Abstract
A major goal of cancer immunology is to stimulate the generation of long-lasting, tumor antigen-specific immune responses that recognize and destroy tumor cells. This article discusses advances in thermal medicine with the potential to improve cancer immunotherapy. Accumulating evidence indicates that survival benefits are accorded to individuals who achieve an increase in body temperature (i.e. fever) following infection. Furthermore, accumulating evidence indicates that physiological responses to hyperthermia impact the tumor microenvironment through temperature-sensitive check-points that regulate tumor vascular perfusion, lymphocyte trafficking, inflammatory cytokine expression, tumor metabolism, and innate and adaptive immune function. Nevertheless, the influence of thermal stimuli on the immune system, particularly the antitum or immune response, remains incompletely understood. In fact, temperature is still rarely considered as a critical variable in experimental immunology. We suggest that more attention should be directed to the role of temperature in the regulation of the immune response and that thermal therapy should be tested in conjunction with immunotherapy as a multi-functional adjuvant that modulates the dynamics of the tumor microenvironment.
Those who cannot be cured by medicine can be cured by surgery.
Those who cannot be cured by surgery can be cured by heat.
Those who cannot be cured by heat are to be considered incurable.
Hippocrates, c. 460–370 BC
A Warm-up to the Field of Thermal Medicine
Tumor immunologists often refer to the work of Dr. William Coley over 100 years ago as representing the dawn of modern efforts to stimulate the immune system to combat tumors. Coley witnessed a significant improvement in tumor-control and overall survival in patients who were inoculated with bacterial cultures. However, as noted many years later by his daughter, Helen Coley Nauts and John McLaren in their retrospective review of his work (1), Coley did not appreciate fully that those patients who achieved the longest duration of remission experienced the highest fevers. Moreover, in the mid-1800s, physicians reported [as reviewed in (2)] significant tumor regression in some cancer patients who experienced high fevers during natural infection by erysipelas, the same bacterial strain that Coley used to inoculate his patients. Unfortunately Dr. Coley’s subsequent experiments using more purified forms of the “toxins”, which elicited less fever, failed to elicit tumor regression in contrast to the less purified inoculum. While fever is a more complex process than simple hyperthermia, (or a warming of tissue or core temperature without a regulated change in the hypothalamic set point), an intriguing question remains regarding the potential contribution of increased body/tumor temperature in antitumor immunotherapy.
The origins of this question are quite old. Long before body temperature could be measured by the first thermometers, clinicians in ancient cultures were aware of the importance of body temperature in health and disease. For example, in ancient Chinese and Ayurvedic medicine, physical or medicinal warming (and cooling) of body temperature was prescribed for ailments including arthritis and cancer. In ancient Greece, Hippocrates too was using heat for treatment of a wide variety of inflammatory diseases and also cancer, and alternatively, he is recognized for prescribing medications derived from the bark and leaves of the willow tree (rich in salicin) to relieve fever; this natural source was used in the 1800s to isolate salicylic acid, the active ingredient in aspirin. Furthermore, the usefulness of external heating to modify vascular function was well-recognized in ancient times. Coley’s work not only represents an early effort demonstrating the importance of immune-based therapy, but also the modern reinvigoration of efforts to determine the role of temperature in cancer therapies. A growing research community in ‘Thermal Medicine’ now focuses on determining the molecular and cellular mechanisms by which temperature manipulation may be used as therapy against cancer and other diseases.
A strong impetus to use hyperthermia in cancer therapy was provided by radiation biologists in the 1970s and 80s, who demonstrated the potent radiation-sensitization caused by hyperthermia; quantifiable cytotoxic effects were induced in tumor cells in vitro after heating to heat-shock range temperatures between 42–45°C. Moreover, in vivo studies demonstrated that heating increased the oxygenation state of tumors (3, 4). Despite these important findings (3) the broader application of hyperthermia to oncology stalled in the 1990s due to poorly designed clinical trials and the lack of suitable equipment to deliver heat locally. Nevertheless, persistence and gradual improvements in engineering led to several randomized clinical trials evaluating the combination of local/regional heating with radiation and/or chemotherapy. In one trial that combined hyperthermia with radiation, improved local tumor control was achieved in several human or canine cancers (3); in a multicenter randomized trial for patients with localized high-risk soft tissue sarcomas, the regimen combining radiation and chemotherapy with hyperthermia demonstrated significant positive clinical outcomes (4).
In the past 20 years the basic concepts in thermal medicine have greatly expanded. For example, the development of thermosensitive drug delivery platforms is rapidly transforming the ability to deliver toxic drugs specifically to tumors, avoiding normal tissue (5). The use of isolated limb, intra-peritoneal or thoracic cavity hyperthermia perfusion protocols is increasing (6,7) while image-guided approaches to heating (e.g., MR-guided thermal ablation strategies) have the potential to expand even further the therapeutic application of thermal medicine. Radiofrequency and ultrasound-based ablation protocols that create highly defined regions of necrotic tumor tissue through the targeted delivery of cytotoxic doses of extreme heat (or cold as in cryotherapy) is being combined with other therapies, including immunotherapy (8). Furthermore, interest in the effects of mild (fever-range) hyperthermia on normal and malignant tissues has also intensified given the impact of this treatment on tumor vascular perfusion, immune function and immunogenicity, lymphocyte trafficking, cytokine activity, metabolism, and gene expression- effects which are all relevant to the tumor microenvironment. Some of these recent discoveries relevant to therapies in the febrile, or mild-to-moderate hyperthermia range (38–42°C), are highlighted here. These discoveries suggest that mild hyperthermia may be an effective noninvasive strategy for manipulating the tumor microenvironment in specific ways that could enhance immunotherapy.
Fever and Hyperthermia: Drivers of Thermoregulatory and Immunological Responses Affecting the Tumor Microenvironment
Thermoregulation is a major homeostatic system in all vertebrates, involving extensive neural and vascular networks in the skin and visceral organs to ensure the maintenance of an optimal range of temperatures within the body. (9,10). Mammals and birds are remarkable for maintaining a much warmer core temperature than their environment, which requires substantial metabolic heat production and behavioral modification designed to minimize heat loss from the body surface. While fever and hyperthermia both involve increases in temperature, they result in and result in major thermoregulatory responses, although there are major differences in impact on physiology.
Recognized since ancient times as one of four cardinal signs of inflammation, fever is a complex component of the acute phase response to infection or injury. The increase in body temperature that accompanies fever varies among animals, but involves shifting body functions toward heat production and conservation. For example, the ‘set point’ for body temperature in the hypothalamus is elevated in most vertebrates during fever, which may result in an animal feeling cold despite an elevated body temperature. Fevers occur in all vertebrates, including those typically considered poikilothermic such as reptiles and amphibians. Even arthropods and annelids attempt to increase temperature in response to injury and infection. The fact that the “fever” response to infection and injury has been maintained throughout evolution for at least 600 million years strongly suggests a positive benefit to immunity and overall survival (11). The range of temperature elevation through which most vertebrates fight infections is remarkably consistent, varying between 1 to 5 degrees C above ambient body temperatures (9,12–14). A central hypothesis of Thermal Medicine is that there are evolutionarily-conserved, highly sensitive, thermal set points that regulate the immune system; these might be manipulated therapeutically to favorably influence the outcome of immunotherapy.
In contrast to fever, hyperthermia results from forced heating of the body or tissues in the absence of pyrogenic agents, and the body responds with vigorous thermoregulatory cooling mechanisms (9,10). Strenuous exercise and fever are two events that impact the body’s ability to maintain a constant temperature, and temporary elevations of several degrees C in core temperature are typically seen in both circumstances. A major concept being explored by researchers in Thermal Medicine is that application of heat to a specific region of the body (in the absence of fever) may result in a significant counter reaction aimed at restoring the normal temperature of the affected region. These counter reactions might alter the physiology of the tumor microenvironment, altering the immune response. Importantly, fever and hyperthermia share a dependence upon activation of heat shock factor 1, (HSF-1) (14) suggesting a common stress induced pathway could account for some of the similar effects of each process on the immune response.
Here we provide several examples of research using concepts driven by knowledge of fever and thermoregulation that should be of interest to cancer immunologists. These examples are schematically illustrated in Figure 1 and highlight mechanisms through which thermal therapy could be strategically used to co-opt the natural activities of fever, or the natural vascular responses to hyperthermia, to enhance immunotherapy. Since temperature is a physical parameter, altering aspects of cells and tissues without the need to use another drug to effect these changes,, it may be used as a complementary therapy to manipulate the tumor microenvironment in ways, (highlighted below) that may enhance the efficacy of immunotherapy.
Figure 1.
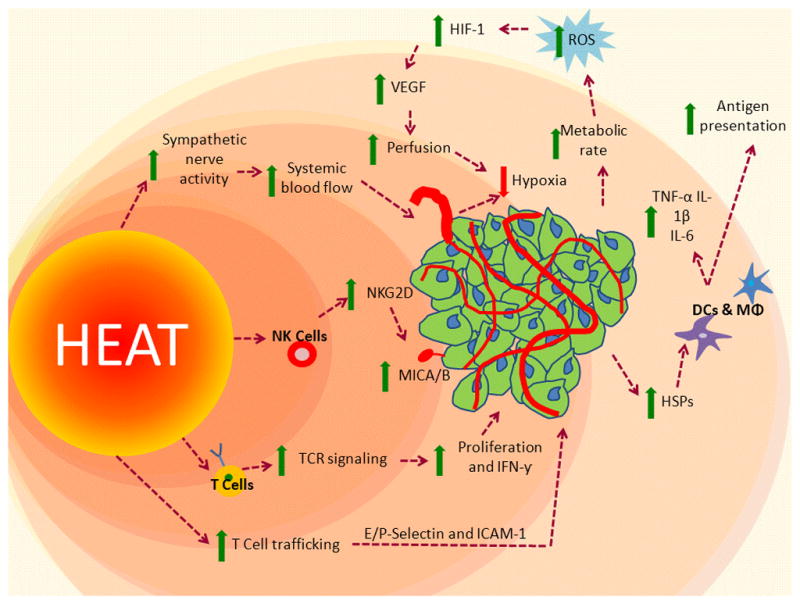
A multi-functional adjuvant: Heating impacts the tumor microenvironment through intrinsic and extrinsic mechanisms, which could enhance immunotherapy.
Increased vascular perfusion and blood flow to the tumor occurs through both thermoregulatory signals, as well as changes in tumor metabolism, leading to increased HIF-1, which leads to increased ROS production and VEGF expression. Increased trafficking of CD8+ T cells occurs through heat-induced increases in E/P selectin and ICAM-1 on tumor blood vessels; increased TCR signaling and differentiation of naïve T cells to effector cells. Increased tumor cell-surface expression of MICA/B and upregulation of the ligand NKG2D on NK cells, thereby increasing NK cell cytotoxic potential. Increased functional activity of macrophages, dendritic cells, Increased release of HSPs into the extracellular environment, which stimulate downstream immune activity, and increased antigen presentation.
Using Heat to Alter Immunosuppressive Hypoxia in the Tumor Microenvironment
There is growing appreciation that the immunosuppressive state of the tumor microenvironment is supported by the hypoxia which develops within tumors (15,16). Hypoxia is a complex , dynamic physiological state that is brought about by multiple dynamic factors within growing tumors, including tumor cell metabolism, and abnormal vascular perfusion. When tumor-bearing mice were subjected to temperatures between 39°C and 43°C, increases in tumor oxygenation were observed up to 24 hours after heating (3, 4). The level of reoxygenation correlates with the radiation sensitivity of the tumor as observed in studies with canine sarcomas (17) and in clinical trials of patients with soft-tissue sarcomas and breast cancer (18). Brizel and colleagues showed that one heat treatment led to reoxygenation of tumors in patients within 24 to 48 hours, whereas no measurable reoxygenation of tumors was observed during a week of standard radiation therapy (18). Jones and colleagues reported that hyperthermia (41–41.5°C ) significantly increased the pO2 in hypoxic, human tumors, but did not appreciably effect the oxygenation state of tumors which were not hypoxic (18). In canine patients, the combination of hyperthermia and radiotherapy led to prolonged improvement in oxygenation in hpoxic tumors. (19). These increases in tumor oxygenation improved tumor response to radiotherapy (20). The reoxygenation potential of mild hyperthermia is not only important for the efficacy of radiation, but isalso important for tumor immunology, since hypoxia can support immunosuppressive programs within tumors that promote tumor growth and interfere with immunotherapy.
Several potential mechanisms by which hyperthermia reduce hypoxia have been identified. At the physiological level, Sen and colleagues (21) recognized that addition of heat to large regions of normal tissues surrounding tumors would activate thermoregulatory responses driven by neural (sympathetic) control of smooth muscle surrounding arterioles, helping to increase blood flow from the heated region to remove excess heat. They hypothesized that blood flow within a tumor would experience at least some of the thermoregulatory ‘pulse’ of blood being actively pumped to the surface during heating. This hypothesis was tested in murine tumor models, which revealed increased perfusion of tumor blood vessels and reduced tumor hypoxia; the interstitial pressure of tumors also decreased following hyperthermia, which could facilitate the uptake of therapeutic molecules, or immuno-effector cells into tumors. . Since the efficacy of radiation therapy was also improved in this model system, these data provide evidence that targeting a normal vascular response (thermoregulation) using mild, systemic heat treatment can indirectly affect the tumor microenvironment such that radiation therapy can be improved.
Tumor cell metabolism can also be a target of heat, influencing hypoxia within tumors. For example, HIF-1 has been linked to heat-induced tumor reoxygenation. Moon and colleagues (22) reported that a brief period of hyperthermia activated HIF-1 and its downstream targets, such as vascular endothelial growth factor (VEGF) and pyruvate dehydrogenase kinase 1 (PDK1), leading to enhanced tumor perfusion/vascularization and oxygenation. Hyperthermia also increased the transcription of NADPH oxidase-1 through the ERK pathway, and the augmented expression of HIF-1 and its downstream targets through NADPH oxidase-1-mediated production of reactive oxygen species (ROS). While much work needs to be done to optimize the timing of heat treatment to achieve the best outcomes when combined with radiation, chemotherapy, or immunotherapy, it is clear that using heat to target either physiological or molecular targets within tumors can be an effective strategy to rapidly change hypoxia and tumor vessel perfusion within tumors.
Thermal Therapy Increases Immune Cell Trafficking into the Tumor and Immune Organs
Several lines of investigation have revealed that targeting the tumor vasculature by heat has the potential to improve the efficacy of immunotherapy. A barrier to CD8+ T cell infiltration in tumor sties frequently exists in preclinical mouse tumor models and patients, leading to speciulation that tumor blood vessels may be primary ‘bottleneck’ limiting extravasation of effector cells. Recent studies have shown that mild hyperthermia strongly promotes infiltration of the tumor microenovnments by tumor specific cytotoxic CD8+ T cells (23–26). Direct examination using intravital microscopy by Fisher and colleagues (24)of the rate of cytotoxic CD8 T cell homing at the tumor vascular junctiure in murin tumor moedls cocumented that tumor vesels fail to support efficient interactions with circulating T cells nder normal, homeostatic conditions. However, these vessels could be converted to a high rate-trafficking site, evidence by an approximate 5-fold increase in intratumoral homing of ctotoxic T cells following administration of mild systemic hyperthermia in several preclinical muring tumor models. Notably, hyperthermia was shown to act at multiple discrete steps in the adhesion cascade, culminating in improved CD8+ effector T cell traicking acresoos tumor vascular barriers. Thermally enhanced T cell trafficking is mediated by thermally –enhanced E-selecin and P-selectin- dependent tethering and rolling interactions within vessels walls as well as stable binding to intercellular adhesion molecule-1 (ICAM-1). A concomitant decrease in the number of intratumoral CD4+, CD25, Foxp3+regulatory T cells (Treg) was observed after heating, resulting in a profound increase in the ratio of CD8 +T cells: Treg after heat treatment. The overall impact of these findings is that newly homed CD8+t cells enterining the tumor microenvironment as a consequence of heating are causally linked to lysis of tumor cells and improved tumor control.
Thermal actions of cytotoxic CD8+ T cell trafficking are remarkably site-specific such that improved trafficking of effecor T cells is only observed in the tumor miceroenvironment. Exclusive trafficking to sites where effector T celsl a is predictive to maximize the probability tht these cells can initiate lytic detstractive programs within tumor tissues. However, prior observations in ono-tumor bearig mice has shown that entry of naïve and central memory T cells into lympoc nodes and Peyer’s patches can also be augmented by heating. Here, heat treatent was shown to enhance naïve/central memory CD4/CD8 T cells trafficking exclusively acresoss specialized high endotherlial venules 9heavs) which are the major portals of T cell entry in lymph nodes and Peyer’s Patches. (27,28).
Other Effects of Mild Hyperthermia on Immune Cells
Effects of mild hyperthermia on functions of innate and adaptive immune cells that play important roles in the tumor microenvironment have been the focus of several recent reviews (29–32). The functions of macrophages, dendritic cells (DC), T, B and natural killer (NK) cells may be enhanced after exposure to elevated temperatures. . Antigen presenting cells have been a major focus in this field. For example, the migration of Langerhans cells to draining lymph nodes is accelerated by mild systemic heating of mice; DC primed with toll-like receptor (TLR)-agonists increase their IL-12p70 production in response to mild heating while increased temperatures appears to increase TLR4 expression on DC and macrophages (29). Cytotoxicity of immune effector cells has also been a major focus of study in this field;. peripheral blood human NK cells exhibit enhanced cytotoxicity upon heating. This is consistent with other studies which show that mild systemic heating of mice resulted in significantly decreased burden of metastatic lung tumors, and NK cells were involved with this increased control (34). Treatment of tumor-bearing mice with systemic heating combined with alpha-galactosylceramide also slowed tumor growth and enhanced survival (35). Ostberg and colleagues studied mild thermal stress on NK cell cytotoxicity and proposed a mechanism by which thermal stimuli enhance NK cell cytotoxic activity, possibly through increased NKG2D expression along with distinct changes in the overall plasma membrane organization of lipid domains (37, 38).
The cytotoxic potential of T cells is also enhanced by heating. Cippitelli and colleagues demonstrated that thermally enhanced cytotoxic activity of T cells occured through modulation of the Fas/Fas Ligand system, and this effect was dependent upon thermal activation of HSF1 (36). This effect was also linked to thermally enhanced expression and translocation of the transcription factors AP-1 and NF-κB, which regulate fas ligand expression. Our group demonstrated that mild systemic heating of mice and in vitro treatment of T cells induce the activation of specific PKC isoforms and their movement, along with receptor-activated c kinase 1 (RACK 1) to the T lymphocyte uropod (37). RACK 1, which is important for maintaining the activated T cell structure, translocates to the cytoskeleton following hyperthermia treatment.
While most previous work has not identified specific, antigen-dependent events associated with thermally-enhanced immune function, mild thermal treatment can affect subsequent antigen-specific, activation-related events of naive CD8+ T cells. Mace and colleagues observed that exposure of CD62LhiCD44lo Pmel-1 CD8+ cells to 39.5°C prior to their antigen-dependent activation with gp10025–33 peptide-pulsed C57BL/6 splenocytes resulted in a greater percentage of cells that differentiated into CD62LloCD44hi effector cells compared with naïve CD8+ T cells incubated at 37°C (38). While studying details of these effects, we observed that mild heating of CD8+ T cells resulted in the reversible clustering of GM1+ CD− microdomains in the plasma membrane (38,39). We observed the same membrane clustering phenomenon in CD8+ T cells isolated from spleen, lymph nodes, and peripheral blood following mild whole-body heating of mice (38,39). In addition, mild heating resulted in the clustering of TCRβ and the CD8 coreceptor and also enhanced the rate of conjugate formation with antigen presenting cells in the spleen. In a pilot study assessing the efficacy of hyperthermic isolated limb perfusion as a treatment regimen for patients with in-transit metastases of malignant melanoma, Olofsson and colleagues found an increase in Melan-A-specific T lymphocytes in a sub-population of patients, demonstrating the potential of thermal therapy in the activation and differentiation of immune effector cells in the tumor microenvironment (40).
A major mechanism by which temperature could regulate the functional activity of a variety of immune cells could be through thermally sensitive organizational features of the plasma membrane. Since it has been well-documented that increases in temperature can increase plasma membrane fluidity in a variety of cells, including immune cells, it is likely that increasing the fluidity of the plasma membrane could be linked to the thermal activation of immune effector cells. (38,39)(41).
Immunogenicity of Tumor Cells is Enhanced by Mild Hyperthermia
There is increasing evidence that exposure of tumor cells to hyperthermia enhances sensitivity to immune cell recognition and killing. For example, hyperthermia may enhance the expression of heat shock proteins now thought to play an important role on the external surface of tumor cells (42). HSP70 family members expressed on the tumor cell surface may activate NK cells via specific receptor interactions. Hsp70 stimulates the proliferation and activation of NK cells while binding granzyme B, rendering tumor cells more sensitive to NK cell-mediated cytotoxicity.
Additional insight into the molecular mechanisms of thermally-enhanced NK cell activity against heated tumor targets comes from studies examining the impact of heat stress on tumor cell expression of the non–classical MHC Class I ligand (MICA), a cell surface protein recognized by an activating receptor (NKG2D) on NK cells (37). The gene for MICA contains heat-shock response elements, which contribute to MICA upregulation upon heat-shock. Even mild (fever range) thermal stress upregulates MICA expression in human colon tumor cells, supporting a role for hyperthermia in increasing the sensitivity of tumor cells to immunological attack.
The role of inducible heat-shock protein 70 (HSP70) in chaperoning MHC Class I epitopes is one mechanism underlying the enhanced sensitivity of tumor cells to immune recognition. HSP70-chaperoned proteins may also stimulate dendritic cells to augment tumor-specific T-cells. A prevailing paradigm is that Hsp70 released from tumor cells may be in a complex with intracellular polypeptide antigens, which are then recognized by antigen presenting cells (APC), leading to generation of antitumor immunity (43). While the nature of the Hsp70-peptide antigen complex and the APC cell surface receptors involved remain a topic of intense study (44), these interactions lead to a pro-inflammatory response.
Thermal Regulation of Pro-inflammatory Cytokines
There are several studies which suggest that the expression and function of cytokines important for the regulation of anti-tumor immunity and inflammation can be significantly impacted by mild hyperthermia. Genomic analyses were performed on tumor biopies from a cohort of 22 client-owned dogs with spontaneous soft tissue sarcomas treated with thermoradiotherapyT(45). Tumor cells were isolated from biopsies obtained prior to and 24 hours after the first hyperthermia treatment. Changes in the diffusion coefficient of water (a surrogate for inflammation) correlated with differences in several genes associated with inflammation, including CCR1, interleukin 1α (IL-1β), IL-6, IL-8, IL-10, and MARCO. These results demonstrate that clinical application of hyperthermia can lead to an inflammatory response. commensurate with what has been observed pre-clinically
Some time ago, Duram and Duff reported that IL-1-induced T cell proliferation was increased by hyperthermia, suggesting that IL-1 function was sensitive to thermal stimuli (46). However, this cytokine also plays a central role in the generation of fever, and is a major player in the regulation of multiple immune pathways (47)(48). Specifically, IL-1 was isolated first as the endogenous fever–producing protein and recombinant IL-1 (either IL-1α or IL-1β) was found to be the most potent pyrogen for humans, producing fever at doses as low as 1 ng/kg. Several reports showed that immunologically active cytokines such as IL-2 result in greater in vitro responses at 39°C than at 37°C. Capitano and colleagues (52) recently demonstrated that mild elevation in body temperature (39.5°C) in mice that had been subjected to total body irradiation exhibited enhanced recovery from neutropenia, involving a thermally-enhanced cytokine cascade in which IL-1 induced IL-17 that in turn induced G-CSF.
Systemic thermal therapy can also alter the activities of the pro-inflammatory cytokine IL-6 (24), which may contribute to tumor pathogenesis. Evans and colleagues (28, 29) discovered that thermal therapy can reverse this pro-tumorigenic function, as IL-6 is involved in the enhanced effector T lymphocyte-trafficking to the tumor microenvironment. Antibody neutralization of IL-6 but not other inflammatory cytokines present in the tumor microenvironment, such as TNF, IL-1β, or IFN-γ, prevented the induction of E/P-selectin- and ICAM-1-dependent trafficking of adoptively-transferred IFN-g, granzme B loaded CD8+ T cells via the tumor vasculature.. Thermal therapy further failed to increase ICAM-1 density on tumor vessels or to induce CD8+ T cell intratumoural accumulation in IL-6-deficient mice. Interestingly, the antitumorigenic activiteis of IL-6 unleased by heating in preclinical mouse models was driven by an atypical non-hematopoietic cell source of IL-6. In the absence of IL-6 signaling, heat was not able to uregulate T cell mediated apoptosis of tumor cell targets. These results highlight the need to test hyperthermia in combination with various forms of immunotherapy, including vaccines and other immunomodulatory agents.
Summary and Future Research Questions
The high grade energy of sunlight becomes the medium grade energy of organic molecules and this in turn is dissipated as the low grade energy of heat, which finally becomes useless as entropy. Thus, the flow of biological energy is irreversible.
Albert L. Lehninger, 1965
Heat is the end product of nearly all of the energy released in the body, and its production is essential for all life. To maintain a very narrow range of core body temperature, the heat produced from metabolic reactions must be constantly balanced with that absorbed from or dissipated to the external environment; this thermal homeostasis is maintained through extensive neural, vascular, and biochemical mechanisms collectively known as thermoregulation.. Given the central role of thermoregulation in the physiology of life, it is not surprising that forced addition of heat to the system through hyperthermia or regulated increases in body temperature through fever should have myriad effects on cellular function. Dissecting those effects that may enhance the antitumor immune response and harnessing the power of strategic applications of heat are the challenges that researchers in Thermal Medicine face as they develop tools that can manipulate the temperature of either normal or tumor tissues. One of the most important aspects of the use of thermal therapy is that it is based upon physical interaction with tumor tissue and can therefore avoid the problems associated with adding another, potentially toxic, drug in various combination strategies. While this brief summary has highlighted some of the immune effects of mild to moderate levels of heating, it is important to recognize that these data may also be highly relevant to heating protocols such as thermal ablation since there are regions of mild hyperthermia at the edges of the ablated field. Several questions must be addressed, however, to maximize the clinical potential of thermal therapies.
One major question is how the heat energy added to living systems is affecting overall metabolism and energy required for antitumor immunity. Recent research shows that there is a large bio-energetic cost associated with generating effective T cell-mediated immune response (49–51). A weakened immune response and an increased incidence of infectious diseases during cold exposure have often been observed in animals in the wild and in experimental situations (52–54). Based on these findings, it has been suggested that when immunological defenses are too energetically costly (55–57), they are selectively “traded off” in favor of higher priority functions such as thermoregulation. Results from a new set of studies in mice (Kokolus et al., submitted (62)) demonstrate that simply not having enough heat available can impair the development of more effective antitumor immunity; these results should lead to additional experiments related to the impact of heat treatments.
A second question involves the detailed relationships between fever, and hyperthermia. The contributions of pyrogen and inflammatory signals from infectious agents to the positive effects of body temperature elevation must still be determined. In this regard, it will be very important to more precisely identify how the inflammatory microenvironment of tumors, as well as chemokine/cytokine signaling which directs immune cell trafficking, is influenced by both conditions.
A third question focuses on determining the optimal dose and temperature. Many studies in Thermal Medicine use temperatures ranging from 38.5 to 42°C. This is a very large range physiologically to study biological impact of temperature, and much more work is needed to identify optimal temperatures for influencing various immune endpoints. Studies examining the duration of heating and determining whether local, regional or systemic heating is most beneficial are also needed.
Finally, development of specific equipment for selective applications of thermal therapy is improving, but much more work is still needed. Furthermore, it is essential that we have rapid, non-invasive techniques for precisely measuring temperatures in various sites in the body.
While these and other questions are important to the applications of thermal medicine, the research highlighted here will hopefully draw attention to temperature as a variable in tumor immunology research. These discoveries have the potential of helping to explain finally, in mechanistic fashion, the natural, long-conserved sensitivity of the immune system to thermal signals and in so doing help to improve immunotherapy in cancer patients.
Acknowledgments
The authors thank Kathleen Kokolus, Jason Eng and John Subjeck for their suggestions on the manuscript. This work was supported by NIH NCI R01 CA135368 (EAR) and NIH NCI R01 CA40355 (MWD).
References
- 1.Nauts HC, McLaren JR. Coley toxins--the first century. Adv Exp Med Biol. 1990;267:483–500. doi: 10.1007/978-1-4684-5766-7_52. [DOI] [PubMed] [Google Scholar]
- 2.Bull JM. Whole body hyperthermia as an anticancer agent. CA Cancer J Clin. 1982 Mar-Apr;32(2):123–128. doi: 10.3322/canjclin.32.2.123. [DOI] [PubMed] [Google Scholar]
- 3.Viglianti B, Stauffer P, Repasky E, Jones E, Vujaskovic Z, Dewhirst M. Hyperthermia. In: Hong W, Bast R, Hait W, Kufe D, Holland J, Pollock R, et al., editors. Holland-Frei Cancer Medicine. 8. Shelton: People's Medical Publishing House; 2010. pp. 528–540. [Google Scholar]
- 4.Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem B, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. The Lancet Oncology. 2010 Jun;11(6):561–570. doi: 10.1016/S1470-2045(10)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDaniel JR, Dewhirst MW, Chilkoti A. Actively targeting solid tumours with thermoresponsive drug delivery systems that respond to mild hyperthermia. Int J Hyperthermia. 2013 Aug 7; doi: 10.3109/02656736.2013.819999. [DOI] [PubMed] [Google Scholar]
- 6.Tilleman TR, Richards WG, Zellos L, Johnson BE, Jaklitsch MT, Mueller J, et al. Extrapleural pneumonectomy followed by intracavitary intraoperative hyperthermic cisplatin with pharmacologic cytoprotection for treatment of malignant pleural mesothelioma: a phase II prospective study. J Thorac Cardiovasc Surg. 2009 Aug;138(2):405–411. doi: 10.1016/j.jtcvs.2009.02.046. [DOI] [PubMed] [Google Scholar]
- 7.Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003 Oct 15;21(20):3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 8.Waitz R, Solomon SB, Petre EN, Trumble AE, Fasso M, Norton L, et al. Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy. Cancer Res. 2012 Jan 15;72(2):430–439. doi: 10.1158/0008-5472.CAN-11-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon CJ. Temperature Regulation in Laboratory Rodents. Cambridge, UK: Cambridge University Press; 1993. [Google Scholar]
- 10.Guyton AC, Hall JE. Textbook of Medical Physiology. 11. Philadelphia, PA: Elsevier Saunders; 2006. [Google Scholar]
- 11.Kluger MJ, Kozak W, Conn CA, Leon LR, Soszynski D. The adaptive value of fever. Infect Dis Clin North Am. 1996 Mar;10(1):1–20. doi: 10.1016/s0891-5520(05)70282-8. [DOI] [PubMed] [Google Scholar]
- 12.Blumberg MS. Body Heat: Temperature and Life on Earth. Cambridge: Harvard University Press; 2002. [Google Scholar]
- 13.Dinarello CA. Review: Infection, fever, and exogenous and endogenous pyrogens: some concepts have changed. J Endotoxin Res. 2004 Aug;10(4):201–222. doi: 10.1179/096805104225006129. [DOI] [PubMed] [Google Scholar]
- 14.Singh IS, Hasday JD. Fever, hyperthermia and the heat shock response. Int J Hyperthermia. 2013 Aug;29(5):423–435. doi: 10.3109/02656736.2013.808766. [DOI] [PubMed] [Google Scholar]
- 15.Sitkovsky MV. T regulatory cells: hypoxia-adenosinergic suppression and re-direction of the immune response. Trends Immunol. 2009 Mar;30(3):102–108. doi: 10.1016/j.it.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol. 2005 Sep;5(9):712–721. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- 17.Vujaskovic Z, Poulson JM, Gaskin AA, Thrall DE, Page RL, Charles HC, et al. Temperature-dependent changes in physiologic parameters of spontaneous canine soft tissue sarcomas after combined radiotherapy and hyperthermia treatment. Int J Radiat Oncol Biol Phys. 2000 Jan 1;46(1):179–185. doi: 10.1016/s0360-3016(99)00362-4. [DOI] [PubMed] [Google Scholar]
- 18.Jones EL, Prosnitz LR, Dewhirst MW, Marcom PK, Hardenbergh PH, Marks LB, et al. Thermochemoradiotherapy improves oxygenation in locally advanced breast cancer. Clin Cancer Res. 2004 Jul 1;10(13):4287–4293. doi: 10.1158/1078-0432.CCR-04-0133. [DOI] [PubMed] [Google Scholar]
- 19.Thrall DE, Larue SM, Pruitt AF, Case B, Dewhirst MW. Changes in tumour oxygenation during fractionated hyperthermia and radiation therapy in spontaneous canine sarcomas. Int J Hyperthermia. 2006 Aug;22(5):365–373. doi: 10.1080/02656730600836386. [DOI] [PubMed] [Google Scholar]
- 20.Thrall DE, Maccarini P, Stauffer P, Macfall J, Hauck M, Snyder S, et al. Thermal dose fractionation affects tumour physiological response. Int J Hyperthermia. 2012;28(5):431–440. doi: 10.3109/02656736.2012.689087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sen A, Capitano ML, Spernyak JA, Schueckler JT, Thomas S, Singh AK, et al. Mild elevation of body temperature reduces tumor interstitial fluid pressure and hypoxia and enhances efficacy of radiotherapy in murine tumor models. Cancer Res. 2011 Jun;71(11):3872–3880. doi: 10.1158/0008-5472.CAN-10-4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moon EJ, Sonveaux P, Porporato PE, Danhier P, Gallez B, Batinic-Haberle I, et al. NADPH oxidase-mediated reactive oxygen species production activates hypoxia-inducible factor-1 (HIF-1) via the ERK pathway after hyperthermia treatment. Proc Natl Acad Sci U S A. 2010 Nov 23;107(47):20477–20482. doi: 10.1073/pnas.1006646107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mikucki ME, Fisher DT, Ku AW, Appenheimer MM, Muhitch JB, Evans SS. Preconditioning thermal therapy: Flipping the switch on IL-6 for anti-tumour immunity. Int J Hyperthermia. 2013 Aug;29(5):464–473. doi: 10.3109/02656736.2013.807440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher DT, Chen Q, Skitzki JJ, Muhitch JB, Zhou L, Appenheimer MM, et al. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J Clin Invest. 2011 Oct;121(10):3846–3859. doi: 10.1172/JCI44952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Q, Wang W, Bruce R, Li H, Schleider DM, Mulbury MJ, et al. Central Role of IL-6 Receptor Signal-Transducing Chain gp130 in Activation of L-Selectin Adhesion by Fever-Range Thermal Stress. Immunity. 2004 Jan;20(1):59–70. doi: 10.1016/s1074-7613(03)00358-3. [DOI] [PubMed] [Google Scholar]
- 26.Vardam TD, Zhou L, Appenheimer MM, Chen Q, Wang WC, Baumann H, et al. Regulation of a lymphocyte-endothelial-IL-6 trans-signaling axis by fever-range thermal stress: hot spot of immune surveillance. Cytokine. 2007 Jul;39(1):84–96. doi: 10.1016/j.cyto.2007.07.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans SS, Fisher DT, Skitzki JJ, Chen Q. Targeted regulation of a lymphocyte-endothelial-interleukin-6 axis by thermal stress. International Journal of Hyperthermia. 2008;24(1):67–78. doi: 10.1080/02656730701772498. [DOI] [PubMed] [Google Scholar]
- 28.Evans SS, Wang WC, Bain MD, Burd R, Ostberg JR, Repasky EA. Fever-range hyperthermia dynamically regulates lymphocyte delivery to high endothelial venules. Blood. 2001 May 1;97(9):2727–2733. doi: 10.1182/blood.v97.9.2727. [DOI] [PubMed] [Google Scholar]
- 29.Frey B, Weiss EM, Rubner Y, Wunderlich R, Ott OJ, Sauer R, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia. 2012;28(6):528–542. doi: 10.3109/02656736.2012.677933. [DOI] [PubMed] [Google Scholar]
- 30.Peer AJ, Grimm MJ, Zynda ER, Repasky EA. Diverse immune mechanisms may contribute to the survival benefit seen in cancer patients receiving hyperthermia. Immunol Res. 2010 Mar;46(1):137–154. doi: 10.1007/s12026-009-8115-8. [DOI] [PubMed] [Google Scholar]
- 31.Ostberg JR, Repasky EA. Emerging evidence indicates that physiologically relevant thermal stress regulates dendritic cell function. Cancer Immunology, Immunotherapy. 2006 Mar;55(3):292–298. doi: 10.1007/s00262-005-0689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muthana M, Multhoff G, Pockley AG. Tumour infiltrating host cells and their significance for hyperthermia. Int J Hyperthermia. 2010;26(3):247–255. doi: 10.3109/02656730903413375. [DOI] [PubMed] [Google Scholar]
- 33.Peng JC, Hyde C, Pai S, O'Sullivan BJ, Nielsen LK, Thomas R. Monocyte-derived DC primed with TLR agonists secrete IL-12p70 in a CD40-dependent manner under hyperthermic conditions. J Immunother. 2006 Nov-Dec;29(6):606–615. doi: 10.1097/01.cji.0000211308.82997.4e. [DOI] [PubMed] [Google Scholar]
- 34.Shen RN, Hornback NB, Shidnia H, Shupe RE, Brahmi Z. Whole-body hyperthermia decreases lung metastases in lung tumor-bearing mice, possibly via a mechanism involving natural killer cells. J Clin Immunol. 1987 May;7(3):246–253. doi: 10.1007/BF00915730. [DOI] [PubMed] [Google Scholar]
- 35.Hattori T, Kokura S, Okuda T, Okayama T, Takagi T, Handa O, et al. Antitumor effect of whole body hyperthermia with alpha-galactosylceramide in a subcutaneous tumor model of colon cancer. Int J Hyperthermia. 2007 Nov;23(7):591–598. doi: 10.1080/02656730701708328. [DOI] [PubMed] [Google Scholar]
- 36.Cippitelli M, Fionda C, Di Bona D, Piccoli M, Frati L, Santoni A. Hyperthermia enhances CD95-ligand gene expression in T lymphocytes. J Immunol. 2005 Jan 1;174(1):223–232. doi: 10.4049/jimmunol.174.1.223. [DOI] [PubMed] [Google Scholar]
- 37.Wang XY, Ostberg JR, Repasky EA. Effect of fever-like whole-body hyperthermia on lymphocyte spectrin distribution, protein kinase C activity, and uropod formation. Journal of immunology (Baltimore, Md : 1950) 1999 Mar;162(6):3378–3387. [PubMed] [Google Scholar]
- 38.Mace TA, Zhong L, Kilpatrick C, Zynda E, Lee CT, Capitano M, et al. Differentiation of CD8+ T cells into effector cells is enhanced by physiological range hyperthermia. J Leukoc Biol. 2011 Nov;90(5):951–962. doi: 10.1189/jlb.0511229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mace TA, Zhong L, Kokolus KM, Repasky EA. Effector CD8+ T cell IFN-gamma production and cytotoxicity are enhanced by mild hyperthermia. Int J Hyperthermia. 2012;28(1):9–18. doi: 10.3109/02656736.2011.616182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olofsson R, Lindberg E, Karlsson-Parra A, Lindner P, Mattsson J, Andersson B. Melan-A specific CD8+ T lymphocytes after hyperthermic isolated limb perfusion: a pilot study in patients with in-transit metastases of malignant melanoma. Int J Hyperthermia. 2013 May;29(3):234–238. doi: 10.3109/02656736.2013.782428. [DOI] [PubMed] [Google Scholar]
- 41.Csoboz B, Balogh GE, Kusz E, Gombos I, Peter M, Crul T, et al. Membrane fluidity matters: Hyperthermia from the aspects of lipids and membranes. Int J Hyperthermia. 2013 Aug;29(5):491–499. doi: 10.3109/02656736.2013.808765. [DOI] [PubMed] [Google Scholar]
- 42.Multhoff G, Hightower LE. Cell surface expression of heat shock proteins and the immune response. Cell Stress Chaperones. 1996 Sep;1(3):167–176. doi: 10.1379/1466-1268(1996)001<0167:cseohs>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milani V, Noessner E, Ghose S, Kuppner M, Ahrens B, Scharner A, et al. Heat shock protein 70: role in antigen presentation and immune stimulation. Int J Hyperthermia. 2002 Nov-Dec;18(6):563–575. doi: 10.1080/02656730210166140. [DOI] [PubMed] [Google Scholar]
- 44.Wang XY, Facciponte J, Chen X, Subjeck JR, Repasky EA. Scavenger receptor-A negatively regulates antitumor immunity. Cancer Res. 2007 May 15;67(10):4996–5002. doi: 10.1158/0008-5472.CAN-06-3138. [DOI] [PubMed] [Google Scholar]
- 45.Chi JT, Thrall DE, Jiang C, Snyder S, Fels D, Landon C, et al. Comparison of genomics and functional imaging from canine sarcomas treated with thermoradiotherapy predicts therapeutic response and identifies combination therapeutics. Clin Cancer Res. 2011 Apr 15;17(8):2549–2560. doi: 10.1158/1078-0432.CCR-10-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duff GW, Durum SK. Fever and immunoregulation: hyperthermia, interleukins 1 and 2, and T-cell proliferation. Yale J Biol Med. 1982 Sep-Dec;55(5–6):437–442. [PMC free article] [PubMed] [Google Scholar]
- 47.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012 Aug;11(8):633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dinarello CA. IL-1: discoveries, controversies and future directions. Eur J Immunol. 2010 Mar;40(3):599–606. doi: 10.1002/eji.201040319. [DOI] [PubMed] [Google Scholar]
- 49.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nature reviews Immunology. 2005 Nov;5(11):844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 50.Pearce EL. Metabolism in T cell activation and differentiation. Curr Opin Immunol. 2010 Jun;22(3):314–320. doi: 10.1016/j.coi.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frauwirth KA, Thompson CB. Regulation of T lymphocyte metabolism. J Immunol. 2004 Apr 15;172(8):4661–4665. doi: 10.4049/jimmunol.172.8.4661. [DOI] [PubMed] [Google Scholar]
- 52.Karp CL. Unstressing intemperate models: how cold stress undermines mouse modeling. J Exp Med. 2012 Jun 4;209(6):1069–1074. doi: 10.1084/jem.20120988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shope RE. The swine lungworm as a reservior and intermediate host for swine influenza virus. V. Provocation of swine influenza by exposure of prepared swine to adverse weather. J Exp Med. 1955 Nov 1;102(5):567–572. doi: 10.1084/jem.102.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moragues V, Pinkerton H. Variation in Morbidity and Mortality of Murine Typhus Infection in Mice with Changes in the Environmental Temperature. J Exp Med. 1944 Jan 1;79(1):41–43. doi: 10.1084/jem.79.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lochmiller RL, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. [Google Scholar]
- 56.Martin LB, Weil ZM, Nelson RJ. Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Philos Trans R Soc Lond B Biol Sci. 2008 Jan 27;363(1490):321–339. doi: 10.1098/rstb.2007.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rauw WM. Immune response from a resource allocation perspective. Front Genet. 2012;3:267–280. doi: 10.3389/fgene.2012.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]


