Abstract
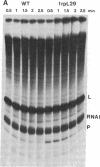
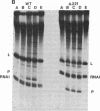
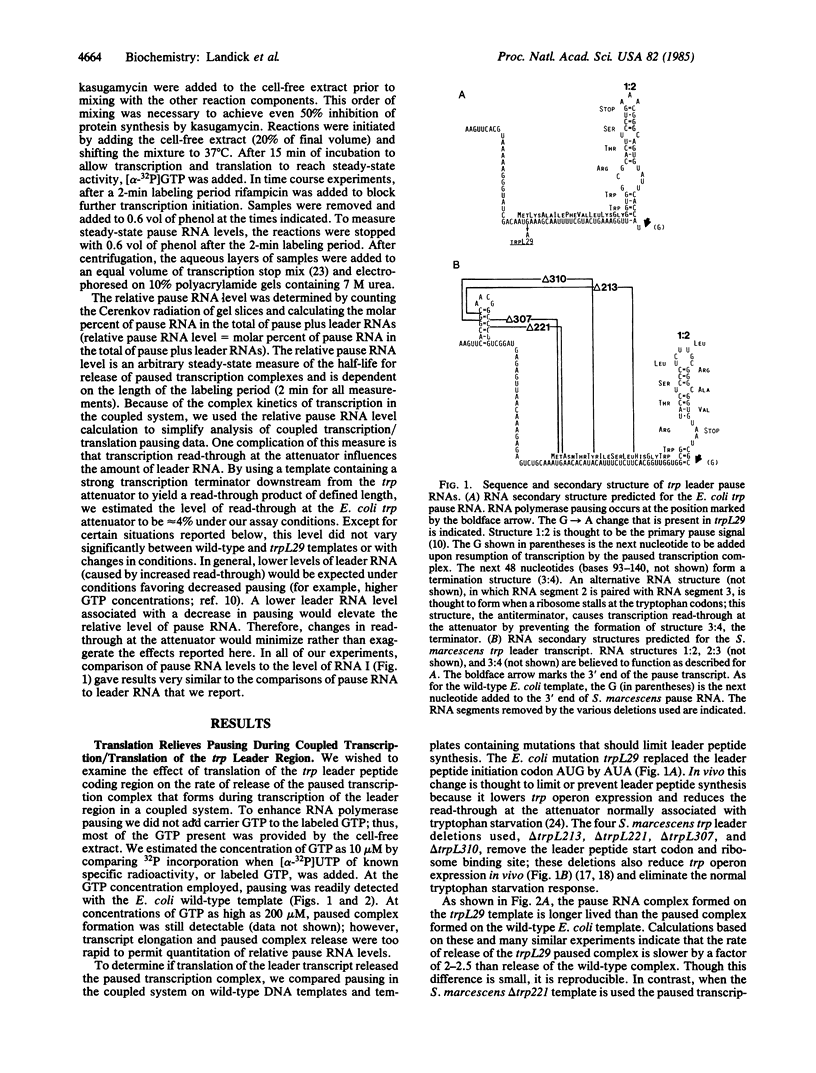
It has been proposed that RNA polymerase pausing in the leader region of the tryptophan (trp) operon of Escherichia coli is responsible for the synchronization of transcription and translation essential to attenuation control. In this report we use an in vitro coupled transcription/translation system to study the effect of trp leader peptide synthesis on RNA polymerase pausing in the trp leader region. Wild-type and translation-defective trp leader templates of E. coli and Serratia marcescens were employed, and pause RNA synthesis and paused complex release (activation) were quantified relative to synthesis of the terminated leader transcript. It was observed that pausing in the trp leader region was prolonged when translation of the leader transcript was reduced by mutations in the leader region or by addition of the translation inhibitor kasugamycin or chloramphenicol. Experiments with S-30 extracts from a mutant strain that is inefficient in translating the tryptophan codons in the leader transcript indicated that ribosome movement to these codons also releases the paused transcription complex. These findings indicate that the paused trp leader transcription complex resumes transcription when released by ribosome movement over the leader peptide coding region. This release would facilitate the coupling of transcription and translation essential to attenuation control.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Crawford I. P., Yanofsky C. Regulation of tryptophan operon expression by attenuation in cell-free extracts of Escherichia coli. J Biol Chem. 1982 Aug 10;257(15):8795–8798. [PubMed] [Google Scholar]
- Doolittle W. F., Yanofsky C. Mutants of Escherichia coli with an altered tryptophanyl-transfer ribonucleic acid synthetase. J Bacteriol. 1968 Apr;95(4):1283–1294. doi: 10.1128/jb.95.4.1283-1294.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnham P. J., Platt T. Rho-independent termination: dyad symmetry in DNA causes RNA polymerase to pause during transcription in vitro. Nucleic Acids Res. 1981 Feb 11;9(3):563–577. doi: 10.1093/nar/9.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. F., Das A., Kolter R., Winkler M. E., Yanofsky C. Analysis of the requirements for transcription pausing in the tryptophan operon. J Mol Biol. 1985 Apr 5;182(3):397–409. doi: 10.1016/0022-2836(85)90199-8. [DOI] [PubMed] [Google Scholar]
- Fisher R. F., Yanofsky C. Mutations of the beta subunit of RNA polymerase alter both transcription pausing and transcription termination in the trp operon leader region in vitro. J Biol Chem. 1983 Jul 10;258(13):8146–8150. [PubMed] [Google Scholar]
- Fisher R., Yanofsky C. A complementary DNA oligomer releases a transcription pause complex. J Biol Chem. 1983 Aug 10;258(15):9208–9212. [PubMed] [Google Scholar]
- Gardner J. F. Initiation, pausing, and termination of transcription in the threonine operon regulatory region of Escherichia coli. J Biol Chem. 1982 Apr 10;257(7):3896–3904. [PubMed] [Google Scholar]
- Gesteland R. F. Isolation and characterization of ribonuclease I mutants of Escherichia coli. J Mol Biol. 1966 Mar;16(1):67–84. doi: 10.1016/s0022-2836(66)80263-2. [DOI] [PubMed] [Google Scholar]
- Hauser C. A., Sharp J. A., Hatfield L. K., Hatfield G. W. Pausing of RNA polymerase during in vitro transcription through the ilvB and ilvGEDA attenuator regions of Escherichia coli K12. J Biol Chem. 1985 Feb 10;260(3):1765–1770. [PubMed] [Google Scholar]
- JULIAN G. R. (14C)LYSINE PEPTIDES SYNTHESIZED IN AN IN VITRO ESCHERICHIA COLI SYSTEM IN THE PRESENCE OF CHLORAMPHENICOL. J Mol Biol. 1965 May;12:9–16. doi: 10.1016/s0022-2836(65)80277-7. [DOI] [PubMed] [Google Scholar]
- Kolter R., Yanofsky C. Attenuation in amino acid biosynthetic operons. Annu Rev Genet. 1982;16:113–134. doi: 10.1146/annurev.ge.16.120182.000553. [DOI] [PubMed] [Google Scholar]
- Kolter R., Yanofsky C. Genetic analysis of the tryptophan operon regulatory region using site-directed mutagenesis. J Mol Biol. 1984 May 25;175(3):299–312. doi: 10.1016/0022-2836(84)90350-4. [DOI] [PubMed] [Google Scholar]
- Landick R., Yanofsky C. Stability of an RNA secondary structure affects in vitro transcription pausing in the trp operon leader region. J Biol Chem. 1984 Sep 25;259(18):11550–11555. [PubMed] [Google Scholar]
- Morita M., Oka A. The structure of a transcriptional unit on colicin E1 plasmid. Eur J Biochem. 1979 Jul;97(2):435–443. doi: 10.1111/j.1432-1033.1979.tb13131.x. [DOI] [PubMed] [Google Scholar]
- Oxender D. L., Zurawski G., Yanofsky C. Attenuation in the Escherichia coli tryptophan operon: role of RNA secondary structure involving the tryptophan codon region. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5524–5528. doi: 10.1073/pnas.76.11.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T., Squires C., Yanofsky C. Ribosome-protected regions in the leader-trpE sequence of Escherichia coli tryptophan operon messenger RNA. J Mol Biol. 1976 May 15;103(2):411–420. doi: 10.1016/0022-2836(76)90320-x. [DOI] [PubMed] [Google Scholar]
- Platt T. Termination of transcription and its regulation in the tryptophan operon of E. coli. Cell. 1981 Apr;24(1):10–23. doi: 10.1016/0092-8674(81)90496-7. [DOI] [PubMed] [Google Scholar]
- Stent G. S. Genetic transcription. Proc R Soc Lond B Biol Sci. 1966 Mar 22;164(995):181–197. doi: 10.1098/rspb.1966.0022. [DOI] [PubMed] [Google Scholar]
- Stroynowski I., Yanofsky C. Transcript secondary structures regulate transcription termination at the attenuator of S. marcescens tryptophan operon. Nature. 1982 Jul 1;298(5869):34–38. doi: 10.1038/298034a0. [DOI] [PubMed] [Google Scholar]
- Winkler M. E., Yanofsky C. Pausing of RNA polymerase during in vitro transcription of the tryptophan operon leader region. Biochemistry. 1981 Jun 23;20(13):3738–3744. doi: 10.1021/bi00516a011. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Comparison of regulatory and structural regions of genes of tryptophan metabolism. Mol Biol Evol. 1984 Feb;1(2):143–161. doi: 10.1093/oxfordjournals.molbev.a040307. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Horn V. Rifampin resistance mutations that alter the efficiency of transcription termination at the tryptophan operon attenuator. J Bacteriol. 1981 Mar;145(3):1334–1341. doi: 10.1128/jb.145.3.1334-1341.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalkin H., Yanofsky C., Squires C. L. Regulated in vitro synthesis of Escherichia coli tryptophan operon messenger ribonucleic acid and enzymes. J Biol Chem. 1974 Jan 25;249(2):465–475. [PubMed] [Google Scholar]
- Zurawski G., Elseviers D., Stauffer G. V., Yanofsky C. Translational control of transcription termination at the attenuator of the Escherichia coli tryptophan operon. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5988–5992. doi: 10.1073/pnas.75.12.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Yanofsky C. Escherichia coli tryptophan operon leader mutations, which relieve transcription termination, are cis-dominant to trp leader mutations, which increase transcription termination. J Mol Biol. 1980 Sep 5;142(1):123–129. doi: 10.1016/0022-2836(80)90210-7. [DOI] [PubMed] [Google Scholar]