Abstract
Precursor-to-product ratios in steroid hormone metabolism may accurately reflect enzymatic activity and production of metabolites relative to their disappearance. The purpose of this study was to explore the use of direct precursor-to-product steroid ratios to discriminate between infants with congenital adrenal hyperplasia (CAH) due to 21- α -hydroxylase deficiency and infants with no disorder, thus characterizing the biochemical phenotype in CAH. Deidentified dried blood spot samples from confirmed CAH cases identified by newborn screen (CAH-positive, N = 8) and from cases with no disorder (CAH-negative, N = 10) were obtained from the California State Newborn Screening Program. Samples (∼6.25 mm circular spots) underwent methanol and water extraction (9:1 ratio). Deuterated steroids served as isotope internal standards. 17-α-hydroxyprogesterone (17-OHP), 11-deoxycortisol (S), androstenedione (A4) and cortisol (F) concentrations were determined by liquid chromatography–tandem mass spectrometry (LC–MS/MS), and the 17-OHP/S, 17-OHP/A4, and S/F ratios were calculated. The mean 17-OHP and A4 concentrations in samples from CAH cases were significantly increased when compared to cases with no disorder (p = 0.003 for both). 17-OHP/S and 17-OHP/A4 ratios were also significantly elevated in CAH cases (p = 0.007 and p < 0.001, respectively). In contrast, S and F concentrations and the S/F ratio were similar between the two groups. In CAH, the elevated 17-OHP/S ratio is a biomarker of diminished 21-α-hydroxylase activity, and the elevated 17-OHP/A4 ratio is a biomarker of adrenal androgen excess via increased 17,20-lyase activity. The similar S/F ratio indicates that the rate of production via 11-β-hydroxylase and disappearance of F is maintained in CAH.
Keywords: Congenital adrenal hyperplasia, Steroid profiling, Precursor-to-product ratios, Newborn screening
1 Introduction
Congenital adrenal hyperplasia (CAH) is the most common pediatric disorder of the adrenal gland, consisting of several autosomal recessive disorders affecting one of the enzymes required for cortisol synthesis. In each disorder, decreased cortisol production leads to increased pituitary secretion of adrenocorticotropin hormone (ACTH) and adrenal insufficiency with hyperandrogenism. In the Caucasian population, 21-α-hydroxylase (P450c21) deficiency (i.e., classical CAH, OMIM #201910), which is often accompanied by severe salt wasting, accounts for over 90 % of cases, while 11-β-hydroxylase (P45011 β) deficiency accounts for 5 % of cases (Joint 2002; Speiser et al. 2010b). In California, the incidence of CAH due to 21-α-hydroxylase deficiency is approximately 1:12,000 live births, thus accounting for 40–50 newborns identified each year with classical CAH (Health 2012).
Individuals with classical CAH are known to have elevated levels of 17-α-hydroxyprogesterone (17-OHP) (17-α-hydroxypregn-4-ene-3,20-dione) due to deficiency of 21-α-hydroxylase. Thus, 17-OHP is the initial step in screening for CAH in many states, and is typically measured by radioimmunoassay (RIA) methods (White 2009). However, elevated 17-OHP may occur in other conditions, such as stress-induced elevation of 17-OHP values in preterm, low birth weight, and sick infants (Ersch et al. 2008; Fingerhut 2009). Adjusting 17-OHP cut-offs for birth weight and gestational age have not allowed for sufficient discrimination between neonates with CAH versus those with transiently elevated 17-OHP (Matern et al. 2007). The advent of liquid chromatography–tandem mass spectrometry (LC–MS/MS) has enabled the application of steroid profiling to different clinical scenarios, such as differentiating the cause of adrenal insufficiency (Holst et al. 2007). LC–MS/MS is more sensitive and specific than radioimmunoassay, and it has the advantage of measuring multiple analytes with a relatively low sample volume (Soldin and Soldin 2009).
Steroid profiling using LC–MS/MS has been used to accurately measure multiple intermediary steroid metabolites. The traditional approach to steroid profiling in CAH involves using absolute concentration cutoffs of steroid metabolites for diagnostic purposes (Minutti et al. 2004). 17-OHP, androstenedione (A4) (androst-4-ene-3,17-dione), and cortisol (F) (11β,17α,21-trihydroxypregn-4-ene-3,20-dione) have been accurately measured in DBS using LC–MS/MS (Minutti et al. 2004). The ratio of (17-OHP + A4)/F, developed by the Mayo Clinic, has been used as a “second-tier” test in neonatal screening to discriminate between true CAH cases and CAH neonates with transient 17-OHP elevation (Lacey et al. 2004; Matern et al. 2007). The calculation of the steroid ratio in the second-tier test is based on the observation that concentrations of steroids behind the enzyme defect (i.e., 17-OHP and A4) are usually elevated, while concentrations of steroid products downstream from the defective enzyme (i.e., F) are relatively low. Thus, the ratio of (17-OHP + A4)/F in samples of affected infants are greater than the ratios in unaffected infants. Janzen et al. have proposed measuring 21-deoxy-cortisol (21-DOF) (11β,17α-dihydroxypregn-4-ene-3,20-dione) alone or calculating an alternative ratio of (17-OHP + 21-DOF/F) to differentiate between neonates with and without CAH, as 21-DOF is produced from 11-β-hydroxylation of 17-OHP as an alternative pathway when conversion of 17-OHP to 11-deoxycortisol (S) (Reichstein's compound S; 17α,21-dihydroxypregn-4-ene-3,20-dione) is impaired (Janzen et al. 2007). However, these previous LC–MS/MS steroid profiling efforts to characterize neonates with CAH have focused on empiric steroid metabolite concentration ratios, in which there is no direct relationship between the numerator(s) and denominator. Peter et al. calculated pre- and post-corticotropin stimulated plasma steroid levels by RIA to identify CAH heterozygotes and found that heterozygotes had significantly higher post-corticotropin 17-OHP/S ratios than normal relatives (Peter et al. 1990). However, this direct precursor-to-product ratio test was not performed on neonates, and the steroids were measured via RIA, which has a high degree of cross-reactivity with antibodies and other steroids (Fingerhut 2009). Furthermore, the implication of the 17-OHP/S precursor-to-product ratio as a marker of 21-α-hydroxylase enzymatic function was not addressed. In a system of connected pathways, the concentration of a metabolic intermediate is determined by its production and disappearance rates. Therefore, the change in steroid (precursor or product) concentration alone cannot be accurately predicted solely on changes in enzyme kinetics of 21-α-hydroxylase.
The direct precursor-to-product ratio can provide useful dynamic information about the network of pathways in cortisol biosynthesis, and has been utilized as an indirect measure of adrenocortical enzyme activity in very low birth weight preterm infants, as compared with term healthy infants (Hingre et al. 1994). For example, at metabolic steady state, the ratio of 17-OHP to S is a measure of the concentration-dependent rate of appearance of S, relative to the rate of its disposal. We hypothesize that since 17-OHP is a substrate of 21-α-hydroxylase, and the precursor of S, the precursor-to-product ratio may accurately reflect the enzyme deficiency. In addition, the ratio of 17-OHP/A4 may reflect 17,20-lyase (P450-17α) function, and the ratio of S/F may reflect 11-β-hydroxylase function, in the presence of 21-α-hydroxylase deficiency. We present here a study of precursor-to-product ratios from steroid profiles of DBS from known CAH-positive and CAH-negative samples. The aim of this study was to clarify the biochemical abnormalities in infants with CAH through use of direct precursor-to-product ratios as an additional discriminatory test between infants with 21-α-hydroxylase deficiency and infants with no disorder.
2 Materials and methods
2.1 Theoretical considerations
The concentration of a metabolic intermediate is generally determined by the balance between its production and its disappearance. The production of a compound B from its precursor A is given by the rate equation [A] × Kp, where Kp is the first order rate constant of production, and [A] is the concentration of the precursor A. The disappearance of compound B is given by [B] × Kd, where Kd is the rate constant of disappearance, and [B] the concentration of the product B. At steady state, the production rate of B is equal to its disappearance rate [A] × Kp = [B] × Kd, such that [A]/[B] = Kd/Kp. In situations where the production rate and disappearance rate are not in balance, the calculated precursor-to-product ratio reflects the inequality. For example, if the production rate is greater than the disappearance rate [A] × Kp > [B] × Kd, then the precursor-to-product ratio [A]/[B] > [Kd] / [Kp]. Finally, when the disappearance of B is independent of its concentration, there is no correlation between precursor and product.
In 21-α-hydroxylase deficiency, the rate of production of is low relative to a normal individual, thus Kd/Kp′ ≫ Kd/Kp. Consequently, the precursor-to-product ratio ([A] / [B]), i.e., 17-OHP/S ratio, should be elevated, thereby reflecting the decrease in enzyme activity. In the conversion of 17-OHP to A4 in CAH, both production and disappearance of A4 are elevated. In this case, an elevated precursor-to-product ratio [A]/[B] > Kd/Kp, would imply that the production of A4 by 17,20-lyase exceeds its disappearance and the system is in quasi-steady state. Since the enzymatic block in CAH is proximal to S, the S/F ratio reflects the production of F by 11-β-hydroxylase in CAH-positive neonates relative to CAH-negative controls. In this study, three precursor-to-product ratios were determined using the steroid profile: 17-OHP/S, 17-OHP/A4, and S/F. These ratios should be informative of the dynamic relationship among the steroid intermediates.
2.2 Samples
The study was conducted with approval from the Human Subjects Committee at the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center. The Helsinki Declaration does not apply to this study, as the samples were deidentified. DBS samples of confirmed positive CAH cases (N = 8) and negative cases (i.e., normal controls, N = 10) from the period 2009–2010 were obtained from the California State Newborn Screening Program. The birth weights of infants in the CAH-positive group ranged between 2,450 and 4,600 g; the female to male ratio (F/M) was 5/3 and the initial values of 17-OHP by FIA (i.e., first-tier test) ranged between 220 and 750 nmol/L. The birth weights of infants in the CAH-negative control group ranged between 1,700 and 4,000 g; the F/M ratio was 1/9 and the initial values of 17-OHP by FIA ranged between 7.0 and 90 nmol/L. Gestational age was not available for the samples, as it is not routinely collected by the Newborn Screening Program. However, the low birth weight samples have a higher likelihood of belonging to preterm neonates.
2.3 Isotope standard mixture
HPLC-grade methanol (MeOH), water (H2O), and formic acid were purchased from Fisher Scientific (Waltham, MA). Reagent-grade 17-OHP, A4, S, and F were purchased from Sigma-Aldrich (St. Louis, MO). Stable isotopelabeled steroids (all >98 % enriched): (2,2,4,6,6,21,21,21-D8)-17-hydroxyprogesterone (d8-17-OHP), (21,21-D2)-11-deoxycortisol (d2-11-S), (2,2,4,6,6,16,16-D7)-4-androstene- 3,17-dione (d7-A4) and (9, 12, 12-D3)-cortisol (d3-F) were purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA). A stock solution containing these labeled steroids was prepared and the internal standards were diluted with MeOH to final concentrations of 20 ng/mL for d8-17-OHP, d7-A4, and d2-S, and 200 ng/mL for d3-F.
2.4 Sample preparation
Extraction of steroids from DBS was carried out using a modified method of (Dhillon et al. 2011). Briefly, circular (∼6.25 mm) discs were punched out from each DBS sample and combined with 20 μL of isotope standard (IS) mixture and 100 μL of 0.9 % saline. Samples were shaken at room temperature for 1 h, 900 μL of MeOH was added, and samples were again shaken at room temperature for an hour. After centrifuging for 15 min at 3,000 rpm, the supernatant was collected and air dried, then reconstituted with 100 μL of 25 % MeOH in H2O with 0.1 % formic acid. Residues were removed using a 0.20 μm ultra-free centrifugal PTFE membrane filter (Millipore, Billerica, MA), and the filtrate was analyzed by LC–MS/MS.
2.5 Internal standards and calibration curves
Two kinds of calibration standards were prepared to determine the efficiency of the extraction method—MeOH and DBS. Seven standards were prepared by dissolving steroids in 25 % MeOH in H2O with 0.1 % formic acid with the following final concentration ranges: 0–50 ng/mL for 17-OHP, 0–25 ng/mL for A4 and S, and 0–375 ng/mL for F. 50 μL of each MeOH calibration standard was pipetted into a glass tube with 20 μL of IS mixture. After vortexing and drying, the mixture was reconstituted with 100 μL of 25 % MeOH in H2O with 0.1 % formic acid, then transferred to an LC autosampler vial for LC—MS/MS analysis.
The DBS calibration standards were prepared according to a method reported by (Lacey et al. 2004) using washed red blood cells and steroid-free serum (stripped serum). The final concentrations of added steroids were the same as those in the MeOH calibration standards. The DBS calibration standards were extracted and treated in the same manner prior to LC–MS/MS analysis as described in the Sample Preparation section above.
2.6 LC–MS/MS method
Steroid analysis was conducted using a Shimadzu HPLC system (Columbia, MD) attached to an Applied Biosystems API-5000 LC–MS/MS (Foster City, CA). A C18 HPLC column (50 mm × 2.1 mm × 2.6 μm) (Cat # 00B-4462-AN, Phenomenex, Torrance, CA) was used with a gradient profile at a flow rate of 0.40 mL/min with a mobile phase of MeOH and 98 % H2O (2 % MeOH, 0.1 % formic acid) (MeOH-FA). The column was equilibrated in 65 % MeOH/35 % MeOH-FA, and steroids were eluted with 10 % MeOH/90 % MeOH-FA, which was later switched to 35 % of MeOH-FA after 6 min. 10 μL of each sample was submitted for analysis. The steroids were ionized using an atmospheric pressure chemical ionization (APCI) source. The parent (Q1) and daughter (Q3) ions as well as the retention times for the steroids are shown in Table 1.
Table 1. LC–MS/MS parameters for the steroid assays.
| Steroid | Retention time (min) | Molecular wt. (Dalton) | Parent ion (Q1) (m/z) | Daughter ion (Q3) (m/z) |
|---|---|---|---|---|
| Cortisol | 3.60 | 362.46 | 363.40 | 121.40 |
| d3-Cortisol | 366.10 | 123.10 | ||
| 11-Deoxycortisol | 4.15 | 346.46 | 347.10 | 97.10 |
| d2-11-Deoxycortisol | 349.20 | 97.20 | ||
| Androstenedione | 4.48 | 286.40 | 287.20 | 97.20 |
| d7-Androstenedione | 294.10 | 113.00 | ||
| 17-α-Hydroxyprogesterone | 4.80 | 330.46 | 331.20 | 97.00 |
| d8-17-α-Hydroxyprogesterone | 339.20 | 100.20 |
2.7 Calculations and data analysis
The ion intensity of each chromatographic peak was first integrated using Analyst® software. The ratio of the area under each steroid analyte and its corresponding isotope standard (A/I ratio) in the calibration standards was calculated. The A/I ratios were then plotted against the concentrations of the corresponding steroid in the calibration standards. The slope of the calibration curve represents the response of the A/I ratio relative to its respective calibration standard concentration (ng/mL) (Table 2). The calculated concentrations of steroids in each newborn screening DBS sample were determined by the observed A/I ratios of the steroids divided by the corresponding slopes (from Table 2) of the matching DBS calibration standards.
Table 2. Comparison between methanol (MeOH) and dried blood spots (DBS) calibration standards.
| MeOH calibration standards | DBS calibration standardsa | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Steroid | Slope (ng/mL) | Intercept | R2 | Slope (ng/mL)b | Intercept | R2 | Ratio of slopes MeOH/DBS |
| A4b | 0.098 ± 0.002 | 0.099 ± 0.019 | 0.999 | 0.022 ± 0.001 | 0.086 ± 0.013 | 0.989 | 4.55 |
| Fc | 0.045 ± 0.001 | 0.054 ± 0.108 | 0.999 | 0.0075 ± 0.0003 | 0.061 ± 0.044 | 0.995 | 6.59 |
| Sd | 0.094 ± 0.002 | 0.018 ± 0.017 | 0.999 | 0.017 ± 0.001 | 0.042 ± 0.005 | 0.997 | 5.42 |
| 17-OHPe | 0.264 ± 0.013 | 0.109 ± 0.295 | 0.991 | 0.032 ± 0.001 | 0.047 ± 0.017 | 0.998 | 8.12 |
The determined slopes from DBS calibration curves were used to calculate the concentrations (ng/mL) of steroids in newborn screening DBS samples
Androstenedione
Cortisol
11-Deoxycortisol
17-α-Hydroxyprogesterone
The means and standard deviations of the steroids in samples from the CAH-negative and CAH-positive groups were calculated. The null hypothesis that there were no differences in means of steroid concentrations and precursor-to-product ratios between the CAH-positive and CAH-negative groups was tested using t tests. The correlation between each precursor-to-product ratio and 17-OHP concentration was determined by linear regression analysis using Microsoft Excel®. A p value <0.05 was considered to be statistically significant for all analyses.
3 Results and discussion
3.1 Calibration curves
The accuracy and precision of quantitative steroid analysis by LC–MS/MS were demonstrated by the calibration curves. The mean and standard deviation of the slope and the respective intercept for each steroid are shown in Table 2. The linearity of each calibration curve was demonstrated by the excellent coefficient of regression (R2 ≥ 0.99) values for both the MeOH and DBS calibration standards. The standard deviations of the slopes were small (∼2–5 %) relative to the estimates of the slopes. The response of the A/I ratio to the changing concentrations of the steroid in the DBS standards, which is the basis for the estimation of the slope, is substantially less than that of the MeOH calibration standards. Consistent with previous reports, the difference in DBS standards (ratio of slopes of MeOH/DBS standards in Table 2) may be attributable to the low extraction efficiency and perhaps protein binding of steroids (Janzen et al. 2007). The backgrounds in DBS calibration standards representing residual concentrations of steroids in washed red blood cells and stripped serum were estimated by dividing the intercepts by the respective slopes, and ranged from 1 to 10 ng/mL. Therefore, only the slope for each steroid from the DBS calibration standard was used in the calculation of steroid concentration in DBS newborn screening samples.
3.2 Steroid concentrations
Consistent with their first-tier immunoassay testing results, the steroid concentrations between CAH-negative and CAH-positive samples were widely separated (Table 3).
Table 3. Means, standard deviations, and ranges of steroid concentrations (ng/mL) for CAH-negative and CAH-positive DBS samples.
| CAH-negative (N = 10) | CAH-positive (N = 8) | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Steroid | Mean ± SD | Range | Mean ± SD | Range | p valuea |
| 17-OHPb | 1.79 ± 0.89 | 0.33–2.68 | 91.23 ± 67.29 | 15.75–198.85 | 0.004 |
| A4c | 4.30 ± 0.46 | 4.01–5.46 | 29.04 ± 18.49 | 8.19–57.33 | 0.003 |
| Sd | 3.25 ± 0.76 | 2.22–4.66 | 4.82 ± 2.35 | 3.30–10.30 | 0.050 |
| Fe | 34.00 ± 29.65 | 2.66–92.12 | 18.85 ± 8.80 | 5.93–30.50 | 0.077 |
| 17-OHP/S | 0.57 ± 0.32 | 0.17–1.22 | 23.93 ± 20.06 | 1.78–60.16 | 0.007 |
| 17-OHP/A4 | 0.41 ± 0.20 | 0.08–0.67 | 3.05 ± 1.20 | 1.47–4.66 | 0.0002 |
| S/F | 0.27 ± 0.36 | 0.05–1.77 | 0.44 ± 0.54 | 0.15–1.74 | 0.54 |
p values are results of t-tests comparing differences between CAH-negative and CAH-positive groups
17-α-Hydroxyprogesterone
Androstenedione
11-Deoxycortisol
Cortisol
The average concentration for 17-OHP was 1.79 ng/mL (5.39 nmol/L)1 for CAH-negative, and 91.23 ng/mL (276 nmol/L), for CAH-positive samples. These values fall into the reported ranges for preterm and healthy term infants with no disorder (3.38–171 nmol/L), and those with CAH (15.8–1105 nmol/L) (Janzen et al. 2007). The average concentration for A4 was 4.30 ng/mL (12.4 nmol/L) for CAH-negative, and 29.04 ng/mL (83.8 nmol/L) for CAH-positive samples. These values also fall into the reported ranges for CAH-negative cases (1.10–79.4 nmol/L) and CAH-positive cases (2.24–427 nmol/L), respectively (Janzen et al. 2007). The 17-OHP and A4 concentrations from CAH-positive samples were significantly increased compared with those from CAH-negative samples (p = 0.004, and p = 0.003, respectively). Consistent with previous reports, the concentrations of S and F in the CAH-positive group overlapped with those in the CAH-negative group (Janzen et al. 2007; Mitchell and Hermos 1998). Of the precursor-to-product ratios, both the 17-OHP/S and 17-OHP/A4 ratios of the CAH-positive infants were significantly increased compared with those of CAH-negative infants. On the other hand, there was no difference in the S/F ratios between the two sample groups.
3.3 Steroid ratios
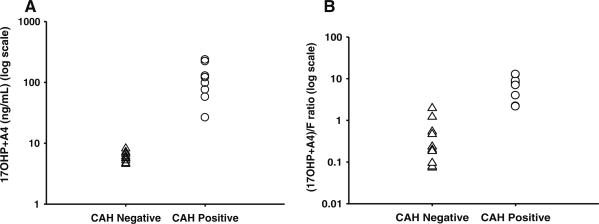
The lack of separation of the F concentrations is propagated into the second-tier ratio (17-OHP + A4)/F (Fig. 1). The distribution of (17-OHP + A4) in CAH-positive samples was clearly separate from CAH-negative samples (Fig. 1a). However, the distribution of (17-OHP + A4)/F in the two groups overlapped (Fig. 1b).
Fig. 1.
Comparison of results from dried blood spots of alternative second-tier test without cortisol, denoted as F (a) versus current second-tier test with F (b). triangle-CAH-negative, circle-CAH positive. Distributions of second-tier newborn screening results in CAH-negative and CAH-positive cases are shown either as 17-OHP + A4 concentrations only (a) or (17-OHP + A4)/F ratio (b). Dividing the sum of 17-OHP and A4 concentrations by the concentration of F does not improve upon the separation between normal CAH-negative and CAH-positive cases
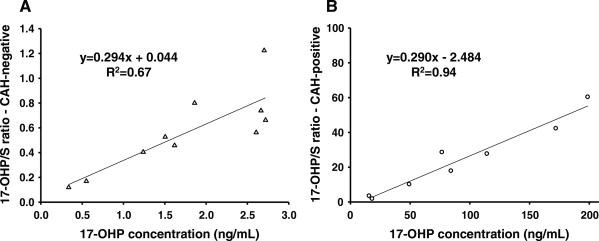
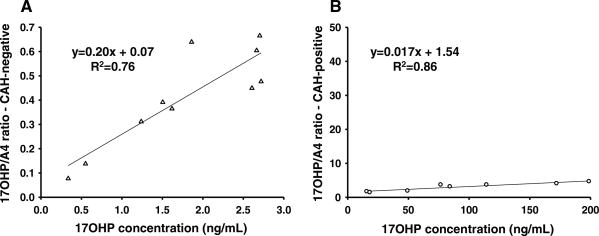
The relationship between precursor-to-product ratio and the 17-OHP concentration was illustrated by plotting the 17-OHP/S and 17-OHP/A4 ratios against 17-OHP concentrations in Figs. 2 and 3. Figure 2 shows the 17-OHP/S ratio relative to varying 17-OHP concentrations in CAH-negative (Fig. 2a) and CAH-positive (Fig. 2b) infants. The slopes of the two graphs were similar. However, the 17-OHP intercepts of the regression equations were different, such that concentrations in CAH-positive samples were much higher (Fig. 2b), and the resulting 17-OHP/S ratios were also much higher. Figure 3 shows the 17-OHP/A4 ratio associated with varying 17-OHP concentrations in CAH-negative (Fig. 3a) and CAH-positive (Fig. 3b) infants. The slope of the regression in the CAH-negative group was much greater than the slope in the CAH-positive group. The two linear regression lines intersect at a 17-OHP concentration of 8.12 ng/mL.
Fig. 2.
The 17-OHP/S ratios plotted against 17-OHP concentrations from DBS in CAH-negative (a) and CAH-positive cases (b). Triangle CAH-negative, Circle CAH positive. The linear regression between 17-OHP/S ratios and 17-OHP concentrations shows a relationship given by the equation y = 0.294x + 0.044; R2 = 0.67 for CAH-negative (a) and y = 0.290x − 2.484; R2 = 0.94 for CAH-positive cases (b). The slopes in the two groups are almost equal, but the intercepts are different. The larger intercept in b suggests that in order to achieve higher ratios in the CAH-positive cases, the 17-OHP concentrations are higher by the amount indicated by the intercept
Fig. 3.
The 17-OHP/A4 ratios plotted against 17-OHP concentrations from DBS in CAH-negative (a) and CAH-positive cases (b). triangle-CAH-negative, circle-CAH positive. The linear regression between 17-OHP/A4 ratios and 17-OHP concentrations shows a relationship given by the equation y = 0.20x + 0.07; R2 = 0.76 for CAH-negative (a) and y = 0.017x + 1.54; R2 = 0.86 for CAH-positive cases (b). The slope in the CAH-positive group is lower, which represents a different response of A4 to increasing concentrations of 17-OHP than in controls
4 Discussion
The classical approach to newborn screening of inborn errors of metabolism is based on the concept that in any enzyme defect, the concentration of the precursor is elevated relative to the product. Therefore, it is expected that in CAH-positive samples, the 17-OHP concentration and the (17-OHP + A4)/F ratios should both be elevated. However, the concentration of a metabolic intermediate is governed by both its production and its disappearance rate. The extensive overlap of F concentrations between the CAH-negative and CAH-positive samples clearly suggests that the assumption that the F level is low in CAH-positive samples may not be valid (Mitchell and Hermos 1998). There is no overlap between the CAH-positive and CAH-negative groups when 17-OHP + A4 sums are compared, but there is overlap when F is included in the denominator.
Thus, the ratio of (17-OHP + A4)/F is less discriminatory than the sum of 17-OHP + A4, as shown in Fig. 1. This observation is consistent with the findings of Sarafoglou et al. that among the missed (false negative) cases in newborn screening for CAH, there were several infants whose samples had high 17-OHP values but normal (below cutoff) second-tier tests (Sarafoglou et al. 2012). Thus, the ratio of (17-OHP + A4)/F does not accurately reflect a distinct biochemical phenotype in CAH-positive infants.
The graphs of the 17-OHP/S ratio relative to varying 17-OHP concentrations in CAH-negative and CAH-positive infants demonstrate their distinct phenotypes. At steady state or quasi-steady state, a change in this ratio corresponds to a change in the system parameter Kd/Kp (see Theoretical Considerations above). In the CAH-negative infants (Fig. 2a), Kp can be assumed to be relatively constant, and any increase in the 17-OHP/S ratio represents an increase in S disappearance due to increased F production, i.e., the product of [S] × Kd is increased. The elevated 17-OHP/S ratios despite a similar S concentration among CAH-positive samples indicate that a greater increase in the 17-OHP concentration is necessary to overcome the decrease in 21-α-hydroxylase enzyme activity so that the production of F can be maintained close to normal levels. The CAH-negative and positive phenotypes are represented by the difference in the intercepts of the regression equations.
The CAH-positive neonates have higher absolute levels of A4 than CAH-negative neonates, but the increased ratio of 17-OHP/A4 in CAH-positive infants suggests that there is a threshold effect in the adrenal androgen production system. The higher slope of the regression of 17-OHP/A4 relative to varying 17-OHP concentrations in the CAH-negative versus the CAH-positive group (Fig. 3) indicates that in normal adrenal function, there is a greater increase in the production of adrenal androgen via 17,20-lyase for an increase in 17-OHP concentration than in CAH. The difference in the slope of the regression analysis suggests that the normal neonatal phenotype is physiologically and biochemically distinct from the state of androgen excess in CAH (Fig. 3a, b). The 17-OHP concentration at the point of intersection (8.12 ng/mL) suggests a phenotypic cutoff value at a lower level than the current second-tier cutoff value (∼12.00 ng/mL, or 38 nmol/L) used in the State of California.
Each ratio is a dynamic parameter of the system of steroid pathways. While the 17-OHP/S ratio reflects the loss of 21-α-hydroxylase enzyme activity, the 17-OHP/A4 ratio reflects the increased production of A4 and its metabolic products via 17,20-lyase. The lack of difference in the S/F ratio between the CAH-positive and negative groups suggests that the rate of production and disappearance of F does not differ between the two groups. Since [S] × Kp = [F] × Kd, such that [S]/[F] = Kd/Kp, equivalent S/F ratios indicate that Kd/Kp is constant in both CAH-positive and CAH-negative groups. Thus, the disappearance of F does not appear to be dependent on its concentration. Furthermore, the usual assumption that the F level is low in CAH is valid only when the S and the F concentrations are both low.
The current study is limited in that only proven CAH-positive and CAH-negative samples were analyzed. Future studies using precursor-to-product ratios may help elucidate the biochemical phenotypes of heterozygous carriers of CYP21A2 gene mutations (Peter et al. 1990) and infants with intermediately and/or transiently elevated 17-OHP. Another limitation is the wide variability in newborn hematocrit concentrations (Jopling et al. 2009), which may affect the accuracy of steroid measurements. However, calculating direct ratios should minimize this error, as it would be the same in both the numerator and denominator. Our study of precursor-to-product ratios in newborn screen DBS samples was not designed to calculate turnover of adrenal steroid metabolites. The application of stable isotope and mass isotopomer determination in steroids may be an additional approach to confirm 21-α-hydroxylase deficiency by actually determining turnover of these compounds.
Infants with CAH due to 21-α-hydroxylase deficiency usually present with signs of androgen excess and diminished capacity for glucocorticoid production, with up to 75 % of cases exhibiting mineralocorticoid deficiency, which may lead to salt-wasting crisis (Speiser et al. 2010a). Ambiguous genitalia due to excessive adrenal androgens may be present in females, whereas genital exam findings are absent in males. The main goals of newborn screening for CAH are to identify infants at risk for salt-wasting crisis and to provide appropriate gender assignment in severely virilized female infants. However, 21-α-hydroxylase enzyme deficiency encompasses four major genotypes corresponding to: mild and severe non-classical, simple virilizing, and salt-wasting phenotypes (Finkielstain et al. 2011). Thus, elevated 17-OHP concentrations are present in CAH, but the results assume a wide range of values that may overlap with the normal phenotype due to variables such as stress and maternal steroid use (Ersch et al. 2008).
We have shown here that in CAH, the elevated 17-OHP/S ratio is a biomarker of abnormal 21-α-hydroxylase activity, and the 17-OHP/A4 ratio is a biomarker of adrenal androgen excess via increased 17,20-lyase activity. We have also shown that the similar S/F ratio in CAH-positive neonates indicates that the rate of production via 11-β-hydroxylase and disappearance of cortisol is maintained in CAH. Previously described applications of steroid profiling in CAH, which rely on absolute steroid concentrations and empiric steroid ratios, do not fully exploit the wealth of information available in DBS that allows further insight into the unique physiological state of CAH. Janzen et al. previously published LC–MS/MS steroid profiles, presenting separate steroid measurements, but not individual ratios of 17-OHP to S (Janzen et al. 2007). However, the ratio of the reported mean 17-OHP to mean S suggests that the 17-OHP to S ratio is on the order of 13:1 for infants with CAH due to 21-α-hydroxylase deficiency, 2.15:1 for preterm infants, and 1.6:1 for healthy term infants, which is consistent with our clinical experience (Hicks et al. 2012). Our results suggest that the elevated 17-OHP/S and 17-OHP/A4 ratios are reflective of the CAH-positive physiologic state.
5 Concluding remarks
The 17-OHP/S and 17-OHP/A4 ratios can distinguish between the biochemical phenotypes of CAH-positive and CAH-negative newborns. Whether the use of precursor-to-product ratios may be used to differentiate between infants affected with CAH versus other conditions with elevated 17-OHP, such as CAH heterozygotes, prematurity, and stress exposure remains to be evaluated.
Acknowledgments
We would like to thank Maria Lajoie and Shu Lim of the CTSI Core Laboratory for their technical assistance, and Seyed Sadjadi of Phenomenex for his help in optimizing the LC–MS/MS method. Steroid analyses were performed at the Biomedical Mass Spectrometry facility at the Los Angeles Biomedical Research Institute at Harbor-UCLA, which is partly supported by the University of California Los Angeles Clinical and Translational Science Institute (UL1 TR000124) and the Metabolomics core of the Center of Excellence for Pancreatic Diseases (P01 AT003960).
Footnotes
The steroid concentrations are presented as ng/mL and nmol/L, the conventional unit of measure used by the Newborn Screening Program in California.
Contributor Information
Rebecca A. Hicks, Email: hicksreb@gmail.com, Division of Endocrinology, Department of Pediatrics, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, 1124 West Carson Street, Bldg RB-1, Box 446, Torrance, CA 90502, USA.
Jennifer K. Yee, Division of Endocrinology, Department of Pediatrics, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, 1124 West Carson Street, Bldg RB-1, Box 446, Torrance, CA 90502, USA
Catherine S. Mao, Division of Endocrinology, Department of Pediatrics, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, 1124 West Carson Street, Bldg RB-1, Box 446, Torrance, CA 90502, USA
Steve Graham, Genetic Disease Screening Program, California Department of Public Health, 850 Marina Bay Parkway, Richmond, CA 94804, USA.
Martin Kharrazi, Genetic Disease Screening Program, California Department of Public Health, 850 Marina Bay Parkway, Richmond, CA 94804, USA.
Fred Lorey, Genetic Disease Screening Program, California Department of Public Health, 850 Marina Bay Parkway, Richmond, CA 94804, USA.
W. P. Lee, Division of Endocrinology, Department of Pediatrics, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, 1124 West Carson Street, Bldg RB-1, Box 446, Torrance, CA 90502, USA
References
- Dhillon K, Ho T, Rich P, Xu D, Lorey F, She J, et al. An automated method on analysis of blood steroids using liquid chromatography tandem mass spectrometry: application to population screening for congenital adrenal hyperplasia in newborns. Clinica Chimica Acta. 2011;412(23–24):2076–2084. doi: 10.1016/j.cca.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Ersch J, Beinder E, Stallmach T, Bucher HU, Torresani T. 17-Hydroxyprogesterone in premature infants as a marker of intrauterine stress. Journal of Perinatal Medicine. 2008;36(2):157–160. doi: 10.1515/JPM.2008.013. [DOI] [PubMed] [Google Scholar]
- Fingerhut R. False positive rate in newborn screening for congenital adrenal hyperplasia (CAH)-ether extraction reveals two distinct reasons for elevated 17alpha-hydroxyprogesterone (17-OHP) values. Steroids. 2009;74(8):662–665. doi: 10.1016/j.steroids.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Finkielstain GP, Chen W, Mehta SP, Fujimura FK, Hanna RM, Van Ryzin C, et al. Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Journal of Clinical Endocrinology and Metabolism. 2011;96(1):E161–E172. doi: 10.1210/jc.2010-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health CDoP. Endocrine diseases. Newborn screening program; State of California: 2012. [Google Scholar]
- Hicks RA, Ferreira BF, Mao CS, Yee JK, Lee WP. The use of 17-α-hydroxyprogesterone to 11-deoxycortisol ratio in newborn screening of congenital adrenal hyperplasia. Journal of Clinical Investigation. 2012;60:222. [Google Scholar]
- Hingre RV, Gross SJ, Hingre KS, Mayes DM, Richman RA. Adrenal steroidogenesis in very low birth weight preterm infants. Journal of Clinical Endocrinology and Metabolism. 1994;78(2):266–270. doi: 10.1210/jcem.78.2.8106610. [DOI] [PubMed] [Google Scholar]
- Holst JP, Soldin SJ, Tractenberg RE, Guo T, Kundra P, Verbalis JG, et al. Use of steroid profiles in determining the cause of adrenal insufficiency. Steroids. 2007;72(1):71–84. doi: 10.1016/j.steroids.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen N, Peter M, Sander S, Steuerwald U, Terhardt M, Holtkamp U, et al. Newborn screening for congenital adrenal hyperplasia: additional steroid profile using liquid chromatography–tandem mass spectrometry. Journal of Clinical Endocrinology and Metabolism. 2007;92(7):2581–2589. doi: 10.1210/jc.2006-2890. [DOI] [PubMed] [Google Scholar]
- Joint, L E C A H W G. Consensus statement on 21-hydroxylase deficiency from the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. Journal of Clinical Endocrinology and Metabolism. 2002;87(9):4048–4053. doi: 10.1210/jc.2002-020611. [DOI] [PubMed] [Google Scholar]
- Jopling J, Henry E, Wiedmeier SE, Christensen RD. Reference ranges for hematocrit and blood hemoglobin concentration during the neonatal period: data from a multihospital health care system. Pediatrics. 2009;123(2):e333–e337. doi: 10.1542/peds.2008-2654. [DOI] [PubMed] [Google Scholar]
- Lacey JM, Minutti CZ, Magera MJ, Tauscher AL, Casetta B, McCann M, et al. Improved specificity of newborn screening for congenital adrenal hyperplasia by second-tier steroid profiling using tandem mass spectrometry. Clinical Chemistry. 2004;50(3):621–625. doi: 10.1373/clinchem.2003.027193. [DOI] [PubMed] [Google Scholar]
- Matern D, Tortorelli S, Oglesbee D, Gavrilov D, Rinaldo P. Reduction of the false-positive rate in newborn screening by implementation of MS/MS-based second-tier tests: the Mayo Clinic experience (2004–2007) Journal of Inherited Metabolic Disease. 2007;30(4):585–592. doi: 10.1007/s10545-007-0691-y. [DOI] [PubMed] [Google Scholar]
- Minutti CZ, Lacey JM, Magera MJ, Hahn SH, McCann M, Schulze A, et al. Steroid profiling by tandem mass spectrometry improves the positive predictive value of newborn screening for congenital adrenal hyperplasia. Journal of Clinical Endocrinology and Metabolism. 2004;89(8):3687–3693. doi: 10.1210/jc.2003-032235. [DOI] [PubMed] [Google Scholar]
- Mitchell ML, Hermos RJ. Cortisol in dried blood screening specimens from newborns with raised 17-hydroxyprogesterone and congenital adrenal hyperplasia. Clinical Endocrinology (Oxf) 1998;48(6):757–760. doi: 10.1046/j.1365-2265.1998.00430.x. [DOI] [PubMed] [Google Scholar]
- Peter M, Sippell WG, Lorenzen F, Willig RP, Westphal E, Grosse-Wilde H. Improved test to identify heterozygotes for congenital adrenal hyperplasia without index case examination. Lancet. 1990;335(8701):1296–1299. doi: 10.1016/0140-6736(90)91185-d. [DOI] [PubMed] [Google Scholar]
- Sarafoglou K, Banks K, Gaviglio A, Hietala A, McCann M, Thomas W. Comparison of one-tier and two-tier newborn screening metrics for congenital adrenal hyperplasia. Pediatrics. 2012;130(5):e1261–e1268. doi: 10.1542/peds.2012-1219. [DOI] [PubMed] [Google Scholar]
- Soldin SJ, Soldin OP. Steroid hormone analysis by tandem mass spectrometry. Clinical Chemistry. 2009;55(6):1061–1066. doi: 10.1373/clinchem.2007.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism. 2010a;95(9):4133–4160. doi: 10.1210/jc.2009-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism. 2010b;95(9):4133–4160. doi: 10.1210/jc.2009-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PC. Neonatal screening for congenital adrenal hyperplasia. Nature Reviews Endocrinology. 2009;5(9):490–498. doi: 10.1038/nrendo.2009.148. [DOI] [PubMed] [Google Scholar]