Abstract
Studies of frailty among patients on hemodialysis have relied on definitions that substitute self-reported functioning for measures of physical performance and omit weight loss or substitute alternate criteria. We examined the association between body composition and a definition of frailty that includes measured physical performance and weight loss in a cross-sectional analysis of 638 adult patients receiving maintenance hemodialysis at 14 centers. Frailty was defined as having three of following characteristics: weight loss, weakness, exhaustion, low physical activity, and slow gait speed. We performed logistic regression with body mass index (BMI) and bioelectrical impedance spectroscopy (BIS)-derived estimates of intracellular water (ICW), fat mass, and extracellular water (ECW) as the main predictors, and age, sex, race, and comorbidity as covariates. Overall, 30% of participants were frail. Older age (odds ratio [OR], 1.31 per 10 years; 95% confidence interval [95% CI], 1.14 to 1.50), diabetes (OR, 1.65; 95% CI, 1.13 to 2.40), higher fat mass (OR, 1.18; 95% CI, 1.02 to 1.37), and higher ECW (OR, 1.33; 95% CI, 1.20 to 1.47) associated with higher odds of frailty. Higher ICW associated with lower odds of frailty (OR, 0.80 per kg; 95% CI, 0.73 to 0.87). The addition of BMI data did not change the area under the receiver operating characteristics curve (AUC; AUC=0.66 versus 0.66; P=0.71), but the addition of BIS data did change the AUC (AUC=0.72; P<0.001). Thus, individual components of body composition but not BMI associate strongly with frailty in this cohort of patients receiving hemodialysis.
Patients on dialysis frequently experience protein energy wasting or loss of protein mass and energy stores, which is likely multifactorial.1 It has recently been appreciated that the same disorders that underlie protein energy wasting as well as muscle wasting itself are also commonly associated with frailty.2–4 Although frailty is generally considered to be a geriatric syndrome, individuals with chronic diseases, such as CKD, may be at risk for premature frailty.5,6 In fact, as many as two thirds to three quarters of patients new to dialysis may be frail by definitions that rely on patient self-report of physical functioning and omit weight loss or substitute alternate criteria for wasting (Table 1).6,7
Table 1.
Definitions of frailty adapted from the Cardiovascular Health Study definition
| Components of Frailty | USRDS DMMS Wave 2 (Incident Dialysis)6 | CDS (Incident Dialysis)7 | REXDP (Prevalent Hemodialysis)8 | NHANES (CKD)9 | Seattle Kidney Study (CKD Stages 1–4)28 |
|---|---|---|---|---|---|
| Slowness/weakness | Rand-36 Physical Function Scale score<75 | SF-12 Physical Function score<75 | Slowness: gait speed over 6 m using cutpoints that correspond to the same speed as the cutpoints from the 15-ft walk in the CHS | Slowness: gait speed over 8 ft with lowest quintile adjusted for sex | Slowness: walking pace assessed over a 4-m course using cutpoints that correspond to the same speed as the cutpoints from the 15-ft walk in the CHS |
| The following items are about activities that you might do during a typical day: does your health now limit you in these activities? If so, how much? | Weakness: patients were asked to stand up and sit down five times; the time for the slowest quartile of the SPPB chair stand based on community-dwelling elderly cohorts was used to define frailty | Weakness: based on self-report and defined as present if participants answered some difficulty, much difficulty, or unable to do when asked how much difficulty they have lifting or carrying something as heavy as 10 lbs (like a sack of potatoes or rice) | Weakness: Grip strength using the same absolute cutoffs as in the CHS | ||
| Vigorous activities, such as running, lifting heavy objects, or participating in strenuous sports | |||||
| Moderate activities, such as moving a table, pushing a vacuum cleaner, bowling, or playing golf | |||||
| Lifting or carrying groceries | |||||
| Climbing several flights of stairs | |||||
| Climbing one flight of stairs | |||||
| Bending, kneeling, or stooping | |||||
| Walking more than 1 mi | |||||
| Walking several blocks | |||||
| Walking 1 block | |||||
| Bathing or dressing yourself | |||||
| Poor endurance/exhaustion | Rand-36 Vitality Scale score<55 | SF-12 Vitality score<55 | SF-36 Vitality score<55 | Defined as present if participants answered some difficulty, much difficulty, or unable to do when asked how much difficulty they have walking from one room to the other on the same level | SF-36 Vitality score<37.5 |
| How much of the time during the last 30 days? | |||||
| Did you feel worn out? | |||||
| Did you feel tired? | |||||
| Did you have a lot of energy? | |||||
| Did you feel full of pep? | |||||
| Physical inactivity | How often do you exercise (do physical activity during your leisure time)? | Lowest quintile based on age- and sex-specific population norms for the Human Activity Profile | Patients who reported no activity beyond self-care and activities required for living were considered inactive | Compared with most (men/women) your age, would you say that you are more active, less active, or about the same? | Self-reported exercise less than one time per week |
| Daily or almost daily | Patients answering less active were classified as inactive | ||||
| Four to five times a week | |||||
| Two to three times a week | |||||
| About one time a week | |||||
| Less than one time a week | |||||
| Almost never or never | |||||
| Patients answering almost never or never were classified as inactive | |||||
| Unintentional weight loss or shrinkage | Undernourished or cachectic (malnourished) as assessed by data abstractor | Not included | BMI≤18.5 kg/m2 | BMI≤18.5 kg/m2 | Self-reported ≥10-lb unintentional weight loss in past 6 months |
See ref2 for Cardiovascular Health Study. USRDS, US Renal Data System; DMMS, Dialysis Morbidity and Mortality Study; CDS, Comprehensive Dialysis Study; REXDP, Renal Exercise Demonstration Project; NHANES, National Health and Nutrition Examination Survey; CHS, Cardiovascular Health Study; SPPB, short physical performance battery.
Low Quételet’s (body mass) index (BMI), expressed in kilograms of body weight divided by height squared, has been substituted for the weight loss criterion of the frailty construct in several studies.8,9 However, BMI is a nonspecific metric of body composition, because the body weight component could reflect adipose tissue or intracellular (muscle) or extracellular (edema) water. The most commonly used definition of frailty, which includes direct measures of gait speed and grip strength (rather than self-reported functioning) as well as weight loss, exhaustion, and level of physical activity,2 has only recently been applied in an ESRD population,10 and the association between frailty and body composition has not been examined systematically in this population.11
In this investigation, we sought to determine the extent to which body composition was associated with frailty in a prevalent hemodialysis cohort. We hypothesized that intracellular water (ICW) estimated by bioelectrical impedance spectroscopy (BIS) would be inversely associated and fat mass would be directly associated with frailty in a cohort of prevalent dialysis patients but that BMI would not be associated.
Results
Of a total of 778 patients enrolled in A Cohort Study to Investigate the Value of Exercise/Analyses Designed to Investigate the Paradox of Obesity and Survival in ESRD (ACTIVE/ADIPOSE), 762 (92.5%) patients provided all of the information necessary to determine frailty status, and 638 (82%) patients also had body composition data available. The average age of participants in the analyzed cohort was 56.8 (14.3) years; 42% of participants were women, 60.3% of participants were black, and 44.8% of participants had diabetes mellitus. Compared with patients without complete data, the cohort with available body composition data had a lower burden of comorbidity and frailty but did not differ significantly based on BMI, sex, or racial distribution from those patients missing body composition data (Table 2). Overall, 190 (30%) patients in the analysis cohort were frail.
Table 2.
Patient characteristics
| Characteristic | All Patientsa (N=778) | Patients with Frailty and Body Composition Dataa (n=638) | Patients Missing Body Composition or Frailty Data (n=140) | P Value |
|---|---|---|---|---|
| Age, yr | 57.1 (14.3) | 56.8 (14.5) | 58.4 (13.0) | 0.21 |
| Women, % | 41.4 | 42.0 | 38.8 | 0.49 |
| Race, % | 0.24 | |||
| White | 23.6 | 24.6 | 18.7 | |
| Black | 61.6 | 60.3 | 67.6 | |
| Other | 14.8 | 15.0 | 13.7 | |
| BMI, kg/m2 | 29.0 (7.1) | 29.0 (7.1) | 29.0 (7.0) | 0.89 |
| Serum albumin, g/dl | 4.0 (0.4) | 4.0 (0.4) | 3.9 (0.4) | 0.02 |
| Serum creatinine, mg/dl | 8.3 (2.8) | 8.4 (2.7) | 7.9 (3.0) | 0.03 |
| Dialysis vintage, yr | 3.2 (0.1–36.6) | 3.0 (0.1–30.1) | 4.8 (0.1–36.6) | <0.001 |
| Comorbidity, % | ||||
| Diabetes | 47.4 | 44.8 | 61.0 | 0.001 |
| CHF | 19.7 | 18.5 | 26.5 | 0.05 |
| CAD | 9.2 | 8.0 | 15.9 | 0.007 |
| Hypertension | 89.3 | 90.3 | 84.2 | 0.05 |
| Frail, % | 31.4 | 29.8 | 39.5b | 0.03 |
CHF, congestive heart failure; CAD, coronary artery disease.
Numbers are mean (SD) or median (25th to 75th).
n=124. Sixteen individuals were missing information that was needed to determine frailty status.
Older participants and those participants with diabetes mellitus were more likely to be frail in multivariable logistic regression analysis without accounting for body composition (Table 3). Sex and race were not statistically significantly associated with frailty. BMI was not significantly associated with odds of frailty when added to the base model as a continuous (odds ratio [OR], 1.02 per kg/m2; 95% confidence interval [95% CI], 0.99 to 1.05) or categorical (P=0.66) variable; moreover, the addition of BMI into the model did not materially change the magnitude of association among other covariates with frailty. In contrast, when data from BIS were added to the base model, higher ICW, a proxy for muscle mass, was associated with lower odds of frailty (OR, 0.80 per kg; 95% CI, 0.73 to 0.87), and fat mass was associated with higher odds of frailty (OR, 1.18 per 10 kg; 95% CI, 1.02 to 1.37). After adjusting for body fat and ICW, higher extracellular water (ECW) was also associated with higher odds of frailty (OR, 1.33; 95% CI, 1.20 to 1.47). In multivariable analyses accounting for body composition, the association of age with frailty was attenuated (OR, 1.16; 95% CI, 1.00 to 1.34), and the association between sex and frailty was magnified (OR, 2.10 for women; 95% CI, 1.35 to 3.70), suggesting the possibilities that the age–frailty association might be explained, in part, by altered body composition with aging and that, after accounting for body composition, frailty was more common among women than men. The associations between body composition and frailty did not differ based on sex (all interaction terms P>0.05).
Table 3.
Multivariable correlates of frailty
| Model 1 (No Body Composition) OR (95% CI) | Model 2 (BMI) OR (95% CI) | Model 3 (BIS) OR (95% CI) | |
|---|---|---|---|
| Age, per 10 yr | 1.31 (1.14 to 1.50) | 1.32 (1.15 to 1.51) | 1.16 (1.00 to 1.34) |
| Women | 1.24 (0.86 to 1.78) | 1.21 (0.84 to 1.74) | 2.10 (1.25 to 3.70) |
| Race | |||
| White | Reference | Reference | Reference |
| Black | 0.98 (0.64 to 1.51) | 0.95 (0.61 to 1.46) | 0.86 (0.54 to 1.35) |
| Other | 1.03 (0.59 to 1.81) | 1.04 (0.59 to 1.83) | 1.29 (0.72 to 2.30) |
| Diabetes | 1.65 (1.13 to 2.40) | 1.58 (1.08 to 2.30) | 1.29 (0.87 to 1.91) |
| CHF | 1.30 (0.83 to 2.00) | 1.26 (0.80 to 1.98) | 1.18 (0.74 to 1.89) |
| CAD | 1.04 (0.55 to 1.97) | 1.03 (0.54 to 1.95) | 1.01 (0.52 to 1.93) |
| BMI, kg/m2 | — | 1.02 (0.99 to 1.05) | — |
| Fat mass, per 10 kg | — | — | 1.18 (1.02 to 1.37) |
| ICW, per kg | — | — | 0.80 (0.73 to 0.87) |
| ECW, per kg | — | — | 1.33 (1.20 to 1.47) |
| −2 Log likelihood | 725.3 | 723.1 | 688.5 |
CHF, congestive heart failure; CAD, coronary artery disease.
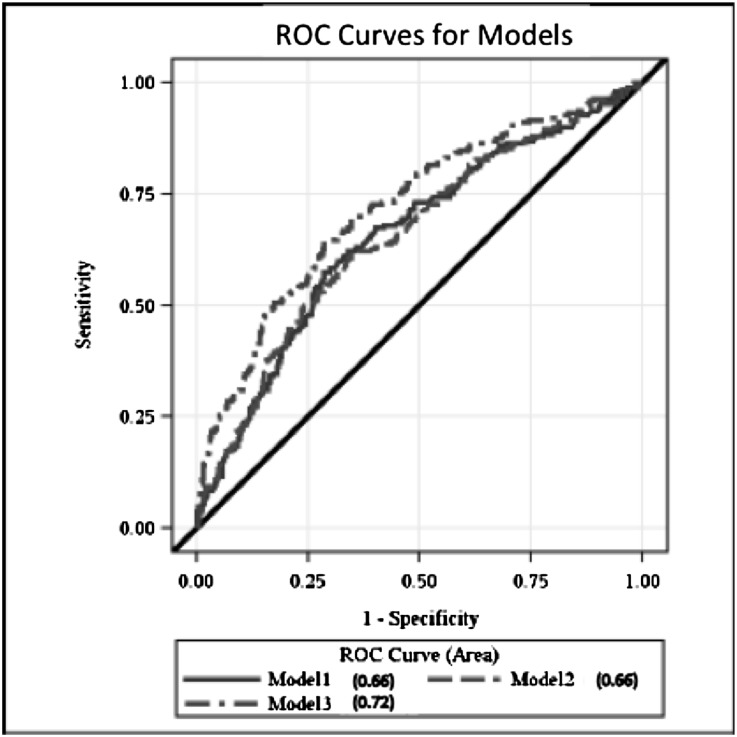
The concordance (c) statistic (area under the receiver operating characteristics [ROC] curve) for predicting frailty for the base model was 0.66 (95% CI, 0.61 to 0.70) (Figure 1, Table 4). Adding BMI to the model (as a continuous or categorical variable) did not effectively increase the c statistic. However, adding ICW, ECW, and body fat to the model significantly improved model discrimination (c statistic=0.72; 95% CI, 0.67 to 0.76; P<0.001 compared with model 1).
Figure 1.
Area under the ROC curves. Model 1 includes age, sex, race, diabetes, heart failure, and coronary artery disease. Model 2 includes variables in model 1 plus BMI. Model 3 includes variables in model 1 plus ICW, fat mass, and ECW estimated by BIS.
Table 4.
Area under the ROC curve (c statistic) for the frailty models
| AUC | 95% CI | P Valuea | |
|---|---|---|---|
| Model 1b | 0.66 | 0.61 to 0.70 | — |
| Model 2c | 0.66 | 0.61 to 0.71 | 0.71 |
| Model 3d | 0.72 | 0.67 to 0.76 | <0.001 |
AUC, area under the ROC curve.
Compared with model 1.
Adjusted for age, sex, race, and comorbidities.
Adjusted for the variables in model 1 plus BMI. The c statistic for a model with BMI in categories was identical.
Adjusted for the variables in model 1 plus body fat mass, ICW, and ECW from BIS.
When we examined the individual components of frailty (Table 5), we found that study participants were least likely to meet the slow walking speed criterion (26%) and most likely to meet the weak grip strength criterion (53%). Higher BMI was associated with slow walking and weak grip strength. There was also a potential association with weight loss, although it did not reach statistical significance (P=0.06). In contrast, measures of body composition using BIS were associated with multiple components of frailty when included simultaneously in a multiple predictor model. Higher ICW, a marker for muscle mass, was associated with lower odds of weight loss, weakness, low physical activity, and slow walking speed. Higher ECW was associated with higher odds of weight loss, weakness, low physical activity, and slow walking speed. Higher fat mass was associated with higher odds of weakness and slow walking speed. None of the body composition variables were significantly associated with exhaustion.
Table 5.
Association of body composition with the components of frailty
| Frailty Component | BMI (kg/m2) | ICW (kg) | Fat Mass (kg) | ECW (kg) | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Weight loss (34.1%) | 0.98 (0.95 to 1.00) | 0.06 | 0.87 (0.81 to 0.94) | <0.001 | 1.00 (0.99 to 1.02) | 0.62 | 1.16 (1.06 to 1.27) | 0.002 |
| Weakness (52.7%) | 1.03a (1.00 to 1.05) | 0.05 | 0.74 (0.68 to 0.81) | <0.001 | 1.03 (1.01 to 1.04) | <0.001 | 1.35 (1.21 to 1.50) | <0.001 |
| Exhaustion (36.2%) | 1.00 (0.98 to 1.02) | 0.91 | 1.00 (0.93 to 1.07) | 0.99 | 0.99 (0.97 to 1.00) | 0.07 | 1.05 (0.96 to 1.15) | 0.26 |
| Low activity (39.4) | 1.01(0.99 to 1.03) | 0.46 | 0.91 (0.85 to 0.98) | 0.01 | 1.01(1.00 to 1.03) | 0.10 | 1.12 (1.03 to 1.22) | 0.01 |
| Slow walking speed (25.7%) | 1.05 (1.02 to 1.08) | 0.001 | 0.77 (0.69 to 0.85) | <0.001 | 1.03 (1.01 to 1.05) | <0.001 | 1.48 (1.31 to 1.67) | <0.001 |
Cutpoints for meeting the weakness criterion differed according to BMI (Supplemental Table 1), which might obscure associations between BMI and grip strength.
Discussion
We found that 30% of this prevalent hemodialysis cohort met the classic definition of frailty based on slow gait speed, weak grip strength, low physical activity, exhaustion, and weight loss. Despite the weight loss criterion, BMI was not statistically significantly associated with frailty in this population. However, lower ICW, higher fat mass, and higher ECW were all associated with frailty.
The inverse association between ICW, a marker of muscle mass, and frailty that we observed is expected given that weakness and slowed performance are central features of the syndrome. Indeed, when we examined the individual components of the frailty syndrome, high ICW was associated most strongly with physical performance (grip strength and walking time).
A direct association between fat mass and frailty is also biologically plausible. Low physical activity is a risk factor for weight gain and obesity. Furthermore, muscle atrophy and low physical activity lead to a lower metabolic rate, further exacerbating the tendency for energy intake to exceed caloric needs among sedentary individuals. There are other possible reasons for higher fat mass to be associated with frailty as well. Adipose tissue can itself produce proinflammatory cytokines and adipokines, which can contribute to muscle wasting. Other hormonal disturbances related to adiposity may contribute to the development of frailty as well. Insulin resistance in obese individuals may promote muscle catabolism, because insulin is an important anabolic signal in muscle. Increased adiposity is also often associated with decreased growth hormone, IGF-1, and testosterone, all of which may contribute to muscle wasting and weakness.12 The strong association of adiposity with poor physical performance would support a possible role for these hormonal derangements. The directionally opposite associations between ICW and frailty and fat mass and frailty underscore the lack of association between BMI and frailty and highlight why measuring or estimating more specific metrics of body composition may be useful in epidemiologic analyses and clinical trials in chronic disease.
The association of higher fat mass with higher odds of frailty is difficult to reconcile with the better survival associated with higher fat mass in dialysis patients.13–15 It is possible that additional fat stores are protective against the catabolism and inflammation that have been associated with ESRD. When individuals are subjected to the stressors associated with ESRD, the protective effect of fat stores may outweigh the adverse effects of high fat mass on survival, including worse physical functioning and frailty. It is also possible that differences in the methods of estimating fat mass could account for some or all of the discrepancy between associations with frailty in our study and survival in other cohorts. Studies relating fat mass to survival have generally relied on BMI,13,14 triceps skin fold thickness,15 or other indirect estimates.13 However, BIS-derived estimates of fat mass correlate closely with fat mass measured by dual-energy x-ray absorptiometry,16 and bioelectrical impedance-based methods are superior to skin fold thickness.17
The association between high ECW and frailty may also be caused by a combination of multiple factors. First, high ECW was associated with weight loss, which could be a result of delayed or inadequate adjustment of dry weight resulting in ECW expansion and tissue loss in the setting of a stable or near stable target weight. Second, the association of ECW with the physical performance components of frailty suggests that overhydration could lead to worse physical function. A recent study of 24 patients receiving dialysis in Norway showed that overhydration assessed by BIS was associated with lower self-reported physical function.18 Although the difference in self-reported physical function among overhydrated and euvolemic patients did not reach statistical significance in that study and self-reported function is not analogous to metrics of physical performance (such as those metrics that we measured), their results are certainly consistent with our results. Possible explanations for this observation include limitations in physical activity and aerobic capacity in the setting of volume overload. Expansion of ECW could also be a more direct effect of frailty in that impaired cardiovascular or neuroendocrine reserve could lead to poor refilling of the vascular compartment and difficulty in removing fluid during dialysis.
Our findings also suggest that estimates of body composition may be more informative than body weight or BMI, particularly when we are interested in physical function or performance rather than nutritional status per se. It is well known that indices that rely solely on height and weight cannot determine the relative amounts of fat and muscle. This shortcoming is particularly important in the ESRD population (and other populations with chronic disease) because of the high likelihood that body composition is altered from what is typically observed in healthy populations. Excess energy intake in the setting of physical inactivity, low-grade inflammation, insulin resistance, and other changes in the hormonal milieu, all of which are common among patients with ESRD, may lead to the loss of muscle mass, even in the setting of excess adiposity or obesity.12 Furthermore, excess extracellular fluid volume among patients with ESRD can lead to a sort of falsely elevated BMI in the sense that the extra weight is not associated with muscle or fat. Nevertheless, it is a reasonable expectation that most patients on dialysis with higher BMI will have more fat than those patients with lower BMI, and therefore, BMI operates relatively well as a gross indicator of nutritional status. However, we have shown that, in the dialysis population, fat mass and muscle mass exhibit distinct associations with frailty. In other words, BMI is less powerful than estimated muscle and fat contributions for distinguishing robust from frail individuals.
The 30% prevalence of frailty in this cohort was more than 4-fold higher than the 7% prevalence in the cohort of community-dwelling elders in which this frailty score was developed, although our patients were considerably younger on average.2 Previous studies of frailty among patients with ESRD were undertaken in incident patients, relied on self-reported functioning (Table 1), and found a higher prevalence of frailty.6,7 It is likely that the differences in prevalence are related to differences in the population as well as important differences in measured rather than self-reported proxies for the physical performance components of the frailty definition. It is possible that patients report difficulty with physical function before their performance deteriorates enough to meet the frailty criterion by objective measures, leading to a higher estimate of the prevalence using self-reported function. In addition, there may be a survivor bias in our prevalent patient sample. Because frailty is associated with higher mortality,6 those patients who survive may be less frail. Indeed, a report from a recent single-center study of prevalent hemodialysis patients designated a similar percentage of individuals as frail (41.8%) using the same scoring system as used in our study.10
Our study has several strengths. We assembled a relatively large and diverse cohort of prevalent dialysis patients on dialysis from two regions of the country and measured physical performance and body composition under standardized conditions in close temporal proximity (usually on the same day). Estimation of body composition using BIS allowed us to separately examine the associations of muscle mass (ICW), tissue edema (extravascular water), and fat mass with frailty. Using trained personnel, we measured physical performance objectively rather than relying on self-reported physical functioning, which we and others have used in previous studies.6,7,19 Finally, comparing ROC curves, we showed that, using BIS-derived metrics of body composition, one could distinguish frail from nonfrail patients significantly better than with demographic characteristics with or without BMI.
However, some important limitations should be acknowledged. First, the association between frailty and body composition does not imply causality in either direction. For example, we could not determine whether large body size limited physical activity or whether limited physical activity led to weight gain. In addition, because weight and body composition were determined at study initiation and we had no prior measurements, we cannot determine whether frail patients had lost muscle mass during earlier stages of CKD or earlier in their dialysis treatment. The cross-sectional nature of the study further limits causal inference. A longitudinal study might be more informative, because one could compare the timing of various changes in body composition with the development of frailty. Second, we did not examine the extent to which associations between body composition and frailty might be mediated by differences in hemoglobin or inflammation, and we did not assess other ways of estimating body composition, such as waist-to-hip ratio. Third, although multifrequency BIS may offer advantages over single-frequency bioelectrical impedance analysis, which are principally related to the ability to better distinguish ICW and ECW, some error in estimating body composition and fluid distribution clearly remains.20–22 Furthermore, the hydration of fat mass was assumed to be 0.73%, which could lead to some overestimation of fat mass in individuals with an expanded ECW.22
In conclusion, our data show the high prevalence of frailty in the hemodialysis population and in particular, the poor performance of BMI as an indicator of frailty among patients on hemodialysis. We highlight the associations among variations in body composition and the odds of frailty. Whereas available evidence suggests that higher adiposity and lean body mass are both associated with better survival in dialysis patients,15 our data suggest that these components of body composition exert opposite effects on physical functioning and frailty. Longitudinal data are needed to determine whether changes in body composition are more strongly associated with physical performance and frailty than cross-sectional measures and whether efforts to preserve or correct unfavorable alterations in body composition might reduce the likelihood of frailty and its attendant complications.
Concise Methods
Study Design and Participants
ACTIVE/ADIPOSE enrolled prevalent adult patients receiving maintenance hemodialysis in seven centers in the San Francisco Bay Area and seven centers in the Atlanta, Georgia metropolitan area during 2009–2011.23 Eligible participants were over 18 years of age, English- or Spanish-speaking, on dialysis for at least 3 months, and able to provide informed consent. Patients provided written informed consent for study participation, and the study was approved by the University of California at San Francisco Committee on Human Research and the Emory University Institutional Review Board.
Study coordinators conducted an interview with participants, measured physical performance (grip strength and walk speed) and body composition (BIS), and abstracted recent clinical and laboratory data from medical records. Patients’ data were also linked to data from the ESRD Medical Evidence Report (Centers for Medicare & Medicaid Services Form 2728) available in the US Renal Data System.
Body Composition
Study coordinators measured height using a stadiometer and recorded postdialysis weight from the last three dialysis sessions in kilograms. BMI was calculated as the average postdialysis weight divided by height in meters squared.
Whole-body BIS was performed before a dialysis session using a device that scans 256 frequencies between 4 and 1000 kHz (SFB7; ImpediMed, San Diego, CA). Patients were placed in a supine position at least 10 minutes before measurement. Electrodes were placed in a tetrapolar configuration using the hand and foot on the side opposite the dialysis access with proximal and distal electrodes 5 cm apart. Ten consecutive measures were performed within a 1-minute period. Total body water was estimated using the resistance extrapolated to infinite frequency, and total body fat mass was calculated by subtracting total body water divided by 0.73 from body weight.22,24 ECW was estimated from resistance extrapolated to zero frequency.
Frailty
We ascertained frailty based on a validated scoring system that incorporates five domains: shrinking, weakness, exhaustion, low physical activity, and slow walking speed.2,25 Each domain was given a dichotomous score of zero or one based on the following five criteria.
Weight loss, referred to in the original description of frailty as shrinking, was defined as unintentional weight loss of ≥10 lb (4.5 kg) in the last 1 year. Patients were asked the following question: “In the past 12 months, have you lost more than 10 lb unintentionally (i.e., not due to dieting or exercise)?”
Weakness was based on measurement of hand grip strength by a handheld dynamometer (Jamar; Lafayette Instrument, Lafayette, IN) immediately before a dialysis session. Patients performed three tests of maximum grip strength with each hand, and the mean of the strongest hand was used to determine frailty. The strength measurement was adjusted by sex and BMI (Supplemental Table 1).
Exhaustion was measured by responses to questions about endurance and energy from the Center for Epidemiologic Studies depression scale.26
Low physical activity was ascertained from the short version of the Minnesota Leisure Time Physical Activity Questionnaire, which asks about the frequency and duration of various activities over a 2-week time period.27
Slow walking speed was scored based on the time to walk 15 ft. Patients were asked to walk 15 ft at their usual pace two times immediately before a dialysis session. Times were measured to the nearest tenth of 1 second, and the faster of the two walking trials was used (Supplemental Table 1).
A score of three or more points was considered frail.
Statistical Analyses
Patient characteristics were described using mean (SD) or median (interquartile range) and proportions for categorical variables. Characteristics of patients included in the analysis were compared with characteristics of those patients with missing frailty or body composition data using t, Wilcoxon ranked sum, or chi-squared tests as appropriate.
We performed multiple predictor logistic regression analyses to determine factors associated with frailty. We examined a base model that included age, sex, race, diabetes mellitus, congestive heart failure, and coronary artery disease (model 1). We then added BMI (model 2) or body composition variables estimated by BIS, including body fat, ICW, and ECW (model 3). BMI was implemented as a predictor using two different specifications as (1) a continuous variable and (2) categories (<20, 20 to <25, 25 to <30, and ≥30 kg/m2) to determine whether there was a nonlinear (and possibly nonmonotonic) relationship between BMI and odds of frailty. We assessed the association of body composition with frailty through logistic regression modeling and tested whether body composition estimated by BIS was more closely related to frailty than BMI by comparing the associated c statistics corresponding to areas under the ROC curves of the models. We tested for interactions between women and frailty in adjusted models. We also used logistic regression to assess the association of body composition with the individual components of the frailty score and adjusted for the same covariates as in the frailty models.
We used SAS version 9.2 (SAS Institute, Cary, NC) for all analyses, and two-tailed nominal P values <0.05 were considered to indicate statistical significance.
Disclosures
None of the authors has any financial conflict of interest with the information presented in this manuscript. K.L.J. serves on the Amgen National Nephrology Advisory Board and is the Deputy Editor of CJASN. G.M.C. serves on the Board of Directors of Satellite Healthcare, Inc. and the Scientific Advisory Board of DaVita Clinical Research.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants N01-DK-0005 (to K.L.J.) and N01-DK-2450 (to G.M.C.).
The interpretation and reporting of the data presented here are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013040431/-/DCSupplemental.
References
- 1.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen GA, Lindholm B, Massy Z, Mitch W, Pineda E, Stenvinkel P, Treviño-Becerra A, Wanner C: A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 73: 391–398, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research Group : Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56: M146–M156, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Hamerman D: Toward an understanding of frailty. Ann Intern Med 130: 945–950, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Kim JC, Kalantar-Zadeh K, Kopple JD: Frailty and protein-energy wasting in elderly patients with end stage kidney disease. J Am Soc Nephrol 24: 337–351, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Onen NF, Agbebi A, Shacham E, Stamm KE, Onen AR, Overton ET: Frailty among HIV-infected persons in an urban outpatient care setting. J Infect 59: 346–352, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Johansen KL, Chertow GM, Jin C, Kutner NG: Significance of frailty among dialysis patients. J Am Soc Nephrol 18: 2960–2967, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL: Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med 172: 1071–1077, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Painter P, Kuskowski M: A closer look at frailty in ESRD: Getting the measure right. Hemodial Int 17: 41–49, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Wilhelm-Leen ER, Hall YN, K Tamura M, Chertow GM: Frailty and chronic kidney disease: The third national health and nutrition evaluation survey. Am J Med 122: 664–671, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McAdams-DeMarco MA, Law A, Salter ML, Boyarsky B, Gimenez L, Jaar BG, Walston JD, Segev DL: Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc 61: 896–901, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delgado C, Doyle JW, Johansen KL: Association of frailty with body composition among patients on hemodialysis. J Ren Nutr 23: 356–362, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L: Sarcopenic obesity: Definition, cause and consequences. Curr Opin Clin Nutr Metab Care 11: 693–700, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansen KL, Young B, Kaysen GA, Chertow GM: Association of body size with outcomes among patients beginning dialysis. Am J Clin Nutr 80: 324–332, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB: Survival advantages of obesity in dialysis patients. Am J Clin Nutr 81: 543–554, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Huang CX, Tighiouart H, Beddhu S, Cheung AK, Dwyer JT, Eknoyan G, Beck GJ, Levey AS, Sarnak MJ: Both low muscle mass and low fat are associated with higher all-cause mortality in hemodialysis patients. Kidney Int 77: 624–629, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molfino A, Don BR, Kaysen GA: Comparison of bioimpedance and dual-energy x-ray absorptiometry for measurement of fat mass in hemodialysis patients. Nephron Clin Pract 122: 127–133, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bross R, Chandramohan G, Kovesdy CP, Oreopoulos A, Noori N, Golden S, Benner D, Kopple JD, Kalantar-Zadeh K: Comparing body composition assessment tests in long-term hemodialysis patients. Am J Kidney Dis 55: 885–896, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lægreid IK, Bye A, Aasarød K, Jordhøy M: Nutritional problems, overhydration and the association with quality of life in elderly dialysis patients. Int Urol Nephrol 44: 1885–1892, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, Masaki K, Murray A, Newman AB, Women’s Health Initiative : Frailty: Emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc 53: 1321–1330, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Dou Y, Zhu F, Kotanko P: Assessment of extracellular fluid volume and fluid status in hemodialysis patients: Current status and technical advances. Semin Dial 25: 377–387, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Dou Y, Liu L, Cheng X, Cao L, Zuo L: Comparison of bioimpedance methods for estimating total body water and intracellular water changes during hemodialysis. Nephrol Dial Transplant 26: 3319–3324, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, Heitmann BL, Kent-Smith L, Melchior JC, Pirlich M, Scharfetter H, Schols AMWJ, Pichard C, Composition of the ESPEN Working Group : Bioelectrical impedance analysis—part I: Review of principles and methods. Clin Nutr 23: 1226–1243, 2004 [DOI] [PubMed] [Google Scholar]
- 23.US Renal Data System : USRDS 2011 Annual Data Report, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2011 [Google Scholar]
- 24.Moissl UM, Wabel P, Chamney PW, Bosaeus I, Levin NW, Bosy-Westphal A, Korth O, Müller MJ, Ellegård L, Malmros V, Kaitwatcharachai C, Kuhlmann MK, Zhu F, Fuller NJ: Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas 27: 921–933, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J, Fried LP: Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 210: 901–908, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Orme JG, Reis J, Herz EJ: Factorial and discriminant validity of the Center for Epidemiological Studies Depression (CES-D) scale. J Clin Psychol 42: 28–33, 1986 [DOI] [PubMed] [Google Scholar]
- 27.Taylor HL, Jacobs DR, Jr., Schucker B, Knudsen J, Leon AS, Debacker G: A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 31: 741–755, 1978 [DOI] [PubMed] [Google Scholar]
- 28.Roshanravan B, Khatri M, Robinson-Cohen C, Levin G, Patel KV, de Boer IH, Seliger S, Ruzinski J, Himmelfarb J, Kestenbaum B: A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis 60: 912–921, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.