myeloma. Haematologia (Budap) 2004;89:826–831. [PubMed] [Google Scholar]
- 8.Cavo M, Zamagni E, Tosi P, et al. Superiority of thalidomide and dexamethasone over vincristine-doxorubicin-dexamethasone (VAD) as primary therapy in preparation for autologous transplantation for multiple myeloma. Blood. 2005;106:35–39. doi: 10.1182/blood-2005-02-0522. [DOI] [PubMed] [Google Scholar]
- 9.Macro M, Divine M, Uzunhan Y, et al. Dexamethasone+thalidomide (dex/thal) compared to VAD as a pre-transplant treatment in newly diagnosed multiple myeloma (MM): A randomized trial. Blood. 2006;108:22a. abstr 57. [Google Scholar]
- 10.Jimenez-Zepeda V, Dominguez-Martinez V. Vincristine, doxorubicin, and dexamethasone or thalidomide plus dexamethasone for newly diagnosed patients with multiple myeloma? Eur J Haematol. 2006;77:239–244. doi: 10.1111/j.1600-0609.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 11.Rajkumar SV, Blood E, Vesole D, et al. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: A clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24:431–436. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- 12.Bladé J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation: Myeloma Subcommittee of the EBMT—European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 13.Durie BG, Harousseau J, Miguel J, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 15.Singhal S, Mehta J, Deskian R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 16.Barlogie B, Desikan R, Eddlemon P, et al. Extended survival in advanced and refractory multiple myeloma after single-agent thalidomide: Identification of prognostic factors in a phase 2 study of 169 patients. Blood. 2001;98:492–494. doi: 10.1182/blood.v98.2.492. [DOI] [PubMed] [Google Scholar]
- 17.Kumar S, Gertz MA, Dispenzieri A, et al. Response rate, durability of response, and survival after thalidomide therapy for relapsed multiple myeloma. Mayo Clin Proc. 2003;78:34–39. doi: 10.4065/78.1.34. [DOI] [PubMed] [Google Scholar]
- 18.Richardson P, Schlossman R, Jagannath S, et al. Thalidomide for patients with relapsed multiple myeloma after high-dose chemotherapy and stem cell transplantation: Results of an open-label multicenter phase 2 study of efficacy, toxicity, and biological activity. Mayo Clin Proc. 2004;79:875–882. doi: 10.4065/79.7.875. [DOI] [PubMed] [Google Scholar]
- 19.Weber DM, Rankin K, Gavino M, et al. Thalidomide with dexamethasone for resistant multiple myeloma. Blood. 2000;96:167a. abstr 719. [Google Scholar]
- 20.Anagnostopoulos A, Weber D, Rankin K, et al. Thalidomide and dexamethasone for resistant multiple myeloma. Br J Haematol. 2003;121:768–771. doi: 10.1046/j.1365-2141.2003.04345.x. [DOI] [PubMed] [Google Scholar]
- 21.Palumbo A. Low-dose thalidomide plus dexamethasone is an effective salvage therapy for advanced myeloma. Haematologica. 2001;86:399–403. [PubMed] [Google Scholar]
- 22.Attal M, Harousseau J-L, Facon T, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–2502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- 23.Harousseau J-L, Attal M, Moreau P, et al. The prognostic impact of complete remission (CR) plus very good partial remission (VGPR) in a double-transplantation program for newly diagnosed multiple myeloma (MM): Combined results of the IFM 99 trials. Blood. 2006;108:A3077. [Google Scholar]
- 24.Facon T, Mary J-Y, Pegourie B, et al. Dexamethasone-based regimens versus melphalan-prednisone for elderly multiple myeloma patients ineligible for high-dose therapy. Blood. 2006;107:1292–1298. doi: 10.1182/blood-2005-04-1588. [DOI] [PubMed] [Google Scholar]
- 25.Osman K, Comenzo RL, Rajkumar SV. Deep venous thrombosis and thalidomide therapy for multiple myeloma. N Engl J Med. 2001;344:1951–1952. doi: 10.1056/NEJM200106213442516. [DOI] [PubMed] [Google Scholar]
- 26.Palumbo A, Bringhen S, Caravita T, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: Randomized controlled trial. Lancet. 2006;367:825–831. doi: 10.1016/S0140-6736(06)68338-4. [DOI] [PubMed] [Google Scholar]
- 27.Knight R, DeLap RJ, Zeldis JB. Lenalidomide and venous thrombosis in multiple myeloma. N Engl J Med. 2006;354:2079–2080. doi: 10.1056/NEJMc053530. [DOI] [PubMed] [Google Scholar]
- 28.Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): A randomised trial. Lancet. 2007;370:1209–1218. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 29.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 30.Richardson PG, Sonneveld P, Schuster MW, et al. Assessment of proteasome inhibition for extending remissions, I: Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 31.Jagannath S, Durie BG, Wolf J, et al. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol. 2005;129:776–783. doi: 10.1111/j.1365-2141.2005.05540.x. [DOI] [PubMed] [Google Scholar]
- 32.Harousseau J, Attal M, Leleu X, et al. Bortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: Results of an IFM phase II study. Haematologica. 2006;91:1498–1505. [PubMed] [Google Scholar]
- 33.Richardson PG, Schlossman RL, Weller E, et al. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood. 2002;100:3063–3067. doi: 10.1182/blood-2002-03-0996. [DOI] [PubMed] [Google Scholar]
- 34.Rajkumar SV, Hayman SR, Lacy MQ, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/dex) for newly diagnosed myeloma. Blood. 2005;106:4050–4053. doi: 10.1182/blood-2005-07-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartlett JB, Tozer A, Stirling D, et al. Recent clinical studies of the immunomodulatory drug (IMiD) lenalidomide. Br J Cancer. 2005;93:613–619. doi: 10.1038/sj.bjc.6602774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruno B, Rotta M, Patriarca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007;356:1110–1120. doi: 10.1056/NEJMoa065464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.