Abstract
During the past decade, evolutionarily conserved non-coding (nc) RNAs, specifically microRNAs (miRNA), have been characterized as regulators of almost every cellular process and signalling pathway. There is now emerging evidence that this new class of regulators also impinges on the DNA damage response (DDR). Both miRNAs and other small ncRNAs are induced at DNA breaks and mediate the repair process. These intriguing observations raise the possibility that crosstalk between ncRNAs and the DDR might provide a means of efficient and accurate DNA repair and facilitate the maintenance of genomic stability.
Introduction
Environmental and endogenous sources constantly trigger DNA damage and, to survive this, cells must rapidly sense and respond to the DNA break. Proliferating cells also have to stop dividing by engaging cell cycle checkpoints. The DDR allows the DNA lesion to be repaired and, if breaks are irreparable, programmed cell death will be induced1. Efficient repair of double-stranded DNA breaks (DSB) is particularly crucial, as it is believed that a single unrepaired DSB is detrimental for cell health2, 3. Two major mechanistically distinct pathways, homologous recombination (HR) and non-homologous end joining (NHEJ) have evolved to deal with DSBs and are regulated by key components that are conserved from yeast to mammals (Box 1)1, 4. These pathways differ in their DNA template requirements, kinetics and the fidelity of the repair process. HR requires an undamaged homologous DNA template to replace an adjacent damaged DNA strand with high fidelity5. In contrast, the untemplated NHEJ pathway is relatively error-prone as it rapidly processes and joins the broken DNA ends6. Although DSB repair is largely constitutive, the relative contribution of the two DSB repair pathways differs in the different cell types, and in different phases of the cell cycle4. NHEJ is favoured in the pre-replicative (G0/G1) phase whereas HR dominates in the replicative (S) phase. Increasing evidence indicates that the microenvironment of a DSB is critical for the choice of repair pathway (Box 1).
BOX1 – The cellular response to DNA double-strand break (DSB).
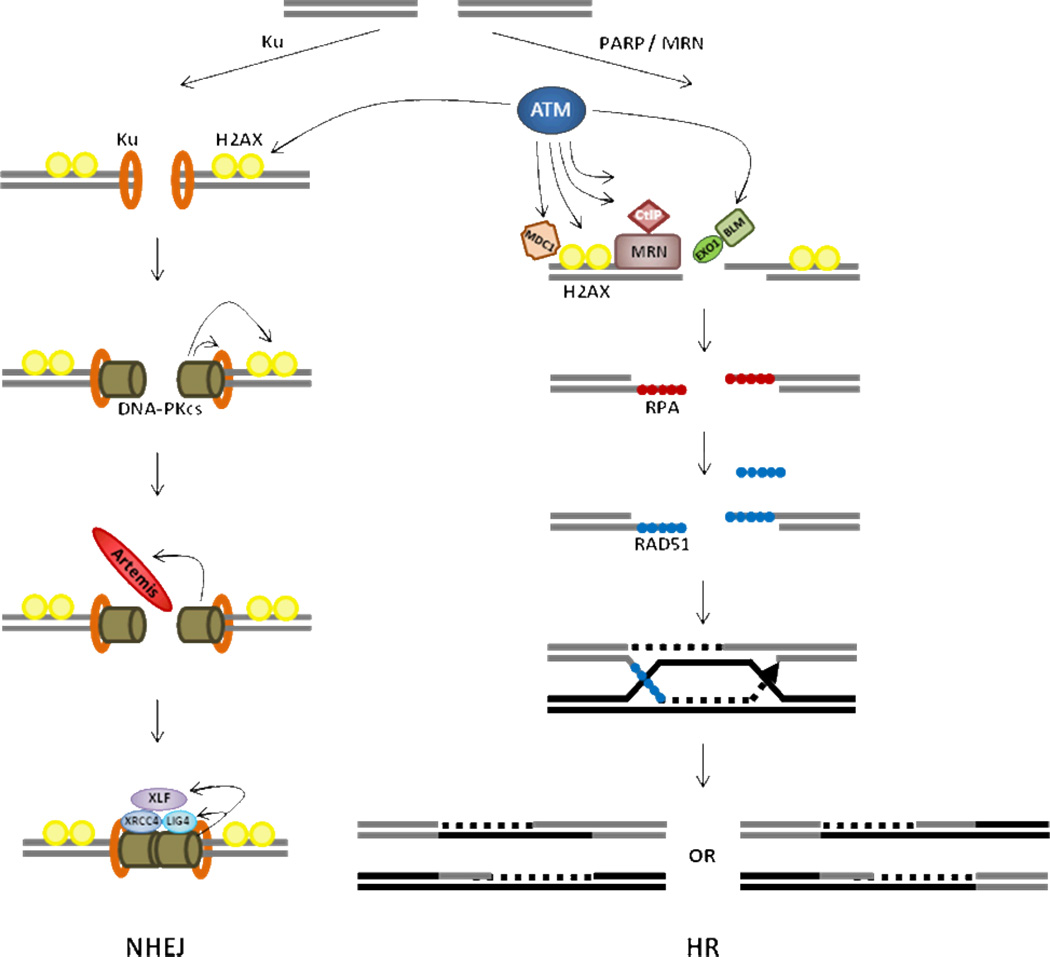
DSBs are produced by various types of genotoxins including ionizing radiation, UV light, reactive oxygen species (ROS), and chemicals and replication fork collapse. Mammalian cells repair DSBs mainly by two DNA repair mechanisms, which are homologous recombination (HR) and non-homologous end joining (NHEJ) [reviewed in Chapman et al, 2012 and Ciccia and Elledge 2010]. Depending on the need for DNA end resection at the damage site, either HR or NHEJ is activated to repair the damage. DSBs are detected by sensor complexes, Mre11-Rad50-Nbs1 (MRN), Ku70/80, and Poly (ADP-ribose) polymerase (PARP). When DNA broken ends can be directly rejoined by NHEJ, Ku70/80 heterodimer is loaded on DSB ends and recruits DNA-PKcs. DNA-PKcs regulates DSB ends stabilization through phosphorylation of ARTEMIS and other substrates. ARTEMIS facilitates end processing and, subsequently, LIG4/XRCC4/XLF ligate the broken ends. Typically replication stress induced DNA lesions are recognized by the MRN complex and the signals are transmitted to the mediators, such as ATM and ATR. The mediators rapidly phosphorylate multiple DNA repair factors including H2AX, CtIP, BRCA1, EXO1 etc. Endonucleolytic cleavage by Mre11 at DSBs allows resection mediated by CtIP and EXO1 in the presence of BRCA1 and BLM. H2AX phosphorylation (γH2AX) spreads around the damage site stabilizing the DNA repair complex. The ssDNA generated by resection is rapidly coated by RPA, and subsequently replaced by RAD51 in the presence of BRCA2. RAD51 nucleofilaments invade the sister chromatid to look for homology and the fidelity of this search is maintained by anti-recombinases (PARI, Srs2 etc). The invading strand is extended by DNA polymerase and ligates to form D loop structures. The final product of the HR-mediated repair is determined by the resolution of the D-loops by anti-recombinases (RTEL1) or resolvases (Mus81/Eme1, Yen1 etc).
DSB Repair Pathway Choice
Cell cycle dependent expression of the key repair proteins could be a mode of regulation, as cellular levels of several HR specific factors like BRCA1, RAD51 and RAD52 increase as cells progress from G1 to S-phase94. Conversely, the absence of certain factors also affects the choice; for example, Ku70 and DNA-PKc deficient ES cells show a sharp increase in HR-mediated repair95. In chicken cells RAD18 and PARP-1 suppress the access of NHEJ to DSBs and facilitates HR96. Resection at the DSB initiates the process of HR, and is also critical for impeding NHEJ. H2AX inhibits CtIP-mediated resection in G1-cells to facilitate NHEJ84. In the same vein, BRCA1 promotes resection and excludes 53BP1 from the DSB site to allow HR85, 86. These results highlight the complexity of the DNA damage response (DDR) and suggest that the microenvironment around a DSB is important for pathway choice.
Only ~2% of our genome accounts for protein-coding genes, but only in the past decade there has been significant advancement in understanding the function and relevance of the rest of the genome7. Now there is convincing evidence that the ‘junk DNA’ produces non-coding transcripts that are critical for maintenance of cell health, and participate in all major cellular processes8. Several classes of ncRNA have been identified, each of which differs in their origin, biogenesis and mode of action (Box 2)9–22. The most widely studied class are microRNAs (miRNAs), are small endogenous ncRNAs that function as post-transcriptional regulators of gene expression23–25. It is predicted that more than 60% of protein-coding mRNAs are directly targeted by miRNAs26. The biogenesis of mature miRNAs from longer primary transcripts involve an intricate pathway that includes cleavage by the RNase III enzymes DROSHA in the nucleus and DICER in the cytoplasm to form a dsRNA, 20–25 nts in length27, 28. Ultimately the mature miRNA is incorporated into the RNA-induced silencing complex (RISC) that includes the Argonaute (AGO) proteins and mediates the interaction of the miRNA with its target transcript (for a comprehensive review, see refs29–31).
BOX 2 Non-coding RNA.
Several classes of non-coding RNAs (ncRNAs), arbitrarily grouped into short (<200nt) and long (>200nt) ncRNAs, have been identified so far and each class differs in their origin, biogenesis and the mode of action. We have highlighted ncRNAs that are relatively well studied and/or have been implicated in maintaining genomic stability.
miRNAs
miRNAs are short ncRNAs that are encoded in intronic regions of protein-coding genes or the intergenic regions of the genome. The primary transcripts (pri-miRNAs) are processed in the nucleus by the DROSHA microprocessor complex to generate ~70bp stem-loop precursor forms (pre-miRNA), exported to the cytoplasm by exportin-5 and further processed by DICER into mature ~22nt products. Mature miRNAs are incorporated in the RNA-induced silencing complex (RISC) which includes Argonaute (AGO) and GW182 families of protein and loaded onto target transcripts. Typically the nucleotides 2–8 from the 5’ end of the miRNA (seed region) pairs perfectly with sequences in the 3’UTR of the target mRNA, and the rest of the miRNA/mRNA interaction is not continuous. miRNAs mediate post-transcriptional gene silencing by inhibiting translation or by inducing degradation of target transcripts.
piRNAs
PIWI-interacting RNAs (piRNAs) are the largest class of small ncRNAs (26–31nt) that act as cofactors for the AGO-family protein PIWI. PIWI-piRNA complexes specifically target and silence transposable element in germline cells to maintain genomic stability. PiRNAs possess a predominant uridine at their 5’ end and are generated in a DICER- and DROSHA-independent manner. They are transcribed from piRNA clusters, intergenic repetitive elements or transposons and processed by still unknown mechanisms. Sense piRNA/AGO3 complex can cleave antisense piRNA transcripts to generate another functional piRNA. Like miRNA, piRNA base-pairs with target mRNAs and induces their degradation, but can also mediate heterochromatin silencing and DNA methylation.
lncRNAs
The class of long (<200 nt) non-coding RNAs (lncRNAs) represents the largest group of mammalian non-coding RNA transcripts with tens of thousands different species. They are involved in a broad range of processes including epigenetic regulation, transcriptional and post-transcriptional regulation, RNA splicing and editing, telomere function, development, cancer and other diseases. lncRNAs are encoded in large integenic loci or regions overlapping protein-coding genes and they resemble mRNAs as most are capped, spliced, polyadenylated, and transcribed by RNA polymerase II. LncRNAs that are transcribed from intergenic regions constitute the specific class of long intergenic non-coding RNA (lincRNAs). Some lncRNAs are transcribed from ultraconserved regions (T-UCR); genomic elements that are completely conserved in mouse, rat and human genomes. T-UCRs are derived from intragenic or intergenic regions with a strong strand preference. Variations in T-UCR expression have been observed in several malignancies and, like miRNAs, T-UCRs are frequently associated with fragile sites and various types of cancer-associated genomic regions.
| Name | Machinery | Roles | References |
|---|---|---|---|
| miRNAs | DROSHA DICER RISC |
mRNA degradation Translational repression |
23–31 |
| piRNAs | PIWI proteins | Transposon repression | 9–12 |
| DDRNAs | DROSHA DICER |
Accumulation of 53BP1 at DSB DNA repair? |
21 |
| diRNAs | RDRs RNA pol IV DCL |
DNA repair? | 20 |
| qiRNAs | QDE-1 QDE-3 DCL1 DCL2 |
DNA repair? | 19 |
| endo-siRNAs | DCR-1 DCR-2 Loqs-D Ago1 Ago2 |
DNA repair? | 22 |
| lncRNAs | Pol II | X-chromosome inactivation Splicing |
8, 13,14 |
| lincRNAs | Pol II | Transcriptional repression Reprogramming of iPS |
15, 16 |
| T-UCRs | Pol II? | Tumorigenesis? | 17, 18 |
There is historical evidence that DSB repair proteins including 53BP132, BRCA133 and Ku7034 bind ncRNA and, more recently, RNA-binding proteins have been shown to be recruited to DSB sites35, 36 and influence the efficacy of repair. Several studies have also shown that miRNA expression is regulated by DNA lesions and in some instances directly by DDR proteins37, 38 (Fig. 1). p53 is important for DNA damage induced expression of miRNAs, and conversely miRNAs regulate cellular levels of p53 (for a comprehensive review, see refs39, 40). From the functional standpoint, it is clear that miRNAs target DNA repair factors and influence DSB repair. There is emerging evidence that other small ncRNAs are induced in response to DSBs in multiple organisms19–22. However there is very limited understanding of how these ncRNAs impacts the DDR. In this Perspective, we highlight the roles of miRNAs and other ncRNAs in the DDR. We speculate on the molecular mechanism of their role in DDR, and propose that ncRNAs directly regulate the process of DSB repair, and broadly influence the choice of DSB repair pathways.
Figure 1. Interplay of miRNAs and the DDR.
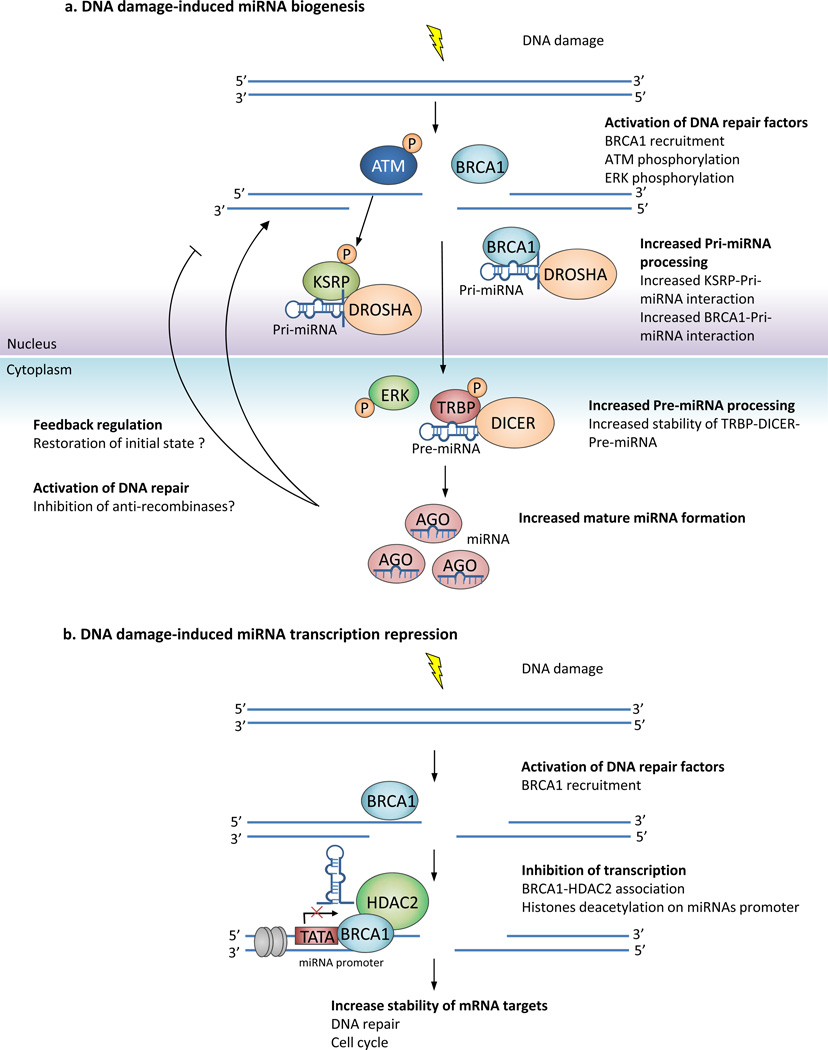
(A) DNA damage-induced miRNA biogenesis. DNA damage activates a signaling cascade which activates the processing of miRNA precursors. DNA-damage induced ATM phosphorylates KSRP and enhances its ability to recruit pri-miRNAs to DROSHA.46. BRCA1 directly interacts with both pri-miRNAs and the DROSHA complex48. Processing by the DROSHA complex allows cytoplasmic export of pre-miRNAs. The MAPK ERK is also phosphorylated after DNA damage80. ERK phosphorylates TRBP and phospho-TRBP stabilizes the TRBP-DICER complex to promote pre-miRNAs processing in the cytoplasm81. Increased levels of mature miRNAs could play a role in the DNA damage response by (i) decreasing the levels of anti-repair genes (such as the anti-recombinases, Srs2, PARI, RTEL14) (ii) down-regulate DDR proteins through a feedback regulation loop to restore pre-DNA damage levels.
(B) DNA damage-induced repression of miRNA transcription. BRCA1 associates with HDAC2 which deacetylates histone H2A and H3 on miR-155 promoter, leading to miR-155 transcriptional repression49. Transcriptional repression of miRNAs could contribute to the DDR by allowing increased expression of target proteins that are involved in DNA repair and checkpoint control.
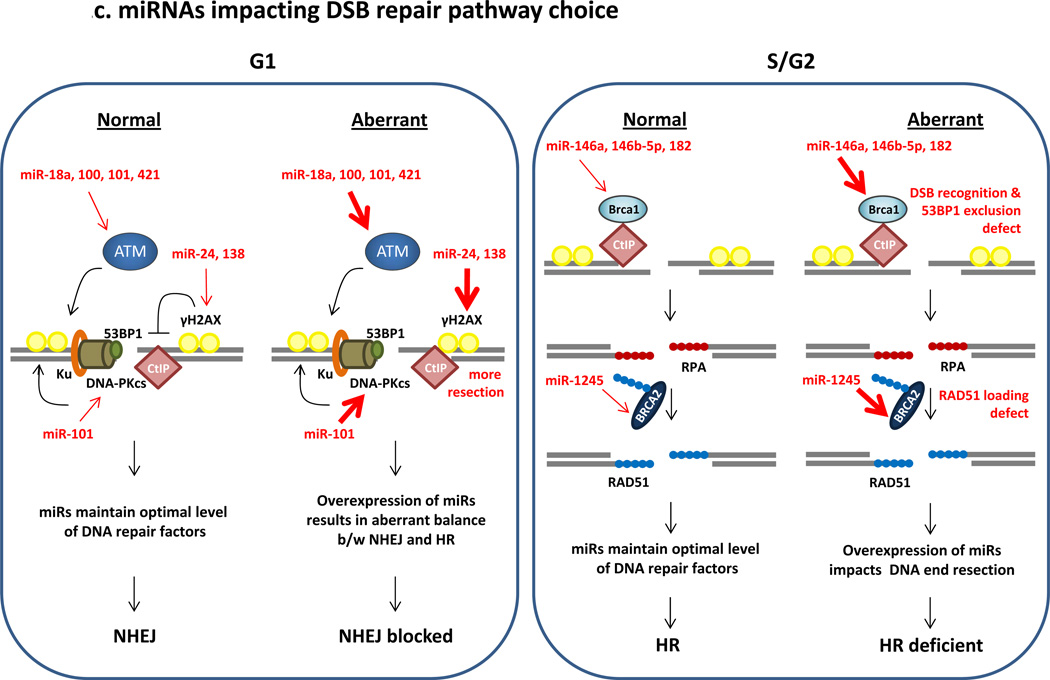
(C) miRNAs impacting DSB repair pathway choice. There is inter-play of NHEJ and HR pathways during the course of the cell cycle, and this is critical for cell health. NHEJ is known to be active throughout the cell cycle phases and HR activity is maximum in S phase and gradually decreases over G2 phase4, 82, 83. In G1, NHEJ is promoted by 53BP1 and H2AX which prevent CtIP-mediated resection of the broken end84, 85. Resection at a DSB impedes NHEJ and allows HR. In S-phase HR is active and BRCA1 is a key player in recruitment of HR proteins to DSBs, thereby excluding NHEJ factors like 53BP185, 86. CtIP promotes ends resection to allow formation of RPA-coated ssDNA at a DSB87, 88. BRCA2 is the mediator protein that is essential for replacing RPA with RAD51 and the formation of the RAD51-ssDNA nucleoprotein filament. In normal cells, miRNAs maintain optimal expression of DNA repair factors allowing efficient repair of DSBs. However, when miRNAs are aberrantly expressed it disrupts the correct choice of DSB repair pathway. For example, over expression of miRNAs targeting H2AX (indicated by bold arrow) may allow CtIP-mediated resection in G1 preventing NHEJ. HR-mediated repair in G1 is detrimental to cell health as it would lead to the loss of heterozygosity. Conversely, in S-phase over-expression of miRNAs targeting BRCA1 will impede HR and allow factors such as 53BP1 to direct the DSB to the NHEJ-mediated repair pathway, which in turn leads to higher mutation rates and chromosomal instability.
miRNA control of the DDR
MiRNAs typically mediate fine regulation of gene expression, ‘tuning’ rather than dramatically altering protein levels. However, any individual miRNA may moderately reduce the amount of multiple proteins and profoundly impact a signaling pathway or cellular process. miRNAs are now being implicated in the DDR, and it is possible that a few miRNAs induced in response to DNA damage regulate the expression of the DDR factors that are necessary for maintaining a specific cell cycle checkpoint, or are involved in a particular DSB repair pathway. Therefore, to gain insight into the cellular response to DNA damage, we need an in-depth understanding of how DNA breaks regulate expression of miRNAs, and how miRNAs influence the DDR.
miRNA induction after DNA damage
The development of miRNA microarrays, RT-PCR platforms and deep sequencing methodologies have resulted in an exponential acquisition of miRNA profiles after the induction of DNA damage from various sources including UV, ionizing radiation (IR), hydrogen peroxide and radiomimetic drugs in an assortment of cell lines38, 41. DNA damage induced change in miRNA expression varies considerably depending on the intensity/type of damaging agents, time after DNA damage, and profiling methods. High doses of damaging agents and longer delays until measurement (6 hours or more) are very likely to have secondary effects on miRNA expression that are not pertinent to the DDR. Keeping these confounding factors in perspective, changes in miRNA expression have been implicated in the initiation of the DDR. When human foreskin fibroblasts are exposed to low doses of radiation (0.1 Gy and 2 Gy of X-rays) and assayed within 30 minutes of exposure42, there is an immediate decrease in miRNAs after 2 Gy, and this corresponds with increased expression of predicted target transcripts involved in cell cycle checkpoints and DNA repair. A large subset of miRNAs, including let-7, is transiently altered by 4 hours after UV damage in human primary fibroblasts43. In prostate tumour lines, 22 miRNAs show threefold altered expression within 4 hours after IR (6 Gy)44. These include miR-521, which is significantly down-regulated by IR. MiR-521 targets the DNA repair factor, Cockayne syndrome protein A (CSA) and manipulating the cellular level of miR-521 impacts the radiosensitivity of the tumour44. In another study, radiation-induced miRNA alteration was assessed in eight patients in complete remission from AML or CML. A common set of 27 miRNAs were differentially expressed more than twofold in all the patients exposed to 1.25 Gy45. Interestingly, there was no obvious overlap between the radiation-induced miRNA expression patterns in the different studies42–45; this may suggest that radiation-induced alterations in miRNA expression are specific to cell lineage. However, in every instance, the miRNAs that respond to radiation are predicted to target factors involved in the DDR.
Direct regulation of miRNA biogenesis by DNA repair factors
The connection between miRNAs and the DDR is supported by the direct role of ATM and BRCA1 in the production of specific miRNAs (Fig. 1A and B). Seventy one miRNAs, representing ~25% of DNA damage induced miRNAs, are upregulated in mouse embryonic fibroblasts (MEFs) after treatment with the radiomimetic drug neocarzinostatin (NCS) in an ATM-dependent manner46. Importantly, ATM does not impact the transcription of these miRNAs, but specifically the processing and biogenesis of the mature miRNAs. KH-type splicing regulatory protein (KSRP), a key component of both DROSHA and DICER complexes, is a substrate of ATM. Phosphorylation of KSRP significantly enhances its activity in recruiting target primary miRNAs (pri-miRNAs) to DROSHA for processing47. By contrast, BRCA1 regulates miRNA processing directly by binding specific pri-miRNAs48. BRCA1 interacts with DROSHA and other proteins in the DROSHA microprocessor complex, facilitating the processing of BRCA1-associated pri-miRNAs. In addition to induction of miRNAs, BRCA1 has also been shown to repress the transcription of particular miRNAs. Specifically, BRCA1 represses miR-155 transcription via its association with HDAC2, which deacetylates histones H2A and H3 at the miR-155 promoter49. DNA damage can also enhance transcription of miRNAs, as DNA damage-induced NF-Kβ is recruited to the promoter of miR-21 where it cooperates with STAT3 to enhance the transcription of miR-2150.
Functional impact of miRNAs on DSB repair
miRNAs regulate the expression of DNA repair proteins and broadly affect the sensitivity of cells to DNA damage. miRNAs can target genes that are involved in the initial steps of DSB recognition (sensors/mediators), or in other cases work downstream on effector genes. This can make a significant difference to the choice of DSB repair pathway and the process of repair itself (Fig. 1C).
DSB sensors and mediators
DSB sensors detect DSBs and are immediately activated to initiate DNA repair pathways. Examples include the Mre11-Rad50-Nbs1 (MRN) complex and Ku70/80, which are crucial for pathway choice. 53BP1 has also now been added to this category4. Transcripts of the DSB sensor genes have long 3’UTRs (for example, the Nbs1 3’UTR is 2246 bases and the Ku80 3’UTR is 1101 bases) with many predicted miRNA binding sites. The high number of miRNA binding sites may suggest that these genes are post-transcriptionally regulated by miRNAs, but it is noteworthy these predictions have not been experimentally validated. Interestingly, a moderate reduction in the ‘sensor’ proteins can have significant physiological relevance. The loss of a single 53BP1 allele in mice leads to aberrant DNA repair and increased incidence of lymphoid malignancies51, 52. Furthermore a subset of human diffuse large B-cell lymphoma (DLBCL)s have a single copy loss of 53BP153. This 53BP1 dosage effect may reflect its structural role as a scaffolding protein during DSB repair, but these results strongly suggest that a miRNA-mediated ‘moderate’ decrease in 53BP1 may significantly impact DSB repair. Indeed, overexpression of miRNAs targeting 53BP1 alters the DNA damage sensitivity of cells, and influences the choice of DSB repair pathways (unpublished data, Y.C.and D.C.).
DSB mediators propagate DNA damage signals primarily via posttranslational modifications, and recruit effector proteins to DNA lesions. Multiple studies demonstrate that miRNAs regulate the mediator proteins ATM and DNA-PK. ATM is a validated target of miRNA-421, miRNA-18a, miRNA-101 and miRNA-10054–57. Overexpression of these miRNAs reduces ATM levels and correlates with aberrant DNA repair, disrupted cell cycle checkpoints, and enhanced radiosensitivity. MiRNA-101 also suppresses the catalytic subunit of DNA-PK(DNA-PKc) in various cancer models in vitro and in vivo, significantly altering the radiosensitivity of tumours56. Another mediator, ATR (a PI3-like kinase) is vital for cell survival and biallelic loss of ATR is not tolerated in any cell type. However, ATR haploinsufficiency results in significant genomic instability, hypersensitivity to genotoxins, and increased cancer predisposition58. Considering its importance for cell health, a modest change in ATR levels could have a profound effect on the DDR, and miRNAs are thus ideal candidates for ‘fine-tuning’ ATR expression.
DSB effectors
DSB effector proteins actively drive DSB repair and include MDC1, H2AX, BRCA1, BRCA2, RAD51, CHK1 and CHK2. There is convincing evidence that miRNAs target some of these effector proteins and impact the DDR. DNA repair is considered to be a ‘house keeping’ function. However, terminally differentiated cells normally down-regulate overall DNA repair and have decreased levels of repair proteins; miRNAs may contribute to this control. In an in vitro model of hematopoietic cell differentiation, miR-24 and miR-182 suppress DSB repair in terminally differentiated blood cells by targeting H2AX and BRCA1, respectively59, 60. Decreased expression of the BRCA1 gene is common in sporadic basal-like breast cancer and sporadic tumours account for more than 90% of the total breast cancer burden61. Since BRCA1-deficiency correlates with increased response to DNA damaging agents, miRNAs downregulating BRCA1 expression may significantly impact the scope of cancer therapy. MiR-182 regulates BRCA1 expression in triple-negative breast tumour lines, and manipulating miR-182 expression influences homologous recombination, and sensitivity of breast tumours in vitro and in vivo to DNA damaging agents60. This is the first example of a miRNA that specifically impacts a DSB repair pathway. BRCA1 is also a critical factor in the genesis and therapy of ovarian tumours, and miR-182 also targets BRCA1 in high-grade serous ovarian carcinoma patients62. Interestingly, miR-96 that is generated from the same polycistronic transcript as miR-182 targets another HR factor, RAD5163. Other miRNAs that target H2AX, BRCA1and BRCA2 and cause genomic instability and aberrant DNA repair include: miR-138 in osteosarcoma cells; miR-146a, miR-146b-5p and miR-1245 in breast tumour lines; and miR-1 in prostate tumours64–67.
The optimum expression of DNA repair proteins is required for efficient DSB repair and the appropriate choice of repair pathways. Although there is no formal evidence that miRNAs directly regulate the choice of homologous recombination versus non-homologous end joining (NHEJ)-mediated repair of a DSB, it is feasible that miRNAs target ‘recognition factors’ for a pathway (for example, BRCA1 for homologous recombination or 53BP1 for NHEJ) and this may significantly affect pathway choice.
Expanded role of non-coding RNAs in DDR
The link between miRNAs and the DDR is just the tip of the iceberg as the connection of other ncRNAs with this process remains largely unexplored. There is long standing evidence that short ncRNAs regulate chromatin structure and transcription in lower organisms68. As the interactions of the DNA repair machinery with chromatin is an intrinsic aspect of the repair process, it is likely that ncRNAs participate in the DDR.
Damage induction of non-coding RNAs
DNA damage induces the production of ncRNAs other than miRNAs. This was first observed in Neurospora Crassa, in which DNA lesions induce expression of the AGO protein, QDE-2 and a novel class of associated small single-strand RNA (20–21 nt), named qiRNAs19 (Fig. 2). QiRNA biogenesis in response to damage requires initial production of a precursor aberrant RNA (aRNA) from the ribosomal DNA (rDNA) locus, a process that depends on the RNA-dependent RNA polymerase QDE-1 and the Werner/Bloom RecQ DNA helicase homologue QDE-3. The aRNAs, which contain both the sense and antisense strands, are processed by DICER-like proteins to produce qiRNAs. The AGO protein, QDE-2 associates with the qiRNAs which have specific nucleotides at both the 5’(uracil) and 3’(adenosine) ends, indicating that they do not arise from non-specific degradation of rRNA. Neurospora mutants in any of these RNA processing proteins exhibit increased sensitivity to DNA damage, suggesting a role for qiRNAs in the DDR19.
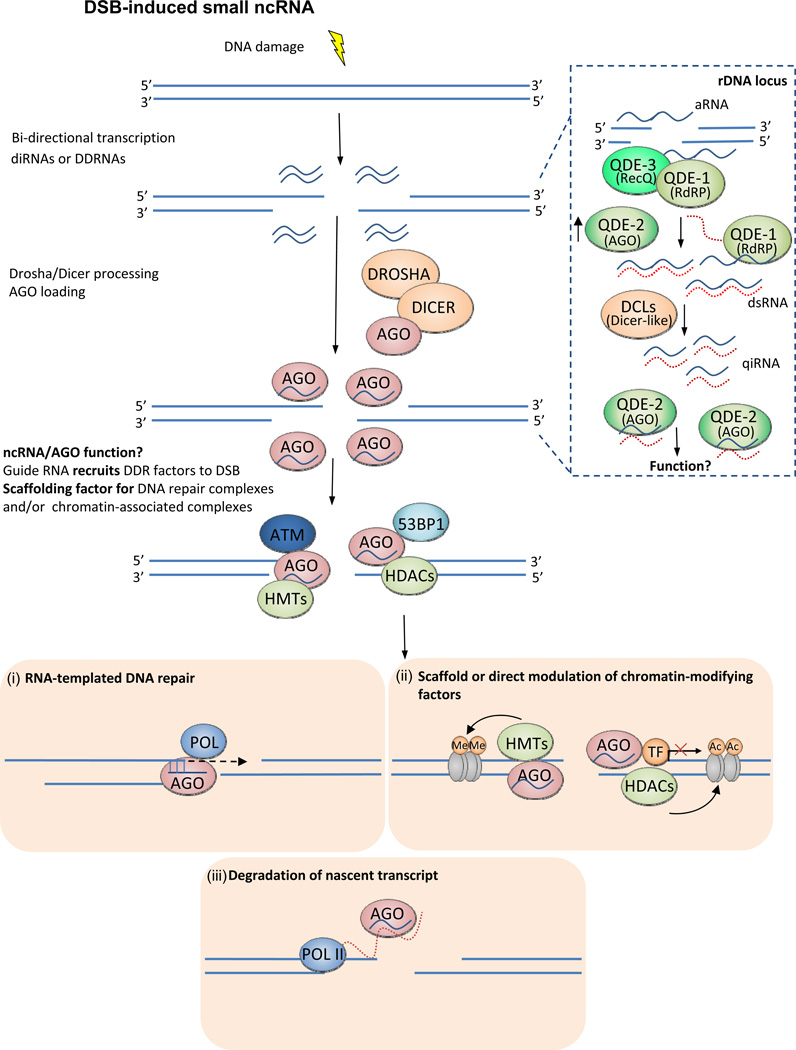
Figure 2. Other noncoding RNAs in DSB repair.
DSBs can trigger the production of short ncRNA at the site of the DNA lesion. The inset is a schematic of the DNA damage induced RdRP-dependent (QDE1) production of qiRNAs in Neurospora Crassa19. qiRNAs were identified in complex with QDE2 (AGO homolog), but their role in DDR remains unknown. In higher eukaryotes lacking RdRPs, it is postulated that antisense transcripts lead to the formation of dsRNAs that are processed by the miRNA biogenesis machinery (DROSHA/DICER). The AGO-bound ncRNAs localizes at the DSB, and potentially facilitates the recruitment of DDR factors (53BP1, p-ATM) to the DSB site. It is feasible that the homology of the ncRNA to sequences proximal to the DSB allows it to serve as a ‘guide’ for recruiting chromatin-modifying proteins/DDR factors to the DSB89. Alternatively, the ncRNA/AGO complex may also serve as a stable scaffold for maintaining the DNA repair foci and facilitating the process of repair90. After the recruitment of the DNA repair machinery to the DSB sites the precise role (if any?) of the short ncRNAs is not known. We speculate that the short ncRNAs could serve as RNA template to fill-in resected DNA during homologous recombination mediated DSB repair76. Induced RNAs might play a role in mediating chromatin silenced state at the breaks either by recruiting associated chromatin factors at the breaks91, 92, or directly by interacting and modulating chromatin associated factor in cis93. The short ncRNA could also act in the conventional siRNA pathway to degrade nascent RNA at the breaks in order to prevent deregulated expression of compromised genes.
The production of non-coding small RNA in response to DSBs is evolutionarily conserved in plants, flies and mammalian cells. Unlike qiRNAs, these small RNAs are produced from sequences near the DSBs20–22. In Arabidopsis thaliana, the biogenesis of DSB-induced small RNAs (diRNAs) requires the PI3-like kinase ATR, RNA polymerase IV (Pol IV), Dicer-like proteins and RNA-dependent RNA polymerases (RdRP)20. AGO2, a component of the RISC complex, binds mature diRNAs, and loss of AGO2 significantly reduces diRNA levels and impedes DSB repair20. However, diRNA expression does not affect RNA-directed DNA methylation or histone H2AX phosphorylation in response to damage20. Nor does it alter the levels of key DNA repair proteins, so the mechanism for its effects on DNA repair is still unclear.
Although flies and mammalian cells lack RdRPs, small dsRNAs are generated from regions close to a single DSB21, 22. In the Drosophila melanogaster S2 cell line, introduction of linearized plasmid (with a blunt end or 5’or 3’overhang) induced the production of small RNAs (~21 nts), termed endo-siRNAs22; by contrast, single strand nicks did not elicit endo-siRNAs. The endo-siRNAs are typically encoded from the transcriptionally active region upstream of the cut site and downstream of annotated transcription start sites. In mammalian cells, a chromosomally integrated reporter system has been used to introduce a single site-specific DSB and study the induction of ncRNAs20, 21. These studies show DICER/DROSHA(DD)-dependent production of small RNAs (termed DDRNAs) in response to a single DSB. The precise genomic origin and role of these small ncRNAs remains unclear. One study21 suggests that the functional form of the DDRNA is encoded proximal to the DSB site, that is, within a few hundred base pairs. In another study20, RNA sequencing data indicates that the peaks of both sense and antisense transcripts are ~5 kb downstream of the site of the DSB. However, it is not yet clear whether the small RNAs produced distal to the DSB site are involved in the DDR.
Impact of non-coding RNAs on DNA repair
There is considerable evidence that ncRNAs contribute to the maintenance of genomic stability69, 70 and there are several intriguing correlations between ncRNAs and DNA repair. For example, several DDR proteins (including 53BP132, BRCA133 and Ku7034) associate with RNA. The activity of DNA-PK can be inhibited by RNA aptamers34 and the activity of ATR is impaired by UV-induced synthesis of human telomerase RNA (hTR)71. Another ncRNA relevant to telomere biology, telomeric repeat-containing RNA (TERRA) has been involved in the maintenance of genomic integrity and stability72, 73. Several DNA repair proteins including DNA-PK, PARP1 and Bloom’s syndrome helicase (BLM) associate with TERRA74. Recently it has been suggested that TERRA functions in maintaining proper telomere function by repressing homologous recombination at the telomeres75. Further evidence that ncRNA pathways affect DNA repair has emerged from data showing that mutations or deletions in factors involved in the biogenesis of small ncRNAs, impact the efficacy of DNA repair and sensitize cells to DNA damage in plants, lower eukaryotes and mammals19–22. These studies are suggestive of a multi-faceted role for ncRNA in the DDR.
A direct role for RNA in DSB repair was first shown in Saccharomyces cerevisiae in which RNA oligonucleotides serve as templates for homologous recombination-mediated repair of a site-specific DSB76. This idea is further supported by RNA-templated DNA rearrangments that occur in another unicellular eukaryote, Oxytricha Trifalla77. Furthermore, in human and mouse cell lines, the idea of a direct role is supported by the fact that formation of 53BP1 foci in response to IR is impaired by RNAse treatment21, and that addition of total RNA can rescue this phenotype. Similarly, elegant experiments that demonstrate RNAse-mediated abrogation of 53BP1 foci from a site-specific DSB, and foci rescue by synthetic dsRNAs from close proximity (within 500 bps) of the cut site convincingly demonstrate the influence of small RNAs on DSB repair21. Interestingly, events preceding the recruitment of 53BP1, such as formation of γ-H2AX, and recognition of the DSB by the MRN complex seem to be independent of RNA21. These observations suggest that DSB recognition, and initial DDR signalling occurs prior to generation of small RNAs. It is feasible that, in organisms that lack RdRP and need to synthesize an antisense transcript, processing at the DSB site is necessary to recruit and spatially accommodate a RNA polymerase. Consistent with this, formation of DSB repair foci in mammalian cells is impeded by the addition of the RNA polymerase II inhibitor, α-amanitin21. However, transcription is also actively suppressed at DSBs by the DDR machinery78, 79, and it is not yet clear how these observations can be reconciled with this model.
In Arabidopsis thaliana and in a human tumour cell line, reporter-based assays suggest that the small RNA biogenesis pathway affects homologous recombination-mediated repair of DSBs20. This is consistent with the notion from fly studies that anti-sense transcripts at DSB sites would require resection, and would temporally fit the profile of homologous recombination22. The idea of RNA-templated DNA repair in Saccharomyces cerevisiae also supports a direct role for small RNAs in this pathway76. However, in mammalian cells, the only direct assay using synthetic small RNAs spanning the cut site was conducted using 53BP1 foci as a read-out for DSB repair21. Formation of 53BP1 foci is not a surrogate marker for a specific DSB repair pathway, and if at all 53BP1 promotes NHEJ, possibly impeding homologous recombination. Therefore it remains to be seen whether which specific repair pathway is promoted by small ncRNAs.
Concluding Remarks
The avalanche of data on ncRNAs and their putative roles in different cellular processes and signalling pathways has finally reached the field of DDR. Although our current understanding of how crosstalk occurs between the DDR and ncRNAs (including miRNAs) barely scratches the surface of a tremendously complex issue, they do provide important glimpses of its relevance. There is clear evidence that miRNAs control the DDR, although most of these studies were conducted in cancer lines with artificial manipulation of miRNA levels. A key question is whether miRNAs mediate DNA repair in ‘normal’ cells. One hypothesis is that having too much of DNA repair proteins is detrimental to the process as the stoichiometry of factors in a DSB repair pathway maybe disrupted. MiRNAs would therefore facilitate DNA repair by maintaining optimum levels of repair proteins. Consistent with this idea, miRNAs may also affect the choice of DSB repair pathways; future studies using conditional ablation of specific miRNAs in animal models should allow this to be tested.
Even less is known about the interplay between other ncRNAs and the DDR. A fundamental question is the source of these small ncRNAs, and the mechanisms that lead to their presence at DSBs in mammalian cells. The limited data suggests that anti-sense transcription at DSBs allows the formation of dsRNA precursors that are then processed by the miRNA biogenesis machinery. So are DSBs in transcriptionally dormant intergenic regions repaired without the induction of ncRNAs? If so, are ncRNAs really required for the repair of breaks in euchromatin, or are they simply by-products of the DDR? A careful kinetic assessment of ncRNA biogenesis at site-specific DSBs in intergenic loci will be very informative. The most important question that needs to be addressed is how ncRNAs function at the site of the DNA lesion. The scarce data available suggests that ncRNAs recruit DNA repair proteins to the DSB site or maintain DNA repair foci. On the basis of observations in yeast and other lower organisms, we speculate on the role of ncRNAs in DSB repair (Fig. 2). ncRNAs may serve as a template for DNA polymerase that ‘fills in’ the resected DNA and/or be involved in regulating chromatin structure at a DSB site. Another possibility is that ncRNAs in complex with AGO proteins rapidly degrade the nascent RNA synthesized from broken DNA templates and prevent aberrant expression of truncated gene products.
ACKNOWLEDGEMENTS
DC is supported by R01CA142698 (NCI), R01 AI101897-01 (NIAID), Basic Scholar Grant (ACS), Ann-Fuller Foundation and start-up funds from DFCI. MEB is supported by a fellowship from the Fonds de la Recherche en Santé du Québec (FRSQ). We apologize to our colleagues whose work we could not discuss due to space constraints.
REFERENCES
- 1.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett CB, Lewis AL, Baldwin KK, Resnick MA. Lethality induced by a single site-specific double-strand break in a dispensable yeast plasmid. Proc Natl Acad Sci U S A. 1993;90:5613–5617. doi: 10.1073/pnas.90.12.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang LC, Clarkin KC, Wahl GM. Sensitivity and selectivity of the DNA damage sensor responsible for activating p53-dependent G1 arrest. Proc Natl Acad Sci U S A. 1996;93:4827–4832. doi: 10.1073/pnas.93.10.4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman JR, Tayloy GRM, Boulton SJ. Playing the End Game: DNA Double-Strand Break Repair Pathway Choice. Mol Cell. 2012;47:495–510. doi: 10.1016/j.molcel.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 5.San Filippo J, Sung P, Klein H. Mechanism of eukaryotic homologous recombination. Annu Rev Biochem. 2008;77:229–257. doi: 10.1146/annurev.biochem.77.061306.125255. [DOI] [PubMed] [Google Scholar]
- 6.Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem. 2010;79:181–211. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander RP, Fang G, Rozowsky J, Snyder M, Gerstein MB. Annotating non-coding regions of the genome. Nat Rev Genet. 2010;11:559–571. doi: 10.1038/nrg2814. [DOI] [PubMed] [Google Scholar]
- 8.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe T, et al. Identification and characterization of two novel classes of small RNAs in the mouse germline: retrotransposon-derived siRNAs in oocytes and germline small RNAs in testes. Genes Dev. 2006;20:1732–1743. doi: 10.1101/gad.1425706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aravin AA, Sachidanandam R, Girard A, Fejes-Toth K, Hannon GJ. Developmentally regulated piRNA clusters implicate MILI in transposon control. Science. 2007;316:744–747. doi: 10.1126/science.1142612. [DOI] [PubMed] [Google Scholar]
- 11.Wang G, Reinke V. A C. elegans Piwi, PRG-1, regulates 21U-RNAs during spermatogenesis. Curr Biol. 2008;18:861–867. doi: 10.1016/j.cub.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siomi MC, Sato K, Pezic D, Aravin AA. PIWI-interacting small RNAs: the vanguard of genome defence. Nat Rev Mol Cell Biol. 2011;12:246–258. doi: 10.1038/nrm3089. [DOI] [PubMed] [Google Scholar]
- 13.Kapranov P, Willingham AT, Gingeras TR. Genome-wide transcription and the implications for genomic organization. Nat Rev Genet. 2007;8:413–423. doi: 10.1038/nrg2083. [DOI] [PubMed] [Google Scholar]
- 14.Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guttman M, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khalil AM, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A. 2009;106:11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calin GA, et al. Ultraconserved regions encoding ncRNAs are altered in human leukemias and carcinomas. Cancer Cell. 2007;12:215–229. doi: 10.1016/j.ccr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 18.Lujambio A, et al. CpG island hypermethylation-associated silencing of non-coding RNAs transcribed from ultraconserved regions in human cancer. Oncogene. 2010;29:6390–6401. doi: 10.1038/onc.2010.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HC, et al. qiRNA is a new type of small interfering RNA induced by DNA damage. Nature. 2009;459:274–277. doi: 10.1038/nature08041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei W, et al. A role for small RNAs in DNA double-strand break repair. Cell. 2012;149:101–112. doi: 10.1016/j.cell.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Francia S, et al. Site-specific DICER and DROSHA RNA products control the DNA-damage response. Nature. 2012;488:231–235. doi: 10.1038/nature11179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michalik KM, Bottcher R, Forstemann K. A small RNA response at DNA ends in Drosophila. Nucleic Acids Res. 2012 doi: 10.1093/nar/gks711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 25.Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12:99–110. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- 26.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis-Dusenbery BN, Hata A. Mechanisms of control of microRNA biogenesis. J Biochem. 2010;148:381–392. doi: 10.1093/jb/mvq096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10:94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fabian MR, Sonenberg N. The mechanics of miRNA-mediated gene silencing: a look under the hood of miRISC. Nat Struct Mol Biol. 2012;19:586–593. doi: 10.1038/nsmb.2296. [DOI] [PubMed] [Google Scholar]
- 30.Czech B, Hannon GJ. Small RNA sorting: matchmaking for Argonautes. Nat Rev Genet. 2011;12:19–31. doi: 10.1038/nrg2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pasquinelli AE. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 2012;13:271–282. doi: 10.1038/nrg3162. [DOI] [PubMed] [Google Scholar]
- 32.Pryde F, et al. 53BP1 exchanges slowly at the sites of DNA damage and appears to require RNA for its association with chromatin. J Cell Sci. 2005;118:2043–2055. doi: 10.1242/jcs.02336. [DOI] [PubMed] [Google Scholar]
- 33.Ganesan S, et al. BRCA1 supports XIST RNA concentration on the inactive X chromosome. Cell. 2002;111:393–405. doi: 10.1016/s0092-8674(02)01052-8. [DOI] [PubMed] [Google Scholar]
- 34.Yoo S, Dynan WS. Characterization of the RNA binding properties of Ku protein. Biochemistry. 1998;37:1336–1343. doi: 10.1021/bi972100w. [DOI] [PubMed] [Google Scholar]
- 35.Adamson B, Smogorzewska A, Sigoillot FD, King RW, Elledge SJ. A genome-wide homologous recombination screen identifies the RNA-binding protein RBMX as a component of the DNA-damage response. Nat Cell Biol. 2012;14:318–328. doi: 10.1038/ncb2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polo SE, et al. Regulation of DNA-end resection by hnRNPU-like proteins promotes DNA double-strand break signaling and repair. Mol Cell. 2012;45:505–516. doi: 10.1016/j.molcel.2011.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landau DA, Slack FJ. MicroRNAs in mutagenesis, genomic instability, and DNA repair. Semin Oncol. 2011;38:743–751. doi: 10.1053/j.seminoncol.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wan G, Mathur R, Hu X, Zhang X, Lu X. miRNA response to DNA damage. Trends Biochem Sci. 2011;36:478–484. doi: 10.1016/j.tibs.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hermeking H. MicroRNAs in the p53 network: micromanagement of tumour suppression. Nat Rev Cancer. 2012 doi: 10.1038/nrc3318. [DOI] [PubMed] [Google Scholar]
- 40.He L, He X, Lowe SW, Hannon GJ. microRNAs join the p53 network--another piece in the tumour-suppression puzzle. Nat Rev Cancer. 2007;7:819–822. doi: 10.1038/nrc2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu H, Gatti RA. MicroRNAs: new players in the DNA damage response. J Mol Cell Biol. 2011;3:151–158. doi: 10.1093/jmcb/mjq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maes OC, An J, Sarojini H, Wu H, Wang E. Changes in MicroRNA expression patterns in human fibroblasts after low-LET radiation. J Cell Biochem. 2008;105:824–834. doi: 10.1002/jcb.21878. [DOI] [PubMed] [Google Scholar]
- 43.Pothof J, et al. MicroRNA-mediated gene silencing modulates the UV-induced DNA-damage response. EMBO J. 2009;28:2090–2099. doi: 10.1038/emboj.2009.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Josson S, Sung SY, Lao K, Chung LW, Johnstone PA. Radiation modulation of microRNA in prostate cancer cell lines. Prostate. 2008;68:1599–1606. doi: 10.1002/pros.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Templin T, et al. Radiation-induced micro-RNA expression changes in peripheral blood cells of radiotherapy patients. Int J Radiat Oncol Biol Phys. 2011;80:549–557. doi: 10.1016/j.ijrobp.2010.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X, Wan G, Berger FG, He X, Lu X. The ATM kinase induces microRNA biogenesis in the DNA damage response. Mol Cell. 2011;41:371–383. doi: 10.1016/j.molcel.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trabucchi M, et al. The RNA-binding protein KSRP promotes the biogenesis of a subset of microRNAs. Nature. 2009;459:1010–1014. doi: 10.1038/nature08025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawai S, Amano A. BRCA1 regulates microRNA biogenesis via the DROSHA microprocessor complex. J Cell Biol. 2012;197:201–208. doi: 10.1083/jcb.201110008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang S, et al. Tumor suppressor BRCA1 epigenetically controls oncogenic microRNA-155. Nat Med. 2011;17:1275–1282. doi: 10.1038/nm.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niu J, et al. DNA damage induces NF-kappaB-dependent microRNA-21 up-regulation and promotes breast cancer cell invasion. J Biol Chem. 2012;287:21783–21795. doi: 10.1074/jbc.M112.355495. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Ward IM, et al. 53BP1 cooperates with p53 and functions as a haploinsufficient tumor suppressor in mice. Mol Cell Biol. 2005;25:10079–10086. doi: 10.1128/MCB.25.22.10079-10086.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ward IM, Minn K, van Deursen J, Chen J. p53 Binding protein 53BP1 is required for DNA damage responses and tumor suppression in mice. Mol Cell Biol. 2003;23:2556–2563. doi: 10.1128/MCB.23.7.2556-2563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takeyama K, et al. Integrative analysis reveals 53BP1 copy loss and decreased expression in a subset of human diffuse large B-cell lymphomas. Oncogene. 2008;27:318–322. doi: 10.1038/sj.onc.1210650. [DOI] [PubMed] [Google Scholar]
- 54.Hu H, Du L, Nagabayashi G, Seeger RC, Gatti RA. ATM is down-regulated by N-Myc-regulated microRNA-421. Proc Natl Acad Sci U S A. 2010;107:1506–1511. doi: 10.1073/pnas.0907763107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bouwman P, et al. 53BP1 loss rescues BRCA1 deficiency and is associated with triple-negative and BRCA-mutated breast cancers. Nat Struct Mol Biol. 2010;17:688–695. doi: 10.1038/nsmb.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan D, et al. Targeting DNA-PKcs and ATM with miR-101 sensitizes tumors to radiation. PLoS One. 2010;5:e11397. doi: 10.1371/journal.pone.0011397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song L, et al. miR-18a impairs DNA damage response through downregulation of ataxia telangiectasia mutated (ATM) kinase. PLoS One. 2011;6:e25454. doi: 10.1371/journal.pone.0025454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fang Y, et al. ATR functions as a gene dosage-dependent tumor suppressor on a mismatch repair-deficient background. Embo J. 2004;23:3164–3174. doi: 10.1038/sj.emboj.7600315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lal A, et al. miR-24-mediated downregulation of H2AX suppresses DNA repair in terminally differentiated blood cells. Nat Struct Mol Biol. 2009;16:492–498. doi: 10.1038/nsmb.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moskwa P, et al. miR-182-mediated downregulation of BRCA1 impacts DNA repair and sensitivity to PARP inhibitors. Mol Cell. 2011;41:210–220. doi: 10.1016/j.molcel.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mueller CR, Roskelley CD. Regulation of BRCA1 expression and its relationship to sporadic breast cancer. Breast Cancer Res. 2003;5:45–52. doi: 10.1186/bcr557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Z, et al. MiR-182 overexpression in tumourigenesis of high-grade serous ovarian carcinoma. J Pathol. 2012 doi: 10.1002/path.4000. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y, Huang JW, Calses P, Kemp CJ, Taniguchi T. MiR-96 downregulates REV1 and RAD51 to promote cellular sensitivity to cisplatin and PARP inhibition. Cancer Res. 2012;72:4037–4046. doi: 10.1158/0008-5472.CAN-12-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y, et al. MicroRNA-138 modulates DNA damage response by repressing histone H2AX expression. Mol Cancer Res. 2011;9:1100–1111. doi: 10.1158/1541-7786.MCR-11-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garcia AI, et al. Down-regulation of BRCA1 expression by miR-146a and miR-146b-5p in triple negative sporadic breast cancers. EMBO Mol Med. 2011;3:279–290. doi: 10.1002/emmm.201100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hudson RS, et al. MicroRNA-1 is a candidate tumor suppressor and prognostic marker in human prostate cancer. Nucleic Acids Res. 2012;40:3689–3703. doi: 10.1093/nar/gkr1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song L, et al. Up-regulation of miR-1245 by c-myc targets BRCA2 and impairs DNA repair. J Mol Cell Biol. 2012;4:108–117. doi: 10.1093/jmcb/mjr046. [DOI] [PubMed] [Google Scholar]
- 68.Lejeune E, Allshire RC. Common ground: small RNA programming and chromatin modifications. Curr Opin Cell Biol. 2011;23:258–265. doi: 10.1016/j.ceb.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 69.van Wolfswinkel JC, Ketting RF. The role of small non-coding RNAs in genome stability and chromatin organization. J Cell Sci. 2010;123:1825–1839. doi: 10.1242/jcs.061713. [DOI] [PubMed] [Google Scholar]
- 70.Brown JD, Mitchell SE, O'Neill RJ. Making a long story short: noncoding RNAs and chromosome change. Heredity (Edinb) 2012;108:42–49. doi: 10.1038/hdy.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kedde M, et al. Telomerase-independent regulation of ATR by human telomerase RNA. J Biol Chem. 2006;281:40503–40514. doi: 10.1074/jbc.M607676200. [DOI] [PubMed] [Google Scholar]
- 72.Azzalin CM, Reichenbach P, Khoriauli L, Giulotto E, Lingner J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science. 2007;318:798–801. doi: 10.1126/science.1147182. [DOI] [PubMed] [Google Scholar]
- 73.Schoeftner S, Blasco MA. Chromatin regulation and non-coding RNAs at mammalian telomeres. Semin Cell Dev Biol. 2010;21:186–193. doi: 10.1016/j.semcdb.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 74.Deng Z, Norseen J, Wiedmer A, Riethman H, Lieberman PM. TERRA RNA binding to TRF2 facilitates heterochromatin formation and ORC recruitment at telomeres. Mol Cell. 2009;35:403–413. doi: 10.1016/j.molcel.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Flynn RL, et al. TERRA and hnRNPA1 orchestrate an RPA-to-POT1 switch on telomeric single-stranded DNA. Nature. 2011;471:532–536. doi: 10.1038/nature09772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Storici F, Bebenek K, Kunkel TA, Gordenin DA, Resnick MA. RNA-templated DNA repair. Nature. 2007;447:338–341. doi: 10.1038/nature05720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nowacki M, et al. RNA-mediated epigenetic programming of a genome-rearrangement pathway. Nature. 2008;451:153–158. doi: 10.1038/nature06452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pankotai T, Bonhomme C, Chen D, Soutoglou E. DNAPKcs-dependent arrest of RNA polymerase II transcription in the presence of DNA breaks. Nat Struct Mol Biol. 2012;19:276–282. doi: 10.1038/nsmb.2224. [DOI] [PubMed] [Google Scholar]
- 79.Shanbhag NM, Rafalska-Metcalf IU, Balane-Bolivar C, Janicki SM, Greenberg RA. ATM-dependent chromatin changes silence transcription in cis to DNA double-strand breaks. Cell. 2010;141:970–981. doi: 10.1016/j.cell.2010.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tang D, et al. ERK activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. J Biol Chem. 2002;277:12710–12717. doi: 10.1074/jbc.M111598200. [DOI] [PubMed] [Google Scholar]
- 81.Paroo Z, Ye X, Chen S, Liu Q. Phosphorylation of the human microRNA-generating complex mediates MAPK/Erk signaling. Cell. 2009;139:112–122. doi: 10.1016/j.cell.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Karanam K, Kafri R, Loewer A, Lahav G. Quantitative Live Cell Imaging Reveals a Gradual Shift between DNA Repair Mechanisms and a Maximal Use of HR in Mid S Phase. Mol Cell. 2012;47:320–329. doi: 10.1016/j.molcel.2012.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shibata A, et al. Factors determining DNA double-strand break repair pathway choice in G2 phase. Embo J. 2011;30:1079–1092. doi: 10.1038/emboj.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Helmink BA, et al. H2AX prevents CtIP-mediated DNA end resection and aberrant repair in G1-phase lymphocytes. Nature. 2011;469:245–249. doi: 10.1038/nature09585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bunting SF, et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell. 2010;141:243–254. doi: 10.1016/j.cell.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chapman JR, Sossick AJ, Boulton SJ, Jackson SP. BRCA1-associated exclusion of 53BP1 from DNA damage sites underlies temporal control of DNA repair. J Cell Sci. 2012 doi: 10.1242/jcs.105353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yun MH, Hiom K. CtIP-BRCA1 modulates the choice of DNA double-strand-break repair pathway throughout the cell cycle. Nature. 2009;459:460–463. doi: 10.1038/nature07955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Limbo O, et al. Ctp1 is a cell-cycle-regulated protein that functions with Mre11 complex to control double-strand break repair by homologous recombination. Mol Cell. 2007;28:134–146. doi: 10.1016/j.molcel.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Verdel A, et al. RNAi-mediated targeting of heterochromatin by the RITS complex. Science. 2004;303:672–676. doi: 10.1126/science.1093686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tattermusch A, Brockdorff N. A scaffold for X chromosome inactivation. Hum Genet. 2011;130:247–253. doi: 10.1007/s00439-011-1027-4. [DOI] [PubMed] [Google Scholar]
- 91.Grosshans H, Johnson T, Reinert KL, Gerstein M, Slack FJ. The temporal patterning microRNA let-7 regulates several transcription factors at the larval to adult transition in C. elegans. Dev Cell. 2005;8:321–330. doi: 10.1016/j.devcel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 92.Bao N, Lye KW, Barton MK. MicroRNA binding sites in Arabidopsis class III HD-ZIP mRNAs are required for methylation of the template chromosome. Dev Cell. 2004;7:653–662. doi: 10.1016/j.devcel.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 93.Wang X, et al. Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature. 2008;454:126–130. doi: 10.1038/nature06992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shrivastav M, De Haro LP, Nickoloff JA. Regulation of DNA double-strand break repair pathway choice. Cell Res. 2008;18:134–147. doi: 10.1038/cr.2007.111. [DOI] [PubMed] [Google Scholar]
- 95.Weinstock DM, Brunet E, Jasin M. Formation of NHEJ-derived reciprocal chromosomal translocations does not require Ku70. Nat Cell Biol. 2007;9:978–981. doi: 10.1038/ncb1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saberi A, et al. RAD18 and poly(ADP-ribose) polymerase independently suppress the access of nonhomologous end joining to double-strand breaks and facilitate homologous recombination-mediated repair. Mol Cell Biol. 2007;27:2562–2571. doi: 10.1128/MCB.01243-06. [DOI] [PMC free article] [PubMed] [Google Scholar]