Abstract
Transgenic mice that over-express connective tissue growth factor (CTGF) in fibroblasts under the control of an enhancer/promoter element of the Colla2 gene (Col1a2-CTGF) recapitulate multiorgan fibrosis similar to fibrosis observed in Scleroderma (SSc). In this study we investigate the regulation of secreted protein acidic and rich in cysteine (Sparc) and Ctgf siRNAs on the expression of several extracellular matrix components in the fibroblasts derived from Col1a2-CTGF transgenic mice. Three fibroblast lines were obtained from each of wide type C57BL/6 and CTGF transgenic C57BL/6, and were transfected with Sparc siRNA or Ctgf siRNA. Real-time quantitative RT-PCR and Western blotting were used to examine the transcription and protein levels of type I collagen, CTGF and SPARC. Student’s t-tests were used to determine the significance of the results. Our results showed that Colla2 and Ctgf increased expression at both transcriptional and translational levels in the fibroblasts from the Col1a2-CTGF transgenic mice compared with those in the fibroblasts from their normal wild-type littermate. The treatment with Sparc siRNA or Ctgf siRNA attenuated the mRNA and/or protein expression of the Colla2, Ctgf and Sparc in these fibroblasts. Sparc and Ctgf siRNAs also showed a reciprocal inhibition at transcript levels. Therefore, our results indicated that both SPARC and CTGF appeared to be involved in the same biological pathway, and they have the potential to serve as a therapeutic target for fibrotic diseases such as SSc.
Keywords: Sparc, Ctgf, fibroblasts, fibrosis, siRNA
Systemic sclerosis (SSc), also known as scleroderma, is a complex autoimmune disease characterized by skin and internal organ fibrosis. Currently, there is neither effective therapy nor effective prevention for this disease. Although the etiology of SSc is still unknown, both in vitro and in vivo studies have indicated that the extensive deposition of collagens, and other extracellular matrix (ECM) proteins by activated fibroblasts is a major pathologic property of SSc (1–2).
To better understand the pathogenic mechanisms and to find potential therapeutic targets of SSc, several kinds of animal models, including genetically modified mice harboring disruptions or manipulation of pivotal signaling pathways, have been established (2–3). Transforming growth factor β (TGFβ) is a fibrotic growth factor. Over-activity of TGFβ signaling has been widely accepted to play important roles in the fibrosis of SSc (4). Connective tissue growth factor (CTGF) is a downstream mediator of TGFβ signaling. Many of the profibrotic properties of TGFβ are induced by the actions of CTGF (5). Col1a2-CTGF transgenic mice that over-express CTGF in fibroblasts under the collogen type 1 (Col1a2) promoter showed an SSc-like fibrotic phenotype (6). The animal models provide a platform for testing potential anti-fibrotic reagents for SSc.
SPARC (secreted protein, acidic and rich in cysteine) is a matricellular component of the ECM. It participates in the modulation of cell-matrix interactions, cell adhesion, wound repair, and angiogenesis (7–9) and possibly plays an important role in fibrosis. Increased expression of SPARC have been found in many fibrotic diseases including SSc, pulmonary fibrosis, renal interstitial fibrosis, hepatic cirrhosis, and atherosclerotic vascular lesions (10–14). SPARC has shown the ability to interact with the TGFβ signaling system through a TGFβ receptor and Smad2/3-dependent pathway (15).
In our previous studies, we observed that SPARC can regulate the expression of type 1 collagen, a major structural protein of the ECM, in normal human fibroblasts (16). Moreover, after exogenous TGFβ stimulation, SPARC siRNA showed a protective role against overexpression of collagen genes (16). Specific inhibition of SPARC expression with siRNA led to a down-regulation of collagen and CTGF gene expression in SSc fibroblasts (17). In order to evaluate the influence of the inhibition of Sparc in the Col1a2-CTGF transgenic mouse model and its potential as a therapeutic target of SSc, an in vitro study was performed using the fibroblasts derived from the novel Col1a2-CTGF transgenic mouse model to investigate the regulation of Sparc siRNA on the expression of several ECM components, and to compare it with that of Ctgf siRNA.
MATERIALS AND METHODS
Cell lines
Two fibroblast lines derived from skin biopsies of Col1a2-CTGF transgenic (heterozygous) mice and wild-type littermate controls (wide type C57BL/6) (6) were used in experiments described here. The cultures were maintained in DMEM with 10% FCS and supplemented with antibiotics (50 U/ml penicillin and 50 μg/ml streptomycin). Fifth-passage fibroblast cells were seeded at a density of 5 × 105 cells in 25-cm2 flasks and grown until confluence.
Transient transfection with siRNA
Double-stranded ON-TARGET plus siRNAs of murine Sparc and Ctgf were purchased from DHARMACON (Lafayette, CO). The corresponding target sequences are 5′-GCACCACACGUUUCUUUG -3′ for Sparc and 5′-GCACCAGUGUGAAGACAUA -3′ for Ctgf, respectively. The culture medium in each culture flask with confluent fibroblasts was replaced with Opti-MEM I medium (Invitrogen, Carlsbad, CA) without FCS and antibiotics. The fibroblasts were transfected with Sparc siRNA or Ctgf siRNA, using Metafectene (Biontex, Munich, Germany) in a concentration of 3 μg siRNA per ml medium. Fibroblasts with Non-Targeting siRNA treatment were used as negative control. After 8 hours, the culture medium was replaced with DMEM. The cells transfected with siRNA were examined after 72 hours of transfection and used for RNA expression and protein assays.
Determination of gene expression by quantitative RT-PCR
Quantitative real-time RT-PCR was performed using an ABI 7900 Sequence Detector System (Applied Biosystems, Foster City, CA). The specific primers and probes for each gene (Col1a2, Ctgf, and Sparc) were purchased from the Assays-on-Demand product line (Applied Biosystems). Total RNA from each sample was extracted from the cultured fibroblasts using RNeasy Mini Kit (Qiagen, Valencia, CA). Complementary DNA (cDNA) was synthesized using MultiScribe™ Reverse Transcriptase (Applied Biosystems). Synthesized cDNAs were mixed with primers/probes in 2 × TaqMan universal PCR buffer and then assayed on an ABI 7900 sequence detector. The data obtained from the assays were analyzed with SDS 2.2 software (Applied Biosystems). The amount of total RNA in each sample was normalized with Gapdh transcript levels.
Western blot analysis
The cellular lysates extracted from the above cultured fibroblasts were used for protein assays. The protein concentration was determined by a spectrophotometer using Bradford protein assay kit (Bio-Rad Laboratories, Hercules, CA). Equal amounts of protein from each sample were subjected to sodium dodecyl sulfate- polyacrylamide gel electrophoresis. Resolved proteins were transferred onto PVDF membrane and incubated with respective primary antibodies, including anti-type I collagen antibody (Biodesign International, Saco, ME), anti-CTGF antibody (GeneTex Inc, San Antonio, TX), and anti-SPARC antibody (R&D Systems Inc, Minneapolis, MN). Mouse β-actin (Alexis Biochemicals, San Diego, CA) was used as an internal control. The secondary antibody was peroxidase-conjugated anti-rabbit, anti-goat, or anti-mouse IgG. Specific proteins were detected by chemiluminescence using an enhanced chemiluminescence system (Amersham, Piscataway, NJ). The intensity of the bands was quantified using ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
RESULTS
Colla2, Ctgf and Sparc expression in Colla2-CTGF transgenic mice fibroblasts
As measured by quantitative real-time RT-PCR, Col1a2, Ctgf and Sparc showed increased gene expression in the fibroblasts from Col1a2-CTGF transgenic mice compared with those from wild- type littermate controls (wild type) (Fig. 1). The fold changes of each gene in transgenic fibroblasts were 2.11±0.01 for Col1a2, 5.77±0.36 for Ctgf, and 1.66±0.18 for Sparc, respectively.
Fig. 1.
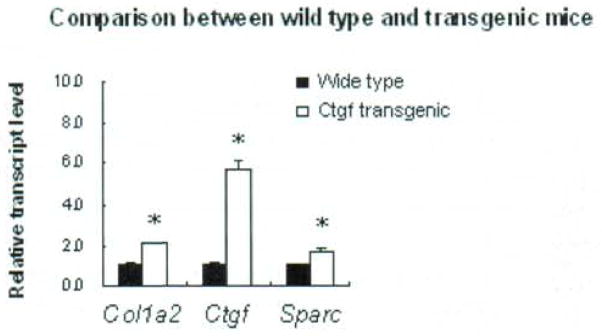
Comparison of gene expression among the wide type and Colla2-CTGF transgenic mice fibroblasts. The expression level of each gene in wild type fibroblasts was normalized to 1. Bars show the mean±SD results of analysis of 3 independent experiments performed in triplicate. * P < 0.05.
Transfection efficiency
Two methods were used for measuring transfection efficiency of siRNA. First, the fibroblasts were transfected with fluorescein-labeled non-silencing siRNA, and then examined under fluorescence microscopy which showed ~80% transfection efficiency by direct cell counting. Second, the fibroblasts from GFP transgenic C57BL/6 mouse (The Jackson Laboratory, Bar Harbor, Maine) were transfected with specific siRNA of GFP, and then examined to see how many cells responded with decreased levels of GFP. A similar efficiency of transfection was seen (Fig. 2).
Fig. 2.
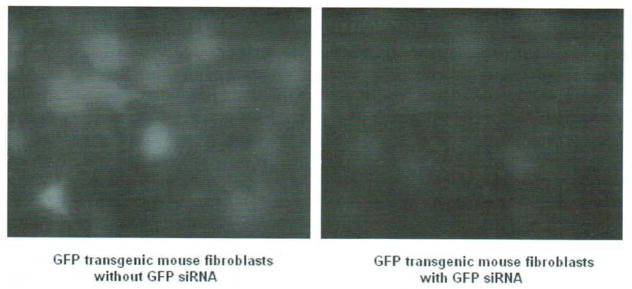
GFP expression in the fibroblasts from GFP transgenic mouse with or without GFP siRNA treatment. Left, without GFP siRNA treatment; right, with GFP siRNA treatment.
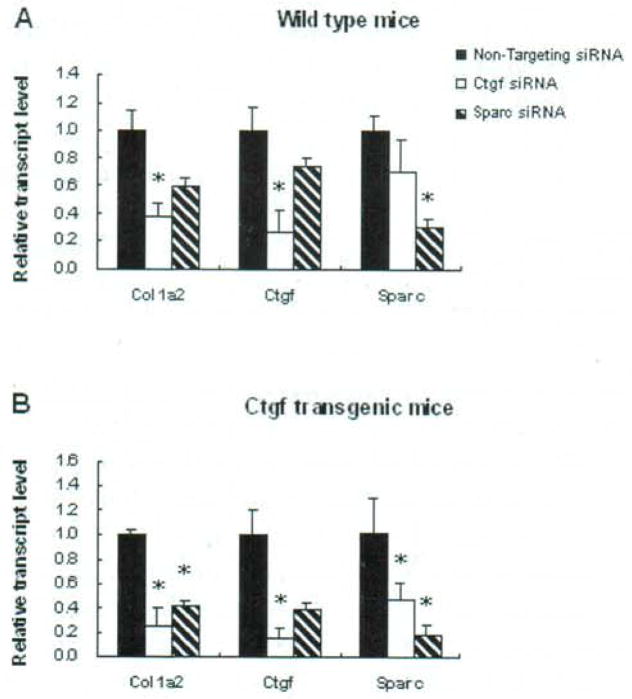
Gene expression of Colla2, Ctgf and Sparc after transfection of siRNAs in wild type and Colla2-CTGF transgenic mice fibroblasts
Seventy-two hours after transfection, the reduction of Ctgf (73% and 85% in wide type and transgenic type, respectively) by Ctgf siRNA and Sparc (69% and 82% in wide type and transgenic type, respectively) by Sparc siRNA were observed in the two fibroblast lines from wide type or Ctgf transgenic mice (Table I and Fig. 3). Interestingly, the expression of Ctgf and Sparc showed a reciprocal down-regulation by Sparc siRNA and Ctgf siRNA (26% and 62% down-regulation of Ctgf by Sparc siRNA in wide type and transgenic, respectively; 29% and 53% down-regulation of Sparc by Ctgf siRNA in wide type and transgenic, respectively) (Table I and Fig. 3). In addition to the expression of Ctgf and Sparc, the Colla2 also showed to be consistently down-regulated in all the fibroblasts after treated either by Ctgf siRNA or Sparc siRNA (Table I and Fig. 3).
Table I.
Inhibition of gene expression by siRNA (assays were performed in triplicates).
| Fibroblast line | Gene name | Relative transcription level (mean±SD) | ||
|---|---|---|---|---|
| Non-Targeting siRNA | Ctgf siRNA | Sparc siRNA | ||
| Wide type mice | Colla2 | 1 | 0.38±0.09 | 0.59±0.06 |
| Ctgf | 1 | 0.27±0.16 | 0.74±0.07 | |
| Sparc | 1 | 0.71±0.22 | 0.31±0.06 | |
| Colla2-CTGF transgenic mice | Colla2 | 1 | 0.26±0.14 | 0.42±0.03 |
| Ctgf | 1 | 0.15±0.08 | 0.38±0.06 | |
| Sparc | 1 | 0.47±0.14 | 0.18±0.08 | |
Fig. 3.
Gene expression in the fibroblasts with and without siRNA transfection. Comparison of gene expression with Ctgf siRNA. Sparc siRNA, or Non-Targeting siRNA treatment in wide type (A) and Colla2-CTGF transgenic mice fibroblasts (B), respectively. The expression level of each gene in each fibroblast line with Non-Targeting siRNA treatment was normalized to 1. Assays were performed in triplicates. * P < 0.05.
Protein expression of COL1, CTGF and SPARC in the fibroblasts with or without siRNA treatment
The expression of the three ECM components at protein level was examined by Western blot analysis. Collagen type I and CTGF showed increased expression in the fibroblasts of Colla2-CTGF transgenic mice compared with those in the cells from their normal littermate (wide type) (Fig. 4), which was consistent with their increased expression at the mRNA level. SPARC protein did not show significant reduction in the fibroblasts of Colla2-CTGF transgenic mice, although its transcriptional level was lower.
Fig. 4.
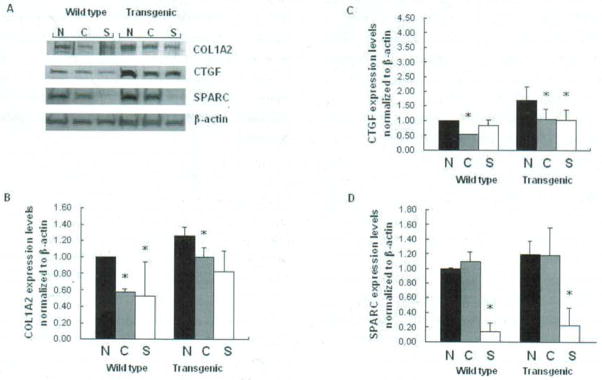
Western blot analysis of type I collagen, Ctgf, and Sparc in wild type and Colla2-CTGF transgenic mice fibroblasts with Ctgf siRNA or Sparc siRNA transfection (A). N: Non-Targeting siRNA treatment: C: Ctgf siRNA treatment; S: Sparc siRNA treatment. Wild type stands for wild type fibroblasts. Densitometric analysis of Western blots for Coll, Ctgf and Sparc are summarized in B, C, and D. Protein expression levels were compared between transgenic mice fibroblasts and wild type fibroblasts with or without Ctgf or Sparc siRNA treatment. Assays were performed in triplicates. * P < 0.05.
Western blots also showed that the expression of Collagen type I, CTGF and SPARC were decreased after Ctgf siRNA or Sparc siRNA treatment in all fibroblast lines except for Sparc expression in the fibroblasts transfected with Ctgf siRNA (Fig. 4). The down-regulation of CTGF protein by Ctgf siRNA was obviously less efficient than that of Sparc protein by Sparc siRNA (27% vs 86%, and 39% vs 92% in wild type and transgenic fibroblasts, respectively), although transcriptional levels of inhibition by their respective siRNAs were similar between Ctgf and Sparc. Sparc siRNA also down-regulated Ctgf, while Ctgf siRNA did not show a reciprocal inhibition to Sparc protein. In addition, the reduction of Colla2 protein by Ctgf siRNA was less than that by Sparc (37% vs 47%, 21% vs 35% in wide type and transgenic fibroblasts, respectively) (Fig. 4.).
DISCUSSION
Scleroderma is a devastating fibrotic disease which confers a high risk of mortality. No optimal therapy for reducing excessive collagen production in this disease is available (17). Animal model studies are crucial in finding therapeutic targets in SSc (18). Recently, Colla2-CTGF transgenic mouse model was generated, that constitutively over-expressed CTGF specifically in fibroblasts (6). These mice displayed features similar to human scleroderma, including dermal fibrosis and lung fibrosis, and thus provided useful tools in the study of fibrogenesis and identification of possible therapeutic targets.
Extensive deposition of collagens and other ECM proteins represent biomarkers for activated fibroblasts of SSc. SPARC and CTGF are two such ECM proteins important in regulating the production of collagen. Several mechanisms of such regulation have been explored, such as through SPARC and/or CTGF directly down-stream regulation or feedback control of TGFβ signaling transduction, and through direct binding to TGFβ receptor or TGFβ itself (15,19–23). In our previous study, it was shown that SPARC siRNA could attenuate the production of some ECM components, such as type 1 and 3 collagens, SPARC and CTGF in human normal and SSc fibroblasts (16–17, 23). We and others also showed that the blockade of CTGF expression either by CTGF siRNA, or its antisense oligonucleotide or corresponding antibody, can block TGFβ-induced collagen production and/or fibronectin expression (23–26). We hereby further validated the anti-fibrotic effect of CTGF and SPARC inhibitors in profibrotic fibroblasts of Colla2-CTGF transgenic mice that constantly over-express CTGF and collagens.
Our studies indicate that, similar to human fibroblasts, Sparc siRNA and Ctgf siRNA down-regulated the expression of collagen in mice fibroblasts of both wild type and Colla2-CTGF transgenic types at both the transcriptional and translational levels (Table I, Figs. 3 and 4). Since parallel expression of Ctgf, Sparc and collagen in Colla2-CTGF transgenic mice fibroblasts suggested that all three genes are involved in Ctgf-driven biological pathways, attenuation of over-expressed collagen type I in Colla2-CTGF transgenic mouse fibroblasts by Sparc siRNA is likely mediated by Ctgf function.
Although mRNA expression levels of Sparc and Ctgf showed a reciprocal inhibition of these two genes by corresponding siRNA treatment in mouse fibroblasts, protein expression of these two appeared different. Sparc siRNA down-regulated CTGF protein in both wild type and transgenic mouse fibroblasts. In contrast, Ctgf siRNA did not show down-regulatory effect on the SPARC protein expression in the fibroblasts. Discordant expression levels of mRNA and protein of Sparc in both wild type and transgenic fibroblasts treated with Ctgf siRNA may reflect translational efficiency in the cells. On the other hand, concordant regulation of mRNA and protein of Ctgf by Sparc siRNA supports our previous finding in human fibroblasts, in which SPARC showed as an upstream regulator of CTGF in response to TGFβ stimulation (23). It is worth noting that an up-regulated gene expression level of Sparc was observed in the fibroblasts of Colla2-CTGF transgenic mice. Along with the reciprocal inhibition of Sparc and Ctgf genes by corresponding siRNA treatment in mouse fibroblasts, these observations further suggested a mutual regulatory effect between SPARC and CTGF in the ECM compartment.
In conclusion, the fibroblasts from Colla2-CTGF transgenic mice showed profibrotic features, which can be ameliorated by Inhibition of Sparc or Ctgf expression using their corresponding siRNAs. Sparc and Ctgf siRNAs showed a reciprocal inhibition in transcript levels, and Sparc siRNA also reduced the protein level of CTGF. The present in vitro study using fibroblasts from Colla2-CTGF transgenic mouse model provides useful and potentially sufficient information for in vivo research to proceed.
Acknowledgments
Supported by UTHSC-H grants from NIH/NIAMS Center of Research Translation (CORT) in Scleroderma (1 P50 AR054144) (F.C.A) and 5RO3AR050517-02 (X.Z.), NIH/NCRR Center for Clinical and Translational Sciences (Houston CTSA Program) (1UL1 RR 024148), NIH PHS NCRR GCRC grant M01RR002558, University of Texas Health Science Center at Houston CreFF (X.Z.), Department of the Army, Medical Research Acquisition Activity: PR064803 (X.Z) and the Scleroderma Foundation (X.Z.), the National Science Foundation of China, grant number 30971594 (J. W), the Major National Science and Technology Program of China, grant number 2008ZX10002-002 and the Major Project on Basic Research, Science and Technology Committee of Shanghai Municipality (10JC1402100 and 09XD1400200) (J. W).
References
- 1.Claman HN, Giorno RC, Seibold JR. Endothelial and fibroblastic activation in scleroderma: the myth of the “uninvolved skin. Arthritis Rheum. 1991;34:1495–501. doi: 10.1002/art.1780341204. [DOI] [PubMed] [Google Scholar]
- 2.Abraham DJ, Varga J. Scleroderma: from cell and molecular mechanisms to disease models. Trends Immunol. 2005;26:587–95. doi: 10.1016/j.it.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Sonnylal S, Denton CP, Zheng B, Keene DR, He R, Adams HP. Postnatal induction of transforming growth factor β signaling in fibroblasts of mice recapitulates clinical, histologic, and biochemical features of scleroderma. Arthritis Rheum. 2007;56:334–44. doi: 10.1002/art.22328. [DOI] [PubMed] [Google Scholar]
- 4.Border WA, Noble NA. Transforming growth factor β in tissue fibrosis. N Engl J Med. 1994;331:1286–92. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 5.Grotendorst GR. Connective tissue growth factor: a mediator of TGF-β action on fibroblasts. Cytokine Growth Factor Rev. 1997;8:171–79. doi: 10.1016/s1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 6.Sonnylal S, Shi-Wen X, Leoni P, et al. Selective expression of connective tissue growth factor in fibroblasts in vivo promotes systemic tissue fibrosis. Arthritis Rheum. 2010;62:1523–532. doi: 10.1002/art.27382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix. Matrix Biol. 2001;19:816–27. doi: 10.1016/s0945-053x(00)00133-5. [DOI] [PubMed] [Google Scholar]
- 8.Lane TF, Sage EH. The biology of SPARC, a protein that modulates cell-matrix interactions. FASEB J. 1994;8:163–73. [PubMed] [Google Scholar]
- 9.Raines EW, Lane TF, Iruela-Arispe ML, Ross R, Sage EH. The extracellular glycoprotein SPARC interacts with platelet-derived growth factor (PDGF)-AB and - BB and inhibits the binding of PDGF to its receptors. Proc Natl Acad Sci USA. 1992;89:1281–85. doi: 10.1073/pnas.89.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou X, Tan FK, Reveille JD, Wallis D, Milewicz DM, Ahn C. Association of novel polymorphisms with the expression of SPARC in normal fibroblasts and with susceptibility to scleroderma. Arthritis Rheum. 2002;46:2990–99. doi: 10.1002/art.10601. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn C, Mason RJ. Immunolocalization of SPARC, tenascin, and thrombospondin in pulmonary fibrosis. Am J Pathol. 1995;147:1759–69. [PMC free article] [PubMed] [Google Scholar]
- 12.Pichler RH, Hugo C, Shankland SJ, Reed MJ, Bassuk JA, Andoh TF. SPARC is expressed in renal interstitial fibrosis and in renal vascular injury. Kidney Int. 1996;50:1978–89. doi: 10.1038/ki.1996.520. [DOI] [PubMed] [Google Scholar]
- 13.Frizell E, Liu SL, Abraham A, Ozaki I, Eghbali M, Sage EH. Expression of SPARC in normal and fibrotic livers. Hepatology. 1995;21:847–54. [PubMed] [Google Scholar]
- 14.Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 15.Schiemann BJ, Neil JR, Schiemann WP. SPARC inhibits epithelial cell proliferation in part through stimulation of the transforming growth factor-β-signaling system. Mol Biol Cell. 2003;14:3977–88. doi: 10.1091/mbc.E03-01-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou X, Tan FK, Guo X, Wallis D, Milewicz DM, Xue S, Arnett FC. Small interfering RNA inhibition of SPARC attenuates the profibrotic effect of transforming growth factor β1 in cultured normal human fibroblasts. Arthritis Rheum. 2005;52:257–61. doi: 10.1002/art.20785. [DOI] [PubMed] [Google Scholar]
- 17.Zhou X, Tan FK, Guo X, Arnett FC. Attenuation of collagen production with small interfering RNA of SPARC in cultured fibroblasts from the skin of patients with scleroderma. Arthritis Rheum. 2006;54:2626–31. doi: 10.1002/art.21973. [DOI] [PubMed] [Google Scholar]
- 18.Eckes B, Hunzelmann N, Moinzadeh P, Krieg T. Scleroderma -- news to tell. Arch Dermatol Res. 2007;299:139–44. doi: 10.1007/s00403-007-0756-7. [DOI] [PubMed] [Google Scholar]
- 19.Francki A, Bradshaw AD, Bassuk JA, Howe CC, Couser WG, Sage EH. SPARC regulates the expression of collagen type I and transforming growth factor-β1 in mesangial cells. J Biol Chem. 1999;274:32145–2152. doi: 10.1074/jbc.274.45.32145. [DOI] [PubMed] [Google Scholar]
- 20.Francki A, McClure TD, Brekken RA, Motamed K, Murri C, Wang T, Sage EH. SPARC regulates TGF-beta1-dependent signaling in primary glomerular mesangial cells. J Cell Biochem. 2004;91:915–25. doi: 10.1002/jcb.20008. [DOI] [PubMed] [Google Scholar]
- 21.Chujo S, Shirasaki F, Kawara S, Inagaki Y, Kinbara T, Inaoki M. Connective tissue growth factor causes persistent proalpha2(I) collagen gene expression induced by transforming growth factor-β in a mouse fibrosis model. J Cell Physiol. 2005;203:447–56. doi: 10.1002/jcp.20251. [DOI] [PubMed] [Google Scholar]
- 22.Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-β. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou XD, Xiong MM, Tan FK, Guo XJ, Arnett FC. SPARC, an upstream regulator of connective tissue growth factor in response to transforming growth factor β stimulation. Arthritis Rheum. 2006;54:3885–89. doi: 10.1002/art.22249. [DOI] [PubMed] [Google Scholar]
- 24.Duncan MR, Frazier KS, Abramson S, Williams S, Klapper H, Huang X, Grotendorst GR. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down-regulation by cAMP. FASEB J. 1999;13:1774–86. [PubMed] [Google Scholar]
- 25.Yokoi H, Mukoyama M, Sugawara A, et al. Role of connective tissue growth factor in fibronectin expression and tubulointerstitial fibrosis. Am J Physiol Renal Physiol. 2002;282:933–42. doi: 10.1152/ajprenal.00122.2001. [DOI] [PubMed] [Google Scholar]
- 26.Amott JA, Nuglozeh E, Rico MC, Arango-Hisijara I, Odgren PR, Safadi FF, Popoff SN. Connective tissue growth factor (CTGF/CCN2) is a downstream mediator for TGF-beta1-induced extracellular matrix production in osteoblasts. J Cell Physiol. 2007;210:843–52. doi: 10.1002/jcp.20917. [DOI] [PubMed] [Google Scholar]