Abstract
Objective
Impaired glucose tolerance and diabetes are risk factors for the development of uterine cancer. Although greater progression free survival among diabetic patients with ovarian and breast cancer using metformin have been reported, no studies have assessed the association of metformin use with survival in women with endometrial cancer (EC).
Methods
We conducted a single-institution retrospective cohort study of all patients treated for uterine cancer from January 1999 through December 2009. Demographic, medical, social, and survival data were abstracted from medical records and the national death registry. Overall survival (OS) was estimated using Kaplan-Meier methods. Cox models were utilized for multivariate analysis. All statistical tests were two-sided.
Results
Of 985 patients, 114 (12%) had diabetes and were treated with metformin, 136 (14%) were diabetic but did not use metformin, and 735 (74%) had not been diagnosed with diabetes. Greater OS was observed in diabetics with non-endometrioid EC who used metformin than in diabetic cases not using metformin and non-endometrioid EC cases without diabetes (log rank test (p=0.02)). This association remained significant (hazard ratio = 0.54, 95% CI: 0.30–0.97, p<0.04) after adjusting for age, clinical stage, grade, chemotherapy treatment, radiation treatment and presence of hyperlipidemia in multivariate analysis. No association between metformin use and OS in diabetics with endometrioid histology was observed.
Conclusion
Diabetic EC patients with non-endometrioid tumors who used metformin had lower risk of death than women with EC who did not use metformin. These data suggest that metformin might be useful as adjuvant therapy for non-endometrioid EC.
Keywords: Metformin, endometrial cancer, non-endometrioid, adjuvant therapy, retrospective cohort study
INTRODUCTION
Cancer of the uterine corpus is the most common gynecologic malignancy and the sixth most frequent cause of cancer death in US women. (1,2) Furthermore, because obesity is a major risk factors for EC, the incidence of these cancers has been predicted to increase as a consequence of the US obesity epidemic. (3) The association with obesity, however, varies by histology and is more strongly associated with risk of endometrioid (also called Type I) than non-endometrioid (Type II) EC. Recent data from our group and others have strongly related the obesity–EC association to higher average circulating insulin and estradiol levels in obese women, and this relationship was specific for endometrioid tumors. (4,5,6)
Metformin, an oral anti-diabetic medication, is recommended first-line pharmacologic therapy for treatment of type 2 diabetes by the American Diabetes Association.(7) Metformin suppresses hepatic gluconeogenesis causing decreased serum levels of glucose and insulin. Use of metformin, has been associated with reduced risk and greater overall survival for several obesity-related cancers, though the results have varied between studies. (8,9,10,11) No studies of EC survival and metformin use have been reported.
Laboratory data have shown that metformin: (i) inhibits growth of endometrial cancer cell lines; (ii) reduces invasion and metastasis of endometrial cancer cell lines by modification of NF-kB, MMP-2/9 AKT and Erk1/2 pathways, and (iii) increases endometrial cancer cell line chemosensitivity to cisplatin and paclitaxel through reduced glyoxylase I expression, and modulation of the mTOR pathway. (12,13,14,15) On a molecular level, the fundamental activity of biguanides inhibits mitochondrial oxidative phosphorylation, and may subject neoplastic cells to energy related stress. (16) Inhibition of oxidative phosphorylation then causes decreased ATP production and triggering of cellular energy regulator AMP-activated protein kinase (AMPK) and its downstream targets including mTOR inhibition. (17) At the whole-organism level, antiproliferative action of metformin may be attributed to decreased insulin levels secondary to decreased hepatic gluconeogenesis in insulin responsive tumors.(18) It is unknown whether the putative anti-neoplastic effects of metformin are attributable to “endocrine” type effects verses direct action on target cells.
MATERIALS AND METHODS
A retrospective cohort investigation of the relationship between metformin use and OS was conducted in a large population of EC patients, who were diagnosed and treated at Montefiore Medical Center (MMC)/Albert Einstein College of Medicine between January 1, 1999 and December 31, 2009. Detailed medical records maintained on all cases were abstracted by trained personnel to obtain the diagnosis, including histology, stage, and grade, details of surgical and adjuvant therapy, age, race/ethnicity, body mass index in kg/m2 (BMI), diagnosis of diabetes, metformin use, hyperlipidemia, hypertension, date of each treatment, and date of death. The date of death was obtained by review of medical records and review of the Social Security Death Index. Entry date for this analysis was defined as date of histopathological diagnosis. Metformin use and diagnosis of diabetes was recorded from medical record documentation at time of surgery or initiation of treatment with chemotherapy/radiation. Tumor stage was determined by the 1988 International Federation of Gynecology and Obstetrics (FIGO) criteria. (19) Approval for this study was obtained from the MMC/Einstein Institutional Review Board.
As part of preliminary data analysis, we summarized demographic and clinical variables at the time of diagnosis, and contrasted these data according to diabetes and use of metformin, as well as tumor histology, using the Chi-square test for categorical and T-test for continuous data. Survival probabilities for overall survival (OS) were estimated using the Kaplan-Meier method for (i) non-diabetics, (ii) diabetics taking metformin, and (iii) diabetics not taking metformin. In keeping with prior publications, non-diabetic EC cases were used as the reference group, since they had the largest sample size of the three groups, providing stability to the estimates. The log rank test was then used to statistically compare OS in the three groups. Univariate and multivariate Cox Proportional Hazard regression analyses were also performed to further assess whether metformin use was an independent risk factor for OS. Previously reported risk factors related to OS in EC patients included in all models were age, stage, grade, and histology. Final multivariate models included these and other factors found to have p<0.10 in univariate analysis, with significance assessed by the partial likelihood ratio test. For purposes of completeness comparison of survival by log rank testing as well as multivariate modeling was performed comparing diabetics using metformin to diabetics not using metformin. All analyses were performed using Stata version 12.0 (StataCorp, College Station, TX).
RESULTS
Of the 985 EC cases 593 (60.2%) and 392 (39.8%) were endometrioid and non-endometrioid tumors, respectively. The histological subtypes represented by the non-endometrioid cancers were: papillary serous 197 (51%), carcinosarcoma 90 (23%), leiomyosarcoma 27 (7%), clear cell 20 (5%), sarcoma 11 (3%) and other 47 (12%). While most patients received full surgical effort (hysterectomy, bilateral salpingoophorectomy and staging), 27 (3%) had a surgical biopsy, followed by no further therapy in 14 women, radiation alone in nine women, chemotherapy alone in three women, and both radiation and chemotherapy in one woman. Patients with non-endometrioid EC had no significant differences from those with endometrioid tumors in terms of the prevalence of diabetes (24.0% versus 26.3%, respectively), or use of metformin (10.2% versus 12.6%), nor were the differences in BMI (30.1 versus 33.9 kg/m2) or age (66.8 versus 61.9) significant. Nonetheless, non-endometrioid EC cases were significantly more likely to be black, to have higher stage and higher grade tumors than those with endometrioid EC.
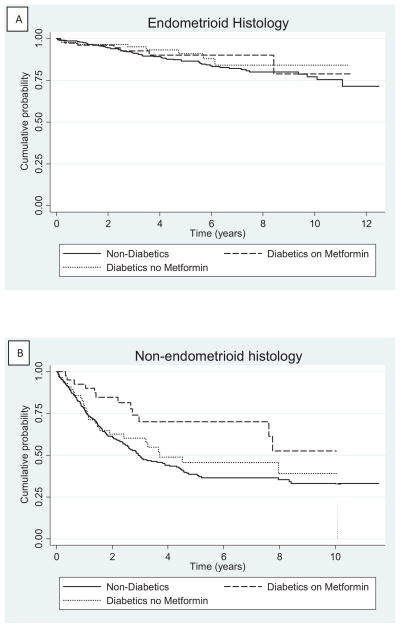
Median follow-up for the entire cohort was 3.34 years [IQ range: 1.6–6.3 years], 4.54 years [IQ range: 2.37–7.16 years] for patients with endometrioid histology and 2.24 years [IQ range: 1.06–4.46 years] in non-endometrioid EC cases. This included 279 patients (28%) who died during follow-up, of whom 198 had non-endometrioid histology (representing 52% of all non-endometrioid cases) and 81 had endometrioid histology (representing 14% of all endometrioid cases). Figure 1 shows OS separately for the two histologic types, according to diabetes status and use of metformin. While no association between endometrioid OS and metformin use was observed (log rank test p = 0.53), diabetic non-endometrioid cases who used metformin had greater OS than either the diabetic cases who did not use metformin or the non-endometrioid cases without diabetes (log rank test p=0.02, Figure 1). Differences in OS in patients with non-endometrioid histology and diabetes taking metformin and those with diabetes not taking metformin was of borderline significance (log rank test p=0.06).
Figure 1.
Kaplan-Meier curve of overall survival in endometrial cancer patients stratified by diabetes and metformin use among cases with (A) endometrioid histology or (B) non-endometrioid histology
Our initial univariate Cox models found several factors associated with OS among endometrioid and non-endometrioid EC cases (Table 2, Table 3). Age, advanced tumor stage, higher tumor grade, and use of chemotherapy alone (without radiation) after surgery were each associated with lower OS (i.e., higher relative hazard [HR] of death) in univariate analysis for patients of non-endometrioid sub-type. Patients with diagnoses of hyperlipidemia and hypertension had lower hazard of death. BMI was not significantly associated with OS in non-endometrioid EC cases. Of primary interest, being a diabetic who used metformin (HR=0.45; 95% CI: 0.25–0.80; P<0.01), but not a diabetic who did not use metformin (HR=0.89; 95% CI: 0.59–1.32; P=0.56), was associated with greater OS relative to non-diabetic women in univariate analysis.
Table 2.
Overall survival analyzed by univariate and multivariate Cox regression models for endometrioid histological subtype
| Variable | Mean (SD) or Frequency (%) | Univariate Hazard Ratio (95% CI, p-value) | Multivariate Hazard Ratio (95% CI, p-value) |
|---|---|---|---|
| Mean Age, years (+/− SD) | 61.9 (11.7) | 1.06 (1.04–1.08, <0.01) | 1.07 (1.04–1.09, <0.01) |
| Mean BMI, kg/m2 (+/−SD) | |||
| <18.5 | 5 (1.0) | Unstable | |
| 18.5–24.9 | 57 (10.9) | 1.00 (ref) | |
| 25–29.9 | 115 (21.9) | 0.80 (0.38–1.66, 0.55) | |
| ≥30 | 348 (66.2) | 0.55 (0.29–1.06, 0.08) | |
| Race | |||
| White | 285 (47.8) | 1.00 | |
| Black | 113 (19.0) | 1.33 (0.76–2.33, 0.32) | |
| Hispanic | 150 (25.2) | 1.21 (0.69–2.12, 0.52) | |
| Other | 45 (8.0) | 0.69 (0.25–1.94, 0.48) | |
| Stage | |||
| I | 489 (84.1) | 1.00 | 1.00 |
| II | 44 (7.6) | 1.66 (0.75–3.67, 0.21) | 1.33 (0.59–2.99, 0.49) |
| III | 36 (6.2) | 2.42 (1.15–5.12, 0.02) | 1.30 (0.56–3.02, 0.55) |
| IV | 12 (2.1) | 7.12 (3.22–15.76, <0.01) | 1.36 (0.47–3.90, 0.57) |
| Grade | |||
| 1 | 380 (64.3) | 1.00 | 1.00 |
| 2 | 137 (23.0) | 2.36 (1.43–3.92, <0.01) | 1.53 (0.87–2.66, 0.14) |
| 3 | 76 (12.7) | 2.63 (1.47–4.72, <0.01) | 1.39 (0.69–2.81, 0.36) |
| Diabetes | |||
| No | 437 (73.7) | 1.00 | 1.00 |
| Yes – Using Metformin | 74 (12.4) | 0.76 (0.35–1.66, 0.49) | 0.79 (0.31–2.00, 0.61) |
| Yes – Not Using Metformin | 82 (13.8) | 0.70 (0.33–1.46, 0.34) | 0.80 (0.36–1.78, 0.58) |
| Hyperlipidemia | |||
| No | 409 (68.9) | 1.00 | 1.00 |
| Yes | 184 (31.1) | 0.61 (0.34–1.09, 0.09) | 0.51 (0.27–0.96, 0.04) |
| Hypertension | |||
| No | 267 (45.0) | 1.00 | |
| Yes | 326 (55.0) | 0.99 (0.63–1.55, 0.97) | |
| Treatment: Radiation | |||
| No | 481 (81.1) | 1.00 | |
| Yes | 112 (18.9) | 1.25 (0.75–2.11, 0.39) | |
| Chemotherapy | |||
| No | 574 (96.8) | 1.00 | 1.00 |
| Yes | 19 (3.2) | 3.33 (1.53–7.25, <0.01) | 7.93 (3.10–20.27, <0.01) |
| Radiation & Chemotherapy | |||
| No | 562 (94.8) | 1.00 | 1.00 |
| Yes | 31 (5.2) | 4.49 (2.42–8.35, <0.01) | 3.82 (1.72–8.46, <0.01) |
Table 3.
Overall survival analyzed by univariate and multivariate Cox regression models, stratified by non-endometrioid histological subtype
| Variable | Mean (SD)or Frequency (%) | Univariate Hazard Ratio (95% CI, p-value) | Multivariate Hazard Ratio (95% CI, p-value) |
|---|---|---|---|
| Mean Age, years (+/− SD) | 66.8 (10.1) | 1.02 (1.00–1.04, 0.01) | 1.02 (1.00–1.03, 0.05) |
| Mean BMI, kg/m2 (+/−SD) | |||
| <18.5 | 9 (2.5) | 1.53 (0.68–3.45, 0.31) | |
| 18.5–24.9 | 59 (16.2) | 1.00 (ref) | |
| 25–29.9 | 119 (32.7) | 0.79 (0.52–1.20, 0.26) | |
| ≥30 | 177 (48.6) | 0.78 (0.53–1.16, 0.22) | |
| Race | |||
| White | 97 (24.7) | 1.00 | |
| Black | 195 (49.7) | 1.01 (0.73–1.39, 0.96) | |
| Hispanic | 82 (20.9) | 0.82 (0.55–1.23, 0.35) | |
| Other | 18 (4.6) | 0.31 (0.11–0.87, 0.03) | |
| Stage | |||
| I | 181 (46.2) | 1.00 | 1.00 |
| II | 34 (8.7) | 1.27 (0.71–2.27, 0.42) | 1.15 (0.64–2.07, 0.63) |
| III | 82 (20.9) | 2.53 (1.72–3.72, <0.01) | 3.12 (2.10–4.64, <0.01) |
| IV | 82 (20.9) | 6.05 (4.23–8.66, <0.01) | 5.74 (3.78–8.71, <0.01) |
| Grade | |||
| 1 | 17 (4.3) | 1.00 | 1.00 |
| 2 | 6 (1.5) | 2.01 (0.37–11.01, 0.42) | 2.60 (0.47–14.44, 0.28) |
| 3 | 365 (93.1.0) | 3.48 (1.29–9.37, 0.01) | 3.81 (1.38–10.53, 0.01) |
| Diabetes | |||
| No | 298 (76.0) | 1.00 | 1.00 |
| Yes – Using Metformin | 40 (10.2) | 0.45 (0.25–0.80, <0.01) | 0.54 (0.30–0.97, 0.04) |
| Yes – Not Using Metformin | 54 (13.8) | 0.89 (0.59–1.32, 0.56) | 0.93 (0.60–1.45, 0.76) |
| Hyperlipidemia | |||
| No | 280 (71.4) | 1.00 | 1.00 |
| Yes | 112 (28.6) | 0.47 (0.33–0.68, <0.01) | 0.45 (0.30–0.66, <0.01) |
| Hypertension | |||
| No | 178 (45.4) | 1.00 | |
| Yes | 214 (54.6) | 0.67 (0.51–0.86, <0.01) | |
| Treatment: Radiation | |||
| No | 348 (88.7) | 1.00 | |
| Yes | 44 (11.3) | 0.69 (0.43–1.10, 0.12) | |
| Chemotherapy | |||
| No | 307 (78.3) | 1.00 | 1.00 |
| Yes | 85 (21.7) | 2.33 (1.72–3.18, <0.01) | 1.00 (0.64–1.55, 0.99) |
| Radiation & Chemotherapy | |||
| No | 218 (55.5) | 1.00 | 1.00 |
| Yes | 174 (44.5) | 0.61 (0.45–0.81, <0.01) | 0.72 (0.51–1.03, 0.08) |
Similar associations with diabetes and metformin use were observed in multivariate Cox models after adjustment for each of the covariates significantly associated with OS. Specifically, diabetics who used metformin (HR = 0.57; 95% CI: 0.31–0.97; P=0.04), but not diabetics who did not use metformin (HR=0.93; 95% CI: 0.60–1.45, P=0.76), had greater OS than non-diabetic women. The effect estimate was essentially unchanged by limiting analysis to just diabetics and comparing risk between those using versus not using metformin, though the result was no longer statistically significant (HR=0.53; 95% CI: 0.24–1.16; P=0.11). Conversely, in women with endometrioid EC, no difference in OS was observed between diabetics using metformin and non-diabetic cases or diabetic cases not using metformin (Table 2).
DISCUSSION
The results of this large retrospective cohort study suggest that the use of metformin among diabetic patients with non-endometrioid EC, but not endometrioid EC, may be associated with improved OS. If correct, this association could be of clinical relevance, especially since non-endometrioid tumors account for 45% of total EC deaths, albeit causing only 15% of all EC in the US. (20) In particular, the findings raise the possibility that metformin might be useful as adjuvant therapy along with standard of care cytotoxic therapy to reduce mortality in non-endometrioid EC patients. The incidence, progression free survival and mortality of several other cancers, including post-menopausal breast cancer, have been shown to be lower among diabetic patients who use metformin and large randomized clinical trials of metformin use as adjuvant therapy for breast cancer are underway. (8, 21, 22, 23, 24, 25) As mentioned, several mechanisms through which metformin may reduce EC mortality have been reported.
It is unexpected and somewhat paradoxical to find an association between metformin use with non-endometrioid but not endometrioid EC OS. That is, insulin resistance is only associated with risk of the incident development of endometrioid EC, and so we expected metformin to have its greatest impact on the OS of those tumors and not non-endometrioid EC. However, the risk factors for recurrence and survival may not be the same as for the initial development of the cancer. For example, obesity is not a risk factor for development of premenopausal breast cancer, but it is a major risk factor for its recurrence. In any event, several sources of evidence have suggested that the impact of metformin may be downstream of its role in reducing glucose and insulin levels; related to its impact on cell cycle (e.g., mitogenic/anti-apoptotic) signaling. (26,27) Furthermore, given the biologic differences between non-endometrioid and endometrioid EC it is not unexpected that different pathways may be relevant to mortality related to the two tumor types. If the antineoplastic effect of metformin is attributable to direct cellular effects such as mitochondrial oxidative phosphorylation and AMPK and its downstream effectors it is not surprising that metformin effect would vary depending on heterogeneous metabolic/genetic characteristics of tumors.(17) While not a primary objective of this study, it is of interest that hyperlipidemia presented a survival advantage in both endometrioid and non-endometrioid type cancers and suggests that statin use may impact overall survival from endometrial cancer. Statin use has been reported to moderate metformin effect on risk of prostate cancer in patients with type 2 diabetes.(28) Further study of statin use in endometrial cancers should be considered.
Limitations of this study were: retrospective data collection, and the inability to adequately address the timing, dose and adherence to metformin in relationship to the disease occurrence. Further, surgical staging and adjuvant treatment data were not standardized, and data regarding adherence to the prescribed adjuvant therapy was too incomplete to include in our analyses. Mortality was reported as all-cause secondary to limitation of collection of data from social security death registry if patient mortality data was not present in the medical record. Additionally, data pertaining to medication adherence, fasting glucose and fasting insulin were not available. These data would be important in defining the role of insulin resistance in cancer recurrence and progression. Pharmacoepidemiology surrounding diabetic medication utilization and cancer presents time related biases including immortal time, time-window bias and time-lag bias (29). Although our study did not include data on metformin use at time of censor or other medication use before metformin, establishment of the metformin cohort as users at time of diagnosis, should have eliminated this type of bias. Other confounding variables that were not accounted for were severity of diabetes and cigarette smoking. It is possible that patients taking metformin had more aggressive medical follow-up, and may have been less likely to be smokers, which could have contributed to decreased mortality.
Meaningful inferences regarding the effect of metformin on endometrioid type cancers may have also been limited by the small number of events in this group (81 deaths). Not surprisingly the majority of deaths were in the non-endometrioid subtype. Overall survival for women with Stage 1 endometrioid type cancers is more than 90% at five years of follow-up (30). In our cohort only 14% of patients with endometrioid versus 52% of patients with non-endometrioid tumors died. The number of deaths required to detect the observed effect size of metformin in the endometrioid subgroup with adequate power and precision would be significantly more than observed.
Epidemiologic, translational and pre-clinical data support the adjunctive role of metformin in EC. There is ongoing study through the Gynecologic Oncology Group (GOG) evaluating biomarkers in patients with EC of insulin resistance, and IGF. Further prospective study is needed to clarify the adjuvant role of metformin for women with EC.
Table 1.
Baseline patient characteristics
| Non-Diabetics | Diabetics Not On Metformin | Diabetics On Metformin | P | |
|---|---|---|---|---|
| N= 735 (%) | N=136 (%) | N=114 (%) | ||
| Mean age, years (+/− SD) | 63.8 (11.8) | 64.1 (10.6) | 64.2 (9.1) | 0.89 |
| Mean BMI, kg/m2 (+/− SD) | 31.5 (8.5) | 34.4 (7.9) | 34.8 (6.7) | <0.001 |
| Histology | 0.57 | |||
| Endometrioid | 437 (59.6) | 82 (60.3) | 74 (64.9) | |
| Non-endometrioid | 298 (40.4) | 54 (39.7) | 40 (35.1) | |
| Stage | 0.02 | |||
| 1 | 489 (66.5) | 92 (67.7) | 87 (76.3) | |
| 2 | 63 (8.6) | 7 (5.2) | 8 (7.0) | |
| 3 | 93 (12.7) | 16 (11.8) | 9 (7.9) | |
| 4 | 75 (10.2) | 11 (8.1) | 8 (8.0) | |
| Biopsy only unstaged | 15 (2.0) | 10 (7.4) | 2 (1.8) | |
| Grade | ||||
| 1 | 299 (40.9) | 58 (42.7) | 42 (36.8) | 0.06 |
| 2 | 97 (13.3) | 18 (13.2) | 27 (23.7) | |
| 3 | 336 (45.8) | 60 (44.1) | 45 (39.5) | |
| Hyperlipidemia | 166 (22.6) | 59 (43.4) | 63 (55.3) | <0.001 |
| Hypertension | 317 (43.1) | 117 (86.0) | 104 (91.2) | <0.001 |
| Race/Ethnicity | ||||
| White | 316 (42.9) | 31 (27.0) | 35 (25.7) | <0.001 |
| Black | 218 (29.6) | 40 (34.8) | 50 (36.8) | |
| Hispanic | 149 (20.2) | 38 (33.0) | 45 (33.1) | |
| Other | 53 (7.2) | 6 (5.2) | 6 (4.4) | |
7. Highlights.
Metformin use is associated with improved overall survival in women with non-endometrioid type uterine cancers.
Metformin may be an important adjuvant therapy for women with endometrial cancer.
Acknowledgments
This work was supported in part by the Albert Einstein Cancer Center through its NCI Cancer Center Support Grant (P30CA013330), and by R01CA1330104 (HS).
Abbreviations
- EC
Endometrial cancer
- OS
overall survival
- MMC
Montefiore Medical Center
Footnotes
A portion of this work was presented at the 2013 Annual Meeting of the Society of Gynecologic Oncologists Los Angeles, CA.
There are no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Bray F, Center M, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegal R, Ward E, Browley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Fader A, Arriba L, Frasure H, Von Gruenigen V. Endometrial cancer and obesity: Epidemiology, biomarkers, prevention and survivorship. Gynecologic Oncology. 2009;114:121–127. doi: 10.1016/j.ygyno.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 4.Gunter M, Hoover D, Yu H, Wassertheil-Smoller S, Manson J, Li J, Harris T, Rohan T, Xue X, Ho G, Einstein M, Kaplan R, Burk R, Wylie-Rosett J, Pollak M, Anderson G, Hoard B, Strickler H. A prospective evaluation of insulin and insulin-like growth factor-I as risk factors for endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(4):921–9. doi: 10.1158/1055-9965.EPI-07-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friberg E, Orsini N, Mantzoros C, Wolk A. Diabetes mellitus and risk of endometrial cancer: a metanalysis. Diabetologia. 2007;50:1365. doi: 10.1007/s00125-007-0681-5. [DOI] [PubMed] [Google Scholar]
- 6.Giovanucci E. Nutrition, insulin, insulin-like growth factors and cancer. Horm Metab Res. 2003;35:694. doi: 10.1055/s-2004-814147. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association. Standards of Medical Care in Diabetes-2009. Diabetes Care. 2009;32(1):S13–S61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chebowski R, McTiernan A, Wactawski-Wende J, Manson J, Aragaki A, et al. Diabetes, metformin, and breast cancer in postmenopausal women. J Clin Oncol. 2012;30(23):2844–52. doi: 10.1200/JCO.2011.39.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noto H, Osame K, Sasuazuki T, Noda M. Substantially increased risk of cancer in patients with diabetes mellitus: a systemic review and meta-analysis of epidemiologic evidence in Japan. J Diabetes Complications. 2010;24:345. doi: 10.1016/j.jdiacomp.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Bodmer M, Becker C, Meier C, Jick S, Meier C. Use of metformin and the risk of ovarian cancer: A case-control analysis. Gynecol Oncol. 2011;123:200–204. doi: 10.1016/j.ygyno.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 11.Sadeghi N, Abbruzzese J, Yeung S, et al. Metformin use is assosciated with better survival of diabetic patients with pancreatic cancer. Clin Ca Res. 2012;18:2905–2912. doi: 10.1158/1078-0432.CCR-11-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantrell L, Zhou C, Mendivil A, Malloy K, Gehrig P, Bae-Jump V. Metformin is a potent inhibitor of endometrial cancer cell proliferation—implications for a novel treatment strategy. Gynecol Oncol. 2010;116(1):92–8. doi: 10.1016/j.ygyno.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan B, Adya R, Chen J, Lehnert H, Saint Cassia L, Randeva H. Metformin treatment exerts antiinvasive and antimetastatic effects in human endometrial carcinoma cells. J Clin Endo and Met. 2012;96(3):808. doi: 10.1210/jc.2010-1803. [DOI] [PubMed] [Google Scholar]
- 14.Dong L, Zhou Q, Zhang Z, Zhu Y, Zuan T, Feng Y. Metformin sensitizes endometrial cancer cells to chemotherapy by repressing glyoxalase I expression. Journal of Obstetrics and Gynaecologic Research. 2012;38:1077–85. doi: 10.1111/j.1447-0756.2011.01839.x. [DOI] [PubMed] [Google Scholar]
- 15.Hanna R, Zhou C, Malloy K, Sun L, Zhong Y, Gehrig P, Bae-Jump V. Metformin potentiates the effects of paclitaxel in endometrial cancer cells through inhibition of cell proliferation and modulation of the mTOR pathway. Gynecol Oncol. 2012;125:458–69. doi: 10.1016/j.ygyno.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owen M, Doran E, Halestrap A. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348:607–14. [PMC free article] [PubMed] [Google Scholar]
- 17.Hardie D, Ross F, Hawley S. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159–69. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 19.Pecorelli S, Benedet J, Creasman W, Shepherd J. FIGO staging of gynecologic cancer. 1994–1997 FIGO Committee on Gynecologic Oncology. International Federation of Gynecology and Obstetrics. Int J Gynecol Obstet. 1999;64(1):5–10. doi: 10.1016/s0020-7292(98)00234-3. [DOI] [PubMed] [Google Scholar]
- 20.Cirisano F, Robboy S, Dodge R, Bently R, Krigman H, Synan I, Soper J, Clarke-Pearson D. Epidemiologic and surgicopathologic findings of papillary serous and clear cell endometrial cancers when compared to endometrioid carcinoma. Gyn Oncol. 1999;74:385–394. doi: 10.1006/gyno.1999.5505. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin P, Stambolic V, Lemieux J, Chen B, Parulekar W, Gelmon K, Hershman D, Hoobday T, Ligibel J, Pritchard M, Whelan T, Rastogi P, Shepherd L. Evaluation of metformin in early breast cancer: a modification of the traditional paradigm for clinical testing of anti-cancer agents. Breast Cancer Res Treat. 2011;126(1):215–20. doi: 10.1007/s10549-010-1224-1. [DOI] [PubMed] [Google Scholar]
- 22.Col N, Ochs L, Springmann V, Aragaki A, Chlebowski R. Metformin and breast cancer risk: a meta-analysis and critical literature review. Breast Cancer Res Treat. 2012;135(3):639–46. doi: 10.1007/s10549-012-2170-x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang P, Li H, Tan X, Chen L, Wang S. Association of metformin use with cancer incidence and mortality: A meta-analysis. Cancer Epi. 2012;37(3):207–218. doi: 10.1016/j.canep.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Romero I, McCormick A, McEwen K, Park S, Karrison T, Yamada D, Pannain S, Lengyel E. Relationship of Type II diabetes and metformin use to ovarian cancer, progression, survival and chemosensitivity. Obstetrics and Gynecology. 2012;119(1):61–67. doi: 10.1097/AOG.0b013e3182393ab3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambe M, Wigertz A, Garmo H, Walldius G, Gungner I, Hammar N. Impaired glucose metabolism and diabetes and the risk of breast, endometrial, and ovarian cancer. Cancer Causes and Control. 2011;22(8):1163–7. doi: 10.1007/s10552-011-9794-8. [DOI] [PubMed] [Google Scholar]
- 26.Landman G, Kleefstra N, van Hateren K, Groenier K, Gans R, Bilo H. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC 16. Diabetes Care. 2010;33:322–6. doi: 10.2337/dc09-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang G, Brouwer-Visser J, Ramirez M, Kim C, Hebert T, Lin J, Arias-Pulido H, Qualis C, Prossantz E, Goldberg G, Smith H, Horwitz S. Insulin-like growth factor 2 expression modulates taxol resistance and is a candidate biomarker for reduced disease-free survival in ovarian cancer. Clin Cancer Res. 2010;16:2999–3010. doi: 10.1158/1078-0432.CCR-09-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehman D, Lorenzo C, Hernandez J, Wang C. Statin use as a moderator of metformin effect on risk for prostate cancer amont type2 diabetic patients. Diabetes Care. 2012;35:1002–7. doi: 10.2337/dc11-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suissa S, Azoulay L. Metformin and the risk of cancer. Diabetes Care. 2012;35:2665–2673. [Google Scholar]
- 30.Creasman W, Odicino F, Mausinneuve P, Quinn M, Beller Y, Benedet J, et al. Carcinoma of the copus uteri. International Journal of Gynecology and Obstetrics. 2006;95:S105–S143. doi: 10.1016/S0020-7292(06)60031-3. [DOI] [PubMed] [Google Scholar]