Abstract
Objectives: This report assesses participant perception of treatment assignment in a randomized, double-blind, placebo-controlled trial of saw palmetto for the treatment of benign prostatic hyperplasia (BCM).
Design: Participants randomized to receive saw palmetto were instructed to take one 320 mg gelcap daily for the first 24 weeks, two 320 mg gelcaps daily for the second 24 weeks, and three 320 mg gelcaps daily for the third 24 weeks. Study participants assigned to placebo were instructed to take the same number of matching placebo gelcaps in each time period. At 24, 48, and 72 weeks postrandomization, the American Urological Association Symptom Index (AUA-SI) was administered and participants were asked to guess their treatment assignment.
Settings: The study was conducted at 11 clinical centers in North America.
Participants: Study participants were men, 45 years and older, with moderate to low severe BPH symptoms, randomized to saw palmetto (N=151) or placebo (N=155).
Outcome measures: Treatment arms were compared with respect to the distribution of participant guesses of treatment assignment.
Results: For participants assigned to saw palmetto, 22.5%, 24.7%, and 29.8% correctly thought they were taking saw palmetto, and 37.3%, 40.0%, and 44.4% incorrectly thought they were on placebo at 24, 48, and 72 weeks, respectively. For placebo participants, 21.8%, 27.4%, and 25.2% incorrectly thought they were on saw palmetto, and 41.6%, 39.9%, and 42.6% correctly thought they were on placebo at 24, 48, and 72 weeks, respectively. The treatment arms did not vary with respect to the distributions of participants who guessed they were on saw palmetto (p=0.823) or placebo (p=0.893). Participants who experienced an improvement in AUA-SI were 2.16 times more likely to think they were on saw palmetto.
Conclusions: Blinding of treatment assignment was successful in this study. Improvement in BPH-related symptoms was associated with the perception that participants were taking saw palmetto.
Introduction
Benign prostatic hyperplasia (BPH), a common condition among older men, is characterized by lower urinary tract symptoms. Severity of BPH symptoms is often assessed by patient self-report of symptom frequency using the American Urological Association Symptom Index (AUA-SI).1 Clinical trials of treatments for BPH have frequently used change in the AUA-SI as an outcome to measure efficacy,2–6 and were double-blinded. Potential risks to unblinding include bias in outcome ascertainment, especially in studies where the outcome measure cannot be objectively measured.7,8
In this study, the participant perception of treatment assignment and its association with outcome measures was evaluated in a double-blind, placebo-controlled trial to evaluate the efficacy of saw palmetto in the treatment of men with BPH: the Complementary and Alternative Medicines for Urological Symptoms (CAMUS) trial.6
Methods
The design and primary findings of the CAMUS trial have been previously described.6,9 Briefly, participants were 45 years of age or older, had an AUA-SI ≥8 and ≥24 (moderate to low severe symptoms), and a peak urinary flow rate >4 mL/sec with a voided volume >125 mL. Participants randomized to saw palmetto were instructed to take one 320 mg gelcap daily for the first 24 weeks, two 320 mg gelcaps daily for the second 24 weeks, and three 320 mg gelcaps daily for the third 24 weeks. Participants randomized to placebo received the same number of matching placebo gelcaps for each time period. Gelcaps were provided to study participants in blister cards so that each gelcap was encapsulated in a plastic shell attached to a card. For saw palmetto, the two batches of saw palmetto extract used were standardized to a reference chromatogram (with 85%–95% fatty acids as marker substances), 30 mg glycerol, 25 mg sorbitol, 10 mg purified water, and 90 mg gelatin. The placebo contained 375 mg polyethylene glycol, 25 mg glycerol, and 75 mg gelatin (matched weight of 475 mg). At 24, 48, and 72 weeks postrandomization, participants were asked to guess their treatment assignment. Responses were as follows: placebo, saw palmetto, or other (don't know, not taking pills). For each time period, study participants were categorized based on whether or not they had an adverse event and whether the change in AUA-SI from baseline was ≤−3 or >−3. A previous study suggested that a decrease of 3 points was the mean decrease among trial participants who rated their improvement as “slight”.10 A total of 369 participants were randomized in CAMUS. The per-protocol population of 306 participants who received treatment for the full 72 weeks was analyzed: 151 assigned to saw palmetto and 155 assigned to placebo.
General estimating equations with a log-binomial model were used to evaluate the association of time and treatment assignment with the occurrence of adverse events, change in AUA-SI, and participant guess adjusting for intrapatient variation, and to estimate the relative risks.11 The model uses the binomial distribution with a log link and was constructed for two binary endpoints: participant guessed he was on placebo (yes, no) and participant guessed he was on saw palmetto (yes, no).
Results
Among participants assigned to saw palmetto, 94%, 99%, and 100% guessed at their treatment at weeks 24, 48, and 72, respectively. Among participants assigned to placebo, 92%, 99%, and 100% guessed their treatment at weeks 24, 48, and 72, respectively. Demographic characteristics and baseline AUA-SI did not vary by treatment assignment (Table 1).
Table 1.
Demographic and Baseline Characteristics of Study Participants
| CAMUS | ||
|---|---|---|
| Treatment assignment | Saw Palmetto | Placebo |
| No. participants | 151 | 155 |
| Age in yearsa | 60.9 (8.5) | 60.8 (8.0) |
| Race, N (%) | ||
| African-American | 17 (11) | 17 (11) |
| White | 128 (85) | 130 (84) |
| Other | 6 (4) | 8 (5) |
| AUA-SIa | 14.4 (4.2) | 14.5 (4.7) |
Mean (standard deviation).
CAMUS, Complementary and Alternative Medicines for Urological Symptoms trial; AUA-SI, American Urological Association Symptom Index.
The proportion of participants who guessed they were on saw palmetto did not vary with study week (p=0.064) or treatment assignment (p=0.823) (Table 2). Similarly, the proportion of participants who guessed they were on placebo did not vary with study week (p=0.182) or treatment assignment (p=0.893). When treatment assignments were combined, 22%, 26%, and 27% of study participants thought they were saw palmetto and 39%, 40%, and 43% of study participants thought they were on placebo at weeks 24, 48, and 72, respectively. Treatment assignment was not associated with the proportions of participants who did not guess at their treatment (p=0.875).
Table 2.
CAMUS Participant Guess of Treatment Assignment
| Assigned to saw palmetto | Assigned to placebo | |||||||
|---|---|---|---|---|---|---|---|---|
| Study week | N | Guess saw palmetto(%) | Guess placebo (%) | Guess other (%) | N | Guess saw palmetto (%) | Guess placebo (%) | Guess other (%) |
| 24 | 142 | 22.5 | 37.3 | 40.1 | 142 | 21.8 | 41.6 | 36.6 |
| 48 | 150 | 24.7 | 40.0 | 35.3 | 153 | 27.4 | 39.9 | 32.7 |
| 72 | 151 | 29.8 | 44.4 | 25.8 | 155 | 25.2 | 42.6 | 32.3 |
The proportions of men who reported adverse events did not differ by treatment assignment (p=0.337) or study week (p=0.856) (Table 3). Adverse events were not associated with participant perceptions of being on saw palmetto (p=0.784) or placebo (p=0.529).
Table 3.
CAMUS Participant Guess of Treatment Assignment by Whether or Not Participant Had an Adverse Event (AE)
| Assigned to saw palmetto | Assigned to placebo | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Guess saw palmetto (%) | Guess placebo (%) | Guess other (%) | N | Guess saw palmetto (%) | Guess placebo (%) | Guess other (%) | |
| Week 24 | ||||||||
| AE | 70 | 24.3 | 35.7 | 40.0 | 70 | 22.9 | 44.3 | 32.8 |
| No AE | 72 | 20.8 | 38.9 | 40.3 | 72 | 20.8 | 38.9 | 40.3 |
| Week 48 | ||||||||
| AE | 79 | 29.1 | 34.2 | 36.7 | 67 | 22.4 | 38.8 | 38.8 |
| No AE | 71 | 19.7 | 46.5 | 33.8 | 86 | 31.4 | 40.7 | 27.9 |
| Week 72 | ||||||||
| AE | 76 | 31.6 | 40.8 | 27.6 | 73 | 23.3 | 45.2 | 31.5 |
| No AE | 75 | 28.0 | 48.0 | 24.0 | 82 | 26.8 | 40.2 | 32.9 |
Neither treatment assignment (p=0.422) nor study week (p=0.098) (Table 4) was associated with the proportions of participants who experienced a decrease in AUA-SI of 3 points of more. A decrease of ≤−3 was positively associated with participants guessing they were on saw palmetto (p<0.001) and negatively associated with participants guessing they were on placebo (p<0.001).
Table 4.
CAMUS Participant Guess of Treatment Assignment by Whether or Not Participant Had a Decrease in AUA-SI of 3 Points or More
| Assigned to saw palmetto | Assigned to placebo | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Guess saw palmetto (%) | Guess placebo (%) | Guess other (%) | N | Guess saw palmetto (%) | Guess placebo (%) | Guess other (%) | |
| Week 24 | ||||||||
| Da | 60 | 33.3 | 23.3 | 43.3 | 65 | 32.3 | 24.6 | 43.1 |
| No Db | 82 | 14.6 | 47.6 | 37.8 | 77 | 13.0 | 55.8 | 31.2 |
| Week 48 | ||||||||
| Da | 71 | 26.8 | 38.0 | 35.2 | 74 | 39.2 | 29.7 | 31.1 |
| No Db | 79 | 22.8 | 41.8 | 35.4 | 79 | 16.5 | 49.4 | 34.2 |
| Week 72 | ||||||||
| Da | 70 | 38.6 | 35.7 | 25.7 | 81 | 38.3 | 28.4 | 33.3 |
| No Db | 81 | 22.2 | 51.9 | 25.9 | 74 | 10.8 | 58.1 | 31.1 |
D, change in AUA-SI from baseline ≤−3.
No D, change in AUA-SI from baseline >−3.
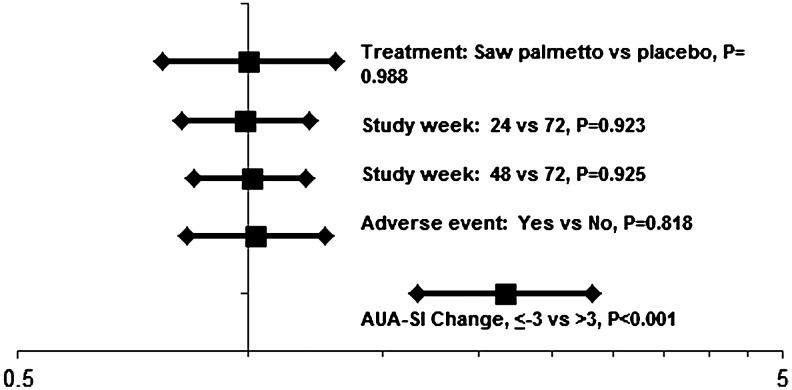
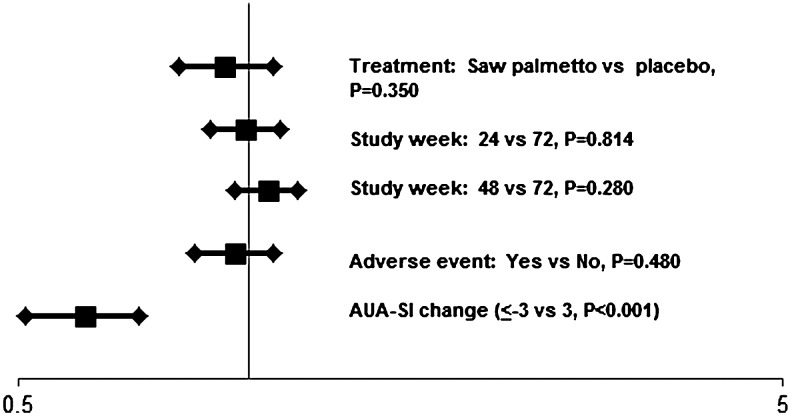
In the multivariate models, treatment assignment, study week, and the occurrence of adverse events were not significantly associated with participant guess of treatment (Figs. 1 and 2). Participants who experienced a decrease in their AUA-SI of 3 points or more were 2.16 times more likely to think they were taking saw palmetto than participants who experienced a smaller decrease, no change, or an increase in AUA-SI (Fig. 1). The relative risk that a participant thought he was on placebo was 0.61 if he experienced an AUA-SI decline of at least 3 points (Fig. 2).
FIG. 1.
Relative risk and its 95% confidence interval for guessing treatment with saw palmetto. AUA-SI, American Urological Association Symptom Index.
FIG. 2.
Relative risk and its 95% confidence interval for guessing treatment with placebo.
Discussion
One of the challenges of blinding saw palmetto is its distinctive odor. Blinding in CAMUS was effective because participant guesses did not vary by treatment assignment, and participants correctly identified their treatment less than half of the time. These observations are similar to those in other clinical trials of dietary supplements.12–14 In a single-center placebo-controlled trial of saw palmetto, the proportions of study participants who guessed they were on saw palmetto did not differ by treatment assignment.3 In a trial that used another natural product with a distinctive odor (fish oil), blinding was also successful since participants could not accurately guess their treatment assignment.12 Although all participants randomized to fish oil detected the fishy odor of the preparation, only half correctly identified their treatment.12 While none of the participants randomized to placebo (olive oil) reported a fishy odor, only 25% guessed that they were on placebo.12 In the current study, the two treatment arms did not differ with respect to the proportion of participants who thought they were on the experimental treatment arm, saw palmetto. Similarly, in a trial of Echinacea as a treatment for rhinovirus infections, the proportions of study participants who thought they were on the active treatment arm were similar in the placebo and Echinacea arms.13 The finding that saw palmetto participants were less likely to correctly guess their treatment than placebo participants is similar to the observations in a trial of St. John's wort for major depression.14 In that study, participants were randomized to Hypericum perforatum (St. John's wort), sertraline as an active comparator, and placebo. Correct guesses were reported for 29%, 66%, and 36% for St. John's Wort, placebo, and sertraline, respectively.14
In CAMUS, the time periods differed by the number of gelcaps taken daily by participants, and were associated with increasing doses of saw palmetto for those assigned to that treatment. The lack of a time period effect suggests that the increase in number of gelcaps did not have an impact on participant perception of treatment assignment. For saw palmetto participants, increasing doses were not reflected in a commensurate increase in accuracy in guessing at treatment assignment.
The lack of correlation between adverse events and participant perception of treatment may be attributed to the fact that almost all adverse events reported in the study were mild to moderate.6 Furthermore, saw palmetto is not associated with specific adverse events, so it is unlikely that study participants would have associated adverse events with its use. The role of adverse events in perception of therapy is varied. In a placebo-controlled trial of amitriptyline in the treatment of chronic pain from spinal cord injuries, 60% of those assigned to amitriptyline and 45% of those assigned to placebo based their treatment guess on side-effects.15 In a study of fluvoxamine in the treatment of pediatric outpatients with anxiety disorder, adverse events were not associated with treatment guesses by patients, their parents, or clinical evaluators.16
The finding that participant perception that they were on the experimental therapy, saw palmetto, was correlated with a greater improvement in AUA-SI, is consistent with other reports that have shown a relationship between a beneficial effect on an outcome measure and an increase in the correct perception of treatment assignment.16–18 In a placebo-controlled trial of bupropion as a smoking-cessation agent, participants who guessed that they were on the active agent were more likely to quit smoking than those who guessed they were on placebo or were unsure.17 In a trial of risperidone in children with autism, health care providers associated the active agent with patient improvement.16 In a study that compared vertebroplasty with a control intervention in the treatment of osteoporotic vertebral compression fractures, participants assigned to the control intervention who guessed that they received vertebroplasty had greater pain relief at days 14 and 30 than those who guessed that they received the control intervention.18
Strengths of this study were that participants were asked to guess at their treatment assignment at multiple fixed timepoints, and were offered the option to indicate uncertainty. There were several limitations to this study. Participants were not asked to assess their confidence in their guess, nor whether their guess was based on adverse events or efficacy. Because time on study was correlated with the number of gelcaps taken, the effects of dosage and time cannot be separately evaluated. Lastly, the perception of clinic staff on treatment assignment was not evaluated.
Conclusions
To the authors' knowledge, this is the first evaluation of blinding in a clinical trial of saw palmetto in patients with BPH. The study showed that blinding was successful since participants correctly guessed their treatment less than half of the time. Participants who experienced a positive treatment outcome were more likely to perceive that they were on saw palmetto and less likely to guess that they were on placebo.
Acknowledgments
This study was funded by grants U01 DK63778, U01 DK63788, U01 DK63795, U01 DK63797, U01 DK63825, U01 DK63831, U01 DK63833, U01 DK63835, U01 DK63840, U01 DK63862, U01 DK63866, and U01 DK63883 from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, National Center for Complementary and Alternative Medicine, and the Office of Dietary Supplements. Saw palmetto fruit extract and matching placebo were donated by Rottapharm/Madaus, Cologne, Germany. The authors wish to express their appreciation to the full CAMUS study team.6
Disclosure Statement
No competing financial interests exist.
References
- 1.Barry MJ, Fowler FJ, Jr, O'Leary MP, et al. . The American Urological Association symptom index for benign prostatic hyperplasia: The Measurement Committee of the American Urological Association. J Urol 1992;148:1549–1557; discussion 1564. [DOI] [PubMed] [Google Scholar]
- 2.McConnell JD, Roehrborn CG, Bautista OM, et al. . The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. NEJM 2003;349:2387–2398 [DOI] [PubMed] [Google Scholar]
- 3.Bent S, Kane C, Shinohara K, et al. . Saw palmetto for benign prostatic hyperplasia. NEJM 2006;354:557–566 [DOI] [PubMed] [Google Scholar]
- 4.Nickel JC, Gilling P, Tammela TL, et al. . Comparison of dutasteride and finasteride for treating benign prostatic hyperplasia: The Enlarged Prostate International Comparator Study (EPICS). BJU Int 2011;108:388–394 [DOI] [PubMed] [Google Scholar]
- 5.Roehrborn CG, Barkin J, Siami P, et al. . Clinical outcomes after combined therapy with dutasteride plus tamsulosin or either monotherapy in men with benign prostatic hyperplasia (BPH) by baseline characteristics: 4-year results from the randomized, double-blind Combination of Avodart and Tamsulosin (CombAT) trial. BJU Int 2011; 107:946–954 [DOI] [PubMed] [Google Scholar]
- 6.Barry MJ, Meleth S, Lee JY, et al. . Effect of increasing doses of saw palmetto extract on lower urinary tract symptoms: A randomized trial. JAMA 2011;306:1344–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altman DG, Schulz KF, Moher D, et al. . The revised CONSORT statement for reporting randomized trials: Explanation and elaboration. Ann Intern Med 2001;134:663–694 [DOI] [PubMed] [Google Scholar]
- 8.Hrobjartsson A, Boutron I. Blinding in randomized clinical trials: Imposed impartiality. Clin Pharmacol Ther 2011;90:732–736 [DOI] [PubMed] [Google Scholar]
- 9.Lee J, Andriole G, Avins A, et al. . Redesigning a large-scale clinical trial in response to negative external trial results: The CAMUS study of phytotherapy for benign prostatic hyperplasia. Clin Trials 2009;6:628–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barry MJ, Williford WO, Chang Y, et al. . Benign prostatic hyperplasia specific health status measures in clinical research: How much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol 1995;154:1770–1774 [DOI] [PubMed] [Google Scholar]
- 11.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 2005;162:199–200 [DOI] [PubMed] [Google Scholar]
- 12.Damico KE, Stoll AL, Marangell LB, et al. . How blind is double-blind? A study of fish oil versus placebo. Prostaglandins Leukot Essent Fatty Acids 2002;66:393–395 [DOI] [PubMed] [Google Scholar]
- 13.Turner RB, Bauer R, Woelkart K, et al. . An evaluation of Echinacea angustifolia in experimental rhinovirus infections. NEJM 2005;353:341–348 [DOI] [PubMed] [Google Scholar]
- 14.Effect of Hypericum perforatum (St John's wort) in major depressive disorder: A randomized controlled trial. JAMA 2002;287:1807–1814 [DOI] [PubMed] [Google Scholar]
- 15.Turner JA, Jensen MP, Warms CA, et al. . Blinding effectiveness and association of pretreatment expectations with pain improvement in a double-blind randomized controlled trial. Pain 2002;99:91–99 [DOI] [PubMed] [Google Scholar]
- 16.Vitiello B, Davis M, Greenhill LL, et al. . Blindness of clinical evaluators, parents, and children in a placebo-controlled trial of fluvoxamine. J Child Adolesc Psychopharmacol 2006;16:219–225 [DOI] [PubMed] [Google Scholar]
- 17.Schnoll RA, Epstein L, Audrain J, et al. . Can the blind see? Participant guess about treatment arm assignment may influence outcome in a clinical trial of bupropion for smoking cessation. J Subst Abuse Treat 2008;34:234–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brinjikji W, Comstock BA, Heagerty PJ, et al. . Investigational Vertebroplasty Efficacy and Safety Trial: Detailed analysis of blinding efficacy. Radiology 2010;257:219–225 [DOI] [PubMed] [Google Scholar]