Abstract
Sirtuin 1 (SIRT1) depletion in vascular endothelial cells mediates endothelial dysfunction and premature senescence in diverse cardiovascular and renal diseases. However, the molecular mechanisms underlying these pathologic effects remain unclear. Here, we examined the phenotype of a mouse model of vascular senescence created by genetically ablating exon 4 of Sirt1 in endothelial cells (Sirt1endo−/−). Under basal conditions, Sirt1endo−/− mice showed impaired endothelium-dependent vasorelaxation and angiogenesis, and fibrosis occurred spontaneously at low levels at an early age. In contrast, induction of nephrotoxic stress (acute and chronic folic acid–induced nephropathy) in Sirt1endo−/− mice resulted in robust acute renal functional deterioration followed by an exaggerated fibrotic response compared with control animals. Additional studies identified matrix metalloproteinase-14 (MMP-14) as a target of SIRT1. In the kidneys of Sirt1endo−/− mice, impaired angiogenesis, reduced matrilytic activity, and retention of the profibrotic cleavage substrates tissue transglutaminase and endoglin accompanied MMP-14 suppression. Furthermore, restoration of MMP-14 expression in SIRT1-depeleted mice improved angiogenic and matrilytic functions of the endothelium, prevented renal dysfunction, and attenuated nephrosclerosis. Our findings establish a novel mechanistic molecular link between endothelial SIRT1 depletion, downregulation of MMP-14, and the development of nephrosclerosis.
One of the vexing questions related to progression of chronic fibrosing diseases is that of the role played by vascular rarefaction, a consistent companion of fibrosis: Does vascular demise underpin whether the disease progresses or whether the loss of microvasculature is the consequence of progressive fibrosis? Stress-induced premature senescence (SIPS) of vascular endothelial cells contributes to microvascular rarefaction and SIPS of endothelial progenitor cells impedes microvascular regeneration.1–11 Microvascular rarefaction, particularly of the peritubular capillary network, has been construed as one of the major factors contributing to the progression of renal diseases.12,13
One of the mechanisms of SIPS has been identified as a depletion of sirtuin 1 (SIRT1), an NAD-dependent deacetylase that undergoes cathepsin-mediated cleavage as a result of stress-induced increase in lysosomal membrane permeability.14 Recent demonstration of beneficial effects resulting from ablation of senescent cells in delaying aging-associated disorders serves as a potent reinforcement of the idea that these cells are major contributors to organ dysfunction.15 However, the role of endothelial senescent cells and their contribution to organ pathology remain tenuous. Most recent evidence indicates that angiocrine signals discharged by endothelial cells and identified as cleavage products of matrix metalloproteinase-14 (MMP-14) (which generates laminin 5 fragments serving as ligands for EGF receptor) are critical for epithelial regeneration, at least after unilateral pneumonectomy.16 Similarly, endothelial progenitors have been shown to support regeneration of liver parenchyma after partial hepatectomy.17 To pursue the search for the consequences of an isolated endothelial cell senescence and/or reduced resistance to stress, we generated mice deficient in SIRT1 in endothelial cells. Previous studies of this model demonstrated impaired angiogenesis,18 and we showed an increased proportion of senescent endothelial and endothelial progenitor cells and their reduced in vitro stress tolerance.14 Curiously, gene expression profiling of endothelial cells pretreated with SIRT1 small interfering RNA revealed downregulation of MMP-14, among other genes.18
Having demonstrated that several relevant cardiovascular risk factors, such as asymmetric dimethylarginine, advanced glycation end products, or hydrogen peroxide, lead to the cathepsin-dependent SIRT1 depletion in endothelial and endothelial progenitor cells,13 we sought to determine the role of SIRT1 deficiency per se, independent of the above stressors, in cell pathology. In this study, we performed in vivo analysis of consequences of endothelial SIRT1 deficiency and a possible relation between reduced expression of MMP-14 and the quality of kidney regeneration after acute and chronic injury.
Results
General Characterization of the Mouse Model: Vascular and Renal Phenotype
Our strategy to delete SIRT1 in endothelial cells, similar to that used by Potente et al.,18 is summarized in Supplemental Figure 1. We cross-bred B6;129-Sirt1tm1Ygu/J mice (homozygous for targeted allele Sirt1co/co, viable and fertile, containing a loxP-flanked neomycin cassette upstream and downstream of exon 4 of the targeted gene) with Tie2-Cre transgenic mice. The offspring thrive as their wild-type, heterozygote, and homozygote littermates (Mendelian distribution) are normotensive, show normal blood counts, and do not develop detectable renal functional abnormalities at 12 weeks of age (data not shown). Endothelial and endothelial progenitor cells isolated from SIRT1endo−/− mice, however, showed an increased proportion of senescent and apoptotic cells under basal conditions and reduced resistance to stress, as recently shown by our group.14 En face aortic preparations stained for the expression of senescence-associated β-galactosidase revealed a dramatic increase in the frequency of senescent endothelial cells already at 12 weeks of age (Supplemental Figure 2, A–C).14 Moreover, immunofluorescence detection of another marker of senescent cells, histone 3 trimethylated on lysine 9 (H3K9Me3), showed that SIRT1endo−/− mice have an increased proportion of senescent endothelial cells in the renal microvasculature (Supplemental Figure 2D). These data confirm that both the macrovascular and microvascular endothelium undergoes premature senescence in SIRT1endo−/− mice.
Ex vivo angiogenesis assays in three-dimensional (3D) matrigel using explant cultures of aortic segments revealed a profound defect in sprouting angiogenesis in SIRT1endo−/− mice (Supplemental Figure 3A). To evaluate endothelial function in the macrovasculature, we next performed studies of acetylcholine-induced vasorelaxation of thoracic aortic ring segments. As shown in Supplemental Figure 3B, at 12 weeks of age, SIRT1endo−/− mice exhibited a mild defect in endothelium-dependent vasorelaxation, which became much more prominent in older mice at 15 months of age (Supplemental Figure 3C). Histomorphologic examination of kidneys from these mice did not reveal any pathologic changes in heterozygote animals, but showed a spontaneously developing mild patchy interstitial fibrosis without glomerular involvement in SIRT1endo−/− mice already at 12 weeks of age, evident at high magnification (Figure 1, A and B). Quantitative PCR data demonstrated significantly higher expression of collagen I and only slightly increased collagen III under basal conditions (Figure 1, C and D). These data suggest that despite normal BP and renal function, vascular functions are already impaired and traces of fibrosis occur in SIRT1endo−/− mice at the early age. These data also highlight a nonessential role of endothelial SIRT1 in the maintenance of vascular and renal functions under basal conditions.
Figure 1.

Morphologic characterization of the endothelial SIRT1-knockout mouse model. (A) Representative Masson’s trichrome staining of kidney sections from 12-week-old SIRT1endo−/− knockout mice, SIRT1endo+/− heterozygote mice, and control animals. Histomorphologic examination does not reveal any noticeable pathologic changes in heterozygote or control animals, but shows a spontaneously developing mild patchy interstitial fibrosis in SIRT1endo−/− mice at the early age. (B) Quantification of the fibrotic area (grid method). (C and D) Expression of collagen I and collagen III, examined by quantitative real-time PCR. n=6 animals per group. Data are the mean ± SEM. *P<0.05. Original magnification, ×600.
Acute Renal Injury and Chronic Nephropathy in Endothelial SIRT1-Deficient Mice
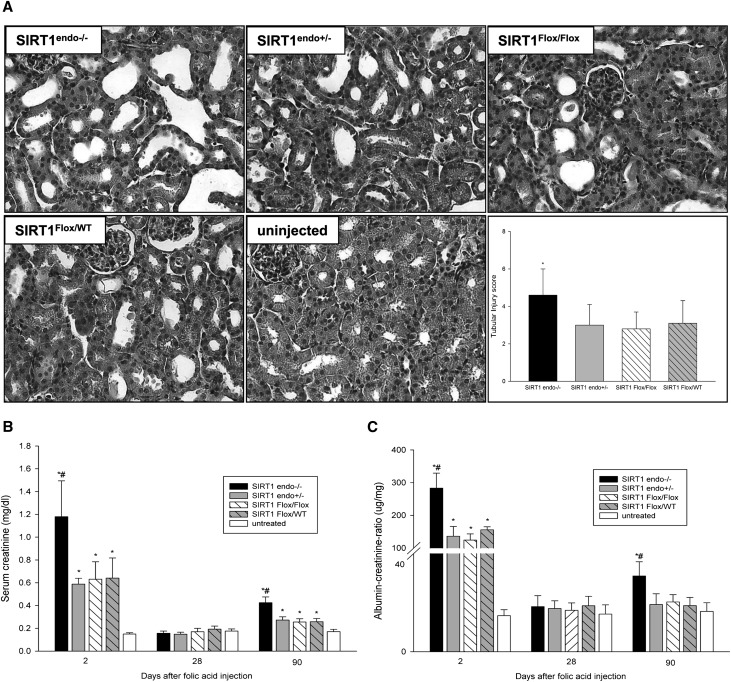
Considering a mild phenotype of SIRT1endo−/− mice under baseline conditions, we next examined renal responses to acute and chronic kidney injury, conditions that apply a selection pressure on angiogenic and regenerative processes. As shown above, SIRT1endo−/− mice exhibit a profound defect in angiogenesis already under normal conditions. Dysfunctional renal vasculature, particularly the peritubular capillary network, has been incriminated in the progression of kidney disease.19,20 Here, we intended to study the effect of SIRT1-depleted endothelium on regenerative processes in an experimental model of kidney injury: folic acid–induced nephropathy. We intentionally used a tubulotoxic model to define the pathogenetic role of endothelium in structural and functional recovery from parenchymal damage. Response to this insult should theoretically report not only on the susceptibility of SIRT1endo−/− mice to injury, but also on the propensity toward resolving injury vis à vis development of fibrotic lesions. Previous studies using this well characterized model of kidney injury showed the peak of collagen I overexpression after 80 days with development of fibrosis within 3 months (a slowly progressive model).21 As summarized in Figure 2, SIRT1endo−/− mice exhibited a much more robust acute response (day 2) to the nephrotoxin than the heterozygote SIRT1endo+/− mice, or SIRT1Flox/Flox and SIRT1Flox/WT control animals. Moreover, 90 days postinsult, SIRT1endo−/− mice were albuminuric and had elevated serum creatinine levels (although both parameters returned to normal 28 days after injection of folic acid) in excess of those seen in SIRT1endo+/− and control mice (Figure 2, B and C). These findings correlated with morphologic observations. Masson’s trichrome staining of kidneys at different time points postinsult showed that 1 month postinjection, at the time when kidney function was unperturbed (based on serum creatinine and albuminuria), and at 3 months, when it was perturbed, kidneys from SIRT1endo−/− knockout mice showed significantly more severe tubulointerstitial fibrosis than heterozygote and control mice (Figure 3, A and B). In accord with this, collagen I and III mRNA expression, as well as the short/long ratio of endoglin isoforms, a marker of senescence, were all elevated in SIRT1endo−/− mice (Figure 3, C–E). Folic acid–treated SIRT1endo−/− mice exhibited microvascular rarefaction (Supplemental Figure 4), which was undetectable under basal conditions (data not shown). These data provide evidence of the exaggerated deterioration of renal function in SIRT1endo−/− mice acutely after injection of folic acid, quasi-recovery of renal function 1 month after acute injury despite the fact that kidneys at the time show unresolved injury, and accelerated progression of renal fibrosis and dysfunction 3 months postinjection.
Figure 2.
Acute and chronic effects of nephrotoxic injury. (A) Representative hematoxylin and eosin–stained kidney sections from 12-week-old SIRT1endo−/− knockout mice, SIRT1endo+/− heterozygote mice, and control mice treated with folic acid. Histomorphologic examination reveals more severe AKI (day 2) in SIRT1endo−/− compared with the other treated groups. Increased serum creatinine levels (B) and higher urinary albumin excretion (C) confirm a much more robust acute response (day 2) in SIRT1endo−/− knockout mice after folic acid injection than in SIRT1endo+/− heterozygote and control mice. Although both parameters have normalized at 28 days after injection of folic acid, SIRT1endo−/− animals show significantly increased serum creatinine levels and albuminuria compared with the other treated groups at 90 days after treatment. The animals in the treatment groups are injected with 250 mg/kg folic acid in 0.3 M NaHCO3 vehicle and euthanized after 2 days (AKI) or 28 or 90 days (chronic fibrotic phase). Untreated mice are injected with vehicle only. There are no significant differences in serum creatinine levels or albuminuria between the untreated groups; therefore, they are presented together as one single group of untreated animals. n=6–7 per group. Data are the mean ± SEM. *P<0.05 treated versus untreated animals; #P<0.05 SIRT1endo−/− versus other treated groups at the same time point. Original magnification, ×400.
Figure 3.
Exaggerated fibrotic response to the nephrotoxin in endothelial SIRT1-knockout mice. (A) Representative Masson’s trichrome staining of kidney sections demonstrate that at 28 days postinjection (when kidney function is laboratory unperturbed) and at 90 days (when it is perturbed), SIRT1endo−/− knockout mice show significantly more tubulointerstitial fibrosis than SIRT1endo+/− heterozygote and SIRT1Flox/Flox/SIRT1Flox/WT control animals. (B) Quantification of the fibrotic area. (C–E) Expression of collagen I and collagen III, as well as the expression ratio of short (S)/long (L) endoglin isoforms, examined by quantitative real-time PCR 90 days after folic acid treatment show significant increase in SIRT1endo−/− mice. The animals in treatment groups are injected with 250 mg/kg folic acid in 0.3 M NaHCO3 vehicle and euthanized after 28 or 90 days. Untreated mice are injected with vehicle only and euthanized after 90 days. n=6–7 per group. Data are the mean ± SEM. *P<0.05 treated versus untreated animals; #P<0.05 SIRT1endo−/− versus other treated groups after 90 days and SIRT1endo+/− versus SIRT1Flox/WT after 90 days. Original magnification, ×100.
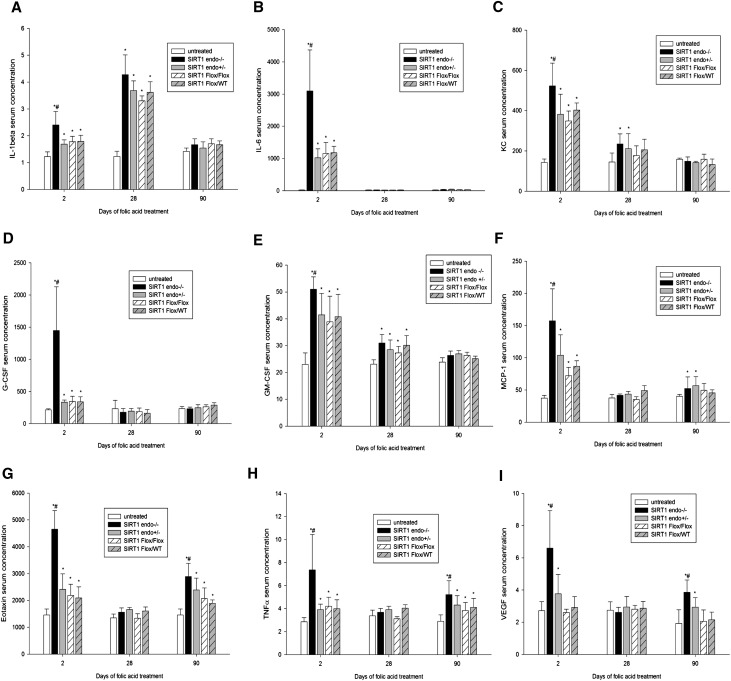
Studies of cytokine and chemokine responses to nephrotoxic stress in these mice revealed two distinct patterns. SIRT1endo−/− knockout mice demonstrated significantly higher levels of several cytokines/chemokines than heterozygote and control animals treated with folic acid (Figure 4). IL-1, IL-6, KC (the murine homolog of human IL-8), granulocyte colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor were predominantly increased in the acute phase, with gradual normalization of the levels at 3 months. The second group, encompassing monocyte chemotactic protein-1, eotaxin, TNF-α, and vascular endothelial growth factor showed a biphasic expression profile with an initial expression peak in the acute phase, followed by temporary normalization after 28 days, and a second peak occurring 3 months after folic acid injection. These data indicate the predominantly proinflammatory serum cytokine profile in SIRT1endo−/− mice during superimposed acute and chronic injury.
Figure 4.
Cytokine and chemokine serum concentrations (picograms per milliliter) in folic acid–treated SIRT1endo−/− knockout mice, SIRT1endo+/− heterozygote mice, and SIRT1Flox/Flox and SIRT1Flox/WT control mice. The following parameters are determined: (A) IL-1β, (B) IL-6, (C) KC, (D) G-CSF, (E) GM-CSF, (F) MCP-1, (G) eotaxin, (H) TNF-α, and (I) VEGF. The animals in treatment groups are injected with 250 mg/kg folic acid in 0.3 M NaHCO3 vehicle and euthanized after 48 hours (AKI) or 28 or 90 days (chronic fibrotic phase). Untreated mice are injected with vehicle only. Cytokine and chemokine concentrations are measured with a Luminex IS100 analyzer and analyzed using appropriate curve-fitting software (Luminex 100IS software version 2.3). There are no significant differences between untreated groups; therefore, they are presented together as one single group of untreated animals. n=5. Data are the mean ± SEM. *P<0.05 treated versus untreated animals; #P<0.05 SIRT1endo−/− versus other treated groups at the same time point. KC, murine homolog of human IL-8; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; MCP-1, monocyte chemotactic protein-1; VEGF, vascular endothelial growth factor.
Downregulation of MMP-14 in SIRT1-Depleted Endothelial Cells
Although the above-mentioned functional manifestations of SIRT1 deficiency in endothelial cells were consistent with the known targets of this deacetylase, the observed exaggerated profibrotic response of SIRT1endo−/− mice did not appear to have a clear molecular explanation. Previous microarray findings of reduced MMP-14 mRNA abundance in cultured endothelial cells subjected to SIRT1 inhibition could represent the potential cause of the exaggerated fibrotic responses in SIRT1endo−/− mice.18 To confirm that similar MMP-14 depletion occurs in SIRT1endo−/− mice, we used immunohistochemistry to examine en face aortic preparations for the expression of the enzyme. SIRT1endo−/− mice exhibited a 5-fold decrease in MMP-14 staining intensity of en face aortic endothelium compared with control animals (Supplemental Figure 5A). Therefore, we further examined expression of MMP-14 protein in cultured endothelial cells exposed to a selective SIRT1 inhibitor. The data presented in Figure 5A demonstrate that inhibition of SIRT1 resulted in a profound suppression of MMP-14 expression. To test whether this defect could lead to impaired matrilytic activity, human umbilical vein endothelial cells (HUVECs) were cultured in 3D matrigel and stained with Coomassie blue. The broad “halo” of degraded matrigel, a functional manifestation of MMP enzymatic activity and consistently seen around intact individual HUVECs (Figure 5B), exhibited retraction or total disappearance in cells treated with SIRT1 inhibitor III. Zymography analyses confirmed the decline in MMP-2 activity (pro–MMP-2 is activated by MMP-14) after inhibition of SIRT1 (Supplemental Figure 5B). Moreover, primary renal endothelial cells isolated (as detailed in Supplemental Figure 6) from kidneys of SIRT1endo−/− mice showed a significant reduction of matrilytic activity (Supplemental Figure 5C). These findings collectively confirm the fact that MMP-14 is a novel target for SIRT1 and the repression of this deacetylase leads to reduced expression and activity of MMP-14.
Figure 5.
MMP-14 is a downstream target of endothelial SIRT1 inhibition. (A) Representative immunoblot using protein lysates isolated from primary HUVECs treated with 50 µM of a specific SIRT1 inhibitor (SIRT1 inhibitor III) for 48 hours. The inhibition of SIRT1 results in a profound decrease in MMP-14 expression levels. HUVECs cultured without addition of SIRT1 inhibitor served as a control. Cell lysates are separated by SDS-PAGE and analyzed by immunoblot using appropriate antibodies. β-Tubulin serves as a loading control. n=5. (B) A broad bright “halo” of proteolytically degraded matrigel, a functional manifestation of MMP enzymatic activity, is consistently seen around individual cultured HUVECs. When cells are cultured in the presence of different concentrations of SIRT1 inhibitor, the halo phenomenon exhibits dose-dependent attenuation or total disappearance. The lower panel depicts the results of line-scan analysis of halo density, whereas the right panels summarize quantitative analyses of capillary length, halo intensity, and integrated intensity. n=30 cells per group. Data are the mean ± SEM. *P<0.05.
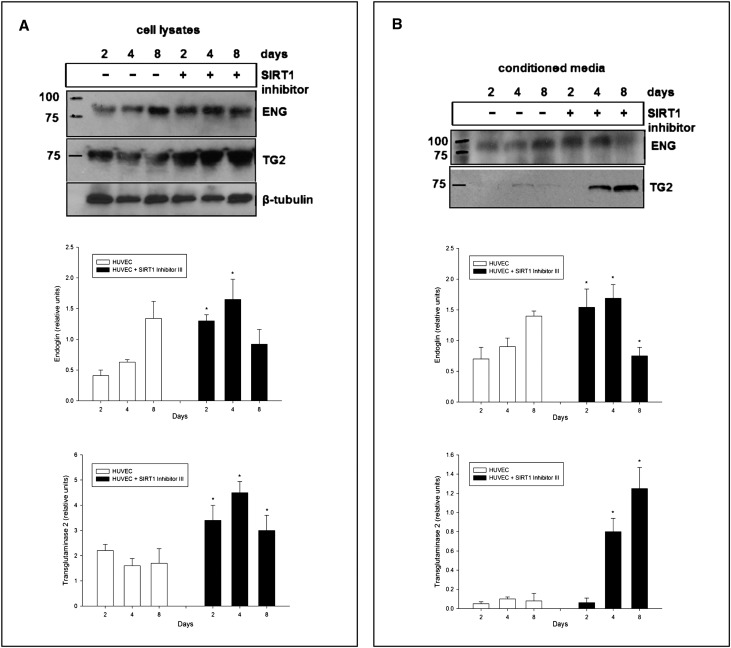
We next examined other putative targets of MMP-14, endoglin and tissue transglutaminase (both normally cleaved by MMP-14), overexpression of both potentially contributing to profibrotic program via type II TGF-β receptor and matrix proteins cross-linking, respectively.22–24 Inhibition of SIRT1 in HUVECs resulted in accumulation of endoglin on days 2 and 4 (but not at 8 days) of treatment (Figure 6A). Unexpectedly, the same direction of changes was observed in the culture medium (Figure 6B). This is most likely due to the enhanced production of a short endoglin (as shown in Figure 3), an antiangiogenic splice variant.23,25 Similarly, expression of cell-associated tissue transglutaminase was elevated in cells treated with SIRT1 inhibitor. Collectively, these findings confirm that MMP-14 represents a novel target for SIRT1 and that depletion of the latter reduces MMP-14 expression with the resulting loss of proteolytic matrix-degrading properties of endothelial cells and subsequently decreased cleavage of endoglin and tissue transglutaminase. For that reason, we sought to establish a model of in vivo induction of MMP-14 concomitant with SIRT1 inhibition, as detailed below.
Figure 6.
Expression of endoglin (ENG) and tissue transglutaminase (TG2) in primary HUVECs treated with 50 µM of specific SIRT1 inhibitor (SIRT1 inhibitor III) for 2, 4, and 8 days. (A) Treatment with SIRT1 inhibitor results in accumulation of endoglin on days 2 and 4 (but not at day 8) compared with untreated control cells. Similarly, expression of tissue transglutaminase is elevated in cells treated with the SIRT1 inhibitor. HUVECs cultured in medium without the addition of the inhibitor serve as a control. The expression of ENG and TG2 is examined in cell lysates separated by SDS-PAGE and analyzed by immunoblot using appropriate antibodies. β-Tubulin serves as a loading control. n=5. (B) Treatment with the SIRT1 inhibitor results in increased levels of endoglin on days 2 and 4 (but not at day 8) in culture media compared with untreated control cells. The concentration of transglutaminase is elevated in media from cells treated with SIRT1 inhibitor on days 4 and 8 (but not at day 2). HUVECs cultured in medium without the addition of SIRT1 inhibitor serve as a control. The expression of ENG and TG2 is examined in conditioned culture supernatants and analyzed by immunoblot using appropriate antibodies. n=5. Data are the mean ± SEM. *P<0.05.
Concanavalin A Induction of MMP-14 in Mice with Pharmacologic Inhibition of SIRT1
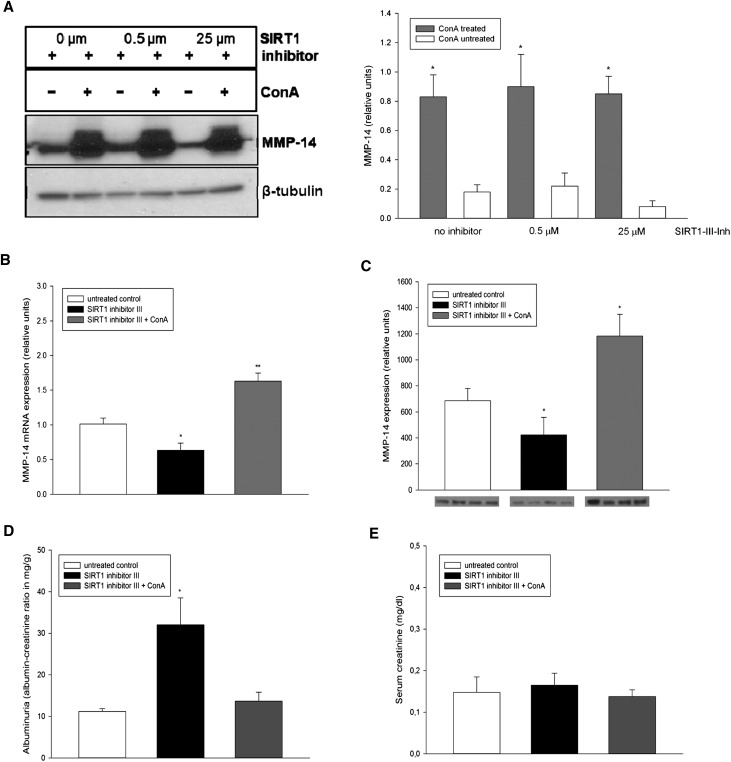
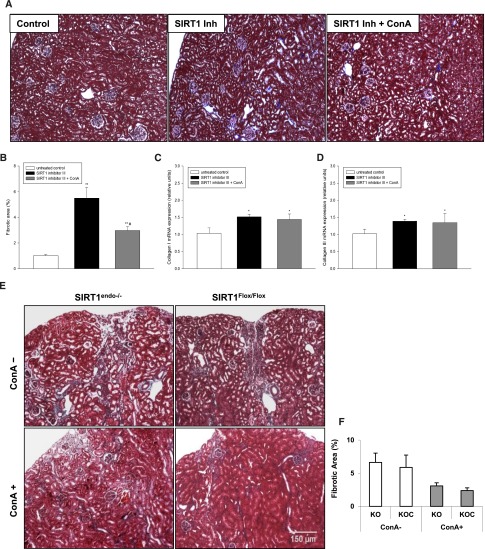
Concanavalin A (ConA) was previously shown to induce MMP-14.26,27 We confirmed this activity of ConA in vitro by pretreating HUVECs overnight with different concentrations of ConA, which consistently resulted in increased expression of MMP-14 (Figure 7A). Importantly, ConA effect was independent on the expression of SIRT1, as it occurred in cells treated with SIRT1 inhibitor III. Therefore, we next treated wild-type B6;129 mice with a highly specific SIRT1 inhibitor III together with weekly intravenous injections of ConA and compared these mice with those receiving SIRT1 inhibitor III alone. Untreated animals served as controls. To validate the efficacy of this model, we performed quantitative real-time PCR and immunoblot analysis, which showed suppression of renal MMP-14 in the inhibitor-treated mice and its induction in ConA-cotreated animals (Figure 7, B and C). In these mice, ConA treatment resulted in the reduction of proteinuria with no changes in serum creatinine (Figure 7, D and E) and significant prevention of fibrosis, as demonstrated by Masson’s trichrome staining (Figure 8, A and B). The mRNA synthesis of collagen I and III did not differ between SIRT1 inhibitor III–treated and SIRT1 inhibitor III/ConA–treated mice (Figure 8, C and D), emphasizing the fact that ConA does not directly affect synthesis of extracellular matrix (ECM), but rather stimulates the cleavage of the ECM excess through induction of MMP-14. We next sought to determine whether the antifibrotic effect of ConA is preserved in mice with endothelial cell–selective deletion of SIRT1, rather than after its global inhibition. Despite the fact that SIRT1endo−/− mice are more brittle than their heterozygote and control counterparts, we attempted to examine the effects of ConA in these animals after folic acid administration, albeit after a shortened follow-up period to avoid the loss of mice. Four weeks after administration of folic acid, SIRT1endo−/− and SIRT1Flox/Flox mice exhibited increased tubulointerstitial fibrosis (Figure 8E, compare with Figure 3 depicting the same time course). Weekly intravenous injections of ConA starting 1 week after induction with folic acid resulted in a significant reduction in tubulointerstitial fibrosis. These findings further substantiate the pathogenetic role of suppressed MMP-14 expression occurring in mice with the targeted endothelial deletion of SIRT1 in the development of exaggerated fibrotic response to the nephrotoxic agent. Moreover, the angiogenic sprouting (Figure 9, A and B) and matrilytic activity of aortic rings (Figure 9, C and D) cultured in 3D matrigel, both suppressed in mice receiving SIRT1 inhibitor alone, showed a dramatic restoration in ConA-treated animals. These data confirm the pathogenetic role of suppressed MMP-14 in exaggerated fibrotic response of SIRT1endo−/− mice.
Figure 7.
ConA as a booster of MMP-14 expression in vitro and in vivo. (A) Expression of MMP-14 in primary HUVECs treated with ConA in the presence of different concentrations of a specific SIRT1 inhibitor (SIRT1 inhibitor III) for 16 hours. Treatment with ConA increases MMP-14 expression independent of SIRT1 activity. HUVECs cultured without addition of ConA serve as a control. The expression of MMP-14 is examined in cell lysates by immunoblot using appropriate antibodies. β-tubulin serves as a loading control. n=5. (B and C) Systemic treatment of mice with SIRT1 inhibitor represses mRNA and protein levels of MMP-14, whereas coadministration of ConA induces the expression of MMP-14. (D) Experimental animals treated with SIRT1 inhibitor show increased albuminuria (expressed as the albumin to creatinine ratio) that is prevented by cotreatment with ConA. (E) There are no differences in serum creatinine levels between the groups. Twelve-week-old mice are fed a selective SIRT1 inhibitor at a daily dose of 1 mg/kg body weight for 4 weeks and treated with additional weekly injections of placebo or ConA at a dose of 5 mg/kg body weight. Mice with no treatment served as the control. n=5–6 per group. Data are the mean ± SEM. *P<0.05.
Figure 8.
Fibrotic markers in the kidneys of mice treated either with a specific SIRT1 inhibitor alone or in combination with ConA. (A) Representative Masson’s trichrome staining of kidney sections from mice treated either with SIRT1 inhibitor alone or in a combination with ConA. (B) Quantification of the fibrotic area (grid method). (C and D) Expression of collagen I and collagen III examined by real-time PCR. The animals are fed a specific SIRT1 inhibitor (SIRT1 inhibitor III) at a daily dose of 1 mg/kg body weight for 4 weeks, and the combination group receives additional weekly ConA injections at a dose of 5 mg/kg body weight. Mice with no treatment serve as the control. Data are the mean ± SEM. *P<0.05; *P<0.01; #combination group versus SIRT1 inhibitor alone; n=6 per group. (E) Representative Masson’s trichrome staining of kidney sections from SIRT1endo−/− and SIRT1Flox/Flox mice treated with either folic acid alone or in combination with ConA. (F) Quantification of the fibrotic area. Mice are injected with folic acid for 4 weeks. ConA treatment starts 1 week after folic acid injection. Data are the mean ± SEM. *P<0.05; n=5 for SIRT1endo−/−, SIRT1endo−/− with ConA, and SIRT1Flox/Flox with ConA; n=7 for SIRT1Flox/Flox. Original magnification, ×100.
Figure 9.
Functional confirmation of improved angiogenic and matrilytic activity of the vasculature obtained from mice receiving SIRT1 inhibitor in combination with ConA. (A and B) Aortic rings embedded in matrigel give a much more robust rise of capillary sprouting in mice cotreated with ConA. The left panels represent images of embedded rings on days 3 and 9 (squared areas are magnified), whereas the right panel shows quantitative analysis of capillary sprouting. (C and D) Matrilytic activity of aortic rings embedded in 3D matrigel. Representative images stained with Coomassie blue and line-scan analysis of stain intensity. (D) Quantitative analysis of results is presented as the “halo” intensity and integrated intensity. The animals are fed a specific SIRT1 inhibitor (SIRT1 inhibitor III) at a daily dose of 1 mg/kg body weight for 4 weeks, and the combination group receives additional weekly injections of ConA at a dose of 5 mg/kg body weight. Mice with no treatment serve as the control. n=5–6 per group. Data are the mean ± SEM. *P<0.05.
Discussion
Endothelial SIRT1 depletion or its inactivation is a frequent companion of many cardiovascular and renal diseases.14 The multitude of risk factors and pathogenetic mechanisms, however, obscures the actual contribution of SIRT1 depletion to the disease progression. Here, we utilized a murine model of genetic deletion of endothelial SIRT1 to demonstrate the development of vasculopathy and premature senescence with patchy tubulointerstitial fibrosis at an early age. Furthermore, imposition of a noxious stimulus unmasked the propensity of these animals to exaggerated fibrotic response, at least in part due to the suppressed expression of the master matrix metalloproteinase, MMP-14. By overcoming MMP-14 deficiency, it has become possible to curtail exaggerated fibrotic response and reduce proteinuria in these animals.
Current understanding of the role of SIRT1 in renal physiology and pathogenesis of renal diseases is limited, as was recently summarized.28 SIRT1 is highly expressed in endothelial cells, where it regulates numerous functions, including nitric oxide synthase, cell senescence, and autophagy, to name a few.29 This spectrum of functions would explain the association of endothelial SIRT1 deletion with impaired vasoreactivity and increased numbers of senescent endothelial cells. However, these known SIRT1 functions alone do not explain the profibrotic phenotype of mice deficient in endothelial SIRT1. The finding that the SIRT1 deficiency leads to suppression of MMP-14 is critical in explaining this phenomenon.
Since its discovery in 1994,30 MMP-14 (also known as membrane type-1 metalloproteinase [MT1-MMP]) has been recognized as a major proteinase in ECM remodeling through degradation of collagens I–III, laminin-1 and laminin-5, gelatin, fibronectin, vitronectin, aggrecan, fibrin, and lumican,31 as well as activation of pro–MMP-2.32 MMP-14 knockout mice are characterized by the loss of collagenolytic activity and exhibit increased mortality by 2 months of age and generalized fibrosis due to inadequate collagen turnover.33 Downregulation of MMP-14 expression, examined by cDNA microarray analysis, has been reported in endothelial cells treated with SIRT1 inhibitors.18 In the light of the above findings, the accelerated nephrosclerosis and exaggerated fibrotic response to nephrotoxic insult in mice with endothelial SIRT1 deletion obtain potential mechanistic significance.
Additional evidence of MMP-14 repression comes from the observed changes of its cleavage substrates. The level of endoglin, an extracellular domain of TGF-β auxiliary receptor on endothelial cells that is cleaved by MMP-14 between glycine-leucine residues 586–587, is increased in mice with endothelial deletion of SIRT1.34 A higher ratio of short/long endoglin isoforms represents a marker of cell senescence,35 and its increase in mice with endothelial deletion of SIRT1 is consistent with the numerous senescent cells observed in en face aortic preparations as well as the increased proportion of another marker of senescence, condensed H3K9Me3, in renal microvasculature of these mice. Tissue transglutaminase, another target of MMP-14–mediated cleavage at His461Leu, Arg458Ala, and Pro375Val,24 promotes deposition of fibronectin and cross-linking of fibronectin, collagen, vitronectin, and osteopontin,36 enhances cell-matrix adhesion, and participates in wound-healing response. However, when overexpressed, it contributes to tissue scarring.36,37 Hence, our observation of cellular accumulation of tissue transglutaminase is consistent with the profibrotic phenotype of SIRT1endo−/− mice.
Recent identification of MMP-14 as a key component of regenerative processes further enhances the significance of observations made in SIRT1endo−/− mice. MMP-14 cleaves heparin-binding EGF,38 rendering it heparin independent. In addition, the angiocrine signals generated by endothelial cells and initiated by MMP-14, which generates laminin-5 fragments serving as EGF receptor ligands, are essential for regeneration of alveolar epithelium after unilateral pneumonectomy.16 It is possible that the lack of this mechanism is involved in the observed functional inferiority of endothelial SIRT1 knockout mice after exposure to the noxious stimulus. In addition, this observation can be explained by the proinflammatory profile of SIRT1endo−/− mice. One of the protective mechanisms of SIRT1 action is associated with its anti-inflammatory properties. SIRT1 acts as a negative regulator of nuclear factor-κB activity through the deacetylation of the p65 subunit at lysine 310 and the nuclear factor-κB–SIRT1 negative loop has been described in several experimental models, confirming its biologic relevance.39 This mechanism may be responsible for the observed renal dysfunction in the acute phase of nephrotoxic insult. Another mechanism of MMP-14 deficiency contributing to the profibrotic phenotype and impaired regeneration is represented by the cellular accumulation of tissue transglutaminase and elevated short/long endoglin, a marker of enhanced senescence, as shown in Figures 3 and 6. It is revealing, in this vein, that a fast-regenerating mouse strain, the MRL mouse, is characterized by the increased expression of MMP-14,40 whereas the opposite occurs in endothelial SIRT1-deficient mice.
Defective SIRT1 function has emerged as a hallmark of diverse diseases such as the metabolic syndrome, cardiovascular and renal abnormalities, and neurodegenerative disorders, to mention a few.41 All of these conditions are associated with an increased accumulation of matrix proteins in affected organs,42 thus further compromising their function. In the vasculature, SIRT1 deficiency impedes angiogenesis.43,44 All of these abnormalities could be explained at least in part by the repressed expression and function of MMP-14, which leads to impaired degradation of matrix proteins, and the reduced ability of endothelial cells to navigate within the interstitial matrix and restore metabolism and regeneration of the affected organ.45,46
The fact that MMP-14 is incriminated in the observed profibrotic phenotype of mice with endothelial SIRT1 deficiency is further buttressed by the observations that enhancing MMP-14 expression by SIRT1-independent means reduces folic acid–induced tubulointerstitial fibrosis in mice treated with SIRT1 inhibitor and in SIRT1endo−/− mice. Sirtuin deficiency is also a common finding in aged animals, as is fibrosis of different organs.47,48 The data presented herein perhaps could be extrapolated to explain the mechanisms of widespread fibrosis seen in aging and the strategies to overcome it.
In conclusion, these data provide a mechanistic molecular link between SIRT1 depletion causing downregulation of MMP-14 and development of the profibrotic phenotype. The latter can be reversed by the repletion of MMP-14. Remarkably, the observed defects in organ function are initiated by endothelial-selective SIRT1 deletion and premature senescence of the endothelium, further emphasizing the primacy of microvascular supply in tissue homeostasis and wound repair.
Concise Methods
Mice with Endothelial SIRT1 Deletion
The endothelial SIRT1-deleted mouse model was created by cross-breeding B6;129-Sirt1tm1Ygu/J with Tie2-Cre transgenic mice expressing cre-recombinase in vascular endothelial cells.49,50 The resulting SIRT1endo+/− mice were mated with SIRT1Flox/Flox mice to obtain endothelial-deleted SIRT1endo−/− mutant mice. Animal protocols were conducted in accordance with the National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee. Male mice aged between 12 and 16 weeks were used in this study.
Cell Culture
Primary HUVECs were maintained in endothelial basal medium-2 (Lonza) supplemented with 2% FBS and growth factors, under conditions of 37°C and 5% CO2. Primary cultures of renal endothelial cells from SIRT1endo−/− mice were isolated using magnetic cell separation and maintained cultured in Iscove’s modified Dulbecco’s medium as detailed in the Supplemental Methods.
Acetylcholine-Induced Vasorelaxation of Aortic Rings
Thoracic aortas were divided into cylindrical segments, mounted on a wire myograph, and bathed in Krebs buffer gassed with 95% O2/5% CO2, for recording of isometric tension.51 The vessels were preconstricted with phenylephrine to 70% of maximal response and used for assessment of acetylcholine-induced vasorelaxation.
Staining of En Face Aortae for Senescence-Associated β-Galactosidase
Senescence-associated β-galactosidase staining of aortas was performed as previously described.1,52 Detection of H3K9Me3 is detailed in the Supplemental Methods.
Aortic Outgrowth Assay
Thoracic aortic rings cross-sectioned with 1-mm intervals were embedded in 3D matrigel in 96-well plates and cultured at 37°C. Newly formed capillary cords in explant cultures were imaged every 24 hours. Quantitative angiogenesis assays were performed according to a previously published protocol.53,54
MMP-14 Expression and Activity in Endothelial Cells
HUVECs were treated with 50 μM SIRT1 inhibitor III for 48 hours. MMP-14 protein expression was examined by immunoblotting. To analyze the in vitro matrix-degrading activity of endothelial MMP and its dependence on SIRT1, HUVECs were cultured in 3D matrigel for 2 weeks with or without the addition of SIRT1 inhibitor III. Cells were fixed with 4% paraformaldehyde, stained with Coomassie Brilliant blue, and destained in acetic acid/methanol solution. Five random images were obtained in each group, and the length of the formed capillary structures, as well as the integrated intensity and intensity (along the line-scan) of the “halo” surrounding each cell, were analyzed using MetaVue software. Activity of MMP-14 was detected zymographically, as detailed in the Supplemental Methods.
Folate Model of AKI and Chronic Progressive Nephropathy
A single intraperitoneal injection of 250 mg/kg body weight folic acid in 0.3 M NaHCO3 vehicle or vehicle alone was administered. The animals were euthanized after 48 hours (AKI phase) or 28 days or 90 days (chronic fibrotic phase). Kidney sections were fixed in 4% paraformaldehyde and embedded in paraffin. Paraffin sections were stained with Masson’s trichrome and quantified using with ImageJ software. The spontaneous patchy fibrosis in untreated mice were assessed under high magnification (×600) using the grid method.
Measurement of Cytokine and Chemokine Levels
Profiling of cytokines and chemokines was accomplished using multiplex analysis of the plasma obtained from experimental animals using Luminex 2 IS100 automated system.
In Vivo Induction of MMP-14 with ConA
Prolonged SIRT1 inhibition in mice was achieved by administration of selective SIRT1 inhibitor III added to drinking water for 4 weeks. Animals were treated with SIRT1 inhibitor with or without additional intravenous injections of ConA. The dose used in our study has been shown to be safe and no signs of hepatic, hematologic, or any overt toxicity were reported.55 The interval between treatments was chosen based on the preliminary studies utilizing fluorescently labeled ConA, which was retained in the renal microvasculature for at least 4 days. In the assessment of aortic matrilytic activity, aortic rings were cultured in 3D matrigel for up to 9 days. After sacrificing the animals, all rings were incubated under the same conditions, and the treatment with SIRT1 inhibitor or ConA was not continued during the ex vivo incubations.
Histologic Studies
The kidneys were perfused in situ with cold PBS and removed. Midcoronal sections were stained with hematoxylin and eosin and an average of three sections per animal were examined. The morphologic evaluation of folic acid–induced AKI was performed using well established criteria in a blind manner.56,57
Statistical Analyses
All experiments were repeated at least three times. Values are given as the mean ± SEM. Data were analyzed using ANOVA with post hoc analysis for multiple-group comparisons using the Bonferroni method. A P value <0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
These studies were supported in part by the grants from the Dr. Werner Jackstaedt Foundation (to R.V.), the American Heart Association (12SDG9080006 to B.B.R.), the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (DK54602, DK052783, DK45462), and the Westchester Artificial Kidney Foundation (to M.S.G.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013010069/-/DCSupplemental.
References
- 1.Brodsky SV, Gealekman O, Chen J, Zhang F, Togashi N, Crabtree M, Gross SS, Nasjletti A, Goligorsky MS: Prevention and reversal of premature endothelial cell senescence and vasculopathy in obesity-induced diabetes by ebselen. Circ Res 94: 377–384, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Huang X, Halicka D, Brodsky S, Avram A, Eskander J, Bloomgarden NA, Darzynkiewicz Z, Goligorsky MS: Contribution of p16INK4a and p21CIP1 pathways to induction of premature senescence of human endothelial cells: Permissive role of p53. Am J Physiol Heart Circ Physiol 290: H1575–H1586, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Patschan S, Chen J, Gealekman O, Krupincza K, Wang M, Shu L, Shayman JA, Goligorsky MS: Mapping mechanisms and charting the time course of premature cell senescence and apoptosis: Lysosomal dysfunction and ganglioside accumulation in endothelial cells. Am J Physiol Renal Physiol 294: F100–F109, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Patschan S, Chen J, Polotskaia A, Mendelev N, Cheng J, Patschan D, Goligorsky MS: Lipid mediators of autophagy in stress-induced premature senescence of endothelial cells. Am J Physiol Heart Circ Physiol 294: H1119–H1129, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Stoessel A, Paliege A, Theilig F, Addabbo F, Ratliff B, Waschke J, Patschan D, Goligorsky MS, Bachmann S: Indolent course of tubulointerstitial disease in a mouse model of subpressor, low-dose nitric oxide synthase inhibition. Am J Physiol Renal Physiol 295: F717–F725, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Addabbo F, Ratliff B, Park HC, Kuo MC, Ungvari Z, Csiszar A, Krasnikov B, Sodhi K, Zhang F, Nasjletti A, Goligorsky MS: The Krebs cycle and mitochondrial mass are early victims of endothelial dysfunction: Proteomic approach. Am J Pathol 174: 34–43, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Li H, Addabbo F, Zhang F, Pelger E, Patschan D, Park HC, Kuo MC, Ni J, Gobe G, Chander PN, Nasjletti A, Goligorsky MS: Adoptive transfer of syngeneic bone marrow-derived cells in mice with obesity-induced diabetes: Selenoorganic antioxidant ebselen restores stem cell competence. Am J Pathol 174: 701–711, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goligorsky MS, Chen J, Patschan S: Stress-induced premature senescence of endothelial cells: A perilous state between recovery and point of no return. Curr Opin Hematol 16: 215–219, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Goligorsky MS: Microvascular rarefaction: The decline and fall of blood vessels. Organogenesis 6: 1–10, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasuda K, Park HC, Ratliff B, Addabbo F, Hatzopoulos AK, Chander P, Goligorsky MS: Adriamycin nephropathy: A failure of endothelial progenitor cell-induced repair. Am J Pathol 176: 1685–1695, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hohenstein B, Kuo MC, Addabbo F, Yasuda K, Ratliff B, Schwarzenberger C, Eckardt KU, Hugo CP, Goligorsky MS: Enhanced progenitor cell recruitment and endothelial repair after selective endothelial injury of the mouse kidney. Am J Physiol Renal Physiol 298: F1504–F1514, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bohle A, Mackensen-Haen S, Wehrmann M: Significance of postglomerular capillaries in the pathogenesis of chronic renal failure. Kidney Blood Press Res 19: 191–195, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Kang DH, Joly AH, Oh SW, Hugo C, Kerjaschki D, Gordon KL, Mazzali M, Jefferson JA, Hughes J, Madsen KM, Schreiner GF, Johnson RJ: Impaired angiogenesis in the remnant kidney model: I. Potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol 12: 1434–1447, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Xavier S, Moskowitz-Kassai E, Chen R, Lu CY, Sanduski K, Špes A, Turk B, Goligorsky MS: Cathepsin cleavage of sirtuin 1 in endothelial progenitor cells mediates stress-induced premature senescence. Am J Pathol 180: 973–983, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM: Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479: 232–236, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding BS, Nolan DJ, Guo P, Babazadeh AO, Cao Z, Rosenwaks Z, Crystal RG, Simons M, Sato TN, Worgall S, Shido K, Rabbany SY, Rafii S: Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell 147: 539–553, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Wang X, Xie G, Wang L, Hill CK, DeLeve LD: Liver sinusoidal endothelial cell progenitor cells promote liver regeneration in rats. J Clin Invest 122: 1567–1573, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, Zeiher AM, Dimmeler S: SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev 21: 2644–2658, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabelink TJ, Wijewickrama DC, de Koning EJ: Peritubular endothelium: The Achilles heel of the kidney? Kidney Int 72: 926–930, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Fine LG, Norman JT: Chronic hypoxia as a mechanism of progression of chronic kidney diseases: From hypothesis to novel therapeutics. Kidney Int 74: 867–872, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Koziolek MJ, Muller GA, Zapf A, Patschan D, Schmid H, Cohen CD, Koschnick S, Vasko R, Bramlage C, Strutz F: Role of CX3C-chemokine CX3C-L/fractalkine expression in a model of slowly progressive renal failure. Nephrol Dial Transplant 25: 684–698, 2010 [DOI] [PubMed]
- 22.Jones RA, Kotsakis P, Johnson TS, Chau DY, Ali S, Melino G, Griffin M: Matrix changes induced by transglutaminase 2 lead to inhibition of angiogenesis and tumor growth. Cell Death Differ 13: 1442–1453, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Mahmoud M, Upton PD, Arthur HM: Angiogenesis regulation by TGFβ signalling: Clues from an inherited vascular disease. Biochem Soc Trans 39: 1659–1666, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Belkin AM, Akimov SS, Zaritskaya LS, Ratnikov BI, Deryugina EI, Strongin AY: Matrix-dependent proteolysis of surface transglutaminase by membrane-type metalloproteinase regulates cancer cell adhesion and locomotion. J Biol Chem 276: 18415–18422, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Pérez-Gómez E, Eleno N, López-Novoa JM, Ramirez JR, Velasco B, Letarte M, Bernabéu C, Quintanilla M: Characterization of murine S-endoglin isoform and its effects on tumor development. Oncogene 24: 4450–4461, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Domoto T, Takino T, Guo L, Sato H: Cleavage of hepatocyte growth factor activator inhibitor-1 by membrane-type MMP-1 activates matriptase. Cancer Sci 103: 448–454, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zucker S, Hymowitz M, Conner C, DeClerck Y, Cao J: TIMP-2 is released as an intact molecule following binding to MT1-MMP on the cell surface. Exp Cell Res 293: 164–174, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Hao CM, Haase VH: Sirtuins and their relevance to the kidney. J Am Soc Nephrol 21: 1620–1627, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borradaile NM, Pickering JG: NAD(+), sirtuins, and cardiovascular disease. Curr Pharm Des 15: 110–117, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M: A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 370: 61–65, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Itoh Y, Seiki M: MT1-MMP: A potent modifier of pericellular microenvironment. J Cell Physiol 206: 1–8, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI: Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem 270: 5331–5338, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H: MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 99: 81–92, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Hawinkels LJ, Kuiper P, Wiercinska E, Verspaget HW, Liu Z, Pardali E, Sier CF, ten Dijke P: Matrix metalloproteinase-14 (MT1-MMP)-mediated endoglin shedding inhibits tumor angiogenesis. Cancer Res 70: 4141–4150, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Blanco FJ, Grande MT, Langa C, Oujo B, Velasco S, Rodriguez-Barbero A, Perez-Gomez E, Quintanilla M, López-Novoa JM, Bernabeu C: S-endoglin expression is induced in senescent endothelial cells and contributes to vascular pathology. Circ Res 103: 1383–1392, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Belkin AM: Extracellular TG2: Emerging functions and regulation. FEBS J 278: 4704–4716, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson TS, El-Koraie AF, Skill NJ, Baddour NM, El Nahas AM, Njloma M, Adam AG, Griffin M: Tissue transglutaminase and the progression of human renal scarring. J Am Soc Nephrol 14: 2052–2062, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Koshikawa N, Mizushima H, Minegishi T, Iwamoto R, Mekada E, Seiki M: Membrane type 1-matrix metalloproteinase cleaves off the NH2-terminal portion of heparin-binding epidermal growth factor and converts it into a heparin-independent growth factor. Cancer Res 70: 6093–6103, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW: Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23: 2369–2380, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tucker B, Klassen H, Yang L, Chen DF, Young MJ: Elevated MMP expression in the MRL mouse retina creates a permissive environment for retinal regeneration. Invest Ophthalmol Vis Sci 49: 1686–1695, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haigis MC, Sinclair DA: Mammalian sirtuins: Biological insights and disease relevance. Annu Rev Pathol 5: 253–295, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ronco P, Chatziantoniou C: Matrix metalloproteinases and matrix receptors in progression and reversal of kidney disease: Therapeutic perspectives. Kidney Int 74: 873–878, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Chun TH, Sabeh F, Ota I, Murphy H, McDonagh KT, Holmbeck K, Birkedal-Hansen H, Allen ED, Weiss SJ: MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J Cell Biol 167: 757–767, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guarani V, Potente M: SIRT1 - a metabolic sensor that controls blood vessel growth. Curr Opin Pharmacol 10: 139–145, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Oh J, Takahashi R, Kondo S, Mizoguchi A, Adachi E, Sasahara RM, Nishimura S, Imamura Y, Kitayama H, Alexander DB, Ide C, Horan TP, Arakawa T, Yoshida H, Nishikawa S, Itoh Y, Seiki M, Itohara S, Takahashi C, Noda M: The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell 107: 789–800, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Riggins KS, Mernaugh G, Su Y, Quaranta V, Koshikawa N, Seiki M, Pozzi A, Zent R: MT1-MMP-mediated basement membrane remodeling modulates renal development. Exp Cell Res 316: 2993–3005, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donmez G, Guarente L: Aging and disease: Connections to sirtuins. Aging Cell 9: 285–290, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Guarente L: Franklin H. Epstein Lecture: Sirtuins, aging, and medicine. N Engl J Med 364: 2235–2244, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF: Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A 100: 10794–10799, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M: Tie2-Cre transgenic mice: A new model for endothelial cell-lineage analysis in vivo. Dev Biol 230: 230–242, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Linder AE, Weber DS, Whitesall SE, D’Alecy LG, Webb RC: Altered vascular reactivity in mice made hypertensive by nitric oxide synthase inhibition. J Cardiovasc Pharmacol 46: 438–444, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J: A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 92: 9363–9367, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen J, Brodsky S, Li H, Hampel DJ, Miyata T, Weinstein T, Gafter U, Norman JT, Fine LG, Goligorsky MS: Delayed branching of endothelial capillary-like cords in glycated collagen I is mediated by early induction of PAI-1. Am J Physiol Renal Physiol 281: F71–F80, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Brodsky S, Chen J, Lee A, Akassoglou K, Norman J, Goligorsky MS: Plasmin-dependent and -independent effects of plasminogen activators and inhibitor-1 on ex vivo angiogenesis. Am J Physiol Heart Circ Physiol 281: H1784–H1792, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Ballerstadt R, Evans C, McNichols R, Gowda A: Concanavalin A for in vivo glucose sensing: A biotoxicity review. Biosens Bioelectron 22: 275–284, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Kelleher SP, Robinette JB, Miller F, Conger JD: Effect of hemorrhagic reduction in blood pressure on recovery from acute renal failure. Kidney Int 31: 725–730, 1987 [DOI] [PubMed] [Google Scholar]
- 57.Noiri E, Peresleni T, Miller F, Goligorsky MS: In vivo targeting of inducible NO synthase with oligodeoxynucleotides protects rat kidney against ischemia. J Clin Invest 97: 2377–2383, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.