Abstract
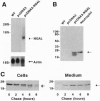
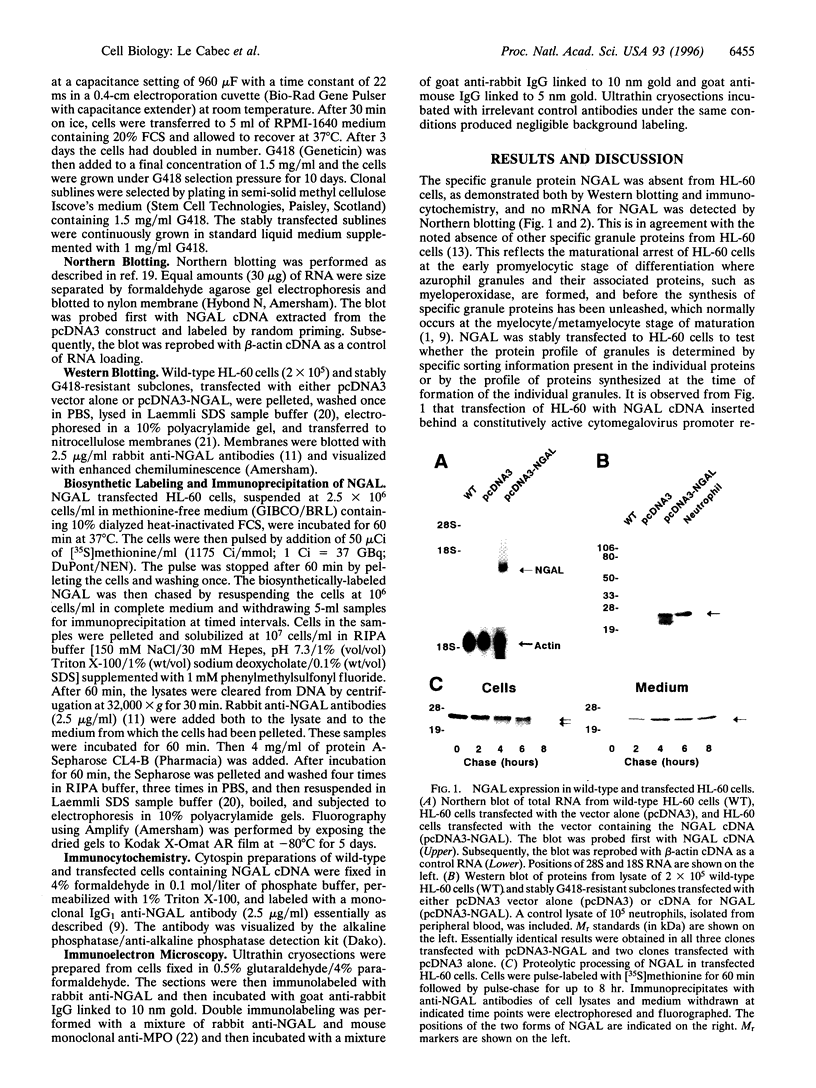
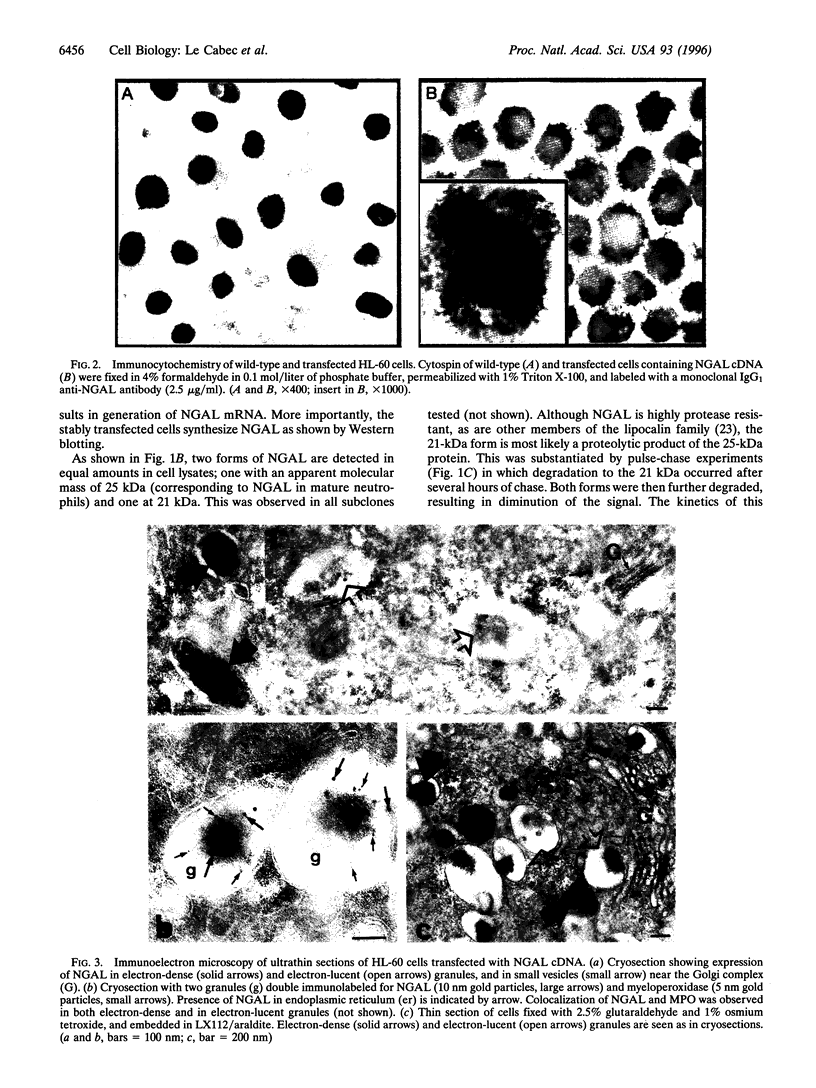
The mechanism of protein targeting to individual granules in cells that contain different subsets of storage granules is poorly understood. The neutrophil contains two highly distinct major types of granules, the peroxidase positive (azurophil) granules and the peroxidase negative (specific and gelatinase) granules. We hypothesized that targeting of proteins to individual granule subsets may be determined by the stage of maturation of the cell, at which the granule proteins are synthesized, rather than by individual sorting information present in the proteins. This was tested by transfecting the cDNA of the specific granule protein, NGAL, which is normally synthesized in metamyelocytes, into the promyelocytic cell line HL-60, which is developmentally arrested at the stage of formation of azurophil granules, and thus does not contain specific and gelatinase granules. Controlled by a cytomegalovirus promoter, NGAL was constitutively expressed in transfected HL-60 cells. This resulted in the targeting of NGAL to azurophil granules as demonstrated by colocalization of NGAL with myeloperoxidase, visualized by immunoelectron microscopy. This shows that targeting of proteins into distinct granule subsets may be determined solely by the time of their biosynthesis and does not depend on individual sorting information present in the proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainton D. F. Neutrophil granules. Br J Haematol. 1975 Jan;29(1):17–22. doi: 10.1111/j.1365-2141.1975.tb01795.x. [DOI] [PubMed] [Google Scholar]
- Berliner N., Hsing A., Graubert T., Sigurdsson F., Zain M., Bruno E., Hoffman R. Granulocyte colony-stimulating factor induction of normal human bone marrow progenitors results in neutrophil-specific gene expression. Blood. 1995 Feb 1;85(3):799–803. [PubMed] [Google Scholar]
- Borregaard N. Current concepts about neutrophil granule physiology. Curr Opin Hematol. 1996 Jan;3(1):11–18. doi: 10.1097/00062752-199603010-00003. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Lollike K., Kjeldsen L., Sengeløv H., Bastholm L., Nielsen M. H., Bainton D. F. Human neutrophil granules and secretory vesicles. Eur J Haematol. 1993 Oct;51(4):187–198. doi: 10.1111/j.1600-0609.1993.tb00629.x. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Sehested M., Nielsen B. S., Sengeløv H., Kjeldsen L. Biosynthesis of granule proteins in normal human bone marrow cells. Gelatinase is a marker of terminal neutrophil differentiation. Blood. 1995 Feb 1;85(3):812–817. [PubMed] [Google Scholar]
- Bundgaard J. R., Sengeløv H., Borregaard N., Kjeldsen L. Molecular cloning and expression of a cDNA encoding NGAL: a lipocalin expressed in human neutrophils. Biochem Biophys Res Commun. 1994 Aug 15;202(3):1468–1475. doi: 10.1006/bbrc.1994.2096. [DOI] [PubMed] [Google Scholar]
- Calafat J., Goldschmeding R., Ringeling P. L., Janssen H., van der Schoot C. E. In situ localization by double-labeling immunoelectron microscopy of anti-neutrophil cytoplasmic autoantibodies in neutrophils and monocytes. Blood. 1990 Jan 1;75(1):242–250. [PubMed] [Google Scholar]
- Collins S. J. The HL-60 promyelocytic leukemia cell line: proliferation, differentiation, and cellular oncogene expression. Blood. 1987 Nov;70(5):1233–1244. [PubMed] [Google Scholar]
- Fouret P., du Bois R. M., Bernaudin J. F., Takahashi H., Ferrans V. J., Crystal R. G. Expression of the neutrophil elastase gene during human bone marrow cell differentiation. J Exp Med. 1989 Mar 1;169(3):833–845. doi: 10.1084/jem.169.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fra A. M., Williamson E., Simons K., Parton R. G. De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8655–8659. doi: 10.1073/pnas.92.19.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabig T. G., Crean C. D., Mantel P. L., Rosli R. Function of wild-type or mutant Rac2 and Rap1a GTPases in differentiated HL60 cell NADPH oxidase activation. Blood. 1995 Feb 1;85(3):804–811. [PubMed] [Google Scholar]
- Gorr S. U., Darling D. S. An N-terminal hydrophobic peak is the sorting signal of regulated secretory proteins. FEBS Lett. 1995 Mar 13;361(1):8–12. doi: 10.1016/0014-5793(95)00142-v. [DOI] [PubMed] [Google Scholar]
- Hayward C. P., Bainton D. F., Smith J. W., Horsewood P., Stead R. H., Podor T. J., Warkentin T. E., Kelton J. G. Multimerin is found in the alpha-granules of resting platelets and is synthesized by a megakaryocytic cell line. J Clin Invest. 1993 Jun;91(6):2630–2639. doi: 10.1172/JCI116502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J. J., Rintels P., Chung J., Sather J., Benz E. J., Jr, Berliner N. Lactoferrin gene promoter: structural integrity and nonexpression in HL60 cells. Blood. 1992 Jun 1;79(11):2998–3006. [PubMed] [Google Scholar]
- Johnston J., Bollekens J., Allen R. H., Berliner N. Structure of the cDNA encoding transcobalamin I, a neutrophil granule protein. J Biol Chem. 1989 Sep 25;264(27):15754–15757. [PubMed] [Google Scholar]
- Kjeldsen L., Bainton D. F., Sengeløv H., Borregaard N. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood. 1994 Feb 1;83(3):799–807. [PubMed] [Google Scholar]
- Kjeldsen L., Bainton D. F., Sengeløv H., Borregaard N. Structural and functional heterogeneity among peroxidase-negative granules in human neutrophils: identification of a distinct gelatinase-containing granule subset by combined immunocytochemistry and subcellular fractionation. Blood. 1993 Nov 15;82(10):3183–3191. [PubMed] [Google Scholar]
- Kjeldsen L., Johnsen A. H., Sengeløv H., Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993 May 15;268(14):10425–10432. [PubMed] [Google Scholar]
- Olsson I., Persson A. M., Strömberg K. Biosynthesis, transport and processing of myeloperoxidase in the human leukaemic promyelocytic cell line HL-60 and normal marrow cells. Biochem J. 1984 Nov 1;223(3):911–920. doi: 10.1042/bj2230911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitsch M. C., Boguski M. S. The first lipocalin with enzymatic activity. Trends Biochem Sci. 1991 Oct;16(10):363–363. doi: 10.1016/0968-0004(91)90149-p. [DOI] [PubMed] [Google Scholar]
- Sengeløv H., Follin P., Kjeldsen L., Lollike K., Dahlgren C., Borregaard N. Mobilization of granules and secretory vesicles during in vivo exudation of human neutrophils. J Immunol. 1995 Apr 15;154(8):4157–4165. [PubMed] [Google Scholar]
- Sengeløv H., Kjeldsen L., Borregaard N. Control of exocytosis in early neutrophil activation. J Immunol. 1993 Feb 15;150(4):1535–1543. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujishita Y., Asaoka Y., Nishizuka Y. Regulation of phospholipase A2 in human leukemia cell lines: its implication for intracellular signaling. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6274–6278. doi: 10.1073/pnas.91.14.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Saffaripour S., Bonfanti R., Sadler J. E., Cramer E. M., Chapman B., Mayadas T. N. Induction of specific storage organelles by von Willebrand factor propolypeptide. Cell. 1991 Jan 25;64(2):403–413. doi: 10.1016/0092-8674(91)90648-i. [DOI] [PubMed] [Google Scholar]