Abstract
Physical activity may counteract metabolic disturbances that promote the progression of CKD. To address this concept, we performed a longitudinal cohort study of 256 participants in the Seattle Kidney Study, a clinic-based study of CKD. Participants with an estimated GFR (eGFR) of 15–59 ml/min per 1.73 m2 at baseline were eligible for the study. Physical activity was quantified using the Four-Week Physical Activity History Questionnaire. We used generalized estimating equations to test associations of physical activity with change in eGFR determined by longitudinal measurements of serum cystatin C. Mean baseline eGFR was 42 ml/min per 1.73 m2. During a median 3.7 years of follow-up, the mean change in eGFRcystatin C was −7.6% per year (interquartile range, −16.8%, 4.9% per year). Participants who reported >150 minutes of physical activity per week had the lowest rate of eGFRcystatin C loss (mean −6.2% per year compared with −9.6% per year among inactive participants). In adjusted analyses, each 60-minute increment in weekly physical activity duration associated with a 0.5% slower decline per year in eGFR (95% confidence interval, 0.02 to 0.98; P=0.04). Results were similar in sensitivity analyses restricted to participants without cardiovascular disease or diabetes, or to participants with moderate/high physical function. After adjustment for eGFR at the time of questionnaire completion, physical activity did not associate with the incidence of ESRD (n=34 events). In summary, higher physical activity levels associated with slower rates of eGFR loss in persons with established CKD.
CKD leads to metabolic disturbances that amplify the risks of cardiovascular diseases, disability, mortality, and progression to dialysis.1–5 Although initial causes of CKD are diverse, metabolic consequences coalesce to include insulin resistance, dyslipidemia, sodium retention, and accumulation of small molecular toxins such as trimethylamine oxide, p-cresyl sulfate, and indoxyl sulfate.6
Physical activity confers diverse biologic benefits that may counteract the adverse metabolic environment of CKD. Conversely, physical inactivity contributes to the development of CKD, particularly through diabetes and hypertension, the most common causes of kidney disease in Western society.7–19 For example, adiposity directly promotes glomerular hypertension, a primary factor in CKD pathogenesis, and stimulates renal interstitial fibrosis and hypoxia, which may contribute to ongoing progression of kidney injury.20–22
We previously demonstrated that greater amounts of leisure-time physical activity were associated with lower rates of kidney function decline in a large, community-based cohort study.23 However, no prospective studies have evaluated associations of physical activity with kidney function outcomes among individuals who have established CKD. Given the overlapping metabolic disturbances of CKD and physical inactivity, it is possible that the beneficial effects of physical activity are particularly strong in the setting of impaired kidney function.
We hypothesized that greater levels of leisure-time physical activity would be associated with slower rates of kidney function decline in an established, prospective cohort study of individuals who have nondialysis CKD.
Results
Participant Characteristics
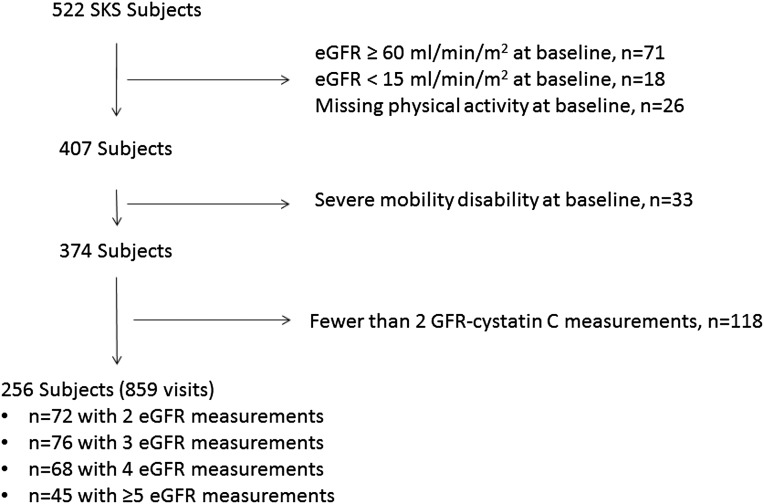
Demographics, comorbid diseases, and laboratory characteristics differed between participants in the highest versus lowest physical activity groups (i.e., ≥150 minutes per week versus 0 minutes per week) (Table 1). The highest physical activity group was characterized by a greater proportion of Caucasians, higher education level, lower body mass, and lower prevalence of coronary disease, peripheral vascular disease, smoking, and diabetes. Baseline estimated GFR (eGFR) did not materially differ by physical activity group. Among participants who reported engaging in any physical activity, 41% reported engaging in walking only and 36% reported engaging in a combination of walking and other leisure-time physical activities. Among the 256-member study cohort, 186 participants had ≥3 eGFR measurements and a median follow-up of 3.7 years (Figure 1).
Table 1.
Baseline characteristics of SKS participants, according to physical activity category (N=256)
| Characteristic | Physical Activity Category | |||
|---|---|---|---|---|
| 0 min/wk | 1–60 min/wk | 60–150 min/wk | > 150 min/wk | |
| Participants (n) | 60 | 74 | 53 | 69 |
| Age at baseline (yr) | 61.8±11.3 | 58.8±12.8 | 61.7±12 | 61.7±12.5 |
| Male sex | 49 (81.7) | 55 (74.3) | 43 (81.1) | 62 (89.8) |
| Race/ethnicity | ||||
| White | 37 (61.7) | 47 (63.5) | 44 (83) | 47 (68.1) |
| Black | 16 (26.7) | 20 (27) | 7 (13.2) | 14 (20.3) |
| Asian/Pacific Islander | 2 (3.3) | 2 (2.7) | 1 (1.9) | 3 (4.3) |
| American Indian/Native Alaskan | 1 (1.7) | 2 (2.7) | 0 (0) | 1 (1.4) |
| Other | 4 (6.7) | 3 (4.1) | 1 (1.9) | 4 (5.8) |
| Study site | ||||
| Harborview Medical Center | 24 (40.0) | 40 (54.1) | 24 (45.3) | 34 (49.3) |
| Veterans Affairs Medical Center | 36 (60.0) | 34 (45.9) | 29 (54.7) | 35 (50.7) |
| Current smoking | 10 (16.7) | 11 (15.1) | 8 (15.1) | 8 (11.8) |
| Any alcohol use | 24 (40.0) | 22 (30.1) | 12 (22.6) | 29 (42.0) |
| College education or higher | 10 (16.7) | 16 (21.1) | 8 (15.1) | 22 (32.8) |
| Body mass index (kg/m2) | 33.9±8 | 32.7±8.1 | 30.4±7.8 | 30.1±6.4 |
| Systolic BP (mmHg) | 141.3±25.3 | 130.2±24.4 | 130.4±20.8 | 135.2±18.9 |
| SPPB | 7.3±3.9 | 9.1±2.8 | 9.6±2.6 | 9.9±2.6 |
| Assistive device use | ||||
| Cane | 17 (28.3) | 9 (11.8) | 11 (20.8) | 6 (8.9) |
| Walker | 5 (5.0) | 5 (6.6) | 2 (3.8) | 4 (6.0) |
| Other (e.g., crutches, etc.) | 2 (3.3) | 3 (3.9) | 0 (0.0) | 2 (3.0) |
| Blood laboratory results | ||||
| Creatinine (mg/dl) | 2.2±1.0 | 2.1±1.4 | 2.1±0.9 | 2±0.9 |
| C-reactive protein (mg/L) | 7.4±8.8 | 5.3±7.3 | 5.1±8.8 | 4.3±6.8 |
| Cystatin C (mg/L) | 2±0.6 | 1.8±0.6 | 1.8±0.5 | 1.7±0.4 |
| Glucose (mg/dl) | 121.7±53.1 | 113.5±31.2 | 123.8±54.7 | 120.9±54.4 |
| Urine laboratory results | ||||
| Albumin to creatinine ratio (mg/g) | 340.4 (45.0, 1035.2) | 68.0 (14.9, 463.2) | 118.0 (11.3, 832.7) | 180.8 (21.8, 677.0) |
| Kidney function (eGFR ml/min per 1.73 m2) | ||||
| Cystatin C | 37.6±12.2 | 41.6±12.9 | 39.9±10.4 | 43.9±10.4 |
| CKD-EPI | 37.8±20.1 | 41.0±18.6 | 37.4±18.2 | 40.5±14.0 |
| Medications | ||||
| Angiotensin-converting enzyme inhibitor | 32 (53.3) | 42 (55.3) | 25 (47.2) | 40 (59.7) |
| Angiotensin II receptor blocker | 25 (41.7) | 30 (39.5) | 18 (34) | 28 (41.8) |
| Statin | 40 (66.7) | 50 (65.8) | 36 (67.9) | 36 (53.7) |
| Prevalent disease | ||||
| Coronary artery diseasea | 35 (58.3) | 36 (48.6) | 23 (43.4) | 24 (34.8) |
| Peripheral vascular diseaseb | 5 (8.3) | 7 (9.2) | 3 (5.7) | 2 (3.0) |
| Cerebrovascular diseasec | 11 (18.3) | 17 (22.3) | 11 (20.8) | 9 (13.4) |
| Diabetesd | 38 (63.3) | 41 (55.4) | 30 (56.6) | 30 (43.5) |
| Hypertensione | 59 (98.3) | 74 (97.4) | 53 (100) | 62 (92.5) |
Data are presented as crude means ± SD for continuous variables, n (proportion) for categorical variables, or median (interquartile range).
Prevalent coronary artery disease was defined as self-reported previous myocardial infarction, cardiac arrest, coronary artery bypass graft, or percutaneous coronary intervention.
Prevalent peripheral vascular disease was defined as self-reported claudication, peripheral vascular surgery, or lower extremity amputation.
Prevalent cerebrovascular disease was defined as self-reported stroke or carotid endarterectomy.
Diabetes was defined by any of the following: use of an oral hypoglycemic medication or insulin, fasting blood sugar ≥126 mg/dl, nonfasting blood sugar ≥200 mg/dl, or hemoglobin A1c ≥6.5%
Hypertension was defined as systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg, or use of antihypertensive medications.
Figure 1.
Participant flow. Note that one participant visit corresponds to a study visit during which GFR was estimated.
Association of Physical Activity with eGFR Decline
The mean relative change in eGFRcystatin C was −7.6% per year (interquartile range, −16.8%, +4.9% per year). Relative eGFR decline was −9.6% per year among inactive participants versus −6.2% per year among participants who were in the highest physical activity group (Table 2). After adjustment for demographic, lifestyle, and clinical patient characteristics, greater durations of physical activity were consistently associated with slower rates of eGFR decline. Each 60-minute greater duration of weekly physical activity was associated with an estimated 0.5% per year slower decline in eGFR (P=0.04).
Table 2.
Association of physical activity and annualized relative change in eGFR cystatin C
| Leisure-Time Physical Activity Level (min/wk) | Percent Annual Change in eGFR Cystatin C (95% Confidence Interval) | |||
|---|---|---|---|---|
| Unadjusted | Model 1a | Model 2b | Model 3c | |
| None | −9.6 (−12.0 to −7.1) | −10.1 (−12.4 to −7.7) | −9.4 (−11.8 to −6.9) | −9.4 (−12.0 to −6.9) |
| 1–59 | −8.2 (−10.3 to −6.1) | −8.0 (−10.0 to −6.0) | −8.1 (−10.2 to −6.0) | −8.7 (−10.9 to −6.5) |
| 60–150 | −6.8 (−9.4 to −4.3) | −7.1 (−9.5 to −4.7) | −7.2 (−9.7 to −4.7) | −8.4 (−10.7 to −6.2) |
| ≥150 | −6.2 (−8.3 to −4.2) | −5.9 (−7.9 to −3.9) | −6.4 (−8.4 to −4.3) | −6.6 (−8.8 to −4.4) |
| P value for trend | 0.02 | 0.02 | 0.03 | 0.05 |
| Per 60 min/week increment | 1.3 (0.23 to 2.32) | 0.61 (0.14 to 1.07) | 0.52 (0.03 to 1.00) | 0.50 (0.02 to 0.98) |
P values for continuous association (per 60 min/wk increment in physical activity) were 0.02, 0.01, 0.03, and 0.04 for the unadjusted model and models 1, 2, and 3, respectively.
Model 1 is adjusted for age, race, sex, and study site.
Model 2 is adjusted for the variables in model 1 plus education, body mass index, diabetes, smoking status, alcohol, and prevalent coronary artery disease.
Model 3 is adjusted for the variables in model 2 plus hemoglobin A1c, systolic BP, angiotensin-converting enzyme inhibitor use, angiotensin-receptor blocker use, statin use, and C-reactive protein.
To evaluate whether observed associations might reflect poorer health status among individuals in the lowest physical activity group, we repeated analyses after removing participants who had prevalent coronary artery disease, prevalent diabetes, and a Short Physical Performance Battery (SPPB)24 score <8 (Table 3). The magnitude of association between the amount of physical activity and eGFR decline was similar in each of these restricted subgroups. The association of physical activity category with eGFR decline was also similar comparing subsets of participants who had baseline eGFR values of 15–30, 30–45, or 45–60 ml/min per 1.73 m2 (P for interaction = 0.72). No statistical interaction of physical activity level with eGFR decline was observed for sex (P=0.42), race (P=0.53), or body mass index (P=0.71).
Table 3.
Association of physical activity with decline in eGFR in subgroups of participants
| Leisure-Time Physical Activity Level (min/wk) | Adjusted Percent Annual Change in eGFR Cystatin Ca | |||||
|---|---|---|---|---|---|---|
| Participants with No Prevalent Coronary Artery Disease at Baseline (n=138)b | Participants with No Prevalent Diabetes at Baseline (n=119)c | Participants with SPPB ≥8 at Baseline (n=173)d | ||||
| n | Percent Annual Change (95% CI) | n | Percent Change per Year (95% CI) | n | Percent Change per Year (95% CI) | |
| None | 25 | −9.8 (−12.2 to −7.4) | 22 | −10.7 (−14.3 to −7.0) | 27 | −8.9 (−12.3 to −5.5) |
| 1–60 | 38 | −8.0 (−10.2 to −5.8) | 33 | −5.3 (−8.4 to −2.2) | 52 | −7.3 (−10.0 to −4.6) |
| 60–150 | 30 | −7.0 (−9.2 to −4.9) | 23 | −5.5 (−8.8 to −2.1) | 42 | −6.5 (−9.3 to −3.7) |
| ≥150 | 45 | −6.2 (−8.3 to −4.1) | 39 | −4.4 (−7.6 to −1.2) | 52 | −6.0 (−8.6 to −3.4) |
| P value for trend | 0.03 | 0.01 | 0.03 | |||
| Per 60 min/week increment | 0.57 (0.11 to 1.04) | 0.66 (0.09 to 1.23) | 0.57 (0.03 to 1.10) | |||
P values for continuous association (per 60 min/wk increment in physical activity) were 0.02, 0.02, and 0.04 for participants with no prevalent coronary heart disease at baseline, no prevalent diabetes at baseline, and SPPB ≥8 at baseline, respectively. 95% CI, 95% confidence interval.
Adjusted for age, race, sex, site, smoking status, alcohol use, angiotensin-converting enzyme inhibitor use, angiotensin-receptor blocker use, and statin use.
Prevalent coronary artery disease was defined as self-reported previous myocardial infarction, cardiac arrest, coronary artery bypass graft, or percutaneous coronary intervention.
Prevalent diabetes was defined by any of the following: use of an oral hypoglycemic medication or insulin, fasting blood sugar ≥126 mg/dl, nonfasting blood sugar ≥200 mg/dl, or hemoglobin A1c ≥6.5%.
A score <8 is associated with disability in lower extremity functioning.24
Analyses defining walking minutes per week as the exposure of interest produced similar effect estimates to those from our primary analyses; however, linearity of the duration-risk association was less clear (Supplemental Table 1). Participants who walked more had slower annualized rates of eGFR decline:– 5.3% per year among participants who self-reported walking ≥150 minutes per week versus– 8.6% per year among participants who reported not walking at all (P for trend = 0.13). Lastly, estimation of GFR using the creatinine-based Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation did not materially alter the observed associations (Supplemental Table 2).
Association of Physical Activity with Incident ESRD
During a median follow-up of 3.0 years, 34 individuals developed ESRD. The unadjusted incidence rate of ESRD was higher among participants who reported no physical activity versus those reporting any (5.7 versus 3.6 events per 100 person-years). However, in fully adjusted models (including eGFR at the time of completion of the questionnaire), neither the presence nor the total amount of physical activity was associated with incident ESRD (Table 4).
Table 4.
Association of physical activity with incident ESRD
| Physical Activity Level | Number of Events (%) | Incidence Rate (per 100 person-years) | Hazard Ratio (95% Confidence Interval) | ||
|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 3c | |||
| None | 60 (10) | 5.68 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Any | 196 (24) | 3.56 | 0.59 (0.28 to 1.24) | 0.59 (0.27 to 1.30) | 1.02 (0.45 to 2.32) |
| P value (any versus none) | 0.19 | 0.21 | 0.93 | ||
| Per 60 min/week increment | 0.90 (0.74 to 1.10) | 0.92 (0.74 to 1.12) | 0.98 (0.85 to 1.14) | ||
P values for continuous association (per 60 minute/week increment in physical activity) were 0.32, 0.41, and 0.88, for models 1, 2, and 3, respectively.
Model 1 is adjusted for age, race, sex, and site.
Model 2 is adjusted for the variables in model 1 plus education, body mass index, diabetes, smoking status, alcohol, and prevalent coronary artery disease.
Model is adjusted for the variables in model 2 plus hemoglobin A1c, systolic BP, angiotensin-converting enzyme inhibitor use, angiotensin-receptor blocker use, statin use, C-reactive protein, and baseline eGFR cystatin C.
Discussion
In this prospective cohort study of patients with stage III–IV CKD, we observed an association of greater self-reported physical activity with slower relative rates of eGFR decline. Specifically, we found a 2.8% difference in annual eGFR decline comparing the highest to lowest category of physical activity after adjustment for sociodemographic factors and prevalent diseases. Associations were quantitatively similar for total leisure-time physical activity and for walking time alone and were robust to the exclusion of the most disabled participants. These findings are consistent with our previous work in a community-based cohort of ambulatory older adults, in which greater physical activity levels were associated with a lower risk of rapid eGFR decline.23 Our results extend these observations from the general population to individuals who have existing kidney disease.
Several small experimental studies have investigated the effects of prescribed exercise regimens on kidney function among patients who have established CKD, with conflicting results. The largest of these studies randomly assigned 30 nondiabetic CKD patients to either daily bicycling for 30 minutes or maintenance of usual lifestyle.25 After the 20-month median intervention time and follow-up, there was no change in the rate of eGFR loss. Similarly, a pilot study that assigned seven obese, diabetic CKD participants to 18 months of aerobic exercise training and four similar participants to no intervention found no subsequent changes in eGFR after the interventional period. A recent study in 21 individuals with stage II–IV CKD randomized participants to either 48 weeks of aerobic exercise training (three times per week, 55 minutes per session) and dietary counseling or standard of care. The linear slope of eGFR at 48 weeks was not different between treatment groups.26 However, two randomized studies did report significant effects of exercise interventions on kidney function. The first assigned 17 adults with CKD to low-intensity aerobic exercise and matched them to 9 control participants who remained sedentary.27 Participants in the exercise group had significantly diminished serum cystatin C levels, whereas no such change was noted in the control group. The second study assigned 10 participants with both CKD and cardiovascular disease to a combination of in-hospital aerobic exercise and at-home daily walking for 12 weeks and compared changes in eGFR to that of nine control participants.28 The exercise intervention significantly improved eGFR (calculated by the Modification of Diet in Renal Disease equation) from 47.0±13.7 at baseline to 55.2±16.9 ml/min per 1.73 m2 at 12 weeks, but no such difference was detected in the control group. Although these results are intriguing, previous interventional studies lack sufficient power and follow-up time to specify effects on kidney function.
Diabetes, obesity, hypertension, and kidney dysfunction itself lead to activation of the renin-angiotensin system, oxidative stress, insulin resistance, endothelial dysfunction, elevated low-grade inflammation, and increased circulating cytokines.29 These metabolic disturbances are highly prevalent in both CKD patients and physically inactive individuals, and augment the risks of micro- and macro-vascular disease.30–36 Aerobic exercise may attenuate or reverse these adverse metabolic processes, which can affect the kidney, in terms of inflammation, fibrosis, and progression, regardless of the primary initiating cause of CKD.
In our study, greater physical activity levels were not associated with a lower risk of incident ESRD, after full adjustment. At baseline, participants who had higher physical activity levels had somewhat better kidney function relative to those who had lower levels, promoting an expected lower risk of falling below the threshold for ESRD initiation. Adjustment for baseline GFR attenuated the association of physical activity with ESRD; however, it is possible that the cumulative effect of physical activity throughout CKD, including that before the baseline assessment, is better reflected by associations not adjusted for baseline GFR. In addition, the fully adjusted regression model included covariates that may be partial or full mediators of the association of physical activity with risk of ESRD. In particular, C-reactive protein and systolic BP may lie in the causal pathway of physical activity and incident ESRD, such that conditioning on these factors would weaken the estimated risk estimates. It is also possible that follow-up time in our study and/or the number of ESRD patients was insufficient to detect associations with incident ESRD.
The most important limitation of our study is the potential for confounding, such that other characteristics that are associated with slower kidney function decline may also be associated with a greater desire and capacity to exercise. To address this concern, we restricted our study population to individuals who had the capacity to exercise and then adjusted for known and measured characteristics that are linked with eGFR decline. We also found similar associations of physical activity with eGFR decline among subgroups of participants who were free of coronary artery disease or diabetes or scored well on the SPPB at baseline. A second limitation is the preponderance of male participants in our study, stemming from the inclusion of a Veterans Affairs study site, which may limit the applicability of our results to other CKD populations.
The Four-Week Physical Activity History Questionnaire (FWH) instrument used in our study has been shown to have a high degree of reliability and validity as a measure of physical activity in CKD.37 Nonetheless, the use of self-report to measure physical activity is expected to lead to misclassification because some participants will underreport their level of activity, whereas others will overreport their level of activity. Because participants were not aware of their future eGFR decline status at the time they completed the baseline physical activity assessment, misclassification in this study is likely to be nondifferential, leading to a bias of results toward the null.
Complete separation of physical activity from related characteristics that may influence kidney function decline requires larger randomized clinical trials than those previously conducted, and these are an appropriate next step. Large trials are required because the predicted effect of exercise on kidney function decline, although numerically modest, is clinically important. For example, the 0.7% difference in eGFR decline associated with just a small increase in physical activity from none to approximately 30 minutes per week in this study would result in an estimated 1-year delay in ESRD initiation (defined by an eGFR of 10 ml/min per 1.73 m2) over 10 years. On the basis of this study and other published work, we believe that future clinical trials should be broadly inclusive, target basic physical activities such as walking, and include ample follow-up time to ascertain relevant changes in kidney function. In the absence of long-term clinical trial data, current evidence suggests that physical activity is likely to be beneficial and not harmful for CKD patients.
In conclusion, our prospective data demonstrate an association of greater physical activity with slower rates of eGFR decline among individuals who have moderate to severe CKD. Associations were independent of measured confounders, consistent across different physical activity types, increased in effect size with greater physical activity levels, and supported by biologic evidence demonstrating effects of exercise on metabolic pathways that directly affect kidney function. Physical activity is emerging as one of the few actionable interventions available to prevent the adverse sequelae of CKD.
Concise Methods
Study Population
The Seattle Kidney Study (SKS) is a clinic-based, prospective cohort study of CKD based in Seattle, Washington.37,38 Briefly, the SKS began recruiting participants in 2004 from outpatient nephrology clinics at Harborview Medical Center and the Veterans’ Affairs Medical Center, affiliated hospitals of the University of Washington. Eligibility criteria are age >18 years and CKD of any stage not requiring dialysis. Major exclusions are kidney transplantation or expectation of starting renal replacement therapy or leaving the area within 3 months. Each site’s institutional review board approved the study, and all participants provided informed consent.
For this analysis, we focused on moderate to severe CKD (stage III–IV; eGFR 15–59 ml/min per 1.73 m2) by excluding 71 participants who had an eGFR ≥60 ml/min per 1.73 m2 and 18 participants who had an eGFR <15 ml/min per 1.73 m2 at baseline. To assess participants who had the capacity to exercise, we further excluded 33 individuals who were unable to ambulate or required use of a wheelchair. We further excluded 118 individuals who had <2 eGFR measurements necessary to calculate a slope and 26 participants who did not complete any component of the physical activity questionnaire, leaving 256 participants for analysis (Figure 1). Excluded participants were of similar age, had a similar baseline eGFR, had a higher prevalence of diabetes and coronary disease, and had similar physical activity levels compared with included participants. Among the excluded participants, 23 (19%) died before scheduled follow-up.
Measurement of Physical Activity
We administered the FWH at baseline to determine self-reported leisure-time physical activity. The FWH queries the frequency and duration in which participants engaged in each of the following activities during the prior month: walking for exercise, jogging, biking, aerobics, golf, tennis, swimming, weight training, running on a treadmill, or using an aerobic machine.39 We calculated the number of minutes per week of each leisure-time activity and then summed these totals across all activities. The FWH questionnaire has been evaluated in the general population against doubly labeled water, heart rate monitoring, changes in maximal oxygen uptake, and accelerometry.40–44 We previously reported that FWH correlates with the percentage of time spent in moderate to vigorous activities assessed by gold standard accelerometry in a subset of 48 SKS participants (intraclass correlation coefficient, 0.38; P=0.02).37
eGFR
SKS study coordinators collect annual serum, plasma, and urine samples from all participants after an overnight fast. We decided, before analysis, to evaluate cystatin C–based estimates of GFR because serum creatinine levels depend on muscle mass, which may be influenced by physical activity.45 In secondary analyses, we assessed GFR using the creatinine-based CKD-EPI equation.46 We measured serum cystatin C levels from frozen serum samples stored at −70°C using either a particle-enhanced immunonephelometric assay (Gentian A/S; Gentian, Moss, Norway) with a clinical chemistry analyzer (DxC600; Beckman Coulter, Miami, FL) or with a nephelometer (BNII; Siemens Healthcare Diagnostics, Inc., Deerfield, IL). We developed a calibration equation to convert nephelometer-based cystatin C concentrations to DxC-based concentrations (gold standard) in a subset of 40 SKS participants who had identical serum samples measured with both assays (r2=0.98). We standardized the DxC measurements by reconstituting the cystatin C reference material (European Reference Material (DA471), International Federation of Clinical Chemistry and Laboratory Medicine) per its certificate of analysis to yield a cystatin C concentration of 5.48 mg/L (uncertainty of 0.15 mg/L). We calculated eGFR at each SKS study examination using the following equation47:
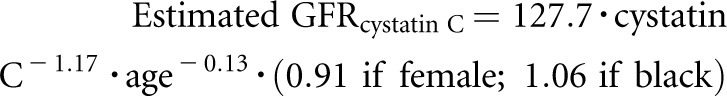 |
Study coordinators identified ESRD events, defined as the first occurrence of initiation of chronic dialysis or kidney transplantation, by 6-month surveillance contacts. We verified self-reported initiations of dialysis or kidney transplantations by medical record review.
Measurement of Covariates
Coordinators measured weight using calibrated scales, height using a wall-mounted tape measure, and waist circumference using a constant-tension tape. We determined prevalent conditions based on participant responses to questionnaires and hospitalizations that occurred after initial SKS enrollment but before the initial assessment for this study. We assessed medications by inventory assessment and completed missing medication data by chart review.48 We defined diabetes by the use of an oral hypoglycemic medication or insulin, fasting blood sugar ≥126 mg/dl, nonfasting blood sugar ≥200 mg/dl, or hemoglobin A1c ≥6.5%. We defined hypertension by the use of any antihypertensive medication, systolic BP ≥140 mmHg, or diastolic BP ≥90 mmHg.49 We measured urine albumin and in spot morning or overnight urine collections.
Statistical Analyses
We tabulated baseline characteristics according to the following leisure-time physical activity categories: none, 1–60, 60–150, and >150 minutes per week. The uppermost physical activity category corresponds to the American Heart Association Physical Activity Guidelines of at least 150 minutes per week of moderate physical activity.50 We used generalized estimating equations, accounting for within-participant clustering across time, to determine whether annualized relative eGFR change differed across physical activity categories after adjusting for potential confounding variables.51 Among participants who initiated dialysis and arrived for a study visit thereafter (n=28), we imputed an eGFR of 10 ml/min per 1.73 m2 at the first postdialysis study visit, and censored all subsequent visits. We evaluated relative change in eGFR based on ongoing work demonstrating minimal dependence of relative eGFR change on baseline eGFR and straightforward interpretation of regression coefficients.
We used Cox proportional hazards regression to estimate the relative hazard of ESRD after adjustment for potential confounding factors. We considered participants to be at risk for ESRD from the date of their initial visit until the first occurrence of dialysis initiation, kidney transplantation, or their data were censored due to death, loss to follow-up, or end of the study data collection period (January 1, 2012), whichever came first.
We selected covariates before analysis based on plausibility that they could confound associations of physical activity with kidney function decline or ESRD. We constructed three multivariable models to assess groups of potential confounding variables. In sensitivity analyses, we determined whether associations of physical activity with eGFR decline were robust to the exclusion of participants who had prevalent coronary disease, prevalent diabetes, and an SPPB score <8, which is associated with lower extremity disability.24
There were 27 participants who did not check any boxes on the physical activity questionnaire but answered all other questions. Missing values for these participants were multiply imputed with chained equations, and the resulting analyses were combined using Rubin’s rules.52,53 Secondary analyses evaluated associations of self-reported physical activity and annualized relative change in eGFR among complete cases only (i.e., restricted to participants with observed values on all predictors), which yielded materially similar results.
In an attempt to characterize the association of walking time alone and eGFR decline, we secondarily examined the association of minutes of walking per week with annualized relative change in eGFR. For these analyses, we additionally adjusted for time spent in other leisure-time physical activities.
All P values were two-tailed (α=0.05). All analyses were performed using STATA software (release 11.2; StataCorp, College Station, TX).
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank all of the SKS study participants and coordinators.
This article is the result of work supported by the Kidney Research Institute (Seattle, WA). This research was also supported by the National Institutes of Health National Heart, Lung, and Blood Institute (Grant 2R01HL070938 to J.H.) and the Department of Veterans Affairs (Rehabilitation Research & Development Career Development Award 6982 to A.J.L.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013040392/-/DCSupplemental.
References
- 1.Khan SS, Kazmi WH, Abichandani R, Tighiouart H, Pereira BJ, Kausz AT: Health care utilization among patients with chronic kidney disease. Kidney Int 62: 229–236, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Fried LF, Shlipak MG, Crump C, Bleyer AJ, Gottdiener JS, Kronmal RA, Kuller LH, Newman AB: Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol 41: 1364–1372, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Coresh J, Astor B, Sarnak MJ: Evidence for increased cardiovascular disease risk in patients with chronic kidney disease. Curr Opin Nephrol Hypertens 13: 73–81, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Weiner DE, Tighiouart H, Stark PC, Amin MG, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ: Kidney disease as a risk factor for recurrent cardiovascular disease and mortality. Am J Kidney Dis 44: 198–206, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Raff AC, Meyer TW, Hostetter TH: New insights into uremic toxicity. Curr Opin Nephrol Hypertens 17: 560–565, 2008 [DOI] [PubMed] [Google Scholar]
- 7.US Renal Data System : USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2011 [Google Scholar]
- 8.Hawkins MS, Sevick MA, Richardson CR, Fried LF, Arena VC, Kriska AM: Association between physical activity and kidney function: National Health and Nutrition Examination Survey. Med Sci Sports Exerc 43: 1457–1464, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Powell KE, Thompson PD, Caspersen CJ, Kendrick JS: Physical activity and the incidence of coronary heart disease. Annu Rev Public Health 8: 253–287, 1987 [DOI] [PubMed] [Google Scholar]
- 10.Paffenbarger RS, Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB: The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med 328: 538–545, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Leon AS, Connett J, Jacobs DR, Jr, Rauramaa R: Leisure-time physical activity levels and risk of coronary heart disease and death. The Multiple Risk Factor Intervention Trial. JAMA 258: 2388–2395, 1987 [PubMed] [Google Scholar]
- 12.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L, INTERHEART Study Investigators : Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 364: 937–952, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Blair SN, Goodyear NN, Gibbons LW, Cooper KH: Physical fitness and incidence of hypertension in healthy normotensive men and women. JAMA 252: 487–490, 1984 [PubMed] [Google Scholar]
- 14.Blair SN, Kampert JB, Kohl HW, 3rd, Barlow CE, Macera CA, Paffenbarger RS, Jr, Gibbons LW: Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA 276: 205–210, 1996 [PubMed] [Google Scholar]
- 15.Nelson L, Jennings GL, Esler MD, Korner PI: Effect of changing levels of physical activity on blood-pressure and haemodynamics in essential hypertension. Lancet 2: 473–476, 1986 [DOI] [PubMed] [Google Scholar]
- 16.Whelton SP, Chin A, Xin X, He J: Effect of aerobic exercise on blood pressure: A meta-analysis of randomized, controlled trials. Ann Intern Med 136: 493–503, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Mayer-Davis EJ, D’Agostino R, Jr, Karter AJ, Haffner SM, Rewers MJ, Saad M, Bergman RN: Intensity and amount of physical activity in relation to insulin sensitivity: The Insulin Resistance Atherosclerosis Study. JAMA 279: 669–674, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Hu FB, Sigal RJ, Rich-Edwards JW, Colditz GA, Solomon CG, Willett WC, Speizer FE, Manson JE: Walking compared with vigorous physical activity and risk of type 2 diabetes in women: A prospective study. JAMA 282: 1433–1439, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M, Finnish Diabetes Prevention Study Group : Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344: 1343–1350, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Johnson RJ, Alpers CE, Yoshimura A, Lombardi D, Pritzl P, Floege J, Schwartz SM: Renal injury from angiotensin II-mediated hypertension. Hypertension 19: 464–474, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I: Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56: 901–911, 2007 [DOI] [PubMed] [Google Scholar]
- 22.O’Seaghdha CM, Hwang SJ, Vasan RS, Larson MG, Hoffmann U, Wang TJ, Fox CS: Correlation of renin angiotensin and aldosterone system activity with subcutaneous and visceral adiposity: The Framingham Heart Study. BMC Endocr Disord 12: 3, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson-Cohen C, Katz R, Mozaffarian D, Dalrymple LS, de Boer I, Sarnak M, Shlipak M, Siscovick D, Kestenbaum B: Physical activity and rapid decline in kidney function among older adults. Arch Intern Med 169: 2116–2123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB: A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 49: M85–M94, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Eidemak I, Haaber AB, Feldt-Rasmussen B, Kanstrup IL, Strandgaard S: Exercise training and the progression of chronic renal failure. Nephron 75: 36–40, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Headley S, Germain M, Milch C, Pescatello L, Coughlin MA, Nindl BC, Cornelius A, Sullivan S, Gregory S, Wood R: Exercise training improves HR responses and V˙O2peak in predialysis kidney patients. Med Sci Sports Exerc 44: 2392–2399, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Pechter U, Ots M, Mesikepp S, Zilmer K, Kullissaar T, Vihalemm T, Zilmer M, Maaroos J: Beneficial effects of water-based exercise in patients with chronic kidney disease. Int J Rehabil Res 26: 153–156, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Toyama K, Sugiyama S, Oka H, Sumida H, Ogawa H: Exercise therapy correlates with improving renal function through modifying lipid metabolism in patients with cardiovascular disease and chronic kidney disease. J Cardiol 56: 142–146, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Amann K, Wanner C, Ritz E: Cross-talk between the kidney and the cardiovascular system. J Am Soc Nephrol 17: 2112–2119, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM: The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int 62: 1524–1538, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Muntner P, Hamm LL, Kusek JW, Chen J, Whelton PK, He J: The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Intern Med 140: 9–17, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, Furberg CD, Psaty BM: Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation 107: 87–92, 2003 [DOI] [PubMed] [Google Scholar]
- 33.DeFronzo RA, Alvestrand A, Smith D, Hendler R, Hendler E, Wahren J: Insulin resistance in uremia. J Clin Invest 67: 563–568, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, Ouedraogo R, Hough K, McCue P, Chan L, Falkner B, Goldstein BJ: Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest 118: 1645–1656, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB: The metabolic syndrome: Prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med 163: 427–436, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pitsavos C, Chrysohoou C, Panagiotakos DB, Skoumas J, Zeimbekis A, Kokkinos P, Stefanadis C, Toutouzas PK: Association of leisure-time physical activity on inflammation markers (C-reactive protein, white cell blood count, serum amyloid A, and fibrinogen) in healthy subjects (from the ATTICA study). Am J Cardiol 91: 368–370, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Robinson-Cohen C, Littman AJ, Duncan GE, Roshanravan B, Ikizler TA, Himmelfarb J, Kestenbaum BR: Assessment of physical activity in chronic kidney disease. J Ren Nutr 23: 123–131, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roshanravan B, Robinson-Cohen C, Patel KV, Ayers E, Littman AJ, de Boer IH, Ikizler TA, Himmelfarb J, Katzel LI, Kestenbaum B, Seliger S: Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol 24: 822–830, 2013 [DOI] [PMC free article] [PubMed]
- 39.Richardson MT, Leon AS, Jacobs DR, Jr, Ainsworth BE, Serfass R: Comprehensive evaluation of the Minnesota Leisure Time Physical Activity Questionnaire. J Clin Epidemiol 47: 271–281, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Racette SB, Schoeller DA, Kushner RF: Comparison of heart rate and physical activity recall with doubly labeled water in obese women. Med Sci Sports Exerc 27: 126–133, 1995 [PubMed] [Google Scholar]
- 41.Miller DJ, Freedson PS, Kline GM: Comparison of activity levels using the Caltrac accelerometer and five questionnaires. Med Sci Sports Exerc 26: 376–382, 1994 [PubMed] [Google Scholar]
- 42.Matthews CE, Freedson PS: Field trial of a three-dimensional activity monitor: Comparison with self report. Med Sci Sports Exerc 27: 1071–1078, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Blair SN, Haskell WL, Ho P, Paffenbarger RS, Jr, Vranizan KM, Farquhar JW, Wood PD: Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol 122: 794–804, 1985 [DOI] [PubMed] [Google Scholar]
- 44.Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN, Paffenbarger RS, Jr: Physical activity assessment methodology in the Five-City Project. Am J Epidemiol 121: 91–106, 1985 [DOI] [PubMed] [Google Scholar]
- 45.Séronie-Vivien S, Delanaye P, Piéroni L, Mariat C, Froissart M, Cristol JP; SFBC “Biology of Renal Function and Renal Failure” Working Group: Cystatin C: Current position and future prospects. Clin Chem Lab Med 46: 1664–1686, 2008 [DOI] [PubMed]
- 46.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith NL, Psaty BM, Heckbert SR, Tracy RP, Cornell ES: The reliability of medication inventory methods compared to serum levels of cardiovascular drugs in the elderly. J Clin Epidemiol 52: 143–146, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ, National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National High Blood Pressure Education Program Coordinating Committee : The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 289: 2560–2572, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Macera CA, Castaneda-Sceppa C: Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 39: 1435–1445, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Zeger SL, Liang KY: Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42: 121–130, 1986 [PubMed] [Google Scholar]
- 52.Royston P: Multiple imputation of missing values. Stata J 4: 227–241, 2004 [Google Scholar]
- 53.Rubin DB: Multiple Imputation for Nonresponse in Surveys, New York, Wiley, 1987 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.