Abstract
Necrotizing and crescentic GN (NCGN) with a paucity of glomerular immunoglobulin deposits is associated with ANCA. The most common ANCA target antigens are myeloperoxidase (MPO) and proteinase 3. In a manner that requires activation of the alternative complement pathway, passive transfer of antibodies to mouse MPO (anti-MPO) induces a mouse model of ANCA NCGN that closely mimics human disease. Here, we confirm the importance of C5aR/CD88 in the mediation of anti-MPO–induced NCGN and report that C6 is not required. We further demonstrate that deficiency of C5a-like receptor (C5L2) has the reverse effect of C5aR/CD88 deficiency and results in more severe disease, indicating that C5aR/CD88 engagement enhances inflammation and C5L2 engagement suppresses inflammation. Oral administration of CCX168, a small molecule antagonist of human C5aR/CD88, ameliorated anti-MPO–induced NCGN in mice expressing human C5aR/CD88. These observations suggest that blockade of C5aR/CD88 might have therapeutic benefit in patients with ANCA-associated vasculitis and GN.
Necrotizing and crescentic GN (NCGN) and vasculitis are associated with ANCA.1,2 ANCAs are specific for myeloperoxidase (MPO) and proteinase 3 (PR3).1 Experimental data indicate that the pathogenesis of ANCA-associated vasculitis (AAV) involves activation of neutrophils by ANCA.1,2 Injection of anti-MPO antibodies into mice causes NCGN and vasculitis, closely mimicking human AAV.3 Alternative complement pathway activation is pivotal in the pathogenesis of anti-MPO NCGN in mice.4–6 The relevance of alternative complement pathway activation to human AAV is supported by immunohistochemical demonstration of alternative complement pathway components at sites of AAV7,8 and by correlation of plasma alternative complement pathway activation fragments with AAV disease activity.9
The complement anaphylatoxin C5a is a potent inflammatory mediator.10,11 The alternative classic and lectin pathways converge at the activation of C5, releasing C5a and C5b. C5a is a powerful chemoattractant for neutrophils, and ligation by C5a of C5aR/CD88 activates neutrophils. Blockade of C5a or C5a receptor (C5aR/CD88) ameliorates anti-MPO NCGN in mice.5,6 ANCA-activated neutrophils activate the alternative complement pathway.4,6,12 Neutrophil priming results in increased availability of ANCA antigens at the surface where they interact with ANCA to activate neutrophils. Human neutrophils activated by human ANCA release factors that activate the alternative complement pathway.4,6,12 In turn, C5a primes neutrophils and increase ANCA antigen expression.6,12 Cleavage of C5 also releases C5b, which joins with C6 to initiate the membrane attack complex (MAC).11
Here we confirm the importance of C5aR/CD88 in mediating anti-MPO NCGN and report that C6 is not required. We also demonstrate that deficiency of another receptor for C5a, C5L2 (C5a-like receptor 2),10 results in more severe disease. This is in accord with earlier studies that have shown an anti-inflammatory effect of C5L2 engagement.10,13,14
Therapeutic implications were investigated using CCX168, an antagonist of human C5aR/CD88 that is undergoing phase 2 evaluation in patients with AAV (EU Clinical Trials Register ID: EUCTR2011–001222–15-GB). Oral administration of CCX168 to humanized mice with knocked-in human C5aR/CD88 ameliorated anti-MPO NCGN.
Results
C5aR/CD88 Deficiency Ameliorates, C5L2 Deficiency Exacerbates, and C6 Deficiency Has No Effect on Anti-MPO–Induced NCGN
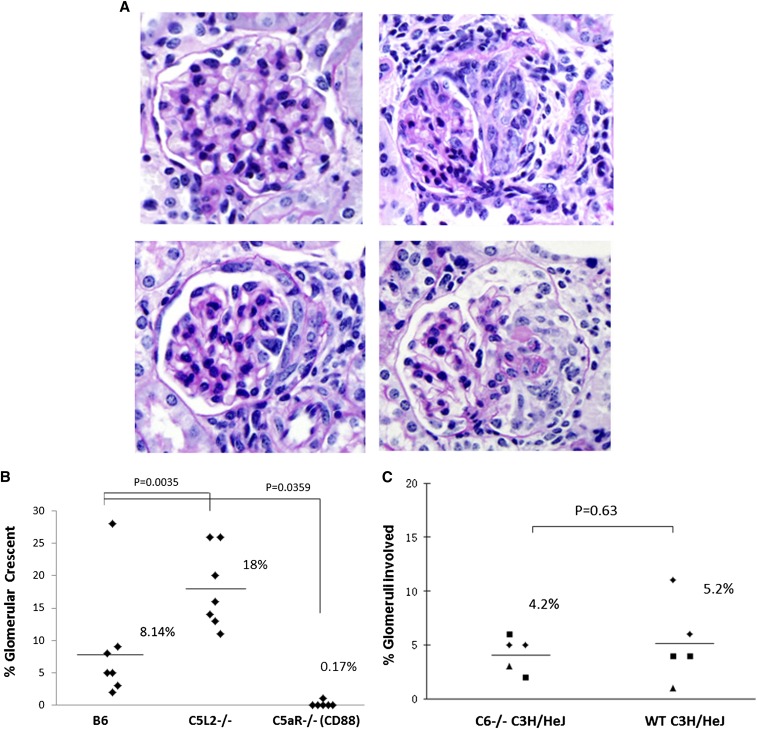
Injection of 50 µg/g mouse antimouse MPO IgG into wild-type (WT) B6 mice resulted in NCGN (Figure 1A) in all mice (n=7) with an average of 8.1% crescents (Figure 1B). B6 mice with knocked-out C5aR/CD88 were protected (P=0.0359), with only 1 of 6 mice developing 1% crescents (Figure 1B). In contrast, B6 mice with knockout of C5L2 (C5a-like receptor 2) had more severe disease (P=0.0035), with 18% crescents (Figure 1B). These observations support a proinflammatory function for C5aR/CD88 and an anti-inflammatory inhibitory function for C5L2, as previously reported.10,13
Figure 1.
C5aR (CD88) deficiency ameliorates, C5L2 deficiency enhances and C6 deficiency has no effect on anti-MPO–induced GN. WT B6, C5L2−/− B6, C5aR/CD88−/− B6, WT C3H/HeJ, and C6−/− C3H/HeJ were injected intravenously with 50 µg/g body weight mouse antimouse MPO IgG and euthanized on day 6. Glomerular crescents were calculated as percentage of glomeruli with crescents by counting all glomeruli in cross-sections of both kidneys, which averaged approximately 80 per cross-section. (A) In contrast to histologically unremarkable glomeruli (upper left, C5aR−/− mouse), glomeruli with crescents had increased extracapillary cells often with adjacent segmental fibrinoid necrosis (upper right, C5L2−/− mouse; lower left, C6−/− mouse; lower right, hC5aR mouse). (B) All WT B6 mice developed glomerular crescents with an average of 8.1% of glomeruli with crescents, B6 mice with knocked-out C5aR/CD88 were protected from induction of GN by anti-MPO IgG, with only 1 of 6 mice developing rare crescents, and B6 mice with knockout of C5L2 had more severe disease, with an average of 18% crescents per mouse (P=0.0035). (C) All WT C3H/HeJ mice and all five C6 knock out C3H/HeJ mice developed NCGN, with an average of 5.2% of glomeruli with crescents in WT mice and 4.4% in C6−/− mice (P=0.63). Thus, the absence of C6 did not influence pathogenicity of anti-MPO. Magnification, ×400, periodic acid-Schiff stain.
C5 activation is required to induce anti-MPO NCGN.5,6s C5 activation generates C5a and C5b. C5a mediates inflammation by attracting and activating neutrophils, whereas C5b joins with C6 to initiate MAC assembly.11 To investigate the pathogenic role of C6, NCGN induction by anti-MPO IgG in WT C3H/HeJ mice was compared with that in C6−/− C3H/HeJ mice. After injection of anti-MPO IgG, all WT C3H/HeJ mice and all C6−/− C3H/HeJ mice developed NCGN, with an average of 5.2% crescents in WT mice and 4.4% in C6−/− mice (P=0.63) (Figure 1C). Thus, C6 and MAC are not important for the pathogenesis of anti-MPO NCGN.
Mouse Anti-MPO IgG Induces NCGN in Mice with Knocked-In Human C5aR/CD88 and Knocked-Out Mouse C5aR/CD88
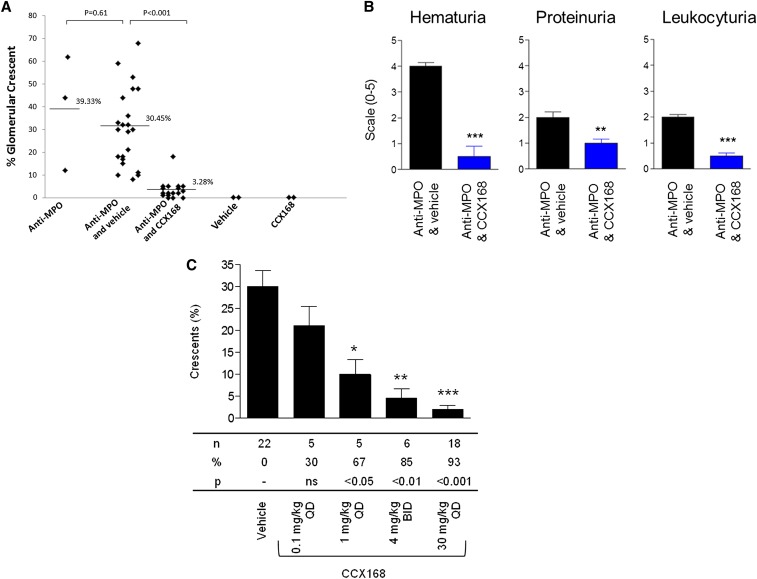
The role for C5aR/CD88 in anti-MPO NCGN in mice has implications for C5aR/CD88 blockade as therapy for AAV. We tested the ability of a small molecule antagonist of human C5aR/CD88 to ameliorate anti-MPO NCGN in mice with the murine C5a receptor knocked out and replaced with the human C5a receptor (hC5aR mice). Injection of mouse anti-MPO into hC5aR mice induced NCGN with an average of 30% crescents, which was similar to induction of disease by anti-MPO in WT littermates that developed 39% crescents (P=0.61) (Figure 2A). The hC5aR mice have a mixed genetic background of 129S6 and B6. The higher percentage of crescents in these mice compared with that in B6 mice is consistent with our observation that 129S6 mice are genetically predisposed to more severe disease and that crosses between 129S6 and B6 mice have intermediate severity.15
Figure 2.
C5aR/CD88 small molecule antagonist CCX168 ameliorates anti-MPO–induced GN. Daily administration of 30 mg/kg CCX168, an orally active small molecule antagonist of human C5aR, markedly reduced the severity of anti-MPO–induced NCGN in hC5aR knock-in mice (7 days after anti-MPO injection). (A) Glomerular crescent formation induced by anti-MPO was reduced from 30.4% with vehicle alone to 3.3% with CCX168 (P<0.0001). (B) Urine hematuria, proteinuria, and leukocyturia were reduced in mice receiving CCX168 (dipstick scale, 0–4+). (C) Glomerular crescent and necrosis formation was reduced in animals receiving CCX168. Data were analyzed by Mann-Whitney t test; **P<0.01 and ***P<0.001. Data are presented as the median with SEM. BID, twice daily; QD, daily.
Mouse Leukocytes with Knocked-In hC5aR/CD88 and Knocked-Out Mouse C5aR/CD88 Respond to an Antagonist of hC5aR/CD88
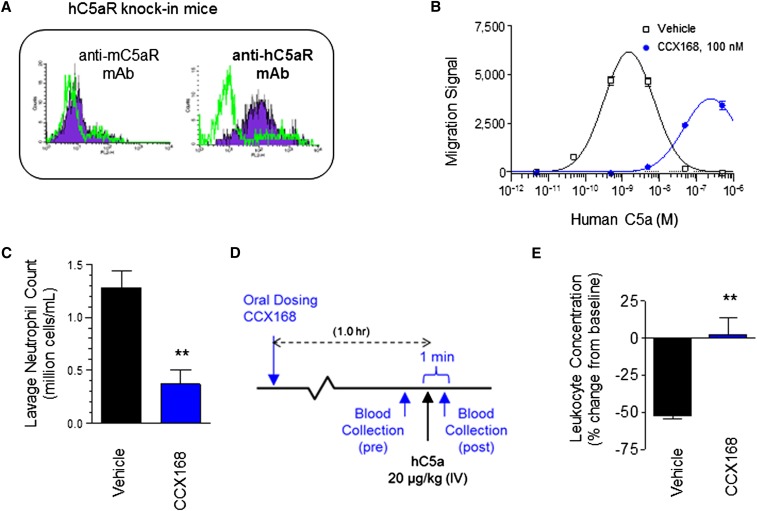
Flow cytometry demonstrated hC5aR but not mC5aR on hC5aR knock-in mice (Figure 3A). CCX168 markedly retarded chemotaxis of leukocytes from hC5aR mice (Figures 3B). Oral pretreatment of hC5aR mice with a single dose of CCX168 inhibited the exudation of peritoneal neutrophils in response to thioglycollate (Figure 3C) as well as transient depletion of blood neutrophils caused by intravenous C5a administration (Figure 3, E and F), thus documenting an inhibitory effect of CCX168 on leukocyte function in hC5aR mice.
Figure 3.
Leukocytes in mice with human C5aR knock-in and mouse C5aR knock-out express human C5aR and respond to human C5a, and CCX168 inhibits activation via human C5aR. (A) Mouse and human C5aR expression in isolated leukocytes from hC5aR knock-in mice. Flow cytometric leukocyte staining with antibodies specific for mouse or human C5aR is shown in blue with isotype controls (green line) shown for comparison. (B) Chemotaxis of hC5aR knock-in cells in response to a dose range of human C5a in the absence (square) or presence (circle) of CCX168 (100 nM) showing inhibition of chemotaxis by CCX168. Migration signal is a measure of cell numbers migrating between ChemoTX chambers based on intensity of fluorescence of a DNA-binding fluorescent marker. (C) Effects of oral pretreatment with vehicle or a single dose of CCX168 on cell count in the peritoneal lavage 24 hours after intraperitoneal thioglycollate injection. (D) Schematic of the C5a-induced leukopenia study in hC5aR knock-in mice. One hour after oral administration of CCX168, blood was drawn 1 minute before and 1 minute after intravenous (IV) administration of C5a (20 μg/kg); leukocyte concentrations were determined in these blood samples. (E) Following the study outline shown in panel D (n=4 mice/group), CCX168 inhibited the transient depletion of blood leukocytes caused by intravenous administration of C5a. Percentage change from baseline is shown (0, no change; 100, no leukocytes in blood). CCX168 was administered as an oral 30 mg/kg dose in these studies. **P<0.01 t test.
A Small Molecule Inhibitor of hC5aR/CD88 (CCX168) in Mice with hC5aR/CD88 Ameliorates Anti-MPO–Induced NCGN
Oral CCX168, 30 mg/kg daily, reduced the severity of anti-MPO NCGN in hC5aR mice. Glomerular crescents were reduced from 30.4% to 3.3% with CCX168 (P<0.0001) (Figure 2A). Urine hematuria, proteinuria, and leukocyturia were reduced in mice receiving CCX168, 30 mg/kg per day (Figure 2B). The protection by CCX168 resulted in reduced crescents (P<0.001) (Figure 2C) and necrosis (P<0.001). In control mice versus CCX168-treated mice, there were more glomeruli with neutrophils (33.2% versus 12.0%; P=0.007) and more neutrophils per glomerulus (1.2 versus 0.2; P=0.003). The amelioration of anti-MPO NCGN by CCX168 was dose dependent (Figure 2C). Thus, an antagonist of human C5aR/CD88 effectively ameliorates anti-MPO induced NCGN in mice with hC5aR/CD88.
Discussion
Acute inflammatory lesions of AAV are extremely destructive and characterized histologically by influx or neutrophils, leukocytoclasia, and necrosis.16,17 These lesions of AAV indicate that the pathogenic inflammatory mechanism involves intense recruitment and activation of leukocytes, especially neutrophils.
The complement system complements inflammation that has been initiated by many inflammatory stimuli. The alternative complement pathway is particularly effective at augmenting and sustaining acute inflammation because it has a self-fueling amplification loop.11
Observations in human AAV and in experimental animal models of AAV support an important role for the alternative complement pathway. The lesions of AAV are characterized by a paucity of immunoglobulin and complement, especially compared with typical immune complex or anti–glomerular basement membrane–mediated GN and vasculitis; however, low-intensity deposition of complement is common, especially at focal sites of inflammation and necrosis.7,8 Xing et al. detected factor B, properdin, MAC, and C3d in glomeruli and small blood vessels with active AAV, which suggested alternative pathway activation.7 Gigante et al. also detected complement components in AAV lesions and observed that the extent of lesional C3c correlated with poor renal outcome.8 In patients with AAV, Gou et al. reported increased plasma levels of C3a, C5a, soluble C5b-9, and Bb in patients with active disease but not remission.9 The plasma Bb correlated with percentage of crescents. Thus, data from tissue specimens and plasma samples support a role for alternative complement pathway activation in AAV.
Animal models that closely mimic human AAV are induced by circulating anti-MPO in mice.3,4 The alternative complement pathway is required for disease induction by anti-MPO.4 Blockade of C5a or C5a receptor (C5aR/CD88) ameliorates anti-MPO–induced NCGN.5,6 Interruption of the C5 axis with anti-C5 effectively ameliorates disease not only when given before but also when given 1 day after injection of anti-MPO.5 The current studies confirm the role of C5aR in mediating anti-MPO NCGN using C5aR-deficient mice as well as a small molecule antagonist of C5aR. Of note, mouse C5a is able to cross-react with human C5aR/CD88.
Deficiency of C5L2 had the opposite effect of C5aR/CD88 deficiency, indicating that C5aR/CD88 engagement enhances inflammation whereas C5L2 engagement suppresses inflammation, which has been proposed before.10,13,14 C5aR/CD88 is a G protein–coupled receptor, whereas C5L2 is structurally homologous but deficient in G protein coupling.10
C5 activation generates not only C5a but also C5b, which complexes with C6 to initiate the membrane attack complex (MAC) (C5b-9d).11 We examined the importance of the C6 in murine anti-MPO NCGN using C6-deficient mice and observed no effect. This C6-deficient strain has been used effectively in other mouse models in which the C6 deficiency ameliorated MAC-mediated injury.18–20 MAC may be present in lesions but is not required to induce injury.
The amelioration of anti-MPO NCGN in mice with humanized C5aR/CD88 not only provides additional support for the important pathogenic role for complement activation in AAV but also supports the possibility that therapy directed at preventing or reducing complement activation might be beneficial in patients with AAV.
Concise Methods
Mice
WT C57B6/6J (B6) and C3H/HeJ mice were obtained from The Jackson Laboratory (Bar Harbor, ME). C6-deficient C3H/HeJ mice (C3H/HeJ.CgC6Q0/Mmmh) and WT C3H/HeJ mice were purchased from Mutant Mouse Regional Resource Centers (Columbia, MO). C5aR−/−, C5L2−/−, and litter mate WT control mice were provided by Dr. Craig Gerard (Harvard Medical School, Boston, MA). MPO−/− mice were maintained by the University of North Carolina Division of Laboratory Animal Medicine. MPO−/− mice (8–10 weeks old) were used for immunization and as donors of anti-MPO antibodies using previously published methods.2 Animal care and animal experiments were conducted in accordance with the Animal Care Committee at the University of North Carolina, Chapel Hill, and National Institutes of Health Guide for Care and Use of Laboratory Animals in Research.
Humanization of Mice with Human C5aR
Standard homologous recombination techniques were used to create mice with the murine C5a receptor replaced with the human C5a receptor. These mice had a mixed genetic background of 129S6 and C57BL/6. In addition to standard confirmation by genotyping, the effectiveness of replacement of the mC5aR with the hC5aR was tested by determining leukocyte expression of mC5aR versus hC5aR on peripheral blood leukocytes by flow cytometry, and by measuring CCX168 suppression of human C5a-induced chemotaxis of thioglycollate-induced peritoneal leukocytes from mC5aR versus hC5aR mice. CCX168 was prepared by the Medicinal Chemistry Department at ChemoCentryx21 and formulated in polyethylene glycol 400/Solutol (70/30). Response to human C5a of hC5aR knock-in mouse leukocytes was tested in vitro using a previously described chemotaxis assay.22 In brief, migration of cells from the upper to the lower ChemoTX chamber (NeuroProbe, Gaithersburg, MD) in response to different concentrations of human C5a was determined by adding CyQUANT solution (Invitrogen) to each lower chamber and measuring the intensity of fluorescence (Migration Signal) of the DNA-binding fluorescent CyQUANT after 120 minutes, which is a relative measure of cell numbers. In vitro, human C5aR responds equally well to murine C5a and human C5a (data not shown), which is in accord with previously reported results.23 The cross-reactivity of CCX168 has been tested against a panel of over 20 chemotactic receptor (including CCR1–10, CXCR1–7, C5L2, C3aR, and ChemR23) and has at least four orders of magnitude less reactivity versus C5aR (data not shown). According to use of a previously described method,24 the effect on in vivo chemotaxis of oral pretreatment 2 hours before intraperitoneal thioglycollate injection with vehicle or a single dose of 30 mg/kg of CCX168 on cell count in peritoneal lavage was measured 24 hours after intraperitoneal injection of thioglycollate. CCX168 effects on C5a-induced leukopenia was studied in hC5aR knock-in mice 1 hour after oral administration of CCX168 by comparing leukocyte counts in blood drawn 1 minute before and 1 minute after intravenous administration of C5a (20 μg/kg).
Preparation of Pathogenic Mouse Antimurine MPO IgG
Purification of native mouse MPO and immunization of MPO−/− mice were performed as previously described.2 Briefly, 8- to 10-week-old MPO−/− mice were immunized intraperitoneally with 20 µg of purified murine MPO in complete Freund adjuvant and boosted twice with 10 µg MPO in incomplete Freund adjuvant. Development of anti-MPO antibodies was monitored by ELISA. Anti-MPO IgG was isolated from serum of MPO−/− mice immunized with MPO by 50% ammonium sulfate precipitation and protein G affinity chromatography. Purity of antibodies was confirmed by SDS-PAGE electrophoresis, and the purified IgG was dialyzed against PBS and sterile-filtered. The protein concentrations were determined by Coomassie protein assay (Pierce, Rockford, IL).
Induction of Experimental GN with Anti-MPO IgG and Amelioration by C5aR Blockade
Induction of experimental necrotizing and crescentic glomerulonephritis (NCGN) with anti-MPO IgG in mice was performed as previously described.2 WT C3H/HeJ (n=5), C6−/− C3H/HeJ (n=5), wild type B6 (n=7), B6 C5aR−/− (n=6), B6 C5L2−/− (n=7) and hC5aR mice were injected intravenously with 50 µg/g body weight mouse anti-mouse MPO IgG and placed in metabolic cages for 12 hours on day 5 to collect urine for analysis, and sacrificed on day 6. Humanized hC5aR mice received varying oral doses of CCX168 C5aR antagonist or vehicle starting on day 1 and ending on day 6. An oral dose of 30 mg/kg b.i.d. CCX168 was selected for the initial proof of concept because preliminary studies showed that this dose is well tolerated in mice and because the resultant plasma concentration of CCX168 was suitable for efficacy studies, peaking one hour after dosing and retaining approximately 140 nM CCX168 in the plasma for 24 hours, which is sufficient to fully inhibit C5aR. Additional dose-response evaluations at 0.1 mg/kg four times daily, 1 mg/kg four times daily, and 4 mg/kg twice daily were used.
Urine was tested by dipstick for hematuria, proteinuria, and leukocyturia (Roche Diagnostics Corp., Indianapolis, IN). Kidney specimens were fixed in 10% buffered formalin and prepared for light microscopy using hematoxylin–eosin and periodic acid-Schiff staining. Glomerular crescents and necrosis were calculated as percentage of glomeruli with crescents or necrosis by counting all glomeruli in cross-sections of both kidneys, which averaged approximately 80 per cross-section. To evaluate neutrophil infiltration, immunohistochemical staining was carried out on paraffin sections using a biotin-labeled rat monoclonal antimouse neutrophil antibody (Cedarlane Laboratories Ltd., Burlington, Ontario, Canada), with detection with peroxidase-conjugated streptavidin (BioGenex, San Ramon, CA) and a DAKO Liquid DAB-chromogen system (Dako, Carpinteria, CA). Results were expressed as the percentage of glomeruli with neutrophils and the number of neutrophils per glomerulus.
Statistical Analysis
All data are presented as mean ± SD. The data were analyzed using a t test and one-way ANOVA with Bonferroni multiple comparison post-test. P<0.05 was considered to indicate a statistically significant difference. All analyses were done using SAS software (version 9.3; SAS Institute, Cary, NC).
Disclosures
A portion of the studies of the effects of the CD88 antagonist on anti-MPO GN in mice with humanized CD88 were funded by ChemoCentryx. D.J.D., J.P.P., L.S.E., T.B., Y.W., L.C.S., M.E.T.P., T.J.S., and J.C.J. are ChemoCentryx shareholders and/or employees.
Acknowledgments
This research was supported by National Institutes of Health grant P01-DK058335 (R.J.F.) and HL051366 (C.G.). Studies of the effects of the CD88 antagonist on anti-MPO GN in mice with humanized CD88 were carried out in collaboration with ChemoCentryx, Inc., Mountain View, CA. Portions of the data in this article have appeared in the following published abstracts: (1) Xiao H, Lu B, Hu P, Falk RJ, Gerard C, Jennette JC: The role of C5a receptors (C5aR and C5L2) and C6 in the pathogenesis of glomerulonephritis induced by myeloperoxidase (MPO) specific antineutrophil cytoplasmic autoantibodies (ANCA) J Am Soc Nephrol 221: 51A, 2011. (2) Xiao H, Dairaghi D, Hu P, Powers JP, Wang Y, Ertl L, Baumgart T, Miao s, Seitz L, Falk RJ, Schall TJ, Jaen JC, Jennette JC: C5a engagement of C5a receptors but not C6 is required for induction of GN by anti-MPO IgG. Clin Exp Immunol 164 (Suppl 1): 127, 2011. (3) Xiao H, Jennette JC, Dairaghi D, Powers JP, Wang Y, Ertl L, Baumgart T, Miao S, Seitz LC, Hu P, Falk RJ, Schall TJ, Jaen JC: Robust efficacy of C5aR antagonist CCX168 in a mouse model of ANCA glomerulonephritis. J Am Soc Nephrol 21: 40A, 2010. (4) Xiao H, Jennette JC, Dairaghi DJ, Ertl L, Baumgart T, Miao S, Powers JP, Seitz LC, Wang Y, Hu P, Falk RJ, Schall TJ, Jaen JC: The human C5a receptor (hC5aR) antagonist CCX168 effectively ameliorates a model of ANCA glomerulonephritis (GN) in hC5aR knock-in mice. Arthritis Rheum 62: S856–S857, 2010.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “With Complements from ANCA Mice,” on pages 207–209.
References
- 1.Jennette JC, Falk RJ, Hu P, Xiao H: Pathogenesis of anti-neutrophil cytoplasmic autoantibody associated small vessel vasculitis. Annu Rev Pathol 8: 139–160, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jennette JC, Falk RJ, Gasim AH: Pathogenesis of antineutrophil cytoplasmic autoantibody vasculitis. Curr Opin Nephrol Hypertens 20: 263–270, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, Maeda N, Falk RJ, Jennette JC: Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest 110: 955–963, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao H, Schreiber A, Heeringa P, Falk RJ, Jennette JC: Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol 170: 52–64, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huugen D, van Esch A, Xiao H, Peutz-Kootstra CJ, Buurman WA, Tervaert JW, Jennette JC, Heeringa P: Inhibition of complement factor C5 protects against anti-myeloperoxidase antibody-mediated glomerulonephritis in mice. Kidney Int 71: 646–654, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Schreiber A, Xiao H, Jennette JC, Schneider W, Luft FC, Kettritz R: C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol 20: 289–298, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xing GQ, Chen M, Liu G, Heeringa P, Zhang JJ, Zheng X, e J, Kallenberg CG, Zhao MH: Complement activation is involved in renal damage in human antineutrophil cytoplasmic autoantibody associated pauci-immune vasculitis. J Clin Immunol 29: 282–291, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Gigante A, Salviani C, Giannakakis K, Rosato E, Barbano B, Moroso A, Gasperini ML, Nofroni I, Salsano F, Cianci R, Pugliese F: Clinical and histological outcome predictors in renal limited pauci-immune crescentic glomerulonephritis: A single centre experience. Int J Immunopathol Pharmacol 25: 287–292, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Gou SJ, Yuan J, Chen M, Yu F, Zhao MH. Circulating complement activation in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Kidney Int 83: 129–137, 2013 [DOI] [PubMed]
- 10.Bamberg CE, Mackay CR, Lee H, Zahra D, Jackson J, Lim YS, Whitfeld PL, Craig S, Corsini E, Lu B, Gerard C, Gerard NP: The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. J Biol Chem 285: 7633–7644, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen M, Daha MR, Kallenberg CG: The complement system in systemic autoimmune disease. J Autoimmun 34: J276–J286, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Hao J, Meng LQ, Xu PC, Chen M, Zhao MH: p38MAPK, ERK and PI3K signaling pathways are involved in C5a-primed neutrophils for ANCA-mediated activation. PLoS ONE 7: e38317, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerard NP, Lu B, Liu P, Craig S, Fujiwara Y, Okinaga S, Gerard C: An anti-inflammatory function for the complement anaphylatoxin C5a-binding protein, C5L2. J Biol Chem 280: 39677–39680, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Scola AM, Johswich KO, Morgan BP, Klos A, Monk PN: The human complement fragment receptor, C5L2, is a recycling decoy receptor. Mol Immunol 46: 1149–1162, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao H, Ciavatta D, Aylor DL, Hu P, de Villena FP, Falk RJ, Jennette JC: Genetically determined severity of anti-myeloperoxidase glomerulonephritis. Am J Pathol 182: 1219–1226, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jennette JC: Nomenclature and classification of vasculitis: lessons learned from granulomatosis with polyangiitis (Wegener’s granulomatosis). Clin Exp Immunol 164[Suppl 1]: 7–10, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jennette JC, Falk RJ: The role of pathology in the diagnosis of systemic vasculitis. Clin Exp Rheumatol 25[Suppl 44]: S52–S56, 2007 [PubMed] [Google Scholar]
- 18.Zhou W, Farrar CA, Abe K, Pratt JR, Marsh JE, Wang Y, Stahl GL, Sacks SH: Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest 105: 1363–1371, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carrera-Marín AL, Romay-Penabad Z, Papalardo E, Reyes-Maldonado E, García-Latorre E, Vargas G, Shilagard T, Pierangeli S: C6 knock-out mice are protected from thrombophilia mediated by antiphospholipid antibodies. Lupus 21: 1497–1505, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banda NK, Hyatt S, Antonioli AH, White JT, Glogowska M, Takahashi K, Merkel TJ, Stahl GL, Mueller-Ortiz S, Wetsel R, Arend WP, Holers VM: Role of C3a receptors, C5a receptors, and complement protein C6 deficiency in collagen antibody-induced arthritis in mice. J Immunol 188: 1469–1478, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan P, Greenman KL, Leleti MR, Li Y, Powers J, Tanaka H, Yang J, Zeng Y: C5aR antagonists. US patent application WO2010075257.2010
- 22.Walters MJ, Wang Y, Lai N, Baumgart T, Zhao BN, Dairaghi DJ, Bekker P, Ertl LS, Penfold ME, Jaen JC, Keshav S, Wendt E, Pennell A, Ungashe S, Wei Z, Wright JJ, Schall TJ: Characterization of CCX282-B, an orally bioavailable antagonist of the CCR9 chemokine receptor, for treatment of inflammatory bowel disease. J Pharmacol Exp Ther 335: 61–69, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Lee H, Zahra D, Vogelzang A, Newton R, Thatcher J, Quan A, So T, Zwirner J, Koentgen F, Padkjaer SB, Mackay F, Whitfeld PL, Mackay CR: Human C5aR knock-in mice facilitate the production and assessment of anti-inflammatory monoclonal antibodies. Nat Biotechnol 24: 1279–1284, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Dairaghi DJ, Zhang P, Wang Y, Seitz LC, Johnson DA, Miao S, Ertl LS, Zeng Y, Powers JP, Pennell AM, Bekker P, Schall TJ, Jaen JC: Pharmacokinetic and pharmacodynamic evaluation of the novel CCR1 antagonist CCX354 in healthy human subjects: implications for selection of clinical dose. Clin Pharmacol Ther 89: 726–734, 2011 [DOI] [PubMed] [Google Scholar]