Abstract
Obesity is an important identifier of cardiovascular disease (CVD) risk, but is challenging to determine accurately in individuals with spinal cord injury (SCI). Body mass index (BMI) is used worldwide as a simple indicator of obesity, but is difficult to measure in individuals with SCI. Furthermore, standard BMI cutoffs underestimate obesity in this population. Therefore, we aimed to identify the best marker of obesity in individuals with SCI, considering both practicality, and ability to detect adiposity and CVD risk. Five anthropometric measures were evaluated: BMI; waist circumference (WC); waist-to-height ratio (WHtR); waist-to-hip ratio; and neck circumference. We evaluated relationships between these measures and abdominal and total body-fat percentage, seven cardiovascular metabolic risk factors (fasting insulin, glucose, glucose tolerance, triglycerides, high-density lipoprotein, low-density lipoprotein, and total cholesterol), and the Framingham risk score. BMI, WC, and WHtR were correlated with abdominal fat percentage. WC and WHtR were correlated with five metabolic risk factors as well as the Framingham risk score. WC is a more practical measure for an SCI population. The optimal cutoff for identifying adverse CVD risk in individuals with SCI was identified as WC ≥94 cm, with 100% sensitivity and 79% specificity. We propose that WC is a simple, more sensitive alternative to BMI in this population that is easy to use in multiple settings. The cutoff provides a simple tool to predict adverse CVD risk profiles that can be used to guide risk management, as well as as a practical aid for individuals with SCI to maintain a healthy body composition.
Key words: : cardiovascular disease risk, obesity, spinal cord injury, waist circumference
Introduction
Great progress has been made in surgical techniques and initial care for individuals who sustain a spinal cord injury (SCI), but secondary complications after injury remain a major concern. Cardiovascular disease (CVD) is one of these secondary complications and is the leading cause of morbidity and mortality in this population, with SCI patients experiencing an earlier onset and faster progression of CVD than in the general population.1,2 Obesity and, in particular, visceral adiposity, are important and well-known risk factors for CVD,3–5 but remain challenging to accurately determine in individuals with SCI.6 This is particularly important because after an SCI, individuals undergo changes in body composition, metabolic rate, and autonomic function. These alterations, coupled with a more sedentary lifestyle after injury, may lead to higher prevalence of obesity in this population.
A first step to address the issue of obesity after SCI is to find an accurate, simple, and meaningful assessment tool that can be used to identify obesity in this population. Two characteristics that are important in the assessment tool are the practicality and accuracy of the tool. Body mass index (BMI; weight/height2) has been used worldwide and is promoted by the World Health Organization as a simple indicator of obesity in the general population.7,8 Although BMI does not specifically measure fat mass, it has been shown to correlate well with measures of body fat at a population level.8 However, in the SCI population, BMI is not a very practical or accurate measure of obesity. For example, to measure weight, a wheelchair scale is needed. Height can be inferred from length, while lying supine with straight legs and flexed feet, but this position can be challenging due to contractures and spasticity. Not only is the use of BMI as a measure of obesity impractical in an SCI population, but also the proposed cutoffs have been shown to underestimate obesity in this population.9–11 This is likely due to decreases in muscle mass below the lesion after injury, such that individuals with SCI with the same height and weight (and thus BMI) as an able-bodied individual will have greater fat mass.
Four other anthropometric measures that have been used as surrogate markers for obesity in the able-bodied population are waist circumference (WC), waist-to-hip ratio (WHR), waist-to-height ratio (WHtR), and neck circumference (NC). WC is simply measured in a standing position, using a tape measure around the abdomen after normal expiration. Although some groups advocate variations in the exact measurement location for WC, the strength of the correlations between WC and both fat percentage and CVD risk factors have been shown to be equally strong for several locations of WC measurements.12 In the able bodied, WC has been shown to be a strong predictor for both total body fat and visceral adipose tissue13–16 and is highly correlated with CVD risk factors directly.13,17,18 In contrast to the standard in the able-bodied population, WC is measured in the supine position in individuals with SCI.9 This is because in the supine position, WC is less dependent on abdominal muscle tone, which may be impaired in individuals with SCI. Several studies suggest strong correlations between WC and adiposity, as well as SCI and CVD risk,19–21 and one study has shown correlations between visceral adiposity, WC, and metabolic risk factors for CVD.22 However, no study to date has examined the relationships between multiple candidate markers of obesity, adiposity, and overall risk of CVD to determine the best index for obesity-related CVD in a SCI cohort. Although WC has been shown to be highly correlated with visceral fat in both the able-bodied population and in individuals with SCI, the latter have 42% more visceral adipose tissue at the same WC, suggesting that the cut-off criteria for obesity should be lowered in this population.22
The disadvantage of WC is that it assumes that CVD risk is the same for everyone with the same WC regardless of their height, or body shape, and this might not be the case.23,24 Accordingly, WHR and WHtR are two parameters that are commonly used to “correct” WC to account for these differences. In the able bodied, both have been shown to be better predictors of CVD than WC in a large meta-analysis, with WHtR being the strongest predictor.24 However, WHR is arguably more suitable for an SCI population because the difficulties with measuring height are avoided. To date, only one study has examined the validity of the corrected WC markers in the SCI population.20 They showed that WHR is strongly correlated with visceral adiposity in individuals with SCI, but not how this relates to the presence of other adverse CVD risk factors.20
Finally, several recent studies in the able bodied identified a strong correlation between NC and visceral adipose tissue, as well as a correlation with CVD risk above and beyond the risk related to visceral fat.25–28 Whether NC is also a predictor for obesity or other adverse cardiovascular risk factors in individuals with SCI is unknown.
Thus, it remains unclear which of these suggested markers is the strongest predictor of obesity in individuals with SCI. Further, because, arguably, the more relevant information is not just whether a marker can detect obesity, but whether this increased adiposity is associated with increased risk of CVD, additional studies examining their relationships with CVD risk factors after SCI are warranted. The aim of this study was, therefore, to identify the best marker for obesity-related CVD risk profile in individuals with SCI, considering both practicality of use and ability to detect 1) adiposity and 2) CVD risk factors.
Methods
This study received ethical approval from the research ethics committee at Simon Fraser University (Burnaby, British Columbia, Canada) as well as the Vancouver Coastal Health Research Institute (Vancouver, British Columbia, Canada) and was performed in association with the Declaration of Helsinki of the World Medical Association.
Participants
Studies were performed on 27 individuals with chronic SCI (>1 year) who gave written informed consent, had no known preexisting (before injury) CVD, and were not taking any cardiovascular medications. Individuals with SCI were recruited from posters displayed at rehabilitation centers as well as through local SCI support groups. All participants abstained from drinking alcoholic beverages from the night before testing and from caffeine on the morning of testing. They did not participate in vigorous exercise on the morning of testing. Females were not tested during their menstrual period. Neurological classification of level and severity of injury was determined using the American Spinal Injury Association (ASIA) Impairment Scale (AIS).29 An AIS A score reflects complete loss of motor and sensory function; the B, C, and D scores reflect progressively less-severe impairments. Level of injury and impairment scored together form the classification of the injury (e.g., T5A). Smoking status was recorded. Arterial blood pressure was determined as a mean of a 30-sec beat-to-beat blood pressure recording using finger plethysmography (Finometer Pro; Finapres Medical Systems, Amsterdam, the Netherlands) after 15 min of supine rest. Physical activity levels were determined from the Physical Activity Scale for Individuals with Physical Disabilities questionnaire.30 This 13-item questionnaire incorporates questions about physical activity during the past 7 days and includes activities such as household work, leisure time activities, and sports. The questionnaire provides a total score in metabolic equivalent (MET) hours per day (MET h/day).
Anthropometric variables
BMI was calculated from weight (kg), determined using a Dual-Energy X-ray Absorptiometry (DEXA) whole-body scan (QDR 4500; Hologic Inc., Bedford, MA), divided by height squared (m2), determined using an electronic ruler (MATLAB 2012b, MathWorks, Natick, MA) on the DEXA images. Length, as determined using a similar method, has been shown to correlate well with measured height in the able-bodied population (Pearson's correlation coefficient, 0.996).11 In cases where participants could not fully stretch their legs because of contractures or spasticity, a self-reported measure of height was used (n=4). WC was measured in cm at the narrowest part of the waist after a normal expiration.9 This site was chosen for ease of localization and measurement when the participant was lying supine. Hip circumference (HC) was measured in cm around the widest portion of the buttocks. Both WC and HC measurements were taken, while lying supine on the DEXA scanner bed, using a stretch-resistant measuring tape with a tensiometer.9 WHR was determined by dividing the measured WC by the measured HC. WHtR was determined by dividing the measured WC by height. NC (in cm) was measured at the mid-point between the jaw and the clavicle. All measurements were conducted by the same investigator.
Body composition
Total body-fat mass (in kg) and total body-fat percentage were determined using the whole-body DEXA scan. Abdominal fat was determined using standardized landmarks to distinguish the trunk region,31 and abdominal fat percentage was determined as abdominal fat mass divided by total mass in the defined region ×100.
Fasting plasma levels of lipids, glucose, and insulin
A venous blood sample was collected after a 12-h overnight fast (excluding water). Samples were centrifuged immediately at 3°C and 3000 rpm for 10 min and the plasma component withdrawn for subsequent analysis. The plasma samples were sent to the clinical laboratory at Vancouver General Hospital, where high-density lipoprotein cholesterol (HDL-C), total cholesterol (TC), triglyceride (TG), and glucose levels were determined by enzymatic assays (Dimension Vista system; Siemens Healthcare Diagnostics Inc, Tarrytown, NY). Low-density lipoprotein cholesterol (LDL-C) levels were calculated using the Friedewald method.32 TC/HDL-C was calculated from TC and HDL-C levels. Plasma insulin was determined at the same laboratory using an immunoassay (ADVIA Centaur assay; Siemens Healthcare Diagnostics). Insulin resistance (IR) was calculated using the homeostasis model assessment (HOMA-IR) method.33
Oral glucose tolerance test
Participants fasted for 12 h overnight before the test, with the exception of drinking water. A finger-prick method was used to determine blood-glucose levels (Contour; Bayer Inc., Toronto, Ontario, Canada) in the fasting state and 30, 60, 90, and 120 min after consumption of a 75-g glucose solution (Glucodex 75g; Rougier Pharma, Mirabel, Quebec, Canada).
Framingham 30-year risk for cardiovascular disease score
We used the Framingham 30-year risk for CVD risk score34 as a measure of overall risk of CVD. This risk score incorporates the following risk factors: HDL-C; TC; age; gender; systolic arterial pressure (SAP) at rest; smoking status; diabetes; and antihypertensive treatment. However, instead of including the measured SAP, we entered a neutral SAP value of 120 mmHg into the risk score formula for all participants. This decision was based on the fact that SCI can impair normal blood-pressure control with lesions above the sixth thoracic level, leading to lower resting blood pressure.35,36 The known relationship between SAP and CVD risk might therefore not exist in the same way in this population. A risk score under 10% is considered low risk, a score between 10 and 20% an intermediate risk, and above 20% a high risk.37
Statistical analyses
All statistical analyses were performed using SigmaPlot statistical software (version 12; Systat Software Inc., San Jose, CA). Data were tested for normality using Shapiro-Wilk's test. Correlations were performed using Pearson's product moment analyses (parametric data) or Spearman's rank-order tests (nonparametric data) to examine the relationships between the anthropometric parameters and abdominal fat percentage, individual risk factors, or the Framingham risk score. Receiver operator characteristic (ROC) curves were generated for the anthropometric measures that were significantly correlated with both abdominal fat percentage and the Framingham risk score. A Framingham 30-year risk score of 10% or more was considered a positive outcome. Significance was assumed at p<0.05; data are reported as mean±standard deviation (SD).
Results
Participants
Twenty-seven individuals (19 men and 8 women) with a range of injury levels from the fourth cervical level to the twelfth thoracic level, including both complete and incomplete injuries, participated in this study. Fifteen individuals had a cervical lesion and 12 had a thoracic lesion. There were 14 individuals with AIS A scores, 6 with AIS B, 5 with AIS C, and 2 with AIS D. Participant characteristics are shown in Table 1.
Table 1.
Participant Characteristics
| Characteristics | Mean (SD) | Number of participants |
|---|---|---|
| Age (years) | 40 (11) | 27 |
| Duration of injury (months) | 166 (116) | 27 |
| BMI (kg/m2) | 23.7 (4.4) | 27 |
| WC (cm) | 87.4 (11.7) | 27 |
| WHtR | 0.51 (0.07) | 27 |
| WHR | 0.87 (0.10) | 27 |
| NC (cm) | 39.4 (3.1) | 22 |
| TC (mmol/L) | 3.80 (0.79) | 23 |
| HDL-C (mmol/L) | 1.13 (0.32) | 23 |
| LDL-C (mmol/L) | 2.23 (0.73) | 23 |
| TGs (mmol/L) | 0.96 (0.44) | 23 |
| Total/HDL-C ratio | 3.60 (1.19) | 23 |
| Insulin (mU/L) | 6.4 (3.3) | 23 |
| IR | 1.3 (0.7) | 23 |
| Glucose (mmol/L) | 4.5 (0.5) | 23 |
| 120-min glucose (mmol/L) | 7.4 (1.4) | 27 |
| Total body fat (%) | 28 (8) | 26 |
| Abdominal fat (%) | 27 (8) | 26 |
| Modified Framingham risk score (%) | 15 (8.3) | 23 |
| Physical activity score (MET h/day) | 16.0 (11.5) | 26 |
We were unable to obtain a blood sample in 4 participants (3 females and 1 male) because of difficulties in obtaining venous access or because the participant declined the blood sample. One participant was unable to complete the DEXA scan. Neck circumference was not measured in 5 participants. None of the participants had evidence of diabetes, but 9 individuals met criteria for impaired glucose tolerance.48
BMI, body mass index; WC, waist circumference; WHtR, waist-to-height ratio; WHR, waist-to-hip ratio; NC, neck circumference; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TGs, triglycerides; IR, insulin resistance; MET, metabolic equivalent; SD, standard deviation.
Anthropometric measures and body composition
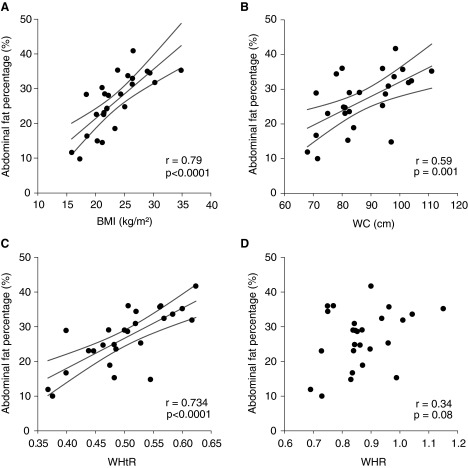
The anthropometric parameters BMI, WC, and WHtR were significantly and positively correlated with abdominal fat and total body fat, both when expressed as absolute fat mass and percentages (Table 2). Correlations between both WHR and NC and measures of adiposity were less strong and, in many cases, did not achieve statistical significance. BMI, WC, and WHtR were the anthropometric measures most strongly correlated with abdominal fat percentage (Fig. 1).
Table 2.
Correlations between Anthropometric Variables and Body Composition
| Total body fat | ||||
|---|---|---|---|---|
| % | g | |||
| r | p | r | p | |
| BMI | 0.73 | 0.0001 | 0.90 | <0.0001 |
| WC | 0.44 | 0.03 | 0.68 | 0.0002 |
| WHtR | 0.63 | 0.0006 | 0.73 | <0.0001 |
| WHR | 0.13 | 0.52 | 0.32 | 0.12 |
| NC | 0.36 | 0.11 | 0.62 | 0.003 |
| Abdominal fat | ||||
|---|---|---|---|---|
| % | g | |||
| r | p | r | p | |
| BMI | 0.79 | <0.0001 | 0.89 | <0.0001 |
| WC | 0.59 | 0.002 | 0.79 | <0.0001 |
| WHtR | 0.74 | <0.0001 | 0.81 | <0.0001 |
| WHR | 0.34 | 0.09 | 0.49 | 0.02 |
| NC | 0.40 | 0.08 | 0.63 | 0.002 |
BMI, body mass index; WC, waist circumference; WHtR, waist-to-height ratio; WHR, waist-to-hip ratio; NC, neck circumference.
FIG. 1.
Correlations between abdominal fat percentage and anthropometric markers for obesity. (A) Body mass index (BMI); (B) waist circumference (WC); (C) waist-to-height ratio (WHtR); and (D) waist-to-hip ratio (WHR). Regression lines and confidence intervals are shown for significant (p<0.05) correlations.
Anthropometric measures and cardiovascular disease risk factors
The correlation coefficients between anthropometric variables and CVD risk factors are shown in Table 3. BMI was positively correlated with only one of the CVD risk factors: fasting TG level. WC was positively correlated with five CVD risk factors: fasting glucose; TG; TC; LDL-C; and TC/HDL-C ratio. WHR correlated positively with four CVD risk factors: fasting glucose; TC; LDL-C; and TC/HDL-C ratio. WHtR was positively correlated with four CVD risk factors: TC; TG; TC/HDL-C ratio; and 120-min glucose. NC was not significantly correlated with any of the CVD risk factors.
Table 3.
Correlation Coefficients between Anthropometric Variables and Individual Risk Factors
| Insulin | Fasting glucose | TG | TC | HDL-C | LDL-C | TC/HDL-C ratio | 120-min glucose | IR | |
|---|---|---|---|---|---|---|---|---|---|
| BMI | |||||||||
| r | 0.29 | 0.37 | 0.48 | 0.33 | 0.12 | 0.12 | 0.23 | 0.11 | 0.34 |
| p | 0.18 | 0.09 | 0.02 | 0.12 | 0.58 | 0.57 | 0.29 | 0.60 | 0.11 |
| WC | |||||||||
| r | 0.10 | 0.46 | 0.46 | 0.57 | −0.11 | 0.43 | 0.56 | 0.32 | 0.20 |
| p | 0.64 | 0.03 | 0.03 | <0.01 | 0.61 | 0.04 | <0.01 | 0.10 | 0.35 |
| WHR | |||||||||
| r | 0.31 | 0.50 | 0.40 | 0.61 | −0.24 | 0.48 | 0.62 | 0.30 | 0.41 |
| p | 0.14 | 0.01 | 0.06 | <0.01 | 0.27 | 0.02 | <0.01 | 0.13 | 0.05 |
| WHtR | |||||||||
| r | 0.20 | 0.36 | 0.43 | 0.54 | 0.07 | 0.33 | 0.44 | 0.39 | 0.26 |
| p | 0.35 | 0.09 | 0.04 | <0.01 | 0.76 | 0.12 | 0.04 | 0.04 | 0.23 |
| NC | |||||||||
| r | −0.10 | 0.41 | 0.36 | 0.30 | −0.10 | 0.38 | 0.35 | 0.33 | −0.01 |
| p | 0.66 | 0.06 | 0.11 | 0.19 | 0.66 | 0.09 | 0.12 | 0.13 | 0.97 |
Statistically significant correlations are shown in bold text.
BMI, body mass index; WC, waist circumference; WHR, waist-to-hip ratio; WHtR, waist-to-height ratio; NC, neck circumference; TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; IR, insulin resistance.
Anthropometric measures and the Framingham 30-year risk score
Of the anthropometric measures, only BMI was not significantly correlated with the Framingham risk score. WC had the strongest correlation (r=0.66; p<0.001) with the risk score, but WHtR (r=0.60; p<0.01), WHR (r=0.56; p<0.01), and NC (r=0.51; p=0.02) also showed significant correlations. ROC curves were generated for WC and WHtR because those measures were most strongly correlated with both the Framingham risk score and measures of adiposity. The area under the curve (AUC) for WC was 0.92 and the optimal cutoff was determined at 94 cm (95% confidence interval [CI], 0.72–0.99; p<0.0001), with a specificity of 100% and a sensitivity of 79%. For WHtR, the AUC was 0.87 (95% CI, 0.66–0.97; p<0.0001) and the optimal cutoff was determined at 0.53, with a specificity of 100% and a sensitivity of 71%. The ROC curves for a subgroup of only male participants showed the optimal cutoff for WC at 94 cm and 0.51 for WHtR. Correlations and ROC curves for WC and WHtR with the Framingham risk score are shown in Figure 2.
FIG. 2.
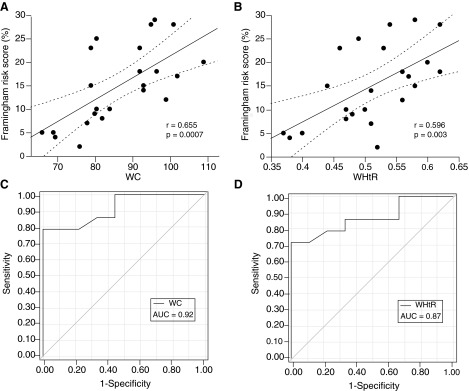
Correlations between anthropometric measures and modified Framingham risk score as well as corresponding receiver operator characteristic (ROC) curves. (A) Correlation with waist circumference (WC); (B) correlation with waist-to-height ratio (WHtR); (C) ROC curve for WC; (D) ROC curve for WHtR. Regression lines and confidence intervals are shown for significant (p<0.05) correlations. The area under the curves (AUC) of the ROC curves are shown.
We used a modified Framingham risk score, with a neutral value for SAP because of the known effect of SCI on blood pressure regulation. However, when the analyses were repeated using the original Framingham risk score (including measured SAP), our results were unchanged. WC still had the strongest correlation with the risk score (r=0.55; p=0.006), and the ROC for WC had an AUC of 0.92 and an optimal cutoff at 94 cm. Similar results were obtained when we restricted these analyses to male participants.
Discussion
The main findings of this study are that BMI, WC, and WHtR were strongly correlated with measures of both total and abdominal adiposity. In addition, WC and its normalized versions, WHtR and WHR, were correlated with several CVD risk factors. Two of these measures, WC and WHtR, were strongly correlated with both adiposity and the Framingham 30-year CVD risk score. Of these two measures, WHtR is the least practical measure for individuals with SCI, because height is challenging to accurately determine in those with contractures or spasticity. We, therefore, suggest that WC is the best marker for obesity-related CVD risk in this population. The stronger correlation with the Framingham risk score and the slightly higher AUC of the ROC curve further support this conclusion. We showed that a cutoff for WC at 94 cm was optimal to predict a positive outcome of >10% risk on the 30-year risk score.
In the present study, we found that BMI was strongly related to body-fat percentage, explaining 63% of the variance in abdominal fat percentage and 52% of the variance in total body fat percentage. Previously, studies in the SCI population showed that only 35–36% of the variance in total body-fat percentage could be explained by BMI.9,38 Our finding is more similar to the 46–79% described in the able-bodied population.9 This might be because BMI was determined by length and weight measured from the DEXA scan in the present study, which may be more robust than using self-reported height or weight as in previous studies in SCI individuals.9,38 Indeed, the only other group that used DEXA measurements of height and weight in people with SCI found that a similar proportion of the variance in body fat was explained by BMI (53%).11 We compared self-reported height and weight to height and weight determined from the DEXA scan (data not shown). Although height from these two methods correlated well (r=0.912; p<0.001), Bland-Altman's analysis showed there was a bias of approximately 2 cm, where self-reported height was greater than that measured from DEXA. Self-reported weight also correlated well with weight from the DEXA scan (r=0.945; p<0.05), but, again, Bland-Altman's analysis showed a bias of 1.7 kg, where self-reported weight was greater than that measured from DEXA.
Although BMI was strongly related to abdominal adiposity and total body fat, it was not correlated with the CVD risk factors studied or the Framingham risk score. This discrepancy has been documented previously,9,39–41 and likely reflects the fact that visceral fat (rather than total body fat or even abdominal fat) is thought to be the main contributor to CVD risk14 and cannot be discerned from BMI measurements.42 Even the “gold standard” of DEXA, which provides specific data on abdominal adiposity, does not have the ability to differentiate between subcutaneous and visceral fat. Previous studies that measured visceral fat using MRI or computed tomography (CT) in the able bodied showed that WC and WHtR are more strongly associated with visceral fat than BMI and so are better predictors of CVD risk.42–44 In the present study, all three measures of waist circumference (WC, WHtR, and WHR) were strongly correlated with the individual risk factors and were also correlated with the Framingham 30-year risk score. Only WC and WHtR were strongly correlated with both adiposity and risk score, suggesting that these represent stronger markers for obesity-related CVD risk. WC was more strongly correlated with all the cardiovascular risk factors and the Framingham risk score than WHtR. On balance, and incorporating practicality of use, we conclude that WC is the best marker for the presence of obesity-related CVD factors in individuals with SCI.
The Framingham risk score is a validated score from the Framingham Heart study, predicting 30-year risk of CVD.34 We adapted the use of the score by entering a neutral value for SAP (120 mmHg) for all participants, effectively eliminating any effect of blood pressure. Ordinarily, an SAP above 120 mmHg would increase the risk score and below this level would lower the risk score. This incorporates the known contribution of hypertension, a component of metabolic syndrome, to obesity-related CVD risk.45 However, whether the presence or absence of hypertension is as tightly related to risk of CVD in those with SCI as in the able-bodied population is unclear. Individuals with injury above the sixth thoracic level can have damage to descending spinal sympathetic pathways controlling the heart and vasculature, leading to resting sympathetic hypoactivity,35 low resting blood pressure, and impaired blood-pressure control.35,36 The lowered SAP in individuals with damage to these autonomic pathways might be hypothesized to have a “protective” effect in the risk score, but the associated blood-pressure dysregulation also manifests in abnormal cardiovascular reflex control46,47 that may be detrimental in terms of CVD risk,1 and this delicate balance would not be captured using the Framingham risk score. Therefore, it is unclear whether hypotension is necessarily cardioprotective in the SCI population. For this reason, we decided to exclude effects of SAP on the CVD risk score entirely. Of note, when the analyses were repeated with SAP incorporated in the risk score, our results were unchanged, despite an obvious change in the absolute value of the score for many individuals.
We excluded individuals from this study with known preexisting (before injury) CVD, because we were interested in the risk of CVD associated with SCI. The average risk score for our sample was 15 (8.3%), indicating a moderate risk for the group as a whole. This elevated risk score fits with the known high morbidity and mortality resulting from CVD after SCI as well as the fact that individuals with SCI experience an earlier onset and faster progression of CVD than in the general population.1,2 However, it is likely that this is an underestimation of the true CVD risk in a larger cohort of individuals with SCI, incorporating those with known preexisting CVD. Although none of our participants had diabetes, 9 met criteria for impaired glucose tolerance.48 This is not surprising, given that individuals with SCI are thought to be at increased risk of metabolic syndrome.49,50
The optimal cutoff for WC was determined to be 94 cm. This cutoff is lower than the 102-cm cutoff for males used in the able-bodied population.37 We did not provide separate criteria for males and females because of the low number of women in our study; the majority of our sample was male. This is representative of the population of individuals with SCI as a whole, in whom the incidence of injury is known to be higher in men than women.51 The ROC curve for a subgroup of just the males revealed the same optimal cutoff of 94 cm for WC, indicating that the cutoff is not artificially lower as a result of inclusion of women in the sample.
The lower cutoff for WC is in line with studies that have shown that general cutoffs for BMI and WC underestimate obesity in individuals with SCI.9,10,22 This underestimation is probably a result of the fact that individuals with SCI lose muscle mass and gain fat mass after injury and thus have a different body composition with the same WC. This has been confirmed using computed tomography to determine visceral fat, where individuals with SCI had more visceral adipose tissue, compared to able-bodied controls matched for WC, BMI, weight, and total abdominal tissue.22 This confirms the need for a lower cutoff for WC in this population. In addition, the WC criteria might be expected to be slightly smaller in our study because we conducted measurements in the supine position, instead of the upright position as in the general population, and this would be associated with diminished gravitational effects on abdominal distension. The concept of using a population-specific cutoff is not new and, in fact, has already been proposed for different ethnicity,52,53 gender, and age categories.54
The main limitation of this study is the fairly small sample size. Studies on obesity indices in the able-bodied population are usually very large, but in a specific population such as this one, the sample size is restricted. However, we were still able to detect substantial correlations between body composition, CVD risk factors, and our anthropometric measures. As a consequence of the small sample size, we were not able to differentiate our optimal cutoff according to age and gender. Another limitation is that we used the presence of an adverse CVD risk profile as the main outcome measure for correlation with our obesity markers, instead of the presence of CVD itself. We, therefore, provide a tool to predict the presence of an adverse risk profile and not CVD per se.
Finally, we would like to stress that although our data support the use of WC as a measure for obesity-related CVD risk after SCI, this should not be constituted to represent a clinical practice guideline at the present time. Further study is required to confirm its utility, as well as examine age- and gender-related cut-off criteria in a large cohort of SCI individuals.
Conclusion
We propose that WC can provide a simple, more-sensitive alternative to BMI that is easy to use in general medical practice, research, or at home. This measure is well suited for use in individuals with SCI because it does not require measures of height or weight that are more challenging to determine in this population. We also provide an optimal WC cutoff for determining the presence of an adverse CVD risk profile at 94 cm. This cutoff provides a simple tool to predict adverse CVD risk profiles related to obesity and, once validated in a larger cohort, can be used to guide risk management as well as as a practical aid for individuals with SCI to maintain a healthy body composition.
Acknowledgments
The authors are grateful to Jessica Inskip, Megan Keenleyside, Inderjeet Sahota, and Maureen McGrath for their assistance with the project. This work was supported by funding from the Christopher and Dana Reeve Foundation and the Heart and Stroke Foundation of Canada. Scott Lear holds the Pfizer Heart and Stroke Foundation Chair in Cardiovascular Prevention Research at St. Paul's Hospital.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Myers J., Lee M., and Kiratli J. (2007). Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am. J. Phys. Med. Rehabil. 86, 142–152 [DOI] [PubMed] [Google Scholar]
- 2.Groah S.L., Weitzenkamp D., Sett P., Soni B., and Savic G. (2001). The relationship between neurological level of injury and symptomatic cardiovascular disease risk in the aging spinal injured. Spinal Cord 39, 310–317 [DOI] [PubMed] [Google Scholar]
- 3.Reeder B.A., Senthilselvan A., Despres J.P., Angel A., Liu L., Wang H., and Rabkin S.W. (1997). The association of cardiovascular disease risk factors with abdominal obesity in Canada. Canadian Heart Health Surveys Research Group CMAJ 157, Suppl. 1, S39–S45 [PubMed] [Google Scholar]
- 4.von Eyben F.E., Mouritsen E., Holm J., Montvilas P., Dimcevski G., Suciu G., Helleberg I., Kristensen L., and von Eyben R. (2003). Intra-abdominal obesity and metabolic risk factors: a study of young adults. Int. J. Obes. Relat. Metab. Disord. 27, 941–949 [DOI] [PubMed] [Google Scholar]
- 5.Leenen R., van der Kooy K., Droop A., Seidell J.C., Deurenberg P., Weststrate J.A., and Hautvast J.G. (1993). Visceral fat loss measured by magnetic resonance imaging in relation to changes in serum lipid levels of obese men and women. Arterioscler. Thromb. 13, 487–494 [DOI] [PubMed] [Google Scholar]
- 6.Rajan S., McNeely M.J., Warms C., and Goldstein B. (2008). Clinical assessment and management of obesity in individuals with spinal cord injury: a review. J. Spinal Cord Med. 31, 361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Institutes of Health, National Heart, Lung and Blood Institute (1998). Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. National Institutes of Health. Obes Res 6, Suppl. 2, 51S–209S [PubMed] [Google Scholar]
- 8.World Health Organization (2000). Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech. Rep. Ser. 894, i–xii, 1–253 [PubMed] [Google Scholar]
- 9.Buchholz A.C., and Bugaresti J.M. (2005). A review of body mass index and waist circumference as markers of obesity and coronary heart disease risk in persons with chronic spinal cord injury. Spinal Cord 43, 513–518 [DOI] [PubMed] [Google Scholar]
- 10.Laughton G.E., Buchholz A.C., Ginis K.A.M., and Goy R.E. (2009). Lowering body mass index cutoffs better identifies obese persons with spinal cord injury. Spinal Cord 47, 757–762 [DOI] [PubMed] [Google Scholar]
- 11.Jones L.M., Legge M., and Goulding A. (2003). Healthy body mass index values often underestimate body fat in men with spinal cord injury. Arch. Phys. Med. Rehabil. 84, 1068–1071 [DOI] [PubMed] [Google Scholar]
- 12.Wang J., Thornton J.C., Bari S., Williamson B., Gallagher D., Heymsfield S.B., Horlick M., Kotler D., Laferrère B., Mayer L., Pi-Sunyer F.X., and Pierson R.N. (2003). Comparisons of waist circumferences measured at 4 sites. Am. J. Clin. Nutr. 77, 379–384 [DOI] [PubMed] [Google Scholar]
- 13.Zhu S., Wang Z., Heshka S., Heo M., Faith M.S., and Heymsfield S.B. (2002). Waist circumference and obesity-associated risk factors among whites in the third National Health and Nutrition Examination Survey: clinical action thresholds. Am. J. Clin. Nutr. 76, 743–749 [DOI] [PubMed] [Google Scholar]
- 14.Lear S.A., Humphries K.H., Kohli S., Frohlich J.J., Birmingham C.L., and Mancini G.B.J. (2007). Visceral adipose tissue, a potential risk factor for carotid atherosclerosis: results of the Multicultural Community Health Assessment Trial (M-CHAT). Stroke 38, 2422–2429 [DOI] [PubMed] [Google Scholar]
- 15.Lemieux S., Prud'homme D., Bouchard C., Tremblay A., and Després J.P. (1996). A single threshold value of waist girth identifies normal-weight and overweight subjects with excess visceral adipose tissue. Am. J. Clin. Nutr. 64, 685–693 [DOI] [PubMed] [Google Scholar]
- 16.Ross R., Léger L., Morris D., de Guise, J., and Guardo R. (1992). Quantification of adipose tissue by MRI: relationship with anthropometric variables. J. Appl. Physiol. 72, 787–795 [DOI] [PubMed] [Google Scholar]
- 17.Dalton M., Cameron A.J., Zimmet P.Z., Shaw J.E., Jolley D., Dunstan D.W., Welborn T.A., and Committee A.S. (2003). Waist circumference, waist-hip ratio and body mass index and their correlation with cardiovascular disease risk factors in Australian adults. J. Intern. Med. 254, 555–563 [DOI] [PubMed] [Google Scholar]
- 18.Han T.S., van Leer E.M., Seidell J.C., and Lean M.E. (1995). Waist circumference action levels in the identification of cardiovascular risk factors: prevalence study in a random sample. BMJ 311, 1401–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demirel S., Demirel G., Tükek T., Erk O., and Yilmaz H. (2001). Risk factors for coronary heart disease in patients with spinal cord injury in Turkey. Spinal Cord 39, 134–138 [DOI] [PubMed] [Google Scholar]
- 20.Emmons R.R., Garber C.E., Cirnigliaro C.M., Kirshblum S.C., Spungen A.M., and Bauman W.A. (2011). Assessment of measures for abdominal adiposity in persons with spinal cord injury. Ultrasound Med. Biol. 37, 734–741 [DOI] [PubMed] [Google Scholar]
- 21.Eriks-Hoogland I., Hilfiker R., Baumberger M., Balk S., Stucki G., and Perret C. (2011). Clinical assessment of obesity in persons with spinal cord injury: validity of waist circumference, body mass index, and anthropometric index. J Spinal Cord. Med. 34, 416–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards L.A., Bugaresti J.M., and Buchholz A.C. (2008). Visceral adipose tissue and the ratio of visceral to subcutaneous adipose tissue are greater in adults with than in those without spinal cord injury, despite matching waist circumferences. Am. J. Clin. Nutr. 87, 600–607 [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Alvarenga J.C., Montesinos-Cabrera R.A., Velazquez-Alva C., and Gonzalez-Barranco J. (2003). Short stature is related to high body fat composition despite body mass index in a Mexican population. Arch. Med. Res. 34, 137–140 [DOI] [PubMed] [Google Scholar]
- 24.Lee C.M.Y., Huxley R.R., Wildman R.P., and Woodward M. (2008). Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J. Clin. Epidemiol. 61, 646–653 [DOI] [PubMed] [Google Scholar]
- 25.Yang L., Samarasinghe Y.P., Kane P., Amiel S.A., and Aylwin S.J.B. (2009). Visceral adiposity is closely correlated with neck circumference and represents a significant indicator of insulin resistance in WHO grade III obesity. Clin. Endocrinol. 73, 197–200 [DOI] [PubMed] [Google Scholar]
- 26.Ben-Noun L., Sohar E., and Laor A. (2001). Neck circumference as a simple screening measure for identifying overweight and obese patients. Obes. Res. 9, 470–477 [DOI] [PubMed] [Google Scholar]
- 27.Preis S.R., Massaro J.M., Hoffmann U., D'Agostino R.B., Sr., Levy D., Robins S.J., Meigs J.B., Vasan R.S., O'Donnell C.J., and Fox C.S. (2010). Neck circumference as a novel measure of cardiometabolic risk: the Framingham Heart Study. J. Clin. Endocrinol. Metab. 95, 3701–3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vallianou N.G., Evangelopoulos A.A., Bountziouka V., Vogiatzakis E.D., Bonou M.S., Barbetseas J., Avgerinos P.C., and Panagiotakos D.B. (2013). Neck circumference is correlated with triglycerides and inversely related with HDL cholesterol beyond BMI and waist circumference. Diabetes Metab. Res. Rev. 29, 90–97 [DOI] [PubMed] [Google Scholar]
- 29.Kirshblum S.C., Burns S.P., Biering-Sorensen F., Donovan W., Graves D.E., Jha A., Johansen M., Jones L., Krassioukov A., Mulcahey M.J., Schmidt-Read M., and Waring W. (2011). International standards for neurological classification of spinal cord injury (revised 2011). J. Spinal Cord Med. 34, 535–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Washburn R.A., Zhu W., McAuley E., Frogley M., and Figoni S.F. (2002). The physical activity scale for individuals with physical disabilities: development and evaluation. Arch. Phys. Med. Rehabil. 83, 193–200 [DOI] [PubMed] [Google Scholar]
- 31.Ketel I.J.G., Volman M.N.M., Seidell J.C., Stehouwer C.D.A., Twisk J.W., and Lambalk C.B. (2007). Superiority of skinfold measurements and waist over waist-to-hip ratio for determination of body fat distribution in a population-based cohort of Caucasian Dutch adults. Eur. J. Endocrinol. 156, 655–661 [DOI] [PubMed] [Google Scholar]
- 32.Friedewald W.T., Levy R.I., and Fredrickson D.S. (1972). Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18, 499–502 [PubMed] [Google Scholar]
- 33.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., and Turner R.C. (1985). Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 [DOI] [PubMed] [Google Scholar]
- 34.Pencina M.J., D'Agostino R.B., Sr., Larson M.G., Massaro J.M., and Vasan R.S. (2009). Predicting the 30-year risk of cardiovascular disease: the Framingham Heart Study. Circulation 119, 3078–3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Claydon V.E., Steeves J.D., and Krassioukov A. (2006). Orthostatic hypotension following spinal cord injury: understanding clinical pathophysiology. Spinal Cord 44, 341–351 [DOI] [PubMed] [Google Scholar]
- 36.Teasell R.W., Arnold J.M., Krassioukov A., and Delaney G.A. (2000). Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch. Phys. Med. Rehabil. 81, 506–516 [DOI] [PubMed] [Google Scholar]
- 37.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (2001). Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 285, 2486–2497 [DOI] [PubMed] [Google Scholar]
- 38.Spungen A.M., Adkins R.H., Stewart C.A., Wang J., Pierson R.N., Jr., Waters R.L., and Bauman W.A. (2003). Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J. Appl. Physiol. 95, 2398–2407 [DOI] [PubMed] [Google Scholar]
- 39.Zlotolow S.P., Levy E., and Bauman W.A. (1992). The serum lipoprotein profile in veterans with paraplegia: the relationship to nutritional factors and body mass index. J. Am. Paraplegia Soc. 15, 158–162 [DOI] [PubMed] [Google Scholar]
- 40.Bauman W.A., Adkins R.H., Spungen A.M., and Waters R.L. (1999). The effect of residual neurological deficit on oral glucose tolerance in persons with chronic spinal cord injury. Spinal Cord 37, 765–771 [DOI] [PubMed] [Google Scholar]
- 41.Janssen T.W., van Oers C.A., van Kamp G.J., TenVoorde B.J., van der Woude L.H., and Hollander A.P. (1997). Coronary heart disease risk indicators, aerobic power, and physical activity in men with spinal cord injuries. Arch. Phys. Med. Rehabil. 78, 697–705 [DOI] [PubMed] [Google Scholar]
- 42.Pouliot M.C., Despres J.P., Lemieux S., Moorjani S., Bouchard C., Tremblay A., Nadeau A., and Lupien P.J. (1994). Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am. J. Cardiol. 73, 460–468 [DOI] [PubMed] [Google Scholar]
- 43.Onat A., Avci G.S., Barlan M.M., Uyarel H., Uzunlar B., and Sansoy V. (2004). Measures of abdominal obesity assessed for visceral adiposity and relation to coronary risk. Int. J. Obes. Relat. Metab. Disord. 28, 1018–1025 [DOI] [PubMed] [Google Scholar]
- 44.Valsamakis G., Chetty R., Anwar A., Banerjee A.K., Barnett A., and Kumar S. (2004). Association of simple anthropometric measures of obesity with visceral fat and the metabolic syndrome in male Caucasian and Indo-Asian subjects. Diabet. Med. 21, 1339–1345 [DOI] [PubMed] [Google Scholar]
- 45.Cardiometabolic Risk Working Group: Executive Committee, Leiter L.A., Fitchett D.H., Gilbert R.E., Gupta M., Mancini G.B.J., McFarlane P.A., Ross R., Teoh H., Verma S., Anand S., Camelon K., Chow C.-M., Cox J.L., Després J.-P., Genest J., Harris S.B., Lau D.C.W., Lewanczuk R., Liu P.P., Lonn E.M., McPherson R., Poirier P., Qaadri S., Rabasa-Lhoret R., Rabkin S.W., Sharma A.M., Steele A.W., Stone J.A., Tardif J.-C., Tobe S., and Ur E. (2011). Identification and management of cardiometabolic risk in Canada: a position paper by the cardiometabolic risk working group (executive summary). Can. J. Cardiol. 27, 124–131 [DOI] [PubMed] [Google Scholar]
- 46.Claydon V.E., and Krassioukov A.V. (2006). Orthostatic hypotension and autonomic pathways after spinal cord injury. J. Neurotrauma 23, 1713–1725 [DOI] [PubMed] [Google Scholar]
- 47.Karlsson A.K. (1999). Autonomic dysreflexia. Spinal Cord 37, 383–391 [DOI] [PubMed] [Google Scholar]
- 48.Alberti K.G., and Zimmet P.Z. (1998). Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 15, 539–553 [DOI] [PubMed] [Google Scholar]
- 49.Bauman W.A., Kahn N.N., Grimm D.R., and Spungen A.M. (1999). Risk factors for atherogenesis and cardiovascular autonomic function in persons with spinal cord injury. Spinal Cord 37, 601–616 [DOI] [PubMed] [Google Scholar]
- 50.Manns P.J., McCubbin J.A., and Williams D.P. (2005). Fitness, inflammation, and the metabolic syndrome in men with paraplegia. Arch. Phys. Med. Rehabil. 86, 1176–1181 [DOI] [PubMed] [Google Scholar]
- 51.Noonan V.K., Fingas M., Farry A., Baxter D., Singh A., Fehlings M.G., and Dvorak M.F. (2012). Incidence and prevalence of spinal cord injury in Canada: a national perspective. Neuroepidemiology 38, 219–226 [DOI] [PubMed] [Google Scholar]
- 52.Lear S.A., Humphries K.H., Frohlich J.J., and Birmingham C.L. (2007). Appropriateness of current thresholds for obesity-related measures among Aboriginal people. CMAJ 177, 1499–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lear S.A., James P.T., Ko G.T., and Kumanyika S. (2010). Appropriateness of waist circumference and waist-to-hip ratio cutoffs for different ethnic groups. Eur. J. Clin. Nutr. 64, 42–61 [DOI] [PubMed] [Google Scholar]
- 54.Dobbelsteyn C.J., Joffres M.R., MacLean D.R., and Flowerdew G. (2001). A comparative evaluation of waist circumference, waist-to-hip ratio and body mass index as indicators of cardiovascular risk factors. The Canadian Heart Health Surveys. Int. J. Obes. Relat. Metab. Disord. 25, 652–661 [DOI] [PubMed] [Google Scholar]