Abstract
Natural tissues, such as bone, tendon, and muscle, have well defined hierarchical structures, which are crucial for their biological and mechanical functions. However, mimicking these structural features still remains a great challenge. In this study, we use ice-templated assembly and UV-initiated cryo-polymerization to fabricate a novel kind of composite hydrogel which have both aligned macroporous structure at micrometer scale and a nacre-like layered structure at nanoscale. Such hydrogels are macroporous, thermoresponsive, and exhibit excellent mechanical performance (tough and high stretchable), attractive properties that are of significant impact on the wide applications of composite hydrogels, especially as tissue-engineering scaffolds. The fabrication method in this study including freeze-casting and cryo-polymerization can also be applied to other materials, which makes it promising for designing and developing smart and multifunctional composite hydrogels with hierar chical structures.
Keywords: bio inspired, hydrogel, composite, nacre, freeze casting
INTRODUCTION
Hydrogels have attracted considerable interest for use in biomedical applications such as drug delivery vehicles,1, 2 sensors and devices,3-5 and especially as tissue-engineering scaffolds.6-8 When hydrogels are used as scaffolds, several of their aspects must be controlled, including the internal porous structure, swelling behavior, mechanical properties, and the ability to adapt to environmental stimuli. The challenge is to optimize all these related and often contradictory properties into one hydrogel. For example, although double-network9 and nanocomposite10 hydrogels have excellent mechanical properties, they both lack macroporous structure and control over pore morphology. In nature, tissues such as bone, tendon, and muscle have well-defined hierarchical structures, assembled from molecular to meso- and macro-level, which are crucial to their biological and mechanical functions. Mimicking these structural features is not trivial and we are so far unsuccessful in applying nature's secrets to our own scaffold designs. Here, by using ice-templated assembly (or freeze casting) and UV-initiated cryo-polymerization, we fabricated a novel kind of bio-inspired composite hydrogel, i.e., poly(N-isopropylacrylamide) (PNIPAAm) and clay platelets, which have both a highly aligned porous structure at micrometer scale and a nacre-like layered structure at nanoscale. With this hierarchical structure, the as-prepared hydrogels exhibit excellent mechanical properties (tough and highly stretchable), and are capable of responding to changes in temperature. The pore size of the composite hydrogels can be tuned by controlling the cooling rate, which in turn affects thermoresponsive and swelling behavior. The mechanical properties could be further improved by incorporating higher clay content in the walls of nacre-like layered structure. This study describes a novel route for achieving smart and multifunctional composite hydrogels with hierarchical structures.
EXPERIMENTAL SECTION
Materials
Monomer (N-isopropylacrylamide [NIPAAm]), initiator (2,2-diethoxyacetophenone [DEAP]), and rhodamine B were purchased from Sigma-Aldrich. Inorganic clay (Laponite XLG [Mg5.34Li0.66Si8O20(OH)4]Na0.66, M.W. = 762.24, ~ 1 nm in thickness and ~ 100 nm in diameter) was purchased from Rockwood Ltd., UK. All materials were used as received.
Preparation of macroporous composite hydrogels
At first, NIPAAm (1 mol/L), DEAP (0.02 mol/L), and various amounts of clay (0.05, 0.1, and 0.15 mol/L) were dissolved into deionized water to get a solution (defined as NC-5, NC-10, and NC-15, according to clay content; see also Supporting Information, Table S1). Next, the solution was poured into a mold (9 × 3 × 5 mm) on a copper stage where it began to freeze from the bottom at a given cooling rate (10, 5, or 1°C/min). The frozen samples were then placed under UV light (λ = 365 nm) for 5 hours to complete the in situ radical cryo-polymerization on a copper stage at −45°C. The samples prepared by random freezing were frozen in a freezer (−25°C) and then cryo-polymerized at the same condition.
Characterization on hierarchical structures of macroporous composite hydrogels
The hydrogel samples were first freeze-dried and then coated with gold by sputtering at 30 mA for 60 s. SEM images of the hydrogels were obtained using a field-emission scanning electron microscopy (JSM 5700F, JEOL, Japan) at an acceleration voltage of 3 kV. The TEM image was obtained on the JEOL 2100H instrument at 200 kV, with a Gatan US1000 camera (fiber-optical charge-coupled device), which shows spatial resolution of 0.2 nm.
Mechanical test on macroporous composite hydrogels
The mechanical properties were tested on dumbbell shape samples (30 mm in overall length, 9 mm in inner width, 3 mm in thickness) in the tensile mode (Instron 5944 testing system, USA). The gauge length was set to 5 mm, and the load speed was 10 mm/min. For each category, at least 4 samples were tested.
Molecular characteristics of PNIPAAm
PNIPAAm was extracted and removed from NC-10 hydrogel by dissolving clay in hydrofluoric acid solution (0.4wt%).111H NMR spectra of NIPAAm and PNIPAAm were recorded on a Bruker Biospin Avance II spectrometer opening at 500 MHz at 25°C after dissolving the samples in D2O. The molecular weight of PNIPAAm was determined by use of Agilent 1200 Series HPLC equipped with refractive index detector. A PLgel 10-μm miniMIX B 250×4.6 mm column (Varian, Inc.) was employed with DMF eluent (0.3 mL/min) and calibrated by using polystyrene standard samples (Agilent Technologies) in DMF with molecular weights ranging from 500 to 6, 870, 000.
Thermoresponsive drug release of macroporous composite hydrogels
Rhodamine B was loaded into the hydrogel when preparing the monomer solution. The hydrogels (in the size of 5 × 9 × 3 mm) were immersed into PBS at 23°C or 37°C for 24 hours. Visible absorption spectra of the solution were tested to indicate the release of rhodamine B, using a Perkin Elmer UV-Vis Lambda 35 Spectrophotometer.
Swelling properties of bio-inspired composite hydrogels
Samples (in the size of 5 × 9 × 3 mm) were first dried in an oven at 50°C for 48 hours and measured for their weight at the dry state (wdry). The dry samples were then soaked in PBS solution and were measured for their weight at the wet state (wwet) after various periods of time. For each category, at least 3 samples were measured.
RESULTS AND DISCUSSION
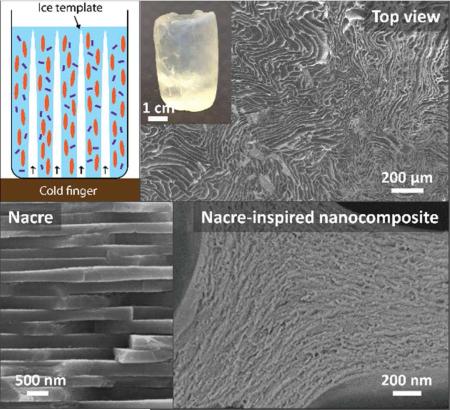
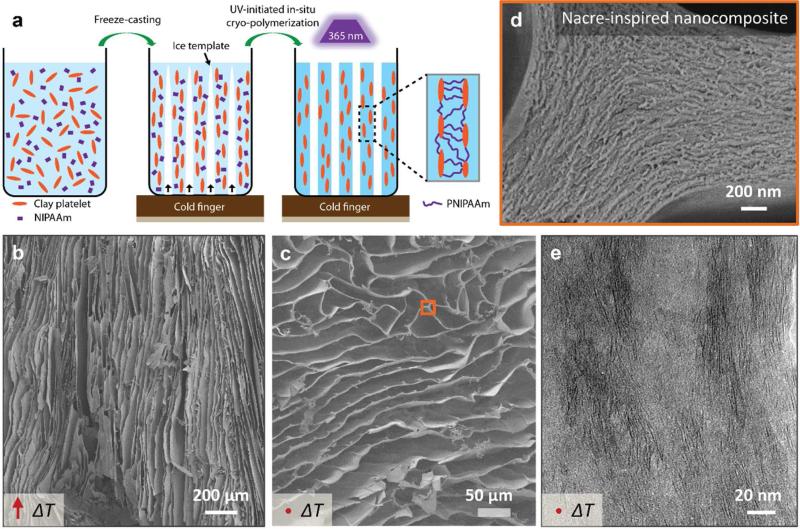
Ice-templated assembly, also known as freeze-casting, is a promising technology for fabricating aligned architectures with various types of materials such as polymers,12-14 ceramics,15 nanoparticles,16 and their composites.17, 18 As shown in Figure 1a, a typical freeze-casting process, followed by a UV-initiated cryo-polymerization step,13, 19 was used as our processing technique (see also the Experimental Section). In general, a given concentration of monomer (NIPAAm), initiator (2,2-diethoxyacetophenone [DEAP]), and crosslinker (inorganic clay platelets, ~ 100 nm in diameter and ~ 1 nm in thickness, see Supporting Information, Figure S1) were mixed with deionized water to obtain a uniform solution. We prepared solutions with the same amount of monomer and water, but different amounts of clay platelets, defined as NC-n (n varies according to the clay content n × 10−2 mol/L) (see also Supporting Information, Table S1). Similar to the mechanism reported in Haraguchi's paper,20, 21 photoinitiator in our solution concentrated also near the clay platelets before polymerization, which was confirmed by the viscosity increases from 22.1 mPaPs to 39.7 mPaPs after adding 0.4wt% of initiator (DEAP) into 4wt% of clay solution after 1 hour. The solution was next poured into a mold placed on a cold finger whose temperature was controlled. During the solidification of the solution from the cold finger, anisotropic ice crystals grew preferentially along the temperature gradient and behaved like templates by expelling the monomer and clay platelets from the solidifying water. After the sample was completely frozen, it was placed under UV light on a copper stage at −45°C to initiate cryo-polymerization from the clay surface and eventually form an organic/inorganic network.21 After polymerization, a hierarchical composite hydrogel was obtained, Figure 1b-e. The as-prepared hydrogel has an aligned porous structure at micrometer scale that replicates the ice crystals. The hydrogel is well aligned parallel to the temperature gradient (ΔT), as illustrated by the SEM images in Figure 1b and c, respectively. The polymer and clay platelets are assembled into a nacre-like layered composite with walls having highly aligned structure, shown by SEM and TEM images in Figure 1d and e, respectively. For comparison, random freezing was performed by placing the solution in a freezer at −25°C and then polymerizing it at the same condition as freeze-cast samples. The monomer conversion of the cryo-polymerization process and the resulting molecular weight of PNIPAAm were investigated after removing clay from the composite in a hydrofluoric acid solution, which also demonstrated clay as crosslinker.11 There is no trace of NIPAAm monomer in 1H NMR spectra, which indicates full conversion of the monomer (Supporting Information, Figure S2).22 From the gel permeation chromatography test (Figure S3), the molecular weight of PNIPAAm (Mw: 1.2 × 105 g/mol, Mn: 5.6 × 104 g/mol) and its polydispersity (Mw/Mn = 2.1) was obtained, which is consistent with the results in similar PNIPAAm/Clay system.11
Figure 1.
a) Schematic illustration of the fabrication method of macroporous composite hydrogels by freeze-casting. A solution, composed of monomer (NIPAAm), initiator (DEAP), and crosslinker (clay platelet) at a given concentration was placed on a cold finger connected to a liquid nitrogen reservoir. During the cooling process, ice crystals grew from the cold finger and templated the assembly of monomer and clay platelets into a nacre-like layered nanocomposite. After freezing, the sample was placed under UV light to initiate cryo-polymerization. The as-prepared nanocomposite hydrogels have an anisotropically aligned structure at micrometer scale, as shown by the SEM images in both the b) parallel and c) perpendicular directions to the temperature gradient (ΔT). In the wall of the aligned structure, clay platelets and PNIPAAm are assembled into nacre-like layered nanocomposites, as shown by d) SEM and e) TEM images.
Because the internal space of hydrogels is important to effectively store cells,23 we tried to control pore size within the composite hydrogels by tuning the cooling rate of the cold finger during the freeze-casting process.15, 16 When the cooling rate increases, the solidifying front grows faster along the temperature gradient and as a result, smaller ice platelets are obtained. The space between the two adjacent walls are in turn smaller (see the structures before polymerization in Supporting Information, Figure S4). The cryo-polymerization method was then used to overcome the challenge of polymerizing monomers without affecting well-defined structures after freeze-casting.13 Because samples are kept frozen during the cryo-polymerization process, the aligned porous structure within the hydrogel shows no obvious difference before (Figure S4) and after (Figure 2) polymerization. For hydrogels (NC-10) fabricated by freeze-casting (Figure 2a-c), the width (D) between two adjacent walls increases with decreasing cooling rate, i.e., 42.7 μm, 48.2 μm, and 107.7 μm, for 10, 5, and 1°C/min, respectively. However, for the hydrogel fabricated by random freezing in a freezer at −25°C, only isotropic pores (79 μm) can be observed.
Figure 2.
SEM images of bio-inspired nanocomposite hydrogels (NC-10) fabricated by freeze-casting at a cooling rate of a) 10, b) 5, and c) 1°C/min, or d) random freezing. The upper and lower rows show the structures at the cross-section perpendicular and parallel to ΔT, respectively. By controlling the cooling rate during freeze-casting, the size of ice crystals and hence the pore size within the hydrogels could be tuned. The width (D) between two adjacent walls in a) to c) is 42.7 μm, 48.2 μm, and 107.7 μm, respectively. d) For the sample fabricated by random freezing, isotropic pores are 79 μm in size.
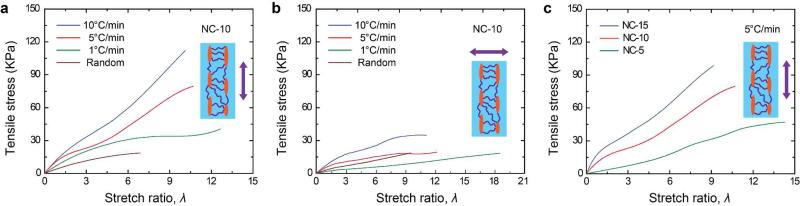
Another issue that limits the application of hydrogels as scaffolds is their poor mechanical properties.9, 10, 24, 25 Modulus and toughness are strongly related to the porosity of the material. As a result, hydrogels fabricated by freeze-casting at various cooling rates will have different mechanical properties due to their distinct hierarchical structure and pore size. Representative stress-strain curves of hydrogels fabricated by freeze-casting and random freezing were obtained via tensile test both parallel and perpendicular to the freezing direction and are shown in Figure 3. The stretch ratio (λ) is defined as the distance between the clamps during stretching divided by the distance before stretching. In the parallel direction, freeze-cast hydrogels have an increasing tensile strength and fracture energy following the order of 10°C/min > 5°C/min > 1°C/min > random freezing. The modulus (calculated from the period of λ = 0 ~ 0.5) of hydrogels fabricated by freeze-casting (41.2 kPa ~ 53.2 kPa) and random freezing (45.6 kPa) shows no obvious difference. However, tensile strength, stretch, and fracture energy of random freezing samples (14.8 kPa, 6.9, 144.9 J/m2, respectively) are much lower than those fabricated by freeze-casting (79.4 ~ 114.5 kPa, 8.8 ~ 11.2, 4124.1 ~ 5092 J/m2); see also Supporting Information, Table S2. In the perpendicular direction, freeze-cast samples showed no obvious advantage over random freezing samples, in terms of tensile strength, stretch, and fracture energy. This illustrates that hydrogels fabricated at a lower cooling rate have larger pore size and show poor mechanical properties in terms of modulus, strength, and fracture energy. The effect of the cooling rate on mechanical properties of composite hydrogels is consistent with that found for ceramics fabricated by freeze-casting.15 Note that all the samples in Figure 3a were prepared from the same solution (NC-10, Supporting Information, Table S1) and at least 4 samples were tested in each category. Although composite hydrogels exhibit better mechanical performance than organic hydrogels, very limited studies4, 7, 26, 27 have investigated their properties when they have large pore size or complex hierarchical structures, as in our samples. Because clay content is one of the key parameters that affect the mechanical properties of composite hydrogels,24, 28 we tried to increase it from 5 to 10, and 15 × 10−2 mol/L, i.e., NC-5, 10, and 15 (Supporting Information, Table S1). Representative stress-strain curves of these hydrogels are shown in Figure 3b. All these samples were fabricated by freeze-casting at 5°C/min. By gradually increasing the clay content, we were able to enhance their tensile strength and modulus, from about 48 kPa and 21 kPa for NC-5, to about 103 kPa and 108 kPa for NC-15. Although at the same time λ decreased from about 14.3 to 9.2, the freeze-cast hydrogels are still quite stretchable and their fracture energy (1055.2 ~ 5462.1 J/m −2) is in the range of cartilage (500 ~ 1500 J/m −2).29 Such unique mechanical properties of these macroporous hydrogels might be attributed to their nacre-like layered structure at nanoscale.15, 17, 24 Nacre achieves high modulus and toughness by alternately incorporating inorganic and organic layers, and reaches an inorganic content as high as 95%.17 Although hydrogel (wet and soft) is different from nacre (dry and hard) in a toughening mechanism,30 it is worth noting that the clay/(clay + polymer) percentage in our specimens has already reached 50% and can be further increased up to 95% as in nacre.
Figure 3.
Mechanical properties of macroporous composite hydrogels from tensile tests. Representative stress-strain curves when stretching a) parallel and b) perpendicular to the freezing direction (inset), of hydrogels fabricated at different cooling rates or random freezing, as labeled. The stretch ratio (λ) is defined as the distance between the clamps during stretching divided by the distance before stretching. In the parallel direction, the hydrogels have an increasing tensile strength and fracture energy following the order of 10°C/min > 5°C/min > 1°C/min > random freezing. Freeze-cast samples show no obvious advantage over random freezing samples in perpendicular direction. c) Representative stress-strain curves of hydrogels with different amounts of clay platelets (NC-5, 10, and 15), all fabricated by freeze-casting at 5°C/min. Hydrogels with higher clay content have higher modulus and tensile strength.
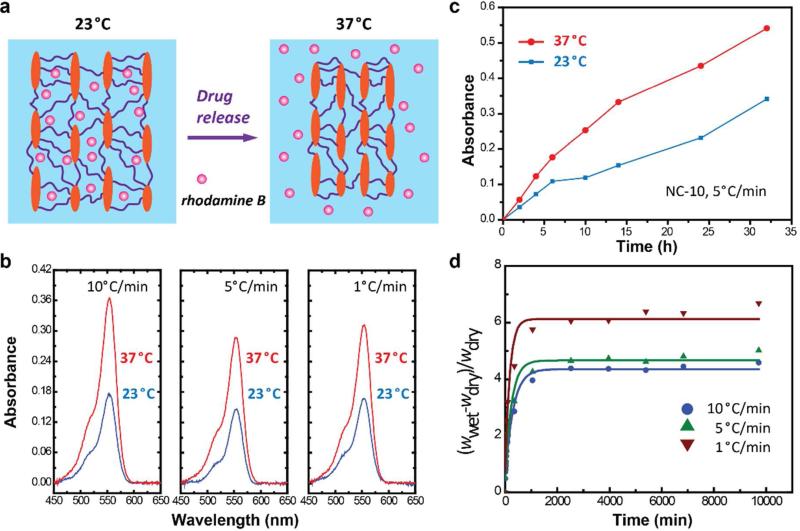
Hydrogel's ability to adapt to environmental stimuli is also important for smart device, cell behavior and drug release.1, 4, 31-35 PNIPAAm, well known for the coil-to-globule transition around its LCST (lower critical solution temperature) at about 32°C (Figure 4a), is widely investigated for its applications as smart drug vehicle and scaffold.26, 31-33, 36 We loaded rhodamine B (as an example of a drug) into the monomer solution before freeze-casting. After cryo-polymerization, hydrogels were immersed into phosphate buffered saline (PBS) solution at 23°C or 37°C to release rhodamine B. PBS solutions were tested for visible absorption spectra after 2, 4, 6, 10, 14, 24, and 32 hours, Figure 4b and c. As shown in Figure 4b, all freeze-cast specimens show enhanced release of rhodamine B at 37°C after 24h. In Figure 4c, the absorption at a wavelength of 550 nm indicate the release profile of NC-10 hydrogel fabricated by freeze-casting at 5°C/min. The figure shows that it has higher release under 37°C, which can be attributed to the thermoresponsive shrinkage of PNIPAAm network (Figure 4a). Although the early release of rhodamine B limited its application in controlled drug release, such hydrogels with thermoresponsive properties at a high clay content might find applications in other chemical and biological processes. It was reported that a high clay content in NC-10 hydrogel restricts its response to temperature.37 However, NC-10 hydrogels fabricated by freeze-casting and cryo-polymerization are thermoresponsive because their porous structure enables easier water permeation.38 This clearly demonstrates the advantage of designing hierarchical structures within composite hydrogels for their application in drug release and as smart scaffolds, especially when high inorganic content is required to increase the mechanical properties of the gel, yet it restricts the gel's response.
Figure 4.
Thermoresponsive drug release and swelling properties of bio-inspired nanocomposite hydrogels. a) Drug release of the hydrogel is largely enhanced by increasing temperature from 23°C to 37°C, because of the coil-to-globule transition of PNIPAAm around its LCST (32°C). b) Visible absorption spectra showing the thermoresponsive release of rhodamine B from hydrogels fabricated by freeze-casting at 10, 5, and 1°C/min. For comparison, hydrogels loaded with rhodamine B were immersed in PBS at 23°C or 37°C for 24 hours. c) Absorption of PBS solution at a wavelength of 550 nm was showing to indicate the release profiles of NC-10 hydrogel (obtained by freeze-casting at 5°C/min) at different releasing temperatures. d) The hydrogels with different pores reach their equilib rium swelling state in PBS in about 20 hours. Hydrogels fabricated by freeze-casting at 1°C/min have a higher final degree of swelling (wwet -wdry)/wdry, compared with those at 5 and 10°C/min, which is consistent with their pore size as shown in Figure 2.
The swelling properties of the composite hydrogels were also investigated. The as-prepared samples (9 × 3 × 5 mm) were dried in a furnace at 50°C for 48 hours to remove water, and were weighed in the dry state (wdry). After soaking the samples in PBS solution to swell, the weight at the wet state (wwet) was measured after a certain period of time. As shown in Figure 4d, all the samples reached their equilibrium state in about 20 hours. The degree of swelling [(wwet -wdry)/wdry] was about 4.58, 5.01, and 6.68 for hydrogels obtained by freeze-casting at 10, 5, 1°C/min, respectively. This is also consistent with the distinct pore size within the hydrogels (Figure 2).
CONCLUSIONS
In conclusion, we have successfully fabricated a novel type of macroporous composite hydrogel (composed of PNIPAAm and clay platelets) by ice-templated assembly (freeze-casting) and UV-initiated cryo-polymerization. The as-prepared composite hydrogels have a unique hierarchical structure with aligned pores at micrometer scale and nacre-like layered structure at nanometer scale. These hydrogels are thermoresponsive and exhibit excellent mechanical performance despite their macroporous structure, which is desirable for biomedical applications in controlled drug release and as smart scaffolds, and other chemical and biological processes. Furthermore, the swelling behavior of these hydrogels in PBS was also investigated. More importantly, the hierarchical structure and in turn the mechanical and thermoresponsive properties as well as swelling behavior could be tuned by controlling the freeze-casting parameters (e.g., cooling rate) and the solution composition. The freeze-casting and cryo-polymerization described here can also be applied to other materials, which makes it promising for designing and developing smart and multifunctional composite hydrogels with hierarchical structures.
Supplementary Material
ACKNOWLEDGMENT
Research reported in this publication was supported by the National Institute of Dental and Craniofacial Research of the National Institutes of Health under award number 1R01DE015633. The authors also thank Ms. Grace Lau, Dr. Wen Yuan, Dr. Sebastien Gottis, Dr. Kaiyang Niu, Dr. Renjia Zhou, Dr. Dong Wang, Lin Wang, and Yuan Chen for their kind help with the experiments.
Footnotes
Supporting Information.
Detailed composition of the solutions used in the work (Table S1), SEM images of clay platelets (S1), 1H NMR spectra of monomer and cryo-polymerized polymer (S2), GPC data of extracted PNIPAAm molecules (S3), SEM images of samples before cryo-polymerization (S4), and a summary of mechanical properties of NC-10 hydrogels (Table S2). This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- 1.Seliktar D. Science. 2012;336:1124–1128. doi: 10.1126/science.1214804. [DOI] [PubMed] [Google Scholar]
- 2.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma MM, Guo L, Anderson DG, Langer R. Science. 2013;339:186–189. doi: 10.1126/science.1230262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tokarev I, Minko S. Adv. Mater. 2010;22:3446–3462. doi: 10.1002/adma.201000165. [DOI] [PubMed] [Google Scholar]
- 5.Hu H, Yuan W, Zhao H, Baker GL. J. Polym. Sci., Part A: Polym. Chem. 2013 doi: 10.1002/pola.26980. [Google Scholar]
- 6.Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Adv. Mater. 2009;21:3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kehr NS, Prasetyanto EA, Benson K, Ergun B, Galstyan A, Galla HJ. Angew. Chem. Int. Ed. 2013;52:1156–1160. doi: 10.1002/anie.201206951. [DOI] [PubMed] [Google Scholar]
- 8.Balakrishnan B, Banerjee R. Chem. Rev. 2011;111:4453–4474. doi: 10.1021/cr100123h. [DOI] [PubMed] [Google Scholar]
- 9.Gong JP, Katsuyama Y, Kurokawa T, Osada Y. Adv. Mater. 2003;15:1155–1158. [Google Scholar]
- 10.Haraguchi K, Takehisa T. Adv. Mater. 2002;14:1120–1124. [Google Scholar]
- 11.Haraguchi K, Xu Y, Li G. Macromol. Rapid Commun. 2010;31:718–723. doi: 10.1002/marc.200900819. [DOI] [PubMed] [Google Scholar]
- 12.Riblett BW, Francis NL, Wheatley MA, Wegst UGK. Adv. Funct. Mater. 2012;22:4920–4923. [Google Scholar]
- 13.Barrow M, Zhang H. Soft Matter. 2013;9:2723–2729. [Google Scholar]
- 14.Wegst UG, Schecter M, Donius AE, Hunger PM. Phil. Trans. R. Soc. A. 2010;368:2099–121. doi: 10.1098/rsta.2010.0014. [DOI] [PubMed] [Google Scholar]
- 15.Deville S, Saiz E, Nalla RK, Tomsia AP. Science. 2006;311:515–518. doi: 10.1126/science.1120937. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Hussain I, Brust M, Butler MF, Rannard SP, Cooper AI. Nat. Mater. 2005;4:787–93. doi: 10.1038/nmat1487. [DOI] [PubMed] [Google Scholar]
- 17.Munch E, Launey ME, Alsem DH, Saiz E, Tomsia AP, Ritchie RO. Science. 2008;322:1516–1520. doi: 10.1126/science.1164865. [DOI] [PubMed] [Google Scholar]
- 18.Yuan W, Zhao H, Hu H, Wang S, Baker GL. ACS applied materials & interfaces. 2013;5:4155–4161. doi: 10.1021/am4001858. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Zhao Q, Sun J, Zhou Q. Soft Matter. 2012;8:3620–3626. [Google Scholar]
- 20.Haraguchi K, Li HJ, Matsuda K, Takehisa T, Elliott E. Macromolecules. 2005;38:3482–3490. [Google Scholar]
- 21.Haraguchi K, Takada T. Macromolecules. 2010;43:4294–4299. [Google Scholar]
- 22.Xu Y, Li G, Haraguchi K. Macromol. Chem. Phys. 2010;211:977–987. [Google Scholar]
- 23.Elisseeff J. Nat. Mater. 2008;7:271–273. doi: 10.1038/nmat2147. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Lin L, Cheng Q, Jiang L. Angew. Chem. Int. Ed. 2012;51:4676–4680. doi: 10.1002/anie.201200267. [DOI] [PubMed] [Google Scholar]
- 25.Sun J-Y, Zhao X, Illeperuma WRK, Chaudhuri O, Oh KH, Mooney DJ, Vlassak JJ, Suo Z. Nature. 2012;489:133–136. doi: 10.1038/nature11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz VB, Thetiot F, Ritz S, Putz S, Choritz L, Lappas A, Forch R, Landfester K, Jonas U. Adv. Funct. Mater. 2012;22:2376–2386. [Google Scholar]
- 27.Gaharwar AK, Schexnailder P, Kaul V, Akkus O, Zakharov D, Seifert S, Schmidt G. Adv. Funct. Mater. 2010;20:429–436. [Google Scholar]
- 28.Haraguchi K, Li HJ. Macromolecules. 2006;39:1898–1905. [Google Scholar]
- 29.Simha NK, Carlson CS, Lewis JL. J. Mater. Sci. -Mater. Med. 2004;15:631–639. doi: 10.1023/b:jmsm.0000026104.30607.c7. [DOI] [PubMed] [Google Scholar]
- 30.Shull KR. Nature. 2012;489:36–37. doi: 10.1038/489036a. [DOI] [PubMed] [Google Scholar]
- 31.Chang C, Wei H, Wu D-Q, Yang B, Chen N, Cheng S-X, Zhang X-Z, Zhuo R-X. Int. J. Pharm. 2011;420:333–340. doi: 10.1016/j.ijpharm.2011.08.038. [DOI] [PubMed] [Google Scholar]
- 32.Chen L, Liu M, Bai H, Chen P, Xia F, Han D, Jiang L. J. Am. Chem. Soc. 2009;131:10467–10472. doi: 10.1021/ja9019935. [DOI] [PubMed] [Google Scholar]
- 33.Burkert S, Bittrich E, Kuntzsch M, Mueller M, Eichhorn K-J, Bellmann C, Uhlmann P, Stamm M. Langmuir. 2010;26:1786–1795. doi: 10.1021/la902505q. [DOI] [PubMed] [Google Scholar]
- 34.Jeong B, Bae YH, Lee DS, Kim SW. Nature. 1997;388:860–862. doi: 10.1038/42218. [DOI] [PubMed] [Google Scholar]
- 35.Nowak AP, Breedveld V, Pakstis L, Ozbas B, Pine DJ, Pochan D, Deming TJ. Nature. 2002;417:424–428. doi: 10.1038/417424a. [DOI] [PubMed] [Google Scholar]
- 36.Shakya AK, Kumar A, Nandakumar KS, Soc JR. Interface. 2011;8:1748–59. doi: 10.1098/rsif.2011.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haraguchi K, Li HJ. Angew. Chem. Int. Ed. 2005;44:6500–6504. doi: 10.1002/anie.200502004. [DOI] [PubMed] [Google Scholar]
- 38.Okay O. Prog. Polym. Sci. 2000;25:711–779. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.