Abstract
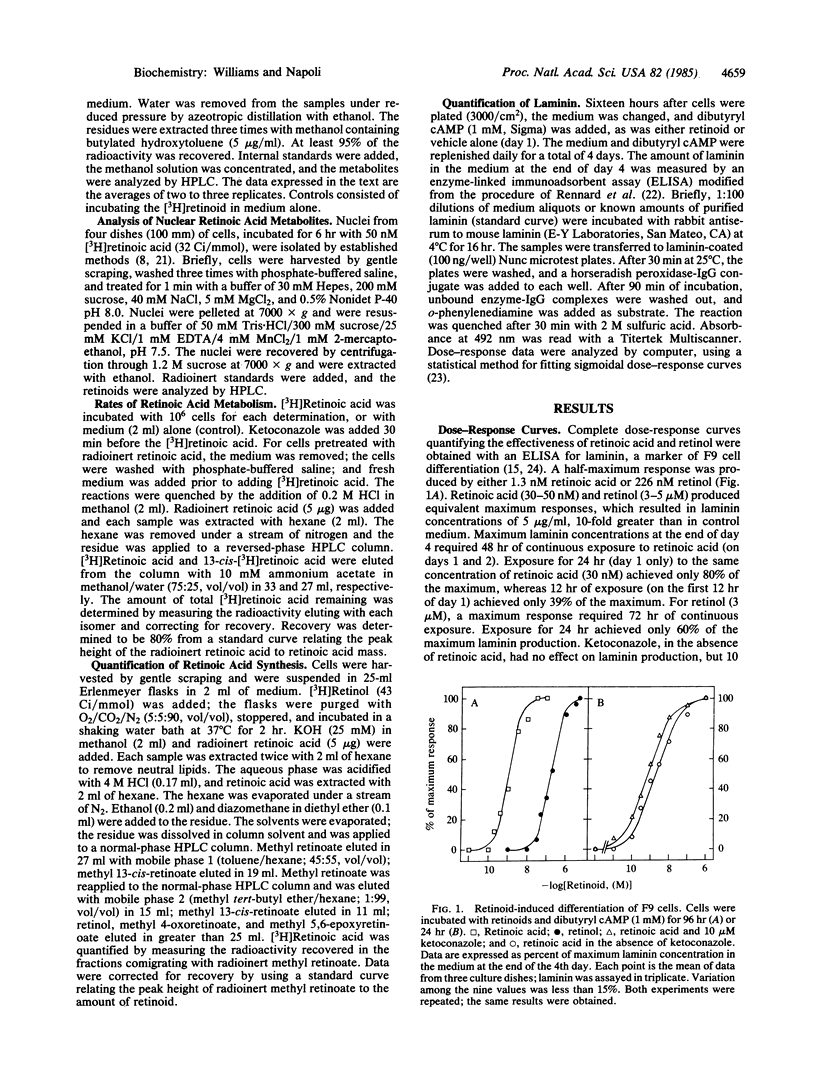
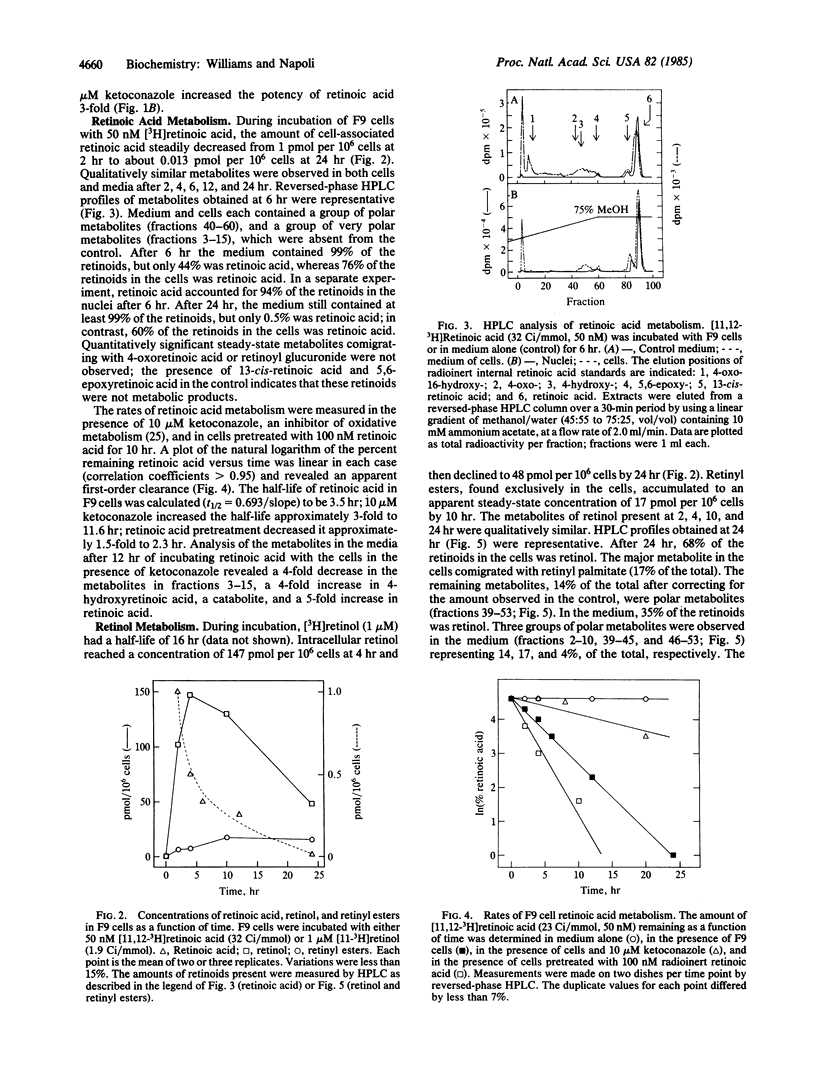
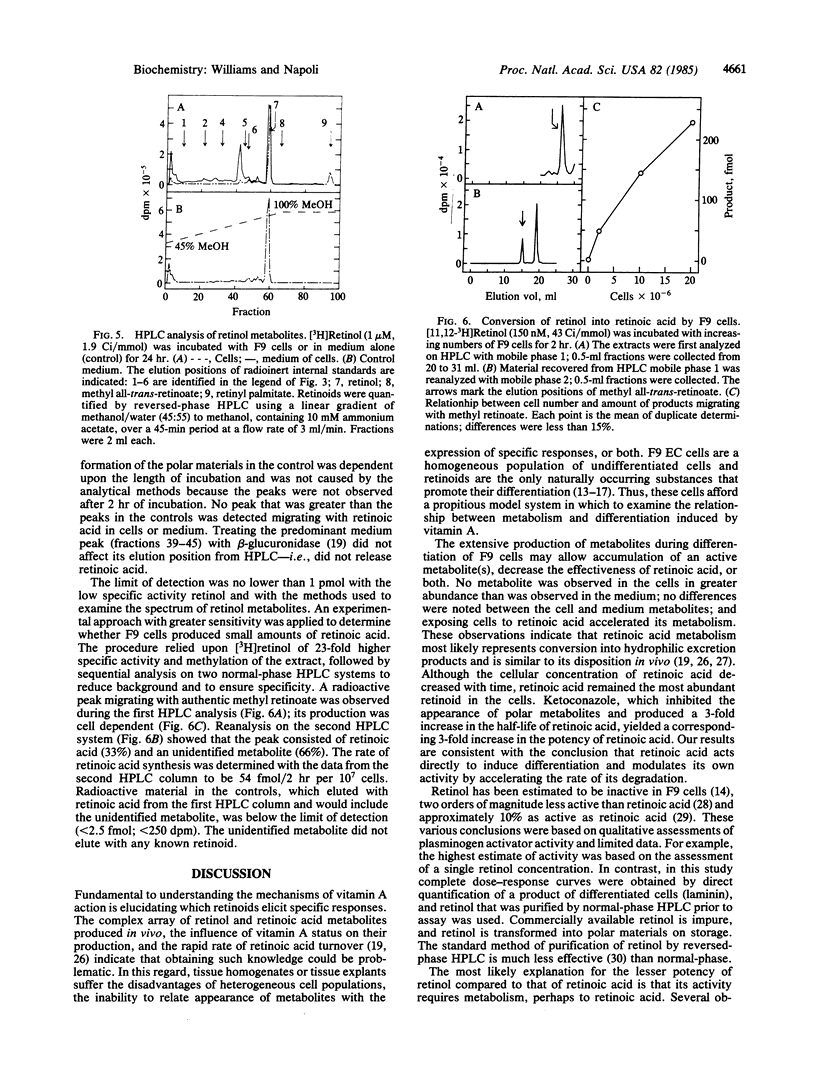
Retinol and retinoic acid dose-response curves were obtained for promotion of the differentiation of F9 murine embryonal carcinoma cells with an enzyme-linked immunoadsorbent assay for laminin, a product of differentiated F9 cells. Retinoic acid produced a half-maximum response at 1.3 nM and a maximum response at about 30 nM; retinol was 1/175th as potent. Maximum differentiation required 48 hr of continuous exposure to retinoic acid, whereas retinol required 72 hr of exposure. The half-time of retinoic acid conversion into polar metabolites was 3.5 hr; metabolism was accelerated by pretreating F9 cells with retinoic acid. An inhibitor of oxidative metabolism, ketoconazole, decreased the rate of retinoic acid metabolism and decreased the concentration of retinoic acid required to produce a half-maximum response. Unchanged retinoic acid was the sole compound isolated from nuclei of F9 cells incubated with retinoic acid. Retinol had a half-life approximately 5-fold longer than retinoic acid, attained greater cell concentrations, and was converted into retinoic acid by F9 cells. These data indicate that retinoic acid itself directs the differentiation of F9 cells and may mediate differentiation induced by retinol.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolmer S. D., Wolf G. Retinoids and phorbol esters alter release of fibronectin from enucleated cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6541–6545. doi: 10.1073/pnas.79.21.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin B. E., Durkin M. E., Bender B., Jaffe R., Chung A. E. Synthesis of laminin and entactin by F9 cells induced with retinoic acid and dibutyryl cyclic AMP. J Biol Chem. 1983 Jun 25;258(12):7729–7737. [PubMed] [Google Scholar]
- Chytil F., Ong D. E. Cellular retinol- and retinoic acid-binding proteins in vitamin A action. Fed Proc. 1979 Oct;38(11):2510–2514. [PubMed] [Google Scholar]
- Dowling J. E., Wald G. THE BIOLOGICAL FUNCTION OF VITAMIN A ACID. Proc Natl Acad Sci U S A. 1960 May;46(5):587–608. doi: 10.1073/pnas.46.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evain D., Binet E., Anderson W. B. Alterations in calcitonin and parathyroid hormone responsiveness of adenylate cyclase in F9 embryonal carcinoma cells treated with retinoic acid and dibutyryl cyclic AMP. J Cell Physiol. 1981 Dec;109(3):453–459. doi: 10.1002/jcp.1041090311. [DOI] [PubMed] [Google Scholar]
- Goodman D. S. Vitamin A metabolism. Fed Proc. 1980 Aug;39(10):2716–2722. [PubMed] [Google Scholar]
- Jetten A. M., De Luca L. M. Induction of differentiation of embryonal carcinoma cells by retinol: possible mechanisms. Biochem Biophys Res Commun. 1983 Jul 29;114(2):593–599. doi: 10.1016/0006-291x(83)90821-5. [DOI] [PubMed] [Google Scholar]
- Kalin J. R., Wells M. J., Hill D. L. Effects of phenobarbital, 3-methylcholanthrene, and retinoid pretreatment on disposition of orally administered retinoids in mice. Drug Metab Dispos. 1984 Jan-Feb;12(1):63–67. [PubMed] [Google Scholar]
- Liau G., Ong D. E., Chytil F. Interaction of the retinol/cellular retinol-binding protein complex with isolated nuclei and nuclear components. J Cell Biol. 1981 Oct;91(1):63–68. doi: 10.1083/jcb.91.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder S., Krondahl U., Sennerstam R., Ringertz N. R. Retinoic acid-induced differentiation of F9 embryonal carcinoma cells. Exp Cell Res. 1981 Apr;132(2):453–460. doi: 10.1016/0014-4827(81)90120-8. [DOI] [PubMed] [Google Scholar]
- Mach M., Ebert P., Popp R., Ogilvie A. Compartmentalization of polyamines in mammalian cells. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1327–1334. doi: 10.1016/0006-291x(82)91395-x. [DOI] [PubMed] [Google Scholar]
- Martin G. R. Teratocarcinomas and mammalian embryogenesis. Science. 1980 Aug 15;209(4458):768–776. doi: 10.1126/science.6250214. [DOI] [PubMed] [Google Scholar]
- McCormick A. M., Napoli J. L. Identification of 5,6-epoxyretinoic acid as an endogenous retinol metabolite. J Biol Chem. 1982 Feb 25;257(4):1730–1735. [PubMed] [Google Scholar]
- Mehta R. G., Cerny W. L., Moon R. C. Nuclear interactions of retinoic acid-binding protein in chemically induced mammary adenocarcinoma. Biochem J. 1982 Dec 15;208(3):731–736. doi: 10.1042/bj2080731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli J. L., Khalil H., McCormick A. M. Metabolism of 5,6-epoxyretinoic acid in vivo: isolation of a major intestinal metabolite. Biochemistry. 1982 Apr 13;21(8):1942–1949. doi: 10.1021/bi00537a038. [DOI] [PubMed] [Google Scholar]
- Napoli J. L., McCormick A. M. Tissue dependence of retinoic acid metabolism in vivo. Biochim Biophys Acta. 1981 Oct 23;666(1):165–175. doi: 10.1016/0005-2760(81)90102-8. [DOI] [PubMed] [Google Scholar]
- Napoli J. L., Pramanik B. C., Williams J. B., Dawson M. I., Hobbs P. D. Quantification of retinoic acid by gas-liquid chromatography-mass spectrometry: total versus all-trans-retinoic acid in human plasma. J Lipid Res. 1985 Mar;26(3):387–392. [PubMed] [Google Scholar]
- Omori M., Chytil F. Mechanism of vitamin A action. Gene expression in retinol-deficient rats. J Biol Chem. 1982 Dec 10;257(23):14370–14374. [PubMed] [Google Scholar]
- Ong D. E., Crow J. A., Chytil F. Radioimmunochemical determination of cellular retinol- and cellular retinoic acid-binding proteins in cytosols of rat tissues. J Biol Chem. 1982 Nov 25;257(22):13385–13389. [PubMed] [Google Scholar]
- Plet A., Evain D., Anderson W. B. Effect of retinoic acid treatment of F9 embryonal carcinoma cells on the activity and distribution of cyclic AMP-dependent protein kinase. J Biol Chem. 1982 Jan 25;257(2):889–893. [PubMed] [Google Scholar]
- Rennard S. I., Berg R., Martin G. R., Foidart J. M., Robey P. G. Enzyme-linked immunoassay (ELISA) for connective tissue components. Anal Biochem. 1980 May 1;104(1):205–214. doi: 10.1016/0003-2697(80)90300-0. [DOI] [PubMed] [Google Scholar]
- Rodbard D., McClean S. W. Automated computer analysis for enzyme-multiplied immunological techniques. Clin Chem. 1977 Jan;23(1):112–115. [PubMed] [Google Scholar]
- Sasak W., De Luca L. M., Dion L. D., Silverman-Jones C. S. Effect of retinoic acid on cell surface glycopeptides of cultured spontaneously transformed mouse fibroblasts (BALB/c 3T12-3 cells). Cancer Res. 1980 Jun;40(6):1944–1949. [PubMed] [Google Scholar]
- Sheets J. J., Mason J. I. Ketoconazole: a potent inhibitor of cytochrome P-450-dependent drug metabolism in rat liver. Drug Metab Dispos. 1984 Sep-Oct;12(5):603–606. [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Strickland S., Sawey M. J. Studies on the effect of retinoids on the differentiation of teratocarcinoma stem cells in vitro and in vivo. Dev Biol. 1980 Jul;78(1):76–85. doi: 10.1016/0012-1606(80)90319-x. [DOI] [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Williams J. B., Pramanik B. C., Napoli J. L. Vitamin A metabolism: analysis of steady-state neutral metabolites in rat tissues. J Lipid Res. 1984 Jun;25(6):638–645. [PubMed] [Google Scholar]



