Abstract
Obstructive sleep apnea is characterized by repeated upper airway obstruction during sleep and affects between 5% and 20% of the population. Epidemiological studies reveal that sleep apnea and associated intermittent hypoxemia increase the risk for hypertension and vascular disease but the mechanisms underlying these effects are incompletely understood. This review reports the results of rodent models of intermittent hypoxia (IH) and relates them to the observed hemodynamic and vascular consequences of sleep apnea. These animal studies have demonstrated that IH exposure in the absence of any other comorbidity causes hypertension, endothelial dysfunction, and augmented constrictor sensitivity, all due at least in part to increased vascular oxidative stress. Animal studies have used a variety of exposure paradigms to study intermittent hypoxia and these different exposure protocols can cause hypocapnia or hypercapnia—or maintain eucapnia—with accompanying alterations in plasma pH. It appears that these different profiles of arterial blood gases can lead to divergent results but the impact of these differences is still being investigated. Overall, the studies in rodents have clearly demonstrated that the vascular and hemodynamic impact of intermittent hypoxia provides a strong rationale for treating clinical sleep apnea to prevent the resulting cardiovascular morbidity and mortality.
Keywords: blood gas, cardiovascular, endothelium, hematocrit, hypercapnia, intermittent hypoxia, sleep apnea, vascular
Rodent Models of Intermittent Hypoxia
Rodent studies provide a tremendous opportunity to explore the mechanisms of cardiovascular changes induced by one of the major consequences of sleep apnea, intermittent hypoxemia. Experimental protocols expose rodents to intermittent hypoxia (IH1) to simulate the blood gas changes that occur in sleep apnea; while they vary in cycle length, severity of hypoxia, number of hypoxic episodes per hour, and number of exposure days, they are quite consistent in reporting mild hypertension (Fletcher 2001; Kanagy et al. 2001; Lesske et al. 1997), augmented vascular contractility (Allahdadi et al. 2005; Julien et al. 2003; Tahawi et al. 2001), and metabolic alterations (Djovkar 1983; Holm et al. 1973; McNulty et al. 1996; Polotsky et al. 2003). Thus, despite slightly different results in the time to onset of cardiovascular changes and the degree of resulting pathology, the similarities are more striking than the differences. However, the different paradigms do produce markedly divergent changes in arterial blood gases, with unclear impacts on arterial pressure, hematocrit (the proportion of red blood cells in the total blood volume), and vascular function.
Blood Gas Differences in Exposure Paradigms
Exposure protocols induce hypoxia by replacing a portion of the inhaled air with nitrogen to periodically briefly reduce the percentage of oxygen (O2) in the inhaled gas mixture to 5–10%. This hypoxic gas mixture alternates with periods of air flush that restore oxygen to normal values (~21%). The hypoxic exposure decreases blood oxygen saturation similar to the desaturation that occurs in patients with moderate to severe apnea (Noda et al. 1998). A major difference between protocols is the resultant partial pressure of carbon dioxide (pCO21) during the hypoxia exposures. In clinical studies of patients with moderate sleep apnea (15 to 30 apneic episodes per hour), pCO2 changes negligibly (Epstein et al. 2001) or increases slightly (Tilkian et al. 1976) during the apneic episodes; an increase may contribute to the severity of the cardiovascular consequences of sleep apnea (Cooper et al. 2005). However, most IH exposure paradigms in rodents do not include CO2 supplementation and the resultant hyperventilation leads to hypocapnia.
In studies comparing hypocapnic, eucapnic, and hypercapnic intermittent hypoxia (IH with abnormally low, optimal, and excessive arterial CO2 pressure, respectively), Eugene Fletcher reported similar blood pressure changes in all protocols (Lesske et al. 1997). Furthermore, his group did not observe increases in hematocrit or in right ventricular weight in any of the subjects, suggesting that the exposures did not cause pulmonary hypertension or erythropoiesis. In contrast, Aidan Bradford and Michelle McGuire found that all three protocols increased hematocrit and caused right ventricular hypertrophy (Bradford 2004; McGuire and Bradford 1999). Later studies from Fletcher’s group also reported either an increase in hematocrit without a change in right ventricular weight (Fletcher et al. 1992b) or an increase in right ventricular weight without an increase in hematocrit (Fletcher et al. 1992a; for a more detailed discussion of cardiovascular impacts, Dematteis et al. 2009).
Studies from our group have shown that hypocapnic but not eucapnic IH increases hematocrit and causes a more profound increase in right ventricular mass than does eucapnic IH (Snow et al. 2008). Rats were exposed for 14 days to either hypocapnic (decreased fraction of inspired oxygen [FiO2] over 90 secs to 5% O2:95% N2, 20 times/hr) or eucapnic IH (decreased FiO2 and increased FiCO2 over 90 secs to 5% O2:5% CO2:90% N2, 20 times/hr). As in other studies, both types of IH caused profound and similar decreases in PO2 during the hypoxic exposure period (68 ± 5 millimeters of mercury [mmHg1] before and 32 ± 6 mmHg during hypoxia). However, IH-exposed rats became significantly hypocapnic with a concurrent alkalosis during the hypoxia exposure whereas those with CO2 supplementation remained eucapnic (pCO2 = 27 ± 3 to 14 ± 2 mmHg and 31 ± 2 to 33 ± 1 mmHg, respectively; Figure 1). Thus both protocols caused a similar degree of arterial hypoxemia but very different levels of pCO2. After 14 days of hypocapnic IH exposure, hematocrit was elevated to the same level as that in rats exposed to chronic hypoxia (Figure 2). In contrast, eucapnic IH elicited a very modest increase in hematocrit, similar to changes observed in patients with obstructive sleep apnea (OSA1) (Hoffstein et al. 1994). It is not clear why the same level of hypoxia produces such markedly different hematocrits or why these results differ from those of Bradford and McGuire, but the striking difference demonstrates that eucapnic exposure more closely mimics the clinical paradigm. levels are accompanied
Figure 1.
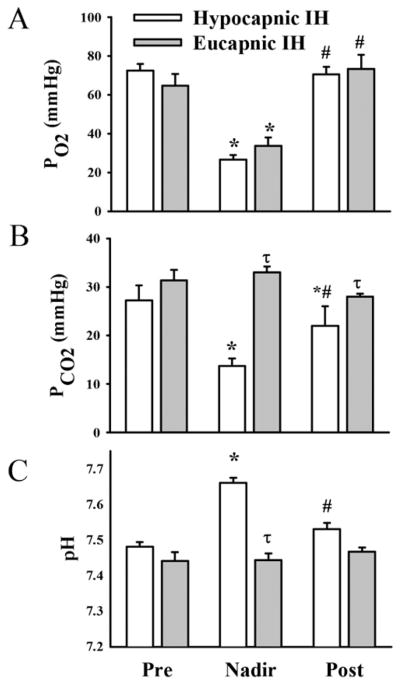
Exposure to hypocapnic intermittent hypoxia (IH) and eucapnic IH had similar effects on arterial oxygen tension (pO2) (A) but very different effects on arterial carbon dioxide tension (pCO2) (B) and on arterial pH (C). Samples were drawn from a surgically implanted arterial line after at least 7 days recovery from surgery before exposure to hypoxia (Pre), during the 10 seconds of minimum (5%) inhaled oxygen content (Nadir), and during the flush with room air (Post). Values are means ± SE of n = 4–5 rats/group. * p < 0.05 vs. respective preexposure value. # p < 0.05 vs. nadir value. τ p < 0.05 vs. hypocapnic IH. mmHg, millimeters of mercury. Reprinted with permission from Snow JB, Kitzis V, Norton CE, Torres, SN, Johnson KD, Kanagy NL, Walker BR, Resta TC. 2008. Differential effects of chronic hypoxia and intermittent hypocapnic and eucapnic hypoxia on pulmonary vasoreactivity. J Appl Physiol 104:110–118.
Figure 2.
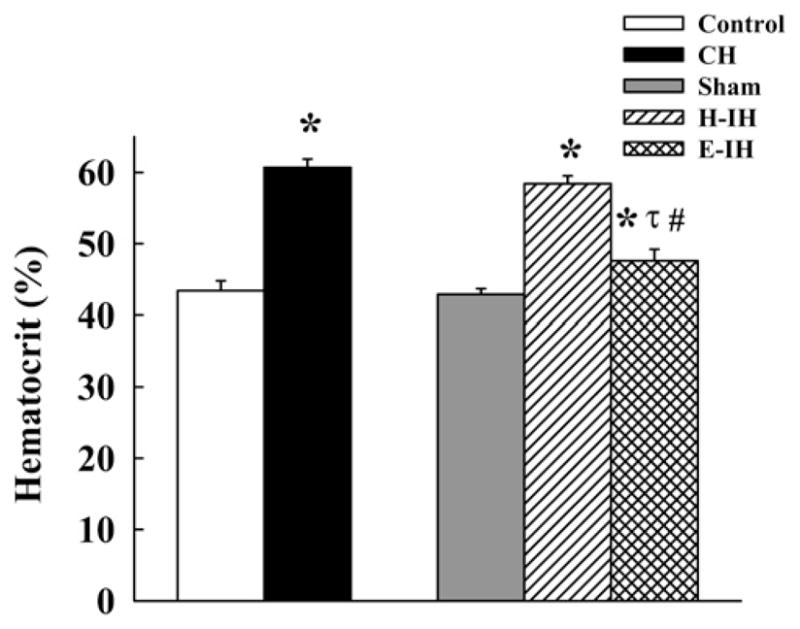
Percent hematocrit in rats exposed for 14 days to normoxia (control), chronic hypoxia (CH; hypobaric hypoxia with barometric pressure maintained at ~380 mmHg), sham-treated, hypocapnic IH (H-IH), and eucapnic IH (E-IH) rats. Values are means ± SE of n = 7–10 rats/group. * p < 0.05 vs. corresponding control group. # p < 0.05 vs. CH. τ p < 0.05 vs. H-IH. Hematocrit is the proportion of red blood cells in the total blood volume. Reprinted with permission from Snow JB, Kitzis V, Norton CE, Torres, SN, Johnson KD, Kanagy NL, Walker BR, Resta TC. 2008. Differential effects of chronic hypoxia and intermittent hypocapnic and eucapnic hypoxia on pulmonary vasoreactivity. J Appl Physiol 104:110–118.
In addition, the different pCO2 by differences in the development of right ventricular hypertrophy, evident in other reported differences in pulmonary function. Pulmonary arterial remodeling and right ventricular hypertrophy accompanied by significant polycythemia occur in rats exposed to either hypocapnic IH or chronic hypoxia but not in rats exposed to eucapnic IH (Fagan 2001; Snow et al. 2008; Thomas and Wanstall 2003). Thus hypercapnia or even eucapnia appears to attenuate the development of both pulmonary hypertension and elevated hematocrit in response to intermittent hypoxia. Indeed, continuous CO2 supplementation appears to interfere with the development of pulmonary hypertension in rats exposed to chronic hypoxia (Kantores et al. 2006; Ooi et al. 2000), suggesting that pCO2 modulates the pulmonary responses to hypoxia.
One possible reason for the differences in hematocrit is that CO2 supplementation can blunt the degree and duration of the fall in pCO2 (compared to hypoxia alone) (Campen et al. 2004). Thus differences in arterial blood gases during exposure to eucapnic and hypocapnic IH appear to differentially affect at least the pulmonary response to IH. Additional studies are needed to determine the ramifications of these differences.
Blood Pressure Changes
Because the hypocapnic intermittent hypoxia exposure protocol is simpler and several studies report no differences in blood pressure response between eucapnic and hypocapnic IH after 35 days (Fletcher et al. 1992b), most animal studies have used this protocol and found a modest increase in arterial pressure within 10 to 35 days (Dematteis et al. 2008; Hinojosa-Laborde and Mifflin 2005; Lesske et al. 1997). Data from our laboratory suggest that eucapnic IH causes a faster and greater increase in arterial pressure than that which occurs with hypocapnic IH exposure (Kanagy et al. 2001; Troncoso Brindeiro et al. 2007). However, there are multiple differences in the study methods in addition to the differences in pCO2, so comparison of the paradigms is difficult. Fletcher’s original studies reporting that neither hematocrit nor blood pressure changed after the two exposure paradigms used catheterized animals with frequent blood sampling that may have prevented such changes. In the Bradford and McGuire study demonstrating that either eucapnic or hypercapnic IH produced a significant increase in arterial pressure and hematocrit, rats were restrained during the exposure period by nose cones and blood was collected weekly from the tail vein rather than from an indwelling catheter; the resulting stress may have played a role in the differing responses reported in these studies. Nonetheless, cumulatively, animal studies indicate that IH causes a modest increase (10 to 20 mmHg) in arterial pressure compared to other experimental models of hypertension (Fletcher 2001; Hinojosa-Laborde and Mifflin 2005; Peng et al. 2006; Unger et al. 1977). This finding is comparable to reports that blood pressure falls from 2 to 10 mmHg with continuous positive airway pressure (CPAP1) treatment of OSA patients (Logan et al. 2003; Robinson et al. 2008). Thus animal models appear to consistently mimic the development of mild hypertension in sleep apnea and suggest that intermittent exposure to periods of hypoxemia mediates the increase in blood pressure.
Animal studies examining the mechanism of IH-related hypertension indicate that it is multifactorial, with substantial contributions by the sympathetic nervous system (Fletcher 2003) and the renin angiotensin system (Fletcher et al. 1999). In addition, there may be contributions from other factors, including reactive oxygen species (ROS1) scavenging of nitric oxide (NO1) (Hayashi et al. 2008), increased levels of endothelin (Allahdadi et al. 2008a; Kanagy et al. 2001), and alterations of baroreflex control of blood pressure (Dematteis et al. 2008; Narkiewicz and Somers 1997).
There is also evidence of augmented sympathetic activity in humans, including elevated urinary and plasma catecholamines (Minemura et al. 1998), elevated muscle sympathetic nerve activity, and decreased sensitivity of the baroreceptor (Logan et al. 2003; Monahan et al. 2006; Narkiewicz and Somers 1997). CPAP treatment generally remedies these alterations (Narkiewicz et al. 1999; Phillips et al. 2007; Somers et al. 1988). Animal models similarly show increases in plasma catecholamines (Zoccal et al. 2007), sympathetic nerve traffic (de Paula et al. 2007), and impaired baroreflex sensitivity (Dematteis et al. 2008), suggesting that IH is the cause of the increased neural activity. In rats exposed to 30 days of intermittent hypoxia, spectral analysis of arterial pressure variability revealed marked elevations in both the low frequency power and the low to high frequency ratio (Lai et al. 2006). In isolated adrenal medullae, 10 days of IH exposure increased catecholamine release compared to control or chronic hypoxia exposure (Kumar et al. 2006). Administration of the ganglion blocker hexamethonium caused a greater fall in arterial pressure in IH-exposed rats than in control rats (Zoccal et al. 2007). In the study by Daniel Zoccal and colleagues, the IH-exposed rats had elevated arterial pressure and increased plasma levels of norepinephrine. Interestingly, the administration of the angiotensin receptor blocker losartan did not affect blood pressure in either group of rats. Together, these studies suggest that chronic IH facilitates cardiovascular sympathetic outflow and decreases baroreflex sensitivity but may not affect the renin angiotensin system.
Sympathetic activity increases renin secretion and some studies demonstrate increased renin levels in OSA patients (Moller et al. 2003). In a small study by Susumu Takahashi and colleagues (2005), plasma levels of angiotensin II were also elevated in OSA patients and appeared to contribute to increases in plasma levels of vascular endothelial growth factor (VEGF). However, according to the findings of other clinical studies, sleep apnea does not elevate renin activity or concentration (Benjamin et al. 2008; Gjorup et al. 2007). Similarly, there are mixed results in animal studies, with some showing a clear activation of the renin-angiotensin-aldosterone system (Fletcher et al. 2002) and others (e.g., Zoccal et al. 2007) suggesting that angiotensin does not contribute to a rise in blood pressure. Additional studies are needed to determine which parameters in the human condition predispose to activation of this system and thus make it a good target for pharmacological intervention (Pimenta et al. 2007).
Vascular Changes
Endothelial Function
Nitric Oxide and Oxidative Stress
One of the most striking and consistent vascular changes reported in studies of sleep apnea is diminished endothelial function, including decreased endothelium-dependent vasodilation in skeletal and cerebral resistance arteries (Phillips et al. 2004) and in cremaster arterioles (Tahawi et al. 2001) in IH-exposed rats. These findings are consistent with the impaired dilation function reported in OSA patients (Carlson et al. 1996; Kraiczi et al. 2001; Lavie 2004) and appear to indicate that the IH component of OSA mediates this aspect of vascular dysfunction. The impaired dilation has been attributed to loss of nitric oxide bioavailability through ROS scavenging as evidenced by increased oxidative stress in OSA patients (Christou et al. 2003; Dyugovskaya et al. 2002; Suzuki et al. 2006) and in animal models of intermittent hypoxia (Jun et al. 2008; Kumar et al. 2006; Prabhakar and Kumar 2004; Seiler et al. 1996; Semenza and Prabhakar 2007). Functional studies demonstrate that scavenging ROS in vivo attenuates the development of IH-induced hypertension in animals (Troncoso Brindeiro et al. 2007) and restores vasodilator function in arteries from exposed rats (Phillips et al. 2004; Tahawi et al. 2001).
Recent studies in patients also report that antioxidant therapy improves endothelial dysfunction and NO bioavailability (El Solh et al. 2006; Grebe et al. 2006; Teramoto et al. 2007). In addition, CPAP treatment of OSA patients improves endothelial function (Cross et al. 2008; El Solh et al. 2007; Jelic et al. 2008). Both animal models and clinical studies suggest that elevated ROS production impairs nitric oxide-dependent vasodilation in sleep apnea. In the study by Sanja Jelic and colleagues (2008), freshly harvested venous endothelial cells from OSA patients showed a significant decrease in both total and phosphorylated endothelial nitric oxide synthase (eNOS) and a fivefold increase in nitrotyrosine and cyclooxygenase (COX1)-2. After 4 weeks of CPAP therapy, expression of eNOS and phosphorylated eNOS were higher whereas expression of inducible NOS (iNOS) and COX-2 were lower. These changes were associated with improved flow-mediated dilation. A study by Jo-Dee Lattimore and colleagues (2006) demonstrated that 3 months of CPAP treatment increased the forearm blood flow response to acetylcholine infusion and augmented the reduction in flow to NOS inhibition, suggesting that CPAP treatment elevates both basal and agonist-stimulated NO production. A recent study by Melanie Cross and colleagues (2008) reported impaired brachial dilation to both acetylcholine infusion and sodium nitroprusside in patients with severe OSA that improved after 6 weeks of CPAP treatment. Thus vascular oxidative stress may scavenge both endogenous and exogenous NO to prevent vasodilation in OSA. Additional studies in animal models will be useful to determine specific molecular targets to reverse the oxidative stress and restore endothelial function.
Cyclooxygenase Products
In addition to diminished NO-dependent vasodilation, studies indicate that the cyclooxygenase pathway may be disrupted in IH-exposed animals. In a study of carotid artery function in IH-exposed rats, enhanced vasoconstriction to endothelin was normalized by treatment with a cyclooxygenase inhibitor (Lefebvre et al. 2006). Studies in nonvascular tissues demonstrate increased COX-2 expression after IH exposure (Kheirandish et al. 2005; Li et al. 2003; Yang et al. 2005), suggesting that COX expression increases in multiple tissues with both vascular and neural effects. Because COX may be a source of superoxide (Kukreja et al. 1986; Rieger et al. 2002) in addition to the vasodilator prostacyclin (Shah 2005), increased COX expression could contribute to elevated vascular ROS generation. Alternatively, increased production of constrictor prostanoids such as thromboxane A2 or PGH2 may also contribute to vascular defects in sleep apnea. In clinical studies, CPAP treatment reduces COX-2 expression in vascular endothelial cells from OSA patients (Jelic et al. 2008). It will be interesting to see whether future studies demonstrate that COX inhibition in vivo prevents or reverses the development of impaired vascular reactivity.
Measures of vascular reactivity in human studies consistently show OSA-related abnormalities including hypoxia-induced impaired dilation (Foster et al. 2007), exacerbation of atherosclerosis (Drager et al. 2009), and impaired cerebral autoregulation (Urbano et al. 2008). Indeed, epidemiological studies show significant independent associations between OSA and hypertension, coronary artery disease, arrhythmias, heart failure, and stroke (Bradley and Floras 2009). However, few studies have addressed COX contributions to these changes in vascular function, although urinary excretion of prostanoid metabolites suggests either decreased production of dilatory versus constrictor prostanoids (Krieger et al. 1991) or a compensatory increase in dilatory prostanoids (Kimura et al. 1998). Direct measures of COX expression and activity in endothelial cells from OSA patients seem to indicate that the reported upregulation of COX-2 in animal studies is paralleled in OSA patients; future studies will determine whether this upregulation affects vasodilatory capacity.
Vascular Smooth Muscle
Researchers have observed increased vasoconstrictor responses to endothelin (Allahdadi et al. 2005; Lefebvre et al. 2006) and to norepinephrine (Julien et al. 2003) in several vascular beds of IH-exposed rats. For example, Blandine Lefebvre and colleagues (2006) found no changes in aortic responses to norepinephrine, angiotensin II, and endothelin but greater endothelin constriction in the carotid artery of IH-exposed rats. In this study, indomethacin normalized the endothelin responses, suggesting that IH increases endothelin stimulation of COX vasoconstrictors. However, increased sensitivity to other constrictor agents has not been consistently observed (Allahdadi et al. 2005; Julien et al. 2003; Lefebvre et al. 2006), and several studies have found a decrease (Phillips et al. 2006) or no change (Tahawi et al. 2001) in constrictor reactivity. The lack of change is somewhat surprising in light of the consistent reports of impaired endothelium-dependent dilation (Tahawi et al. 2001) and increased levels of vascular ROS (Troncoso Brindeiro et al. 2007), both of which should augment constrictor versus dilator function. However, chronic exposure to elevated sympathetic activity and increases in circulating angiotensin II may decrease vascular sensitivity to these agonists, and this desensitization may explain the modest increase in arterial pressure in animal models that show increases in sympathetic activity (Lai et al. 2006; Zoccal et al. 2007), plasma renin activity (Morgan 2007), and plasma endothelin (Kanagy et al. 2001).
The consistent reports of endothelial dilation and greater endothelin constriction suggest that endothelin may be an important mediator of the vascular pathology of this condition. Indeed, recent studies from our laboratory indicate that endothelin antagonists can normalize the increased blood pressure in IH-exposed rats (Allahdadi et al. 2008a). Furthermore, studies from our group reveal that the intracellular signaling cascade that mediates endothelin vasoconstriction is greatly altered after IH exposure (whereas mechanisms that mediate phenylephrine constriction are unaltered; Allahdadi et al. 2005); the changes—increased activation of protein kinase C and decreased activation of calcium influx—result in a profound increase in calcium sensitization (Allahdadi et al. 2008b). Thus studies to determine the mechanism of these alterations in vascular signaling may identify new targets to prevent vascular damage in sleep apnea patients.
Unlike studies of agonist-induced constriction, few reports include discussion of IH effects on myogenic tone. One study of myogenic tone described its decrease in skeletal muscle arterioles after exposure to 14 days of hypocapnic IH (Phillips et al. 2006). The development of myogenic tone was restored by treating rats with the antioxidant tempol during the IH exposure period, suggesting the effect may have been caused by increases in vasodilatory ROS. We have observed that mesenteric arteries from rats exposed to 14 days of eucapnic IH have augmented myogenic tone that is endothelium-dependent (unpublished observations). Thus there may be differences in the response of skeletal versus mesenteric vascular beds; additional studies are needed to determine the mechanisms for these disparate results.
Alternatively, there may also be differences in the effects of eucapnic and hypocapnic IH. After exposure to hypocapnic IH, there is decreased or unchanged sensitivity to vasoconstrictors (Koistinen et al. 2000; McGuire and Bradford 1999; Snow et al. 2008). This result is similar to the profound inhibition of peripheral vasoconstrictor responsiveness that follows exposure to continuous chronic hypoxia (Caudill et al. 1998; Doyle and Walker 1991; Earley and Walker 2002). In contrast, eucapnic IH appears to augment vasoconstrictor sensitivity, similar to the effect of sleep apnea in humans (Kraiczi et al. 2000). Thus vascular reactivity, pulmonary arterial pressure, and hematocrit may be influenced by the exposure paradigms, with the degree of hypocapnia influencing the cellular changes. Further studies delineating the systemic responses to hypocapnic and eucapnic IH will determine how each of the paradigms mimics the effects of sleep apnea in OSA patients.
Conclusions
Animal models of sleep apnea have added substantially to scientists’ understanding of the vascular consequences of this condition. The most important contribution may be the consistent observation that sleep apnea leads to a small but significant increase in arterial pressure independent of comorbidities. The observation that systemic hemodynamics and peripheral vascular function are also altered strengthens the justification for aggressive treatment of apneas and oxygen desaturations with continuous positive airway pressure (CPAP) devices. In addition, the confirmed presence of endothelial pathologies suggests that the long-term consequences of untreated sleep apnea include damage in multiple organ systems. Ongoing studies indicate that endocrine factors and oxidative stress are important players in the vascular pathologies of sleep apnea. Future studies in these areas are expected to identify potential therapeutic targets for the prevention of sleep apnea–induced vascular disease.
Acknowledgments
The author receives funding from the Environmental Protection Agency (EPA STAR award 83186001) and the National Institutes of Health (HL82799) and is an established investigator of the American Heart Association.
Footnotes
Abbreviations used in this article: COX, cyclooxygenase; CPAP, continuous positive airway pressure; IH, intermittent hypoxia; mmHg, millimeters of mercury; NO, nitric oxide; OSA, obstructive sleep apnea; pCO2, partial pressure of carbon dioxide; ROS, reactive oxygen species
References
- Allahdadi KJ, Cherng TW, Pai H, Silva AQ, Walker BR, Nelin LD, Kanagy NL. Endothelin type A receptor antagonist normalizes blood pressure in rats exposed to eucapnic intermittent hypoxia. Am J Physiol Heart Circ Physiol. 2008a;295:H434–H440. doi: 10.1152/ajpheart.91477.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahdadi KJ, Duling LC, Walker BR, Kanagy NL. Eucapnic intermittent hypoxia augments endothelin-1 vasoconstriction in rats: Role of PKCdelta. Am J Physiol Heart Circ Physiol. 2008b;294:H920–H927. doi: 10.1152/ajpheart.01264.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahdadi KJ, Walker BR, Kanagy NL. Augmented endothelin vasoconstriction in intermittent hypoxia-induced hypertension. Hypertension. 2005;45:705–709. doi: 10.1161/01.HYP.0000153794.52852.04. [DOI] [PubMed] [Google Scholar]
- Benjamin JA, Moller M, Ebden P, Bartle I, Lewis KE. Serum angiotensin converting enzyme and the obstructive sleep apnea hypopnea syndrome. J Clin Sleep Med. 2008;4:325–331. [PMC free article] [PubMed] [Google Scholar]
- Bradford A. Effects of chronic intermittent asphyxia on haematocrit, pulmonary arterial pressure and skeletal muscle structure in rats. Exp Physiol. 2004;89:44–52. doi: 10.1113/expphysiol.2003.002656. [DOI] [PubMed] [Google Scholar]
- Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- Campen MJ, Tagaito Y, Li J, Balbir A, Tankersley CG, Smith P, Schwartz A, O’Donnell CP. Phenotypic variation in cardiovascular responses to acute hypoxic and hypercapnic exposure in mice. Physiol Genomics. 2004;20:15–20. doi: 10.1152/physiolgenomics.00197.2003. [DOI] [PubMed] [Google Scholar]
- Carlson JT, Rangemark C, Hedner JA. Attenuated endothelium-dependent vascular relaxation in patients with sleep apnoea. J Hypertens. 1996;14:577–584. doi: 10.1097/00004872-199605000-00006. [DOI] [PubMed] [Google Scholar]
- Caudill TK, Resta TC, Kanagy NL, Walker BR. Role of endothelial carbon monoxide in attenuated vasoreactivity following chronic hypoxia. Am J Physiol. 1998;275:R1025–R1030. doi: 10.1152/ajpregu.1998.275.4.R1025. [DOI] [PubMed] [Google Scholar]
- Christou K, Markoulis N, Moulas AN, Pastaka C, Gourgoulianis KI. Reactive oxygen metabolites (ROMs) as an index of oxidative stress in obstructive sleep apnea patients. Sleep Breath. 2003;7:105–110. doi: 10.1007/s11325-003-0105-9. [DOI] [PubMed] [Google Scholar]
- Cooper VL, Pearson SB, Bowker CM, Elliott MW, Hainsworth R. Interaction of chemoreceptor and baroreceptor reflexes by hypoxia and hypercapnia: A mechanism for promoting hypertension in obstructive sleep apnoea. J Physiol. 2005;568:677–687. doi: 10.1113/jphysiol.2005.094151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross MD, Mills NL, Al Abri M, Riha R, Vennelle M, Mackay TW, Newby DE, Douglas NJ. Continuous positive airway pressure improves vascular function in obstructive sleep apnoea/hypopnoea syndrome: A randomised controlled trial. Thorax. 2008;63:578–583. doi: 10.1136/thx.2007.081877. [DOI] [PubMed] [Google Scholar]
- de Paula PM, Tolstykh G, Mifflin S. Chronic intermittent hypoxia alters NMDA and AMPA-evoked currents in NTS neurons receiving carotid body chemoreceptor inputs. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2259–R2265. doi: 10.1152/ajpregu.00760.2006. [DOI] [PubMed] [Google Scholar]
- Dematteis M, Julien C, Guillermet C, Sturm N, Lantuejoul S, Mallaret M, Levy P, Gozal E. Intermittent hypoxia induces early functional cardiovascular remodeling in mice. Am J Respir Crit Care Med. 2008;177:227–235. doi: 10.1164/rccm.200702-238OC. [DOI] [PubMed] [Google Scholar]
- Dematteis M, Godin-Ribuot D, Arnaud C, Ribuot C, Stanke-Labesque F, Pépin JL, Lévy P. Cardiovascular consequences of sleep-disordered breathing: Contribution of animal models to understanding the human disease. ILAR J. 2009;50:262–281. doi: 10.1093/ilar.50.3.262. [DOI] [PubMed] [Google Scholar]
- Djovkar A. Influence of intermittent hypoxia on intravenous glucose tolerance and insulin sensitivity in anaesthetized normal rats. Horm Metab Res. 1983;15:254–255. doi: 10.1055/s-2007-1018685. [DOI] [PubMed] [Google Scholar]
- Doyle MP, Walker BR. Attentuation of systemic vasoreactivity in chronically hypoxic rats. Am J Physiol. 1991;260:R1114–R1122. doi: 10.1152/ajpregu.1991.260.6.R1114. [DOI] [PubMed] [Google Scholar]
- Drager LF, Bortolotto LA, Krieger EM, Lorenzi-Filho G. Additive effects of obstructive sleep apnea and hypertension on early markers of carotid atherosclerosis. Hypertension. 2009;53:64–69. doi: 10.1161/HYPERTENSIONAHA.108.119420. [DOI] [PubMed] [Google Scholar]
- Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med. 2002;165:934–939. doi: 10.1164/ajrccm.165.7.2104126. [DOI] [PubMed] [Google Scholar]
- Earley S, Walker BR. Endothelium-dependent blunting of myogenic responsiveness after chronic hypoxia. Am J Physiol Heart Circ Physiol. 2002;283:H2202–H2209. doi: 10.1152/ajpheart.00125.2002. [DOI] [PubMed] [Google Scholar]
- El Solh AA, Saliba R, Bosinski T, Grant BJ, Berbary E, Miller N. Allopurinol improves endothelial function in sleep apnoea: A randomised controlled study. Eur Respir J. 2006;27:997–1002. doi: 10.1183/09031936.06.00101005. [DOI] [PubMed] [Google Scholar]
- El Solh AA, Akinnusi ME, Baddoura FH, Mankowski CR. Endothelial cell apoptosis in obstructive sleep apnea: A link to endothelial dysfunction. Am J Respir Crit Care Med. 2007;175:1186–1191. doi: 10.1164/rccm.200611-1598OC. [DOI] [PubMed] [Google Scholar]
- Epstein LJ, Jervis OJ, Jr, Henderson JH, Sullivan M, Mohsenifar Z. Measurement of gastric P(CO2) as an index of tissue hypoxia during obstructive sleep apnea. Respiration. 2001;68:28–34. doi: 10.1159/000050459. [DOI] [PubMed] [Google Scholar]
- Fagan KA. Pulmonary hypertension in mice following intermittent hypoxia. J Appl Physiol. 2001;90:2502–2507. doi: 10.1152/jappl.2001.90.6.2502. [DOI] [PubMed] [Google Scholar]
- Fletcher EC. Physiological consequences of intermittent hypoxia: Systemic blood pressure. J Appl Physiol. 2001;90:1600–1605. doi: 10.1152/jappl.2001.90.4.1600. [DOI] [PubMed] [Google Scholar]
- Fletcher EC. Sympathetic over activity in the etiology of hypertension of obstructive sleep apnea. Sleep. 2003;26:15–19. doi: 10.1093/sleep/26.1.15. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Bao G, Li R. Renin activity and blood pressure in response to chronic episodic hypoxia. Hypertension. 1999;34:309–314. doi: 10.1161/01.hyp.34.2.309. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Lesske J, Behm R, Miller CC, III, Stauss H, Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol. 1992a;72:1978–1984. doi: 10.1152/jappl.1992.72.5.1978. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Lesske J, Qian W, Miller CC, III, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension. 1992b;19:555–561. doi: 10.1161/01.hyp.19.6.555. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Orolinova N, Bader M. Blood pressure response to chronic episodic hypoxia: the renin-angiotensin system. J Appl Physiol. 2002;92:627–633. doi: 10.1152/japplphysiol.00152.2001. [DOI] [PubMed] [Google Scholar]
- Foster GE, Hanly PJ, Ostrowski M, Poulin MJ. Effects of continuous positive airway pressure on cerebral vascular response to hypoxia in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2007;175:720–725. doi: 10.1164/rccm.200609-1271OC. [DOI] [PubMed] [Google Scholar]
- Gjorup PH, Sadauskiene L, Wessels J, Nyvad O, Strunge B, Pedersen EB. Abnormally increased endothelin-1 in plasma during the night in obstructive sleep apnea: Relation to blood pressure and severity of disease. Am J Hypertens. 2007;20:44–52. doi: 10.1016/j.amjhyper.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Grebe M, Eisele HJ, Weissmann N, Schaefer C, Tillmanns H, Seeger W, Schulz R. Antioxidant vitamin C improves endothelial function in obstructive sleep apnea. Am J Respir Crit Care Med. 2006;173:897–901. doi: 10.1164/rccm.200508-1223OC. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Yamashita C, Matsumoto C, Kwak CJ, Fujii K, Hirata T, Miyamura M, Mori T, Ukimura A, Okada Y, Matsumura Y, Kitaura Y. Role of gp91phox-containing NADPH oxidase in left ventricular remodeling induced by intermittent hypoxic stress. Am J Physiol Heart Circ Physiol. 2008;294:H2197–H2203. doi: 10.1152/ajpheart.91496.2007. [DOI] [PubMed] [Google Scholar]
- Hinojosa-Laborde C, Mifflin SW. Sex differences in blood pressure response to intermittent hypoxia in rats. Hypertension. 2005;46:1016–1021. doi: 10.1161/01.HYP.0000175477.33816.f3. [DOI] [PubMed] [Google Scholar]
- Hoffstein V, Herridge M, Mateika S, Redline S, Strohl KP. Hematocrit levels in sleep apnea. Chest. 1994;106:787–791. doi: 10.1378/chest.106.3.787. [DOI] [PubMed] [Google Scholar]
- Holm J, Bjorntorp P, Schersten T. Metabolic activity in rat skeletal muscle. Effect of intermittent hypoxia. Eur J Clin Invest. 1973;3:279–283. doi: 10.1111/j.1365-2362.1973.tb00352.x. [DOI] [PubMed] [Google Scholar]
- Jelic S, Padeletti M, Kawut SM, Higgins C, Canfield SM, Onat D, Colombo PC, Basner RC, Factor P, LeJemtel TH. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation. 2008;117:2270–2278. doi: 10.1161/CIRCULATIONAHA.107.741512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien C, Bayat S, Levy P. Vascular reactivity to norepinephrine and acetylcholine after chronic intermittent hypoxia in mice. Respir Physiol Neurobiol. 2003;139:21–32. doi: 10.1016/j.resp.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Jun J, Savransky V, Nanayakkara A, Bevans S, Li J, Smith PL, Polotsky VY. Intermittent hypoxia has organ-specific effects on oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1274–R1281. doi: 10.1152/ajpregu.90346.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagy NL, Walker BR, Nelin LD. Role of endothelin in intermittent hypoxia-induced hypertension. Hypertension. 2001;37:511–515. doi: 10.1161/01.hyp.37.2.511. [DOI] [PubMed] [Google Scholar]
- Kantores C, McNamara PJ, Teixeira L, Engelberts D, Murthy P, Kavanagh BP, Jankov RP. Therapeutic hypercapnia prevents chronic hypoxia-induced pulmonary hypertension in the newborn rat. Am J Physiol Lung Cell Mol Physiol. 2006;291:L912–L922. doi: 10.1152/ajplung.00480.2005. [DOI] [PubMed] [Google Scholar]
- Kheirandish L, Row BW, Li RC, Brittian KR, Gozal D. Apolipoprotein E-deficient mice exhibit increased vulnerability to intermittent hypoxia-induced spatial learning deficits. Sleep. 2005;28:1412–1417. doi: 10.1093/sleep/28.11.1412. [DOI] [PubMed] [Google Scholar]
- Kimura H, Niijima M, Abe Y, Edo H, Sakabe H, Kojima A, Hasako K, Masuyama S, Tatsumi K, Kuriyama T. Compensatory excretion of prostacyclin and thromboxane metabolites in obstructive sleep apnea syndrome. Intern Med. 1998;37:127–133. doi: 10.2169/internalmedicine.37.127. [DOI] [PubMed] [Google Scholar]
- Koistinen PO, Rusko H, Irjala K, Rajamaki A, Penttinen K, Sarparanta VP, Karpakka J, Leppaluoto J. EPO, red cells, and serum transferrin receptor in continuous and intermittent hypoxia. Med Sci Sports Exerc. 2000;32:800–804. doi: 10.1097/00005768-200004000-00012. [DOI] [PubMed] [Google Scholar]
- Kraiczi H, Hedner J, Peker Y, Carlson J. Increased vasoconstrictor sensitivity in obstructive sleep apnea. J Appl Physiol. 2000;89:493–498. doi: 10.1152/jappl.2000.89.2.493. [DOI] [PubMed] [Google Scholar]
- Kraiczi H, Caidahl K, Samuelsson A, Peker Y, Hedner J. Impairment of vascular endothelial function and left ventricular filling: Association with the severity of apnea-induced hypoxemia during sleep. Chest. 2001;119:1085–1091. doi: 10.1378/chest.119.4.1085. [DOI] [PubMed] [Google Scholar]
- Krieger J, Benzoni D, Sforza E, Sassard J. Urinary excretion of prostanoids during sleep in obstructive sleep apnoea patients. Clin Exp Pharmacol Physiol. 1991;18:551–555. doi: 10.1111/j.1440-1681.1991.tb01490.x. [DOI] [PubMed] [Google Scholar]
- Kukreja RC, Kontos HA, Hess ML, Ellis EF. PGH synthase and lipoxygenase generate superoxide in the presence of NADH or NADPH. Circ Res. 1986;59:612–619. doi: 10.1161/01.res.59.6.612. [DOI] [PubMed] [Google Scholar]
- Kumar GK, Rai V, Sharma SD, Ramakrishnan DP, Peng YJ, Souvannakitti D, Prabhakar NR. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol. 2006;575:229–239. doi: 10.1113/jphysiol.2006.112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CJ, Yang CC, Hsu YY, Lin YN, Kuo TB. Enhanced sympathetic outflow and decreased baroreflex sensitivity are associated with intermittent hypoxia-induced systemic hypertension in conscious rats. J Appl Physiol. 2006;100:1974–1982. doi: 10.1152/japplphysiol.01051.2005. [DOI] [PubMed] [Google Scholar]
- Lattimore JL, Wilcox I, Skilton M, Langenfeld M, Celermajer DS. Treatment of obstructive sleep apnoea leads to improved microvascular endothelial function in the systemic circulation. Thorax. 2006;61:491–495. doi: 10.1136/thx.2004.039164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie L. Sleep apnea syndrome, endothelial dysfunction, and cardiovascular morbidity. Sleep. 2004;27:1053–1055. [PubMed] [Google Scholar]
- Lefebvre B, Godin-Ribuot D, Joyeux-Faure M, Caron F, Bessard G, Levy P, Stanke-Labesque F. Functional assessment of vascular reactivity after chronic intermittent hypoxia in the rat. Respir Physiol Neurobiol. 2006;150:278–286. doi: 10.1016/j.resp.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia: Influence of chemoreceptors and sympathetic nervous system. J Hypertens. 1997;15:1593–1603. doi: 10.1097/00004872-199715120-00060. [DOI] [PubMed] [Google Scholar]
- Li RC, Row BW, Gozal E, Kheirandish L, Fan Q, Brittian KR, Guo SZ, Sachleben LR, Jr, Gozal D. Cyclooxygenase 2 and intermittent hypoxia-induced spatial deficits in the rat. Am J Respir Crit Care Med. 2003;168:469–475. doi: 10.1164/rccm.200211-1264OC. [DOI] [PubMed] [Google Scholar]
- Logan AG, Tkacova R, Perlikowski SM, Leung RS, Tisler A, Floras JS, Bradley TD. Refractory hypertension and sleep apnoea: Effect of CPAP on blood pressure and baroreflex. Eur Respir J. 2003;21:241–247. doi: 10.1183/09031936.03.00035402. [DOI] [PubMed] [Google Scholar]
- McGuire M, Bradford A. Chronic intermittent hypoxia increases haematocrit and causes right ventricular hypertrophy in the rat. Respir Physiol. 1999;117:53–58. doi: 10.1016/s0034-5687(99)00047-x. [DOI] [PubMed] [Google Scholar]
- McNulty PH, Ng C, Liu WX, Jagasia D, Letsou GV, Baldwin JC, Soufer R. Autoregulation of myocardial glycogen concentration during intermittent hypoxia. Am J Physiol. 1996;271:R311–R319. doi: 10.1152/ajpregu.1996.271.2.R311. [DOI] [PubMed] [Google Scholar]
- Minemura H, Akashiba T, Yamamoto H, Akahoshi T, Kosaka N, Horie T. Acute effects of nasal continuous positive airway pressure on 24-hour blood pressure and catecholamines in patients with obstructive sleep apnea. Intern Med. 1998;37:1009–1013. doi: 10.2169/internalmedicine.37.1009. [DOI] [PubMed] [Google Scholar]
- Moller DS, Lind P, Strunge B, Pedersen EB. Abnormal vasoactive hormones and 24-hour blood pressure in obstructive sleep apnea. Am J Hypertens. 2003;16:274–280. doi: 10.1016/s0895-7061(02)03267-3. [DOI] [PubMed] [Google Scholar]
- Monahan KD, Leuenberger UA, Ray CA. Effect of repetitive hypoxic apnoeas on baroreflex function in humans. J Physiol. 2006;574:605–613. doi: 10.1113/jphysiol.2006.108977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan BJ. Vascular consequences of intermittent hypoxia. Adv Exp Med Biol. 2007;618:69–84. doi: 10.1007/978-0-387-75434-5_6. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Kato M, Phillips BG, Pesek CA, Davison DE, Somers VK. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation. 1999;100:2332–2335. doi: 10.1161/01.cir.100.23.2332. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Somers VK. The sympathetic nervous system and obstructive sleep apnea: Implications for hypertension. J Hypertens. 1997;15:1613–1619. doi: 10.1097/00004872-199715120-00062. [DOI] [PubMed] [Google Scholar]
- Noda A, Ito R, Okada T, Yasuma F, Nakashima N, Yokota M. Twenty-four-hour ambulatory oxygen desaturation and electrocardiographic recording in obstructive sleep apnea syndrome. Clin Cardiol. 1998;21:506–510. doi: 10.1002/clc.4960210710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi H, Cadogan E, Sweeney M, Howell K, O’Regan RG, McLoughlin P. Chronic hypercapnia inhibits hypoxic pulmonary vascular remodeling. Am J Physiol Heart Circ Physiol. 2000;278:H331–H338. doi: 10.1152/ajpheart.2000.278.2.H331. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol. 2006;577:705–716. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips CL, Yang Q, Williams A, Roth M, Yee BJ, Hedner JA, Berend N, Grunstein RR. The effect of short-term withdrawal from continuous positive airway pressure therapy on sympathetic activity and markers of vascular inflammation in subjects with obstructive sleep apnoea. J Sleep Res. 2007;16:217–225. doi: 10.1111/j.1365-2869.2007.00589.x. [DOI] [PubMed] [Google Scholar]
- Phillips SA, Olson EB, Morgan BJ, Lombard JH. Chronic intermittent hypoxia impairs endothelium-dependent dilation in rat cerebral and skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol. 2004;286:H388–H393. doi: 10.1152/ajpheart.00683.2003. [DOI] [PubMed] [Google Scholar]
- Phillips SA, Olson EB, Lombard JH, Morgan BJ. Chronic intermittent hypoxia alters NE reactivity and mechanics of skeletal muscle resistance arteries. J Appl Physiol. 2006;100:1117–1123. doi: 10.1152/japplphysiol.00994.2005. [DOI] [PubMed] [Google Scholar]
- Pimenta E, Calhoun DA, Oparil S. Mechanisms and treatment of resistant hypertension. Arq Bras Cardiol. 2007;88:683–692. doi: 10.1590/s0066-782x2007000600009. [DOI] [PubMed] [Google Scholar]
- Polotsky VY, Li J, Punjabi NM, Rubin AE, Smith PL, Schwartz AR, O’Donnell CP. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol. 2003;552:253–264. doi: 10.1113/jphysiol.2003.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR, Kumar GK. Oxidative stress in the systemic and cellular responses to intermittent hypoxia. Biol Chem. 2004;385:217–221. doi: 10.1515/BC.2004.015. [DOI] [PubMed] [Google Scholar]
- Rieger JM, Shah AR, Gidday JM. Ischemia-reperfusion injury of retinal endothelium by cyclooxygenase- and xanthine oxidase-derived superoxide. Exp Eye Res. 2002;74:493–501. doi: 10.1006/exer.2001.1156. [DOI] [PubMed] [Google Scholar]
- Robinson GV, Langford BA, Smith DM, Stradling JR. Predictors of blood pressure fall with continuous positive airway pressure (CPAP) treatment of obstructive sleep apnoea (OSA) Thorax. 2008;63:855–859. doi: 10.1136/thx.2007.088096. [DOI] [PubMed] [Google Scholar]
- Seiler KS, Kehrer JP, Starnes JW. Exogenous glutathione attenuates stunning following intermittent hypoxia in isolated rat hearts. Free Radic Res. 1996;24:115–122. doi: 10.3109/10715769609088007. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Prabhakar NR. HIF-1-dependent respiratory, cardiovascular, and redox responses to chronic intermittent hypoxia. Antioxid Redox Signal. 2007;9:1391–1396. doi: 10.1089/ars.2007.1691. [DOI] [PubMed] [Google Scholar]
- Shah BH. Estrogen stimulation of COX-2-derived PGI2 confers atheroprotection. Trends Endocrinol Metab. 2005;16:199–201. doi: 10.1016/j.tem.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Snow JB, Kitzis V, Norton CE, Torres SN, Johnson KD, Kanagy NL, Walker BR, Resta TC. Differential effects of chronic hypoxia and intermittent hypocapnic and eucapnic hypoxia on pulmonary vasoreactivity. J Appl Physiol. 2008;104:110–118. doi: 10.1152/japplphysiol.00698.2005. [DOI] [PubMed] [Google Scholar]
- Somers VK, Mark AL, Abboud FM. Sympathetic activation by hypoxia and hypercapnia: Implications for sleep apnea. Clin Exp Hypertens A. 1988;10(Suppl 1):413–422. doi: 10.3109/10641968809075998. [DOI] [PubMed] [Google Scholar]
- Suzuki YJ, Jain V, Park AM, Day RM. Oxidative stress and oxidant signaling in obstructive sleep apnea and associated cardiovascular diseases. Free Radic Biol Med. 2006;40:1683–1692. doi: 10.1016/j.freeradbiomed.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahawi Z, Orolinova N, Joshua IG, Bader M, Fletcher EC. Altered vascular reactivity in arterioles of chronic intermittent hypoxic rats. J Appl Physiol. 2001;90:2007–2013. doi: 10.1152/jappl.2001.90.5.2007. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Nakamura Y, Nishijima T, Sakurai S, Inoue H. Essential roles of angiotensin II in vascular endothelial growth factor expression in sleep apnea syndrome. Respir Med. 2005;99:1125–1131. doi: 10.1016/j.rmed.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Teramoto S, Kume H, Yamaguchi Y, Yamamoto H, Hanaoka Y, Ishii M, Ishii T, Ouchi Y. Improvement of endothelial function with allopurinol may occur in selected patients with OSA: Effect of age and sex. Eur Respir J. 2007;29:216–217. doi: 10.1183/09031936.00104806. [DOI] [PubMed] [Google Scholar]
- Thomas BJ, Wanstall JC. Alterations in pulmonary vascular function in rats exposed to intermittent hypoxia. Eur J Pharmacol. 2003;477:153–161. doi: 10.1016/j.ejphar.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Tilkian AG, Guilleminault C, Schroeder JS, Lehrman KL, Simmons FB, Dement WC. Hemodynamics in sleep-induced apnea: Studies during wakefulness and sleep. Ann Intern Med. 1976;85:714–719. doi: 10.7326/0003-4819-85-6-714. [DOI] [PubMed] [Google Scholar]
- Troncoso Brindeiro CM, da Silva AQ, Allahdadi KJ, Youngblood V, Kanagy NL. Reactive oxygen species contribute to sleep apnea-induced hypertension in rats. Am J Physiol Heart Circ Physiol. 2007;293:H2971–H2976. doi: 10.1152/ajpheart.00219.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger M, Atkins M, Briscoe WA, King TK. Potentiation of pulmonary vasoconstrictor response with repeated intermittent hypoxia. J Appl Physiol. 1977;43:662–667. doi: 10.1152/jappl.1977.43.4.662. [DOI] [PubMed] [Google Scholar]
- Urbano F, Roux F, Schindler J, Mohsenin V. Impaired cerebral auto-regulation in obstructive sleep apnea. J Appl Physiol. 2008;105:1852–1857. doi: 10.1152/japplphysiol.90900.2008. [DOI] [PubMed] [Google Scholar]
- Yang L, Sameshima H, Yamaguchi M, Ikenoue T. Expression of inducible nitric oxide synthase and cyclooxygenase-2 mRNA in brain damage induced by lipopolysaccharide and intermittent hypoxia-ischemia in neonatal rats. J Obstet Gynaecol Res. 2005;31:185–191. doi: 10.1111/j.1341-8076.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- Zoccal DB, Bonagamba LG, Oliveira FR, Antunes-Rodrigues J, Machado BH. Increased sympathetic activity in rats submitted to chronic intermittent hypoxia. Exp Physiol. 2007;92:79–85. doi: 10.1113/expphysiol.2006.035501. [DOI] [PubMed] [Google Scholar]


