Abstract
Objective
To evaluate 8-year weight losses achieved with intensive lifestyle intervention (ILI) in the Look AHEAD (Action for Health in Diabetes) study.
Design and Methods
Look AHEAD assessed the effects of intentional weight loss on cardiovascular morbidity and mortality in 5,145 overweight/obese adults with type 2 diabetes, randomly assigned to ILI or usual care (i.e., diabetes support and education [DSE]). The ILI provided comprehensive behavioral weight loss counseling over 8 years; DSE participants received periodic group education only.
Results
All participants had the opportunity to complete 8 years of intervention before Look AHEAD was halted in September 2012; ≥88% of both groups completed the 8-year outcomes assessment. ILI and DSE participants lost (mean±SE) 4.7±0.2% and 2.1±0.2% of initial weight, respectively (p<0.001) at year 8; 50.3% and 35.7%, respectively, lost ≥5% (p<0.001), and 26.9% and 17.2%, respectively, lost ≥10% (p<0.001). Across the 8 years ILI participants, compared with DSE, reported greater practice of several key weight-control behaviors. These behaviors also distinguished ILI participants who lost ≥10% and kept it off from those who lost but regained.
Conclusions
Look AHEAD’s ILI produced clinically meaningful weight loss (≥5%) at year 8 in 50% of patients with type 2 diabetes and can be used to manage other obesity-related co-morbid conditions.
Trial Registration
clinicaltrials.gov Identifier: NCT00017953
The Look AHEAD (Action for Health in Diabetes) study was designed to assess the effects of an intensive lifestyle intervention (ILI) on clinically important health outcomes in overweight/obese individuals with type 2 diabetes.1 The trial’s primary outcome was cardiovascular morbidity and mortality, on which no significant differences were observed between participants assigned to ILI or a usual care group (i.e., diabetes support and education [DSE]) after a mean follow-up of 9.6 years.2 Investigators currently are examining changes in secondary outcomes in Look AHEAD including mood,3 quality of life,4 sleep apnea,5 and physical function,6 all of which were improved by the ILI during the trial’s initial years.
Look AHEAD provides the largest and longest randomized evaluation to date of an ILI for weight reduction. The trial offers invaluable information about the feasibility of inducing and maintaining clinically significant weight loss, defined as a ≥5% reduction in initial body weight.7–10 Weight loss of this size confers additional health benefits beyond those described above. These include prevention and resolution of type 2 diabetes,11,12 reduction in blood pressure and lipids,13–14 amelioration of non-alcoholic fatty liver disease,15 and improvements in urinary incontinence and sexual dysfunction.16,17
The present report provides a detailed analysis of changes in body weight in ILI and DSE participants over the first 8 years of the intervention, which all participants had the opportunity to complete before the trial was halted. ILI participants were provided frequent treatment visits the first year to induce weight loss, followed by a comprehensive weight loss maintenance program in years 2–8.18 Our primary hypotheses were that the ILI group, as compared with DSE, would achieve significantly greater mean weight losses at all years and be more successful in achieving reductions ≥5% and ≥10% of initial weight. Consistent with these hypotheses, we predicted that ILI participants, compared with DSE, would report greater practice of numerous weight control behaviors (e.g., reducing energy intake, increasing physical activity) recommended by the intervention. The study’s large sample size (N=5,145) allowed us to examine the effect on 8-year weight loss of age, gender, and race/ethnicity, as evaluated previously for years 1 and 4.19 The large sample also allowed us to examine, within the ILI group, the percentage of participants that lost ≥10% of initial weight the first year and sustained the loss at year 8. Behavioral characteristics of these weight-loss maintainers were compared with those of participants who initially lost ≥10% but regained it.
METHODS AND PROCEDURES
Participants
A total of 5,145 men and women were enrolled in Look AHEAD at 16 centers throughout the U.S.1,2 Eligible individuals had type 2 diabetes, were 45–76 years of age, and had a body mass index (BMI) ≥25 kg/m2 (or ≥27 kg/m2 if taking insulin). Additional eligibility criteria have been reported and included applicants’ completing a graded exercise test and a test of behavioral adherence.1,2 Those who remained eligible were randomly assigned, with equal probability, to ILI or DSE. All participants signed a consent form approved by their center’s institutional review board.
Interventions
The ILI and DSE interventions have been described previously and are only briefly summarized here.18,20 Participants in both groups received all usual medical care from their own primary care providers.
ILI
In year 1, ILI participants received a comprehensive lifestyle intervention designed to induce an average, study-wide loss ≥7% of initial weight (with an individual goal of losing ≥10%).1,19 The intervention was adapted from the Diabetes Prevention Program (DPP)11,21 and delivered to groups of approximately 10–20 persons by experienced interventionists. In months 1–6, participants attended group sessions (of 60–75 minutes) for the first 3 weeks of each month; the fourth week, they met individually with their interventionist (for 20–30 minutes), and group sessions were not held. In months 7–12, they continued to have a monthly individual meeting, but group sessions were reduced to two per month. Participants were prescribed 1200–1800 kcal/day (depending on initial body weight) with ≤30% of calories from fat (<10% from saturated fat) and ≥15% of calories from protein. Structured meal plans22 and meal replacements18 were provided (free of charge) for the first 4 months, with patients encouraged to replace two meals and one snack daily with liquid shakes and meal bars. From months 5–12, they were instructed to replace one meal and one snack daily. Participants were prescribed ≥175 minutes/week of moderate intensity physical activity, to be achieved by month 6, with a further increase to ≥200 minutes/week for those who met this goal. The activity program relied on unsupervised exercise that, for most, consisted of brisk walking.1,18 Participants were instructed to keep daily records of their food intake, physical activity, and other targeted behaviors.
In years 2–8, the intervention focused principally on maintaining the weight losses and duration of physical activity achieved during year 1, as well as helping unsuccessful individuals achieve the study goals.18,19 Lifestyle counseling was provided primarily in individual sessions to allow tailoring to participants’ specific treatment needs. Each month, participants had an individual, on-site meeting (20–30 minutes), with a second individual contact by telephone or e-mail, approximately 2 weeks later. (This second contact was discontinued beginning in year 5.) Participants had individualized calorie goals, based on their desire to maintain their weight loss, lose more (if their BMI was >23 kg/m2), or reverse weight gain. All were encouraged to continue to use meal replacements (at no charge) to replace one meal or snack per day, to exercise ≥200 minutes/week, and to monitor weight weekly or more often.
During years 2–8, all sites offered a monthly group meeting at which members weighed-in, reviewed diet and activity records, and participated in a lifestyle modification session. Each year sites also offered at least one Refresher Group and one National Campaign, as used in the DPP.21 Refresher Groups typically lasted 6–8 weeks and were organized around a weight loss and/or physical activity theme.19 National Campaigns were similar in providing a group experience for 8–10 weeks but challenged participants to meet a specific goal (e.g., losing 5 lb), for which they received a small prize. Participants were strongly encouraged but not required to attend the various group offerings.
Interventionists included registered dietitians, psychologists, and exercise specialists, all of whom were certified annually. In addition to cognitive behavioral therapy, they incorporated elements of problem solving, motivational interviewing, and cultural tailoring in their counseling.23–25 They also could select more intensive interventions from a toolbox, described previously.18,19
DSE
For the first 4 years, DSE participants were provided three 1-hour group meetings per year that discussed diet, physical activity, and social support, respectively.20 These sessions offered information but not specific behavioral strategies for adhering to the diet and physical activity recommendations. Years 5 to 8 provided one such session per year. Persons who desired more help with weight loss were referred to their PCPs, who were free to recommend whatever interventions they considered appropriate.
Assessments
Weight was measured at baseline and annually thereafter with a digital scale (model BWB-800; Tanita, Willobrook, IL), by certified staff who were masked to intervention assignment. Physical activity was assessed at baseline and years 1, 4, and 8 by the Paffenbarger Activity Questionnaire (PAQ),26 which provides an estimate of weekly energy expenditure from moderate intensity physical activity (e.g., climbing stairs, walking, and other fitness, sport, and recreational activities). At baseline and years 1 and 4, only participants at eight centers (i.e., the same each year) completed the questionnaire; in year 8, participants at all sites completed it. All participants also reported at baseline and years 1–4, as well as at year 8, the number of weeks in the prior year that they engaged in behaviors previously determined to be associated with long-term weight control: 1) increasing physical activity;27–29 2) monitoring body weight;30,31 3) reducing calorie and fat intake;32,33 and 4) using meal replacements.33 These items were included in a questionnaire developed by study investigators (available upon request).
Statistical Analyses
The present analyses focused on the first 8 years of intervention, which all participants had the opportunity to complete before the trial was halted in September 2012. (Weight data reported in the primary endpoint paper included participants at different stages of intervention, ranging from 8.2 to 11 years.) Differences between the DSE and ILI groups in changes in weight over the 8 years were analyzed using a mixed effects analysis of covariance, which included baseline weight, clinical center, and treatment arm. The analyses followed the intention-to-treat principle in which participants were grouped according to intervention assignment, regardless of adherence, and all follow-up data were included. The percentage of participants in each group who met different categorical weight losses (e.g., ≥5% or ≥10% loss) at years 1 and 8 were compared using generalized estimating equations (GEE). For behavioral outcomes (e.g., physical activity), differences between groups on continuous measures were analyzed using analysis of covariance (adjusting for clinical center and baseline value of the outcome) and using GEE for categorical measures. Within the two intervention groups, a mixed effects analysis of covariance was used to examine the relationship between weight loss (at years 1, 4, and 8) and gender, age, and race/ethnicity (controlling for baseline weight, clinical center, and interactions of each of the subgroup variables with year and intervention group). All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Body weight data for participants who died during the 8-year trial were included in the analyses until censored at the time of death. In addition, all analyses were conducted with and without censoring weights of participants (in both groups) who underwent bariatric surgery. In year 1, 5 ILI and 10 DSE participants had surgery, which increased by year 8 to a total of 76 and 84, respectively (the latter values representing 3.0% and 3.3% of participants randomized to ILI and DSE, respectively). The two sets of analyses yielded the same statistical conclusions concerning differences between groups. Mean weight losses for both sets of analyses are reported; however, we focus primarily on weight changes in which bariatric surgery patients were censored (at the time of surgery) to provide the most accurate estimate of the efficacy of the lifestyle intervention.
For the ILI participants only, weight loss trajectories over the 8 years were determined for participants who at year 1 had lost ≥10% of initial weight, 5 to <10%, or <5%. Logistic regression was used to determine the odds of achieving a loss ≥10% or ≥5% at year 8, based upon having achieved these categorical losses at year 1. Data for the 8-year trajectories comprised a completers’ sample as a result of not including participants who had been censored or had failed to provide a measured body weight at both years 1 and 8.
RESULTS
Participants’ baseline characteristics
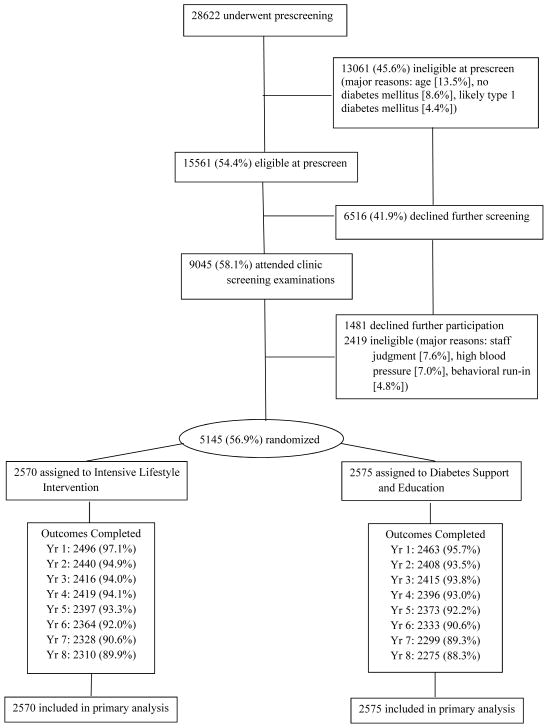
ILI and DSE participants did not differ significantly on baseline characteristics, as reported previously (see Table 1).2,33 Overall, average (±SD) age was 58.7±6.8 years, BMI was 36.0±5.9 kg/m2, and duration of type 2 diabetes was 6.8±6.5 years. Figure 1 shows that 89.9% and 88.3% of ILI and DSE participants, respectively, completed the 8-year outcomes assessment (p=0.077). Year-8 retention was 94.0% (across the two groups) when deceased participants were removed from the denominator.
Table 1.
Baseline characteristics of participants in the ILI and DSE groups.
| Characteristic | ILI N = 2,570 |
DSE N = 2,575 |
P Value |
|---|---|---|---|
| Sex (no. of subjects) | |||
| Female | 1526 (59.3) | 1537 (59.6) | 0.85* |
| Male | 1044 (40.7) | 1038 (40.4) | |
| Ethnicity | |||
| African American | 399 (15.5) | 404 (15.7) | 0.28* |
| American Indian/Alaskan Native | 130 (5.1) | 128 (5.0) | |
| Asian/Pacific Islander | 29 (1.1) | 21 (0.8) | |
| Hispanic/Latino | 339 (13.2) | 338 (13.2) | |
| Non-Hispanic White | 1618 (63.1) | 1628 (63.3) | |
| Other/multiple | 48 (1.9) | 50 (1.9) | |
| Use of insulin | 381 (14.8) | 408 (15.8) | 0.31* |
| Age (yr) | 58.6 ± 6.8 | 58.9 ± 6.9 | 0.12† |
| Weight (kg) | |||
| Females | 94.8 ± 17.9 | 95.4 ± 17.3 | 0.34† |
| Males | 108.9 ± 19.0 | 109.0 ± 18.0 | 0.94† |
| Body mass index (kg/m2) | |||
| Females | 36.3 ± 6.2 | 36.6 ± 6.0 | 0.15† |
| Males | 35.3 ± 5.7 | 35.1 ± 5.2 | 0.41† |
| Body mass index (kg/m2) | |||
| <30 | 403 (15.7) | 362 (14.1) | 0.13 |
| 30 to <35 | 918 (35.7) | 899 (34.9) | |
| 35 to <40 | 672 (26.1) | 740 (28.7) | |
| ≥40 | 577 (22.5) | 574 (22.3) | |
Values shown are means ± SDs or frequency counts (with percentages).
Figure 1.
Flowchart for screening, randomization, and follow-up of participants. Participants who did not complete outcome assessments in years 1 through 8 had either died, withdrawn from the study, or missed the assessment.
Weight loss
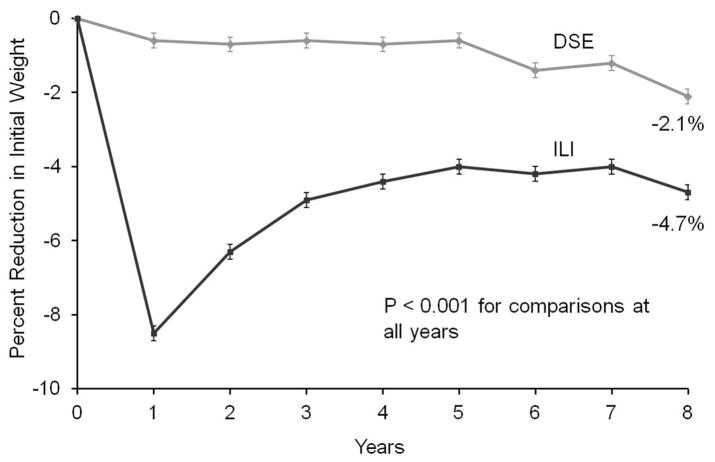
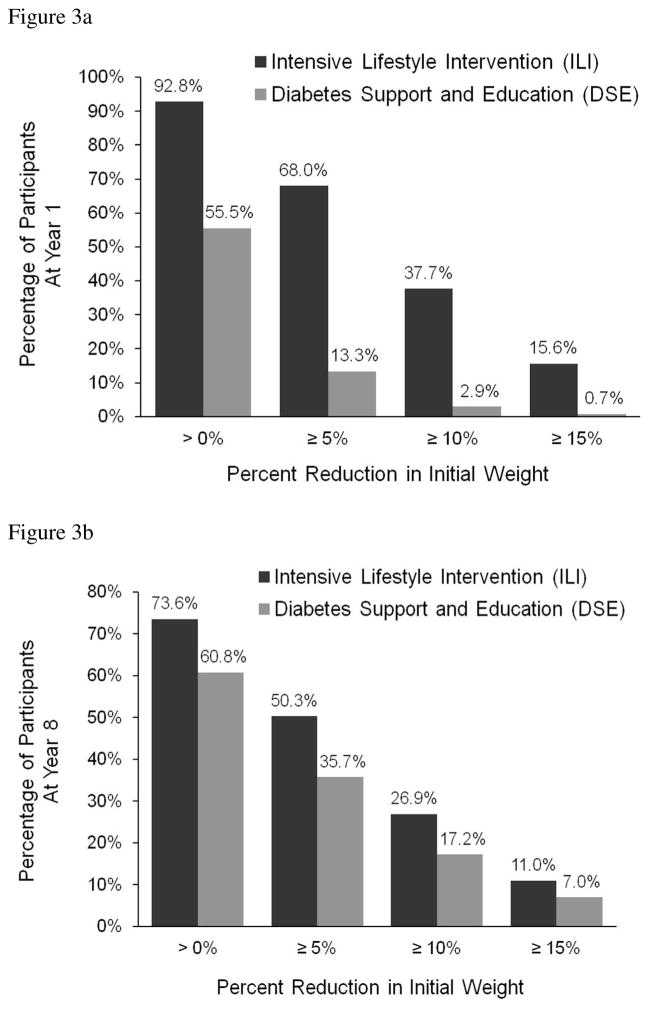
At year 1, ILI participants lost a mean (±SE) of 8.5±0.2% of initial weight, compared with 0.6±0.2% for DSE (p<0.001; see Figure 2). (Mean losses, not censored for bariatric surgery, were 8.6±0.2 and 0.6±0.2%, respectively.) As reported previously,33 more ILI than DSE participants lost ≥5% of initial weight (68.0% vs. 13.3%, p <0.001), as well as ≥10% (37.7% vs. 2.9%, p<0.001). (The percentage of participants who lost ≥5% includes those who lost ≥10%.) Figure 3a shows that ILI was superior to DSE on other measures of categorical weight loss.
Figure 2.
Figure shows mean (±SE) weight losses over 8 years for participants randomly assigned to an intensive lifestyle intervention (ILI) or diabetes support and education (DSE; usual care group). Differences between groups were significant (p<0.001) at all years.
Figure 3.
Figure 3(a). Percentage of participants in the ILI and DSE groups who achieved different categorical weight losses at year 1. The percentages are cumulative such that the 68% of ILI participants who lost 5% or more of initial weight includes those who also lost ≥10% and ≥15%. P<0.001 for all comparisons between treatment groups.
Figure 3(b). Percentage of participants in the ILI and DSE groups who achieved different categorical weight losses at year 8. The percentages are cumulative as described for Figure 1(A). P<0.001 for all comparisons between treatment groups.
At year 8, ILI participants lost 4.7±0.2% of initial weight, compared with 2.1±0.2% for DSE (p<0.001). (Mean losses, including participants who had bariatric surgery, were 5.3±0.2 and 2.7±0.2%, respectively.) More ILI than DSE participants met the ≥5% (50.3% vs. 35.7%; p<0.001) and ≥10% (26.9% vs. 17.2%; p<0.001) categorical losses. Fewer participants in ILI than DSE (26.4% vs. 39.2%; p<0.001) exceeded their baseline weight at year 8 (see Figure 3b). Figure 2 shows that ILI produced greater mean weight loss than DSE at all years and that weight regain in ILI, following maximum weight loss at year 1, plateaued between years 4–6.
Weight loss according to demographic characteristics
Table 2 presents weight loss according to gender, age, and race/ethnicity at years 1, 4, and 8. (Data not censored for bariatric surgery are presented in supplementary Table 1 on-line.) At all years, participants in ILI lost significantly more weight than their corresponding peers in DSE (see Table 2 for means and p values). Within the ILI group, men and women achieved comparable weight losses over the 8 years, with mean differences between them ranging from 0.3% (year 1) to 0.9% (year 8) (Table 2). By contrast, the oldest ILI individuals (65–76 years at baseline) consistently lost more weight than the youngest participants (45–54 years), with differences ranging from 0.9% (year 1) to 3.0% (year 8). Non-Hispanic white participants in ILI lost significantly more weight at year 1 than participants who self-identified as African American, Hispanic, and American Indian/Other. However, at years 4 and 8, mean weight losses were comparable among the four racial/ethnic groups, as they were among DSE participants at all years. Older DSE participants lost more weight than their younger counterparts, with the greatest difference (1.9%) between age groups occurring at year 8.
Table 2.
Percent reduction in initial weight in the ILI and DSE groups at years 1, 4, and 8 according to gender, age, and race/ethnicity.
| Characteristic | Year 1 | Year 4 | Year 8 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ILI | DSE | p value | ILI | DSE | p value | ILI | DSE | p value | |
| Total Sample | 8.5 ± 0.2 | 0.6 ± 0.2 | <.001 | 4.4 ± 0.2 | 0.7 ± 0.2 | <.001 | 4.7 ± 0.2 | 2.1 ± 0.2 | <.001 |
| Gender | |||||||||
| Female | 8.4 ± 0.2a | 0.8 ± 0.2a | <.001 | 4.3 ± 0.2a | 1.1 ± 0.2a | <.001 | 5.1 ± 0.2a | 2.6 ± 0.2a | <.001 |
| Male | 8.7 ± 0.3a | 0.3 ± 0.3a | <.001 | 4.6 ± 0.3a | 0.1 ± 0.3b | <.001 | 4.2 ± 0.3b | 1.3 ± 0.3b | <.001 |
| Age | |||||||||
| 45–55 yr | 8.2 ± 0.3a | 0.3 ± 0.3a | <.001 | 3.6 ± 0.3a | 0.1 ± 0.3a | <.001 | 3.8 ± 0.3a | 1.2 ± 0.3a | <.001 |
| 56–65 yr | 8.6 ± 0.2ab | 0.6 ± 0.2a | <.001 | 4.4 ± 0.2b | 0.7 ± 0.2b | <.001 | 4.7 ± 0.2b | 2.2 ± 0.2b | <.001 |
| 66–76 yr | 9.1 ± 0.4b | 0.9 ± 0.4a | <.001 | 6.0 ± 0.4c | 1.8 ± 0.4c | <.001 | 6.8 ± 0.4c | 3.1 ± 0.4c | <.001 |
| Race/Ethnicity | |||||||||
| Non-Hispanic White | 9.4 ± 0.2a | 0.8 ± 0.2a | <.001 | 4.6 ± 0.2a | 0.8 ± 0.2a | <.001 | 4.7 ± 0.2a | 2.1 ± 0.2a | <.001 |
| Hispanic | 7.2 ± 0.5b | −0.0 ± 0.5a | <.001 | 4.6 ± 0.5a | 0.4 ± 0.5a | <.001 | 4.8 ± 0.5a | 2.1 ± 0.5a | <.001 |
| African American | 6.4 ± 0.4bc | −0.0 ± 0.4a | <.001 | 3.8 ± 0.4a | 0.7 ± 0.4a | <.001 | 5.0 ± 0.4a | 1.8 ± 0.4a | <.001 |
| American Indian/Other | 5.3 ± 0.6c | 0.2 ± 0.6a | <.001 | 3.9 ± 0.6a | 0.2 ± 0.6a | <.001 | 5.0 ± 0.6a | 1.9 ± 0.6a | <.001 |
Note: Values shown are means ± SEs for the intention-to-treat population (ILI=2,570, DSE = 2,575). Weights of participants who died were censored at the time of death. Data for participants who underwent bariatric surgery also were censored (at the time of surgery). In ILI, the cumulative numbers of participants who had surgery in years 1, 4, and 8 were 5, 30, and 76, respectively. Corresponding values in DSE were 10, 45, and 84, respectively. Within columns and demographic groupings, values with different superscripts (i.e., a, b, c, etc.) differ significantly from each other. For example, at year 1, ILI participants 45–55 years of age lost significantly less weight than those 66–76 years (“a” vs. “b”), whereas weight loss of those 56–65 year did not differ significantly from either group (as shown by sharing a superscript “ab” with each group). At year 1, there were no significant differences between men and women in ILI, as shown by the shared superscript “a.” All analyses were adjusted for clinical site and baseline weight. Each family of comparisons, at each assessment time, was conducted using the Bonferonni correction. This yielded p<0.05 for comparison of gender, p<0.017 for comparison of different age groups, and p<0.008 for comparison of racial/ethnic groups.
Physical activity and weight control behaviors
At baseline, participants in both groups reported expending approximately 860 kcal/week in moderate intensity physical activity, as determined by the PAQ. ILI participants, compared with DSE, achieved significantly greater increases on this measure at all subsequent assessments (see Table 3). Reported energy expenditure more than doubled in ILI participants the first year (1737.8±47.6 kcal/week) but declined in subsequent years. Table 3 also presents the number of weeks in the past year that participants reported engaging in selected weight control behaviors. Groups did not differ at baseline on any measures; however, at all subsequent assessments, ILI participants, compared with DSE, significantly increased the number of weeks in which they reported exercising, reducing their calorie and fat intake, and using meal replacements (see Table 3). Practice of all behaviors increased the most during the first year. At years 1, 4, and 8, significantly more ILI than DSE participants also reported measuring their body weight weekly or more often, as well as daily or more often.
Table 3.
ILI and DSE participants’ reports of their physical activity, calorie restriction, and other weight control behaviors.
| Variable | Baseline | Year 1 | Year 4 | Year 8 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ILI | DSE | p value | ILI | DSE | p value | ILI | DSE | p value | ILI | DSE | p value | |
|
|
|
|
|
|
||||||||
| Physical activity (kcal/wk) | 859.5 ± 31.9 | 862.4 ± 34.3 | 0.376 | 1737.8 ± 47.6 | 968.9 ± 40.9 | <.001 | 1245.4 ± 43.2 | 974.7 ± 36.0 | <.001 | 1040.2 ± 35.3 | 853.2 ± 27.3 | 0.001 |
| Increased exercise (no. wk/yr) | 10.3 ± 0.3 | 10.1 ± 0.3 | 0.696 | 35.6 ± 0.4 | 12.2 ± 0.4 | <.001 | 13.8 ± 0.4 | 10.2 ± 0.4 | <.001 | 10.9 ± 0.4 | 8.7 ± 0.4 | <.001 |
| Reduced kcal (no. wk/yr) | 8.8 ± 0.3 | 9.3 ± 0.3 | 0.222 | 40.1 ± 0.4 | 12.6 ±0.4 | <.001 | 21.0 ± 0.4 | 12.1 ± 0.4 | <.001 | 17.9 ± 0.4 | 11.0 ± 0.4 | <.001 |
| Reduced fat (no. wk/yr) | 12.6 ± 0.4 | 12.8 ± 0.4 | 0.627 | 41.2 ± 0.4 | 16.4 ± 0.4 | <.001 | 24.4 ± 0.5 | 16.2 ± 0.5 | <.001 | 20.8 ± 0.5 | 14.1 ± 0.5 | <.001 |
| Meal replacements (no. wk/yr) | 1.6 ± 0.1 | 1.6 ± 0.1 | 0.720 | 39.0 ± 0.3 | 3.5 ± 0.3 | <.001 | 24.3 ± 0.4 | 2.0 ± 0.4 | <.001 | 17.2 ± 0.4 | 1.8 ± 0.4 | <.001 |
| Monitored weight | ||||||||||||
| ≥ Weekly, N (%) | 1041 (40.5) | 1052 (40.9) | 0.798 | 2290 (91.1) | 1085 (43.7) | <.001 | 1769 (73.1) | 1045 (43.5) | <.001 | 1555 (67.2) | 1027 (45.1) | <.001 |
| ≥ Daily, N (%) | 301 (11.7) | 318 (12.3) | 0.479 | 1162 (46.2) | 318 (12.8) | <.001 | 822 (34.0) | 315 (13.1) | <.001 | 754 (32.6) | 319 (14.0) | <.001 |
Values shown for physical activity are raw means ± SE; the p value is from a model that uses the log-transformed value of the measure. P values are adjusted for clinical site and the log of the baseline value (for years 1, 4, and 8). All other values are LS means ± standard error or frequency count (percentage). P values are adjusted for clinical site and baseline value (for years 1, 4, and 8). (As noted in the Methods section, at baseline and years 1 and 4, only participants at eight centers [i.e., the same each year] completed the Paffenbarger Activity Questionnaire27; in year 8, participants at all sites completed it.)
Achievement of categorical weight losses in ILI over 8 years
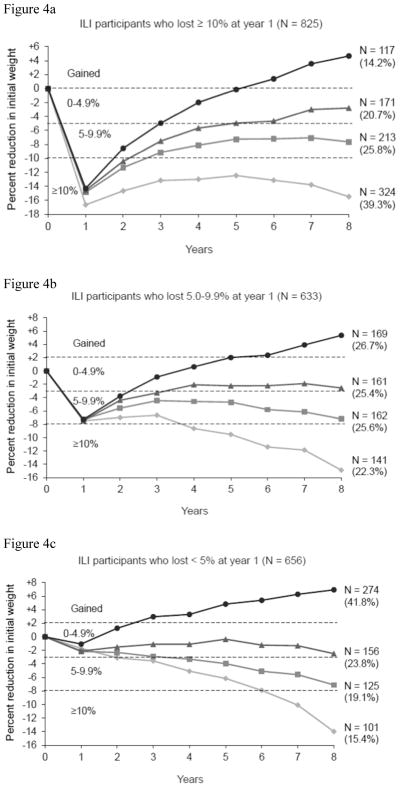
Figure 4a presents the weight loss trajectories of 825 ILI participants who lost ≥10% of weight at year 1 and qualified for data analysis at year 8 (as described in the Methods). Of these 825 participants, 324 (39.3%) achieved a ≥10% loss at year 8, 213 (25.8%) a loss of 5 to <10%, 171 (20.7%) a loss of 0 to <5%, and 117 (14.2%) gained above their baseline weight. A comparison of participants who at year 8 maintained the ≥10% loss versus gained above baseline weight revealed that maintainers reported (at year 8) a higher activity-related energy expenditure (1471.9±121.1 vs. 799.9±100.9 kcal/week, p<0.001) and a greater number of weeks (in the prior year) reducing their calorie and fat intake (both p values <0.001; see Table 4). Weight maintainers also were more likely than full regainers to weigh themselves daily or more often (47.8% vs. 28.4%), as well as weekly or more (82.4% vs. 69.8%) (both ps<0.001).
Figure 4.
Figure 4(a). Weight loss trajectories over 8 years in 825 participants in the intensive lifestyle intervention (ILI) who, at year 1, lost ≥10% of initial weight and, at year 8, provided a measured body weight. The figure shows the number of participants who, at year 8, maintained a loss of 10% or more of initial weight (N=324), of 5.0–9.9% (N=213), of 0–4.9% (N=171), or who gained above their baseline weight (N=117). The percentages shown in parentheses are based on the sample size for the subgroup. Thus, the 324 of 825 participants who maintained a ≥10% loss at year 8 comprised 39.3% of this subgroup of participants.
Figure 4(b). Weight loss trajectories over 8 years in 633 ILI participants who, at year 1, lost 5.0–9.9% of initial weight and, at year 8, provided a measured body weight. The four categories of weight change that these participants achieved at year 8 are presented in the same manner as in Figure 4(A).
Figure 4(c). Weight loss trajectories over 8 years in 656 ILI participants who, at year 1, lost <5% of initial weight and, at year 8, provided a measured body weight. The four categories of weight change that these participants achieved at year 8 are presented in the same manner as in Figure 4(A).
Table 4.
Weight control behaviors at Year 8 for ILI participants who maintained (N=324) versus regained (N=117) their ≥10% weight loss, achieved at Year 1.
| Year 8 Behaviors | Year 8 Weight Change | P Value | |
|---|---|---|---|
| Maintained ≥10% Loss | Gained Above Baseline Weight | ||
| Physical activity (kcal/wk) | 1471.9 ± 121.2 | 799.9 ± 100.9 | 0.001 |
| Reduced Kcal (no. wk/yr) | 20.4 ± 1.4 | 11.9 ± 2.1 | <.001 |
| Reduced fat (no. wk/yr) | 24.2 ± 1.5 | 15.6 ± 2.2 | <.001 |
| Increased exercise (no. wk/yr) | 12.9 ± 1.3 | 8.2 ± 1.8 | 0.013 |
| Meal replacements (no. wk/yr) | 22.8 ± 2.0 | 17.3 ± 2.9 | 0.072 |
| Monitored weight | |||
| ≥Weekly, N (%) | 262 (82.4) | 81 (69.8) | 0.001 |
| ≥Daily, N (%) | 152 (47.8) | 33 (28.4) | <.001 |
Values shown are LS means (raw means for Paffenbarger) ± standard error or frequency count (percentage).
P values are adjusted for clinical site and baseline value.
Figure 4b presents weight loss trajectories of the 633 participants who at year 1 lost 5 to <10% of initial weight and shows that 303 (47.9%) had a loss ≥5% at year 8. Figure 4c presents similar results for the 656 participants who lost <5% at year 1, of whom 226 (34.5%) achieved a ≥5% loss at year 8. The odds of achieving a ≥10% weight loss at year 8 were 2.3 (95% CI: 1.83, 2.97) times greater for participants who at year 1 lost ≥10%, compared to those who lost 5 to <10%, and 3.9 (95% CI: 2.99, 5.15) times greater compared to those who lost <5% at year 1. The odds of achieving ≥5% loss at year 8 were 2.1 (95% CI: 1.70, 2.64) times greater for participants who at 1 year lost ≥10%, compared to those who lost 5 to <10%, and 3.9 (95% CI: 3.10, 4.92) times greater compared to participants who lost <5% at year 1.
DISCUSSION
Look AHEAD is the longest randomized controlled evaluation to date of an intensive lifestyle intervention for weight management. Overweight/obese individuals with type 2 diabetes lost 4.7% of initial body weight at year 8, compared with 2.1% for participants assigned to DSE (i.e., usual care). Fifty percent of ILI participants lost ≥5% of initial weight, a common criterion of clinically meaningful weight loss,7–9 and 26.9% lost ≥10%. The same statistical differences were observed between the ILI and DSE groups when including or censoring weight losses of participants who had bariatric surgery. The Diabetes Prevention Program Outcomes Study34 reported 10 years of follow-up of a lifestyle weight-loss intervention, but both the lifestyle group and the comparison groups received weight loss counseling after a mean of 3.2 years since randomization.
Look AHEAD’s lifestyle intervention was effective over 8 years in both men and women and across an ethnically and racially diverse population. Weight losses of African Americans were among the largest reported in the literature. Older participants also lost more weight than their younger counterparts (as also observed in the DPP35). Some of their weight loss, however, probably was attributable to the combined effects of diabetes36 and aging, since it also occurred in older participants in Look AHEAD’s DSE group and in the original placebo-treated group of the Diabetes Prevention Program Outcomes Study.34
Look AHEAD’s long-term weight loss maintenance intervention, which included monthly or twice monthly individual contacts combined with periodic group meetings (i.e., refresher groups and national campaigns), was modeled on the DPP11,21 and similar protocols.30,37 Look AHEAD, however, has demonstrated that the extended provision of a weight loss maintenance intervention can facilitate clinically meaningful weight loss for up to 8 years – two to three times the duration examined in previous randomized trials.30,37,38 Sustaining weight loss for longer periods may further improve the prevention of type 2 diabetes in at-risk individuals,34 as well as maintain improvements in sleep apnea,5 physical mobility,6 and other health conditions ameliorated by weight loss.7–9
ILI participants achieved their maximum weight loss (of 8.5%) in the first year, when they received the most intensive intervention and reported their highest adherence to the prescribed weight control behaviors, compared with DSE. The ILI group, on average, regained weight from years 1 to 5, at which time body weight stabilized at approximately a 4.0–4.7% loss for the remainder of the trial (compared with 0.7–2.1% for DSE at this time). Look AHEAD’s large sample size permitted examination of patterns of long-term weight change among subgroups of participants, rather than simply the mean change. This analysis revealed that of 825 individuals who lost ≥10% of initial weight at year 1, 39% maintained this degree of weight loss at year 8, and another 26% maintained a loss of 5.0 to <10%. Those who maintained the full ≥10% loss at year 8, compared with those who regained above baseline, reported greater practice of several weight-maintenance behaviors, including high levels of physical activity, reduced calorie intake, and frequent monitoring of body weight. These behaviors have been identified in prior studies of successful weight loss maintainers.28–32
The lifestyle intervention’s strengths are offset by findings that 32% of ILI participants did not lose at least 5% of initial weight in the first year, and only 34.5% of these individuals achieved this goal at year 8. Logistic regression analyses clearly revealed the importance of successful first-year weight loss for achieving a clinically meaningful loss at year 8. This finding highlights the need for research on methods of inducing weight loss in persons who do not have an early, favorable response to lifestyle modification.
Nearly 36% of DSE participants achieved a ≥5% weight loss at year 8, compared with 13.3% at year 1. Several factors may have contributed to more of these participants reaching this criterion over time. DSE participants were permitted, in consultation with their PCPs, to pursue whatever weight loss options they wished. Their weight loss also could have been unintentional39 and reflect the effects of aging40 or illness39 (including diabetes36), as discussed with weight loss in older ILI participants.
In summary, Look AHEAD advances the management of obesity by showing that a comprehensive, long-term lifestyle intervention produced ≥5% weight loss at 8 years in 50% of participants. While efforts clearly are needed to translate the current treatment approach into clinical practice, Look AHEAD provides new optimism for the long-term management of obesity and its many co-morbid conditions that are ameliorated by weight loss.
Supplementary Material
What is already known about this subject?
The intensive lifestyle intervention (ILI) used in the Look AHEAD study was designed to induce a mean loss of 7% or more of initial weight in overweight/obese individuals with type 2 diabetes and to maintain this loss for up to 11.5 years of follow-up.
As reported previously in Obesity, ILI participants achieved a mean loss of 8.6% of initial weight at year 1 and 4.7% at year 4, compared with losses for the usual care group, referred to as diabetes support and education (DSE), of 0.7% and 1.0%, respectively.
At year 4, 46% of ILI participants maintained a loss ≥5% of initial weight (considered a clinically significant reduction), compared with 25% for DSE.
What does this study add?
The present study reports weight loss at year 8 -- the longest period for a randomized controlled trial -- when ILI participants lost a mean of 4.7% of initial weight, compared with 2.1% for ILI.
At year 8, 50% of ILI and 37% of DSE participants achieved a mean loss ≥5% of initial weight.
Nearly 40% of ILI participants who lost ≥10% of initial weight at year 1 maintained this loss at year 8, revealing that long-term weight control is possible with continued practice of skills taught in intensive lifestyle interventions.
Acknowledgments
This study was supported by the Department of Health and Human Services through the following cooperative agreements from the National Institutes of Health: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. The following federal agencies have contributed support: National Institute of Diabetes and Digestive and Kidney Diseases; National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; NIH Office of Research on Women’s Health; and the Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (I.H.S.) provided personnel, medical oversight, and use of facilities. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the I.H.S. or other funding sources.
Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01RR01066); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056), the Clinical Translational Research Center (CTRC) funded by the Clinical & Translational Science Award (UL1 RR 024153) and NIH grant (DK 046204); the VA Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs; and the Frederic C. Bartter General Clinical Research Center (M01RR01346). Some of the information contained herein was derived from data provided by the Bureau of Vital Statistics, New York City Department of Health and Mental Hygiene.
Federal support: National Institute of Diabetes and Digestive and Kidney Diseases (to Mary Evans, PhD; Barbara Harrison, MS; Van S. Hubbard, MD, PhD; and Susan Z. Yanovski, MD); National Heart, Lung, and Blood Institute (to Lawton S. Cooper, MD, MPH; Peter Kaufman, PhD, FABMR; and Mario Stylianou, PhD); and the Centers for Disease Control and Prevention (to Edward W. Gregg, PhD; Ping Zhang, PhD).
The following organizations committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST® of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America.
The authors thank Ms. Patricia Hong for her editorial assistance in preparing the manuscript.
Appendix
Authors: Thomas A. Wadden, PhD; John P. Bantle, MD; George Blackburn, MD, PhD; Paula Bolin, RN, MC; Frederick L. Brancati, MD, MHS; George A. Bray, MD; Jeanne M. Clark, MD, MPH; Mace Coday, PhD; Gareth R. Dutton, PhD; Caitlin Egan, MS; Mary Evans, PhD; John P. Foreyt, PhD; Siran Ghazarian Sengardi, MD; Edward W. Gregg, PhD; Helen P. Hazuda, PhD; James O. Hill, PhD; Edward S. Horton, MD; Van S. Hubbard, MD, PhD; John M. Jakicic, PhD; Robert W. Jeffery, PhD; Karen C. Johnson, MD, MPH; Steven E. Kahn MB, ChB; Abbas E. Kitabchi, PhD, MD; William C. Knowler, MD, DrPH; Cora E. Lewis, MD, MSPH; Barbara J. Maschak-Carey, MSN, CDE; Maria G. Montez, RN, MSHP, CDE; Brenda Montgomery, RN, MS, CDE; David M. Nathan, MD; Julie Nelson, RD; Jennifer Patricio, MS; Anne Peters, MD; Xavier Pi-Sunyer, MD; Henry Pownall, PhD; Amy D. Rickman, PhD, RD, LDN; Mara Vitolins, DrPH; Michael P. Walkup, MS; Delia S. West, PhD; Donald Williamson, PhD; Rena R. Wing, PhD; Holly Wyatt, MD; Susan Z. Yanovski, MD
Footnotes
A list of study authors and collaborators is shown in the appendix.
Author Contributions: Study concept and design: All authors. Acquisition of data: All authors. Analysis and interpretation of data: MPW, TAW, ESH JMJ, WCK, FXP, MPW, DSW, RRW, and SZY. Drafting of the manuscript: TAW, ESH, JMK, WCK, FXP, MPW, DSW, RRW, and SZY. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: MPW
Conflict of Interest Disclosures: All authors will submit the ICMJE Form for disclosure of potential conflicts of interest.
References
- 1.Ryan DA Look AHEAD Research Group. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–28. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 2.Wing RR Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faulconbridge LF, Wadden TA, Rubin RR, et al. One-year changes in symptoms of depression and weight in overweight/obese individuals with type 2 diabetes in the Look AHEAD study. Obesity. 2012;20:783–793. doi: 10.1038/oby.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williamson DA, Rejeski J, Lang W, et al. Impact of a weight management program on health-related quality of life in overweight adults with type 2 diabetes. Arch Intern Med. 2009;169:163–171. doi: 10.1001/archinternmed.2008.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuna ST, Reboussin DM, Borradaile KE, et al. Long-term effect of weight loss on obstructive sleep apnea severity in obese patients with type 2 diabetes. Sleep. 2013;36:641–649. doi: 10.5665/sleep.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rejeski WJ, Ip EH, Bertoni AG, et al. Look AHEAD Research Group. Lifestyle change and mobility in obese adults with type 2 diabetes. N Engl J Med. 2012;366:1209–17. doi: 10.1056/NEJMoa1110294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Institutes of Health/National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Obes Res. 1998;6(Suppl2):51S–210S. [PubMed] [Google Scholar]
- 8.Institute of Medicine. Weighing the options: Criteria for evaluating weight management programs. Washington, DC: National Academy Press; 1995. [PubMed] [Google Scholar]
- 9.World Health Organization. Obesity: Preventing and managing the global epidemic. Geneva, Switzerland: Author; 1998. [Google Scholar]
- 10.Wing RR, Lang W, Wadden TA, et al. Look AHEAD Research Group. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–6. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knowler WC, Barrett-Connor E, Folwer SE, Hamman RF, Lachin JM, Walker EA, Nathan DM Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gregg EW, Chen H, Wagenknecht LE, et al. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA. 2012;308:2489–96. doi: 10.1001/jama.2012.67929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevens VJ, Obarzanek E, Cook NR, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134:1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 14.Poobalan A, Aucott L, Smith WC, et al. Effects of weight loss in overweight/obese individuals and long-term lipid outcomes—a systematic review. Obes Rev. 2004;5:43–50. doi: 10.1111/j.1467-789x.2004.00127.x. [DOI] [PubMed] [Google Scholar]
- 15.Shah K, Stufflebam A, Hilton TN, et al. Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity. 2009;17:2162–8. doi: 10.1038/oby.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subak LL, Wing R, West DS, et al. Weight loss to treat urinary incontinence in overweight and obese women. N Engl J Med. 2009;360:481–90. doi: 10.1056/NEJMoa0806375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wing RR, Bond DS, Gendrano IN, 3rd, et al. Effect of intensive lifestyle intervention on sexual dysfunction in women with type 2 diabetes: Results from an ancillary Look AHEAD study. Diabetes Care. 2013;36:2937–44. doi: 10.2337/dc13-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wadden TA Look AHEAD Research Group. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14:737–52. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wadden TA, Neiberg RH, Wing RR, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity. 2011;19:1987–1998. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wesche-Thobaben JA Look AHEAD Research Group. The development and description of the comparison group in the Look AHEAD trial. Clin Trials. 2011;8:320–9. doi: 10.1177/1740774511405858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wing RR, Jeffrey RW, Burton LR, Thorson C, Nissinoff KS, Baxter JE. Food provision vs structured meal plans in the behavioral treatment of obesity. Int J Obes Relat Metab Disord. 1996;20:56–62. [PubMed] [Google Scholar]
- 23.Perri MG, Nezu AM, McKelvey WF, et al. Relapse prevention training and problem-solving therapy in the long-term management of obesity. J Conult Clin Psychol. 2001;69:722–726. [PubMed] [Google Scholar]
- 24.West DS, DiLillo V, Bursac Z, Gore SA, Greene PG. Motivational interviewing improves weight loss in women with type 2 diabetes. Diabetes Care. 2007;30(5):1081–1087. doi: 10.2337/dc06-1966. [DOI] [PubMed] [Google Scholar]
- 25.Kumanyika SK. Obesity treatment in minorities. In: Wadden TA, Stunkard AJ, editors. Handbook of Obesity Treatment. Guilford Press; New York: 2002. pp. 416–446. [Google Scholar]
- 26.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 27.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK American College of Sports Medicine. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–71. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 28.Jeffery RW, Wing RR, Sherwood NE, Tate DF. Physical activity and weight loss: does prescribing higher physical activity goals improve outcome? Am J Clin Nutr. 2003;78:684–689. doi: 10.1093/ajcn/78.4.684. [DOI] [PubMed] [Google Scholar]
- 29.Jakicic JM, Marcus BH, Lang W, Janney C. Effect of exercise on 24-month weight loss maintenance in overweight women. Arch Intern Med. 2008;168:1550–1559. doi: 10.1001/archinte.168.14.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355:1563–71. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 31.Butryn ML, Phelan S, Hill JO, Wing RR. Consistent self-monitoring of weight: a key component of successful weight loss maintenance. Obesity. 2007;15:3091–3096. doi: 10.1038/oby.2007.368. [DOI] [PubMed] [Google Scholar]
- 32.Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;21:323–41. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- 33.Wadden TA, West DS, Neiberg RH, Wing RR, Ryan DH, Johnson KC, Foreyt JP, Hill JO, Trence DL, Vitolins MZ Look AHEAD Research Group. One-year weight losses in the Look AHEAD study: factors associated with success. Obesity. 2009;17:713–722. doi: 10.1038/oby.2008.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knowler WC, Fowler SE, Hamman RF, et al. Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–86. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wing RR, Hamman RF, Bray GA, et al. Diabetes Prevention Program Research Group. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12:1426–34. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Looker HC, Knowler WC, Hanson RL. Changes in body mass index and weight before and after the development of type 2 diabetes. Diabetes Care. 2001;24:1917–22. doi: 10.2337/diacare.24.11.1917. [DOI] [PubMed] [Google Scholar]
- 37.Svetkey LP, Stevens VJ, Brantley PJ, et al. Weight Loss Maintenance Collaborative Research Group. Comparison of strategies for sustaining weight loss: The weight loss maintenance randomized controlled trial. JAMA. 2008;299:1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 38.Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155–61. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- 39.French SA, Folsom AR, Jeffery RW, Williamson DF. Prospective study of intentionality of weight loss and mortality in older women: the Iowa Women’s Health Study. Am J Epidemiol. 1999;149:504–14. doi: 10.1093/oxfordjournals.aje.a009844. [DOI] [PubMed] [Google Scholar]
- 40.Villareal DT, Apovian CM, Kushner RF, Klein S American Society for Nutrition; NAASO, The Obesity Society. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes Res. 2005;13:1849–1863. doi: 10.1038/oby.2005.228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.